Abstract
Kettin is a giant muscle protein originally identified in insect flight muscle Z-discs. Here, we determined the entire nucleotide sequence of Drosophila melanogaster kettin, deduced the amino acid sequence of its protein product (540 kD) along with that of the Caenorhabditis elegans counterpart, and found that the overall primary structure of Kettin has been highly conserved in evolution. The main body of Drosophila Kettin consists of 35 immunoglobulin C2 domains separated by spacers. The central two thirds of spacers are constant in length and share in common two conserved motifs, putative actin binding sites. Neither fibronectin type III nor kinase domains were found. Kettin is present at the Z-disc in several muscle types. Genetic analysis showed that kettin is essential for the formation and maintenance of normal sarcomere structure of muscles and muscle tendons. Accordingly, embryos lacking kettin activity cannot hatch nor can adult flies heterozygous for the kettin mutation fly.
Keywords: immunoglobulin domain, titin family, Caenorhabditis elegans, Z-disc, flight activity
Introduction
The Z-discs of striated muscles serve as linkers of thin (actin) filaments from adjacent sarcomeres, and may play fundamental roles in the transmission of forces (Vigoreaux 1994). In Drosophila larval somatic muscles, Z-discs appear late in embryogenesis (Bernstein et al. 1993); they are perforated and thick (myosin) filaments frequently penetrate them with muscular contraction. In contrast to Z-discs in larval muscles, the counterparts in Drosophila indirect flight muscles (IFMs) are regular in shape and show greater similarity to the Z-discs of vertebrate skeletal muscles, although there is an apparent difference in the lattice structure (Crossley 1978; Bernstein et al. 1993; Vigoreaux 1994).
Kettin is one of the Z-disc proteins and was initially identified in muscles of giant waterbug, Lethocerus (Lakey et al. 1990, Lakey et al. 1993). Drosophila Kettin was identified in a cross reaction with antibody raised against Lethocerus Kettin (Lakey et al. 1993). IFMs in Drosophila exclusively include a 500-kD major isoform of Kettin; a minor isoform of Drosophila Kettin is 700 kD in molecular mass (Lakey et al. 1993). A partial amino acid sequence of Drosophila Kettin (∼10% of the total) has shown that Kettin possesses repeating units including immunoglobulin C2 (Ig) domains separated by linker sequences (Lakey et al. 1993). Biochemical analysis indicated that an Ig domain flanked by two linkers could bind to actin and α-actinin but not to myosin (Lakey et al. 1993). Furthermore, plots of the binding data gave a maximum binding of 0.036 mol of Kettin per 1 mol of actin monomer or 1 mol of Kettin per 28 mol of actin monomer (Straaten et al. 1999), leading to the speculation that there are ∼30 modules consisting of Ig domain plus a linker sequence and each capable of binding to the actin monomer.
Immunoelectron microscopic observations of IFM showed that Kettin is oriented with the NH2 terminus in the Z-disc and the COOH terminus outside (Straaten et al. 1999), suggesting possible head-to-head interactions of Kettin molecules at the center of Z-discs. Anti–Kettin antibody signals were restricted to the vicinity of the Z-disc and the length of individual 500-kD Kettin molecules was less than one tenth of the sarcomere length (Straaten et al. 1999), suggesting that it is unlikely that Kettin serves as a molecular ruler to determine thick filament length as proposed for vertebrate titin/connectin (Trinick 1994). In vertebrates, titin/connectin molecules are anchored at the Z-disc and M-line through their NH2 and COOH termini, respectively (Labeit and Kolmerer 1995).
In developing Drosophila muscles, thin filaments appear to grow through the addition of actin molecules to filaments already incorporated into Z-discs (Reedy and Beall 1993). Kettin and tropomyosin compete for actin, and Kettin appears to prevent tropomyosin from binding to actin filaments in the vicinity of Z-discs in IFM (Straaten et al. 1999). Kettin is susceptible to calpain, a calcium-activated protease (Lakey et al. 1993). Myofibrils treated with calpain lose dense materials of Z-discs and release α-actinin from myofibrils, possibly suggesting that Kettin is required for α-actinin localization in Z-discs (Lakey et al. 1993). Thus, as proposed by Straaten et al. 1999, Kettin may reinforce the anchorage of actin filaments through associating with the barbed end of growing actin filaments and promoting the antiparallel assembly of actin filaments, which would be followed by cross-linking with α-actinin and elongation of filaments by the addition of actin monomers. Muscle proteins similar in property to Drosophila Kettin have been found in the crayfish and silkworm (Maki et al. 1995; Suzuki et al. 1999).
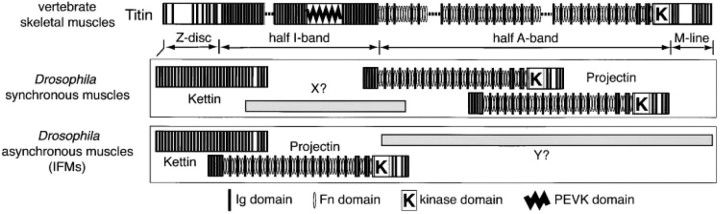
Ig domain repeats similar to those of Kettin are present in other giant muscle proteins such as titin/connectin in vertebrate striated muscles and, accordingly, Kettin may belong to the titin family (for review see Benian et al. 1999). Projectin is the first titin family member identified in Drosophila and most closely related in sequence to Twitchin, a nematode muscle protein (for review see Benian et al. 1999). A mutation in the Twitchin gene (unc-22) causes twitching; that is, in mutant worms, rapid, finely localized movement is frequently observed throughout the body. Muscle structure is disrupted or abnormal in appearance in some mutant worms, particularly those showing abnormal movement (Waterston et al. 1980). Twitchin may be involved in regulating myosin activity (for review see Benian et al. 1999). Projectin appears involved in the connection of myofilaments (Benian et al. 1999). In IFM, Projectin is detected in the I-band, whereas, in other muscles, it is situated solely in the A-band, suggesting that Projectin may have different functions depending on muscle types (Vigoreaux et al. 1991). As with titin, Projectin and Twitchin possess fibronectin type III and protein kinase domains (for reviews see Benian et al. 1999; Kolmerer et al. 1999). Super-repeats consisting of fibronectin type III and Ig domains may be important for the putative ruler function of titin, whereas the kinase domain may be involved in regulatory processes (for reviews see Gautel et al. 1999; Kolmerer et al. 1999).
To further clarify the structure and possible functions of Kettin, we determined the entire amino acid sequences of Kettin of Drosophila melanogaster and its counterpart in Caenorhabditis elegans in the present study. Both proteins were found to be very similar in overall structure and largely composed of Ig domain repeats separated by spacer sequences. Neither fibronectin type III nor kinase domains were detected, indicating that Kettin is a muscle protein distinct in function and structure from other titin family members. In Drosophila embryos lacking kettin activity, normal sarcomere structures failed to be formed and, consequently, hatching does not occur. kettin is also required for the maintenance of normal sarcomere structures, and adult flies heterozygous for kettin cannot fly.
Materials and Methods
Fly Strains and Genetics
Canton-S (CS; wild type [WT]) and v36f;ry506were our laboratory stocks. Gene trap experiments were carried out as follows. A hedgehog fragment (nucleotide position 11,206–11,353; Mohler and Vani 1992), which includes a putative lariat site, splice acceptor, and a 5′ portion of the second exon, was connected in-frame to the 5′ end of the Escherichia coli lacZ coding sequence lacking the initiation codon (a BamHI fragment of pMC1871; Amersham Pharmacia Biotech). The resultant construct was inserted into a P element vector with a vermilion marker and pBluescript (Stratagene; Fridell and Searles 1991) to generate IT1 (see Fig. 1 A). Germline transformation was carried out as described previously (Spradling and Rubin 1982). A transformant line (132) exhibiting muscle-specific lacZ expression was selected and analyzed in the present study. When stocked, 132 and derivatives were balanced with TM6. As described in the text, 132 is a hypomorphic kettin mutant. Using a P[ry; Δ2-3] chromosome (Robertson et al. 1988) the 132 P element was imprecisely excised and two additional kettin mutants, ket5 and ket14, were obtained; they cannot complement with 132. Note that the kettin locus is on the third chromosome and all kettin mutant chromosomes examined here are marked with ry506. Dp(3;Y)B21/+;ket14 /TM6 flies used in the flight assay were made by crossing ket14/TM3 Sb flies with T(Y;3)B21, B, y +/TM6;C(1)RM, y/C(1;Y)1, y flies, obtained from the Bloomington stock center.
Figure 1.
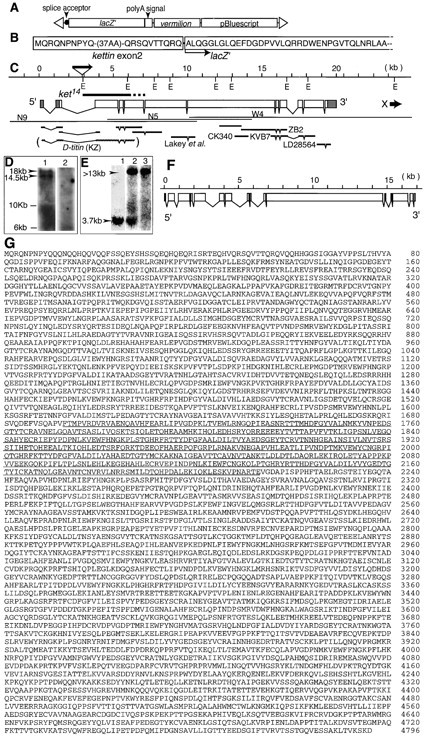
Structures of Drosophila and C. elegans kettin loci and the deduced amino acid sequence of Drosophila Kettin. (A) The structure of IT1, a P element vector used for gene trapping in Drosophila is schematically shown. Filled circle indicates a hedgehog fragment (nucleotide position 11,206–11,341; Mohler and Vani 1992), including a putative lariat site and splice acceptor within intron 1. lacZ′ is E. coli lacZ in which the initiation codon is replaced with an in-frame 5′-terminal sequence of hedgehog exon 2 (nucleotide position 11,342–11,353; Mohler and Vani 1992). A 255-bp fragment of hsp70 (nucleotide position 1,918–2,172; Ingolia et al. 1980) was used as a poly(A)+ signal–containing fragment. Triangles show P element terminal sequences. (B) A part of the deduced amino acid sequence of the polypeptide encoded by the kettin-lacZ′ chimeric RNA found in 132 mutants (E). A cDNA fragment including the kettin-lacZ′ junction was recovered as an RT-PCR product of 132/TM3 larval RNA. (C) Drosophila kettin gene structure. EcoRI sites on the genomic DNA are shown by Es. The triangle associated with a horizontal arrow shows the location of 132 P insertion and the direction of lacZ′ transcription. A thick bar labeled ket14 indicates a kettin locus region deleted in ket14. Open boxes show the sizes and locations of coding exons, whereas stippled boxes, noncoding exons. A thick horizontal arrow labeled X shows a putative gene adjacent to kettin. Thin lines labeled N9, N5, and W4 show the sizes and locations of genomic DNA clones. ZB2 and KVB7 are cDNA clones isolated from cDNA libraries, whereas CK340 and LD28564 are EST clones obtained from Berkeley Drosophila Genome Project. The size and location of cDNA isolated by Lakey et al. 1993 is also shown. Unlabeled cDNA clones are those isolated using RT-PCR. The cDNA enclosed with parentheses and labeled D-titin (KZ) is the size and location of fragment KZ, which was reported to represent a part of a hypothetical gene, D-titin (Machado et al. 1998). (D) High resolution pictures of Northern blots showing the presence of two high molecular weight kettin RNA species (lane 1) and the absence of putative D-titin RNA (lane 2). The molecular sizes of two kettin RNA were determined using RNA molecular markers and λ/HindIII DNA markers. Arrowheads indicate kettin RNA, 14.5 and 18 kb in length. To detect D-titin signals in lane 2, fragment NB2 was used as a probe. (E) Northern blotting for the detection of wild-type and mutant kettin RNA in 132. (lane 1) 132/TM3 larval RNA hybridized with a lacZ cDNA probe. (lane 2) 132/TM3 larval RNA and lane 3, v;ry larval RNA hybridized with a 5-kb kettin genomic DNA probe. The results shown indicate that RNA bands 3.7-kb long and more than 13-kb long, respectively, correspond to a kettin-lacZ composite RNA and authentic kettin RNA. (F) A putative structure of the C. elegans kettin gene. Only the sizes and locations of coding exons are shown. Using published genomic DNA sequences at or near the putative C. elegans kettin locus (R05D8; The C. elegans Sequencing Consortium, 1998), the entire kettin coding sequence was predicted. We assumed that true exon combinations should give the maximum amino acid sequence homology to the Drosophila counterpart. The accession number of the resultant C. elegans kettin is AB026846. (G) Deduced amino acid sequence of Drosophila Kettin, which is 4,796 amino acids in length. A partial amino acid sequence previously reported (Lakey et al. 1993) was underlined. The sequence data of Drosophila kettin are available from GenBank/EMBL/DDBJ under accession number AB026845.
Molecular Cloning, Reverse Transcriptase (RT)–PCR, and Northern Blotting
Drosophila genomic DNA fragments at the kettin locus were isolated by plasmid rescue and subsequent screening of a Drosophila cloned DNA library. cDNA clones, ZB2 and KVB7, were obtained from a randomly primed ZAP cDNA library (8–21-h embryo) and Kauvar's cDNA library (12–24-h embryo; Poole et al. 1985), respectively. CK340 and LD28564 are expressed sequence tag (EST) clones from the Berkeley Drosophila Genome Project EST project. RNA was prepared from embryos or larvae using an mRNA purification kit (Amersham Pharmacia Biotech). The nucleotide sequence of genomic DNA was initially determined, and then splicing sites were determined using the nucleotide sequences of relevant cDNAs, which include EST and RT-PCR clones. NB2, identical in sequence to D-titin NB (Machado et al. 1998), was prepared by PCR using the total Drosophila genomic DNA and suitable primers. Northern blotting was carried out essentially as described by Sambrook et al. 1989. As kettin-specific probes, ZB2 or a 5′ genomic DNA fragment (a 5-kb EcoRI fragment), which includes exon 1 and 2, were used (see Fig. 1 C). The coding sequence of C. elegans kettin was determined based on the genomic DNA sequence recently published (The C. elegans Sequencing Consortium, 1998). We presumed that true combinations of splicing acceptor and donor sequences of C. elegans kettin give the maximum amino acid sequence homology to Drosophila Kettin.
Antibody Staining and In Situ Hybridization
Antibody staining and in situ hybridization were carried out as described by Shishido et al. 1997. When larvae or adult muscle tissues were subjected to antibody staining, larvae and adults were anesthetized, dissected, and fixed with 4% paraformaldehyde before antibody treatment. Primary antibodies used were as follows: rat monoclonal anti–Kettin antibody (1:20; MAC 155; Lakey et al. 1993), rabbit anti–MHC (myosin heavy chain) antibody (1:500; Kiehart and Feghali 1986), and rabbit anti–LacZ antibody (1:2,000; anti–β-Gal; Cappel). MAC 155 epitopes should be located in the central region of Kettin (see the underlined sequence in Fig. 1 G). FITC-phalloidin and SYTOX (Molecular Probes Inc.) were used to detect actin and DNA, respectively. Both sense and antisense RNA probes for in situ hybridization were prepared using ZB2 (kettin) and NB2 (D-titin) DNA as templates; lacZ RNA probes were similarly prepared.
Flight Assay
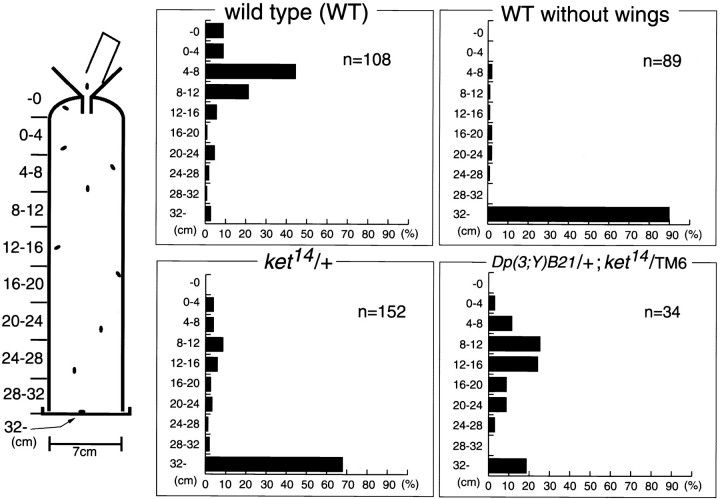
Flight ability was measured essentially as described by Koana and Hotta 1978. A plastic cylinder with a diameter of 7 cm and a height of 32 cm was used as a flight tester (see Fig. 6). Flies, sorted out soon after eclosion and kept for 2–3 d at 22°C, were subjected to the flight assay.
Figure 6.
Requirements of kettin for acquiring flying capability. Results of flight test of wild-type (Canton S) flies with or without wings, and ket14/+ flies with or without Dp(3;Y)B21, which includes the kettin locus. An apparatus used for flight test is schematically shown in the left margin. Flies were dropped through a funnel into a column 32 cm in height and coated with liquid paraffin. Virtually all flies capable of flying are expected to stick to the wall. Note that ∼70% of ket14/+ flies dropped to the bottom while 80% of those with Dp(3;Y)B21 stuck to the wall, indicating kettin to be essential for acquiring flying capability.
Electron Microscopy
Somatic embryonic/larval muscles and IFMs were examined by electron microscopy. Late stage 17 embryos were collected during 20–24 h after egg laying (AEL) at 25°C. First instar wild-type larvae were collected during 24–48 h AEL. ket14 mutant counterparts, collected during the same period, were incapable of hatching but capable of moving inside the egg shell. Second instar larvae homozygous for 132, lacking balancer phenotypes (Tb or Act-GFP), were collected during 48–72 h AEL. IFMs (adult thoraxes) were prepared 7–10 d after eclosion at 22°C. Pharate adult IFMs were collected from pupae that had developed red eyes. Larvae and thoraxes were dissected, fixed in a mixture of 2.5% glutaraldehyde, 2% formaldehyde, and 0.1 M sodium cacodylate, pH 7.4, for 2 h at room temperature, postfixed with 1% osmium tetroxide in the same buffer for 2 h on ice, stained en bloc with 2% uranyl acetate overnight, and dehydrated in a graded ethanol series. After ethanol was replaced with methyl methacrylate, samples were embedded in Rigolac mixture (Kushida 1960). Thin sections were stained with uranyl acetate and lead citrate and subjected to electron microscopic observation.
Results
Isolation and Entire-Nucleotide-Sequence Determination of Drosophila kettin Encoding a Giant Muscle Protein
A trap line of kettin (132) was isolated during gene-trap screening of lethal muscle/nervous system genes in Drosophila. A hedgehog fragment containing a putative lariat signal, 3′ splice site and 5′ end of exon 2 was connected to the 5′ end of the lacZ open reading frame (ORF) associated with a polyadenylation signal (Fig. 1 A), and the resultant construct was introduced into Drosophila by germline transformation. LacZ protein expression occurs only when lacZ, inserted in an intron, is in-frame with an upstream exon. In general, a gene-trap fly line is a hypomorphic or null mutant of the target gene in which lacZ is inserted.
In embryos, 132-LacZ was strongly expressed solely in muscle cells and/or precursors (see Fig. 3 I), suggesting that the gene trapped in 132 is a muscle gene. In situ hybridization to polytene chromosome indicated 132 insertion to occur in or near 62C on the left arm of chromosome III (data not shown), where two muscle-related genes, kettin and D-titin, have been reported to be mapped (Machado et al. 1998; Straaten et al. 1999). Genomic DNA fragments flanking 132-P were isolated by plasmid rescue, followed by screening of a Drosophila cloned library. Also isolated were two relevant cDNA clones (ZB2 and KVB7) using as probes genomic DNA fragments showing positive in situ hybridization signals. Nucleotide sequence analysis showed that ZB2 and KVB7 together represented only a portion of the target gene (132 gene; Fig. 1 C) and, thus, the structure of the remaining portion of the 132 gene was first predicted based on the nucleotide sequence of a 20-kb-long genomic DNA region, and then confirmed by those of RT-PCR and EST clones spanning putative splicing junctions (Fig. 1 C). The cDNA information previously published by others (Lakey et al. 1993) was also used.
Figure 3.
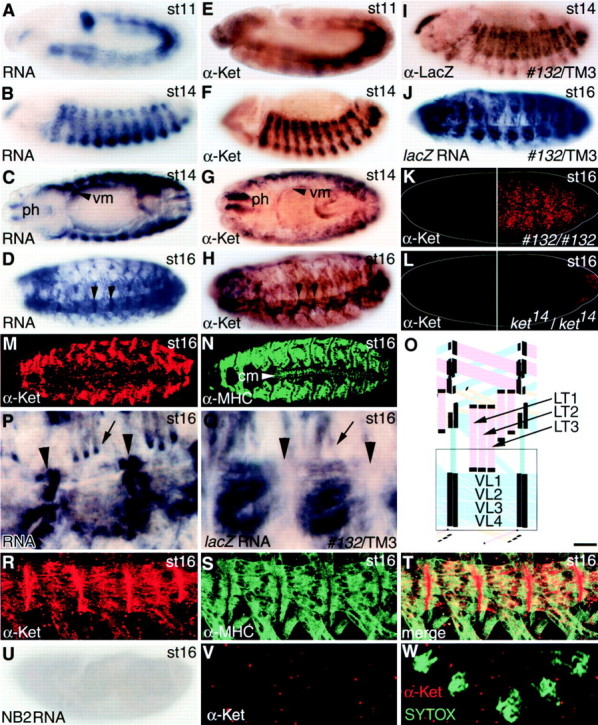
kettin RNA and protein expression in embryos. (A–D) kettin RNA expression occurred in muscle cells or precursors from late stage 11 onwards. As a probe for in situ hybridization, a fragment ZB2 (see Fig. 1 C) was used. At stage 14, not only somatic muscles, but also pharyngeal (ph) and visceral (vm) muscle cells produce kettin RNA. Arrowheads indicate the localization of kettin RNA at muscle attachment sites (see P for enlarged images). (E–H) Occurrence of Kettin protein expression in embryonic muscle cells or precursors. Arrowheads in H show the accumulation of Kettin at muscle attachment sites. I and J, respectively, show lacZ protein and RNA expression in muscle cells heterozygous for 132 insertion. (K) Kettin protein expression in embryos homozygous for 132, a hypomorph of kettin. Kettin protein signals in muscles were detected only after signal amplification (right half). (L) Kettin expression in embryos homozygous for ket14, a strong loss-of-function or null allele of kettin. No or little Kettin signals could be detected even after signal amplification (right half). (M and N) Confocal images of wild-type embryos stained for Kettin (M) and myosin heavy chain (MHC) (N). Kettin signals in cardiac muscle cells (cm) are marginal, if any. (O) Embryonic muscles are schematically shown (modified from Bate 1993). Black bars indicate localization of Kettin signals at muscle attachment sites. The open box may correspond to regions shown in P and Q. LT1-3, lateral transverse 1-3; VL1-4, ventral longitudinal 1-4. (P) kettin RNA localization at muscle attachment sites at stage 16. Arrowheads and arrow, respectively, show kettin RNA signals at indirect and direct muscle attachment sites. (Q) lacZ RNA expression in stage 16 embryos heterozygous for 132 insertion. Note that there is no or little accumulation of lacZ RNA at muscle attachment sites labeled with arrowheads and a arrow. (R–T) Accumulation of Kettin along muscle attachment sites, visualized by confocal microscopy. R, Kettin; S, MHC; and T, a merged picture of R and S. (U) The absence of D-titin RNA expression stage in 16 embryos. Whole-mount in situ hybridization was carried out using NB2 (a D-titin specific fragment) as a probe. In contrast to strong kettin expression (see D), no or little D-titin signals were detected. (V and W) The absence of chromosomal Kettin signals in early Drosophila embryos. SYTOX was used for the detection of DNA. Kettin and SYTOX signals are colored in red and green, respectively. No or little overlapping between Kettin and SYTOX signals was detected, indicating that Kettin is not chromosomal protein. All embryos are oriented with anterior to the left. For embryos shown in lateral view, dorsal is up. Stages are as described (Campos-Ortega and Hartenstein 1985). Bar: (A–N and U) 45 μm; (P and Q) 10 μm; (R–T) 18 μm; (V and W) 7 μm.
The presumed RNA start of the 132 gene is 5′ TTCAGTC and not associated with a TATA box (for a consensus RNA start, see Hultmark et al. 1986). Although no cDNA with poly(A)+ RNA has been isolated to date, two putative polyadenylation signals, AATAAAs, were found ∼400 bp downstream of the predicted termination codon, which is situated in the region corresponding to LD28564 cDNA (Fig. 1 C). There is no apparent splicing acceptor signal between the predicted termination codon and polyadenylation signals. In situ hybridization disclosed the presence of a second transcriptional unit unrelated in sequence to kettin in the vicinity of the predicted 3′ end (Fig. 1 C).
As shown in Fig. 1 C, the 132 gene contains 12 exons with predicted cDNA size of 15 kb. Northern blotting showed that there are two 132-related RNAs in embryos, major 14.5 kb long and minor 18 kb long (Fig. 1 D, lane 1). Thus, the former may correspond to the transcriptional unit analyzed here. The predicted 132 protein is composed of 4,796 amino acids (Fig. 1 G) with molecular weight of ∼5.4 × 105. Since a partial amino acid sequence of Drosophila Kettin previously determined (Lakey et al. 1993) perfectly matches a central region of the deduced amino acid sequence of the 132 protein (see underlines in Fig. 1 G) and the size of the 132 protein is very close to the major 500-kD component of Drosophila Kettin determined by SDS-PAGE (Lakey et al. 1993), we conclude that the 132 gene encodes the major Kettin isoform. Hereafter, we refer to the 132 gene as kettin. Since the minor 18-kb RNA has a capacity to encode a 660-kD protein at maximum, it may encode the minor Kettin isoform found in Drosophila by SDS-PAGE (Lakey et al. 1993), although we do not know its entire molecular structure yet.
Production of kettin-lacZ Fusion Transcripts in 132
RNA was extracted from 132/TM3,Sb,ry larvae and subjected to Northern hybridization. A 5′ genomic DNA kettin fragment including exons 1 and 2 and a 3-kb lacZ fragment were used as probes. As shown in Fig. 1 E, the 132/TM3,Sb,ry larvae contained a 3.7-kb component that hybridized with both kettin and lacZ probes and a component of >13 kb in length and detectable solely with the kettin probe; the latter appears to correspond to a mixture of 14.5- and 18-kb wild-type kettin RNAs (Fig. 1 D). Nucleotide sequence analysis of RT-PCR products indicated that the 3.7-kb component is a composite of a 0.5-kb exon I/II sequence of kettin and the 3.2-kb lacZ ORF (Fig. 1 B), confirming the insertion of a P-lacZ to occur in the second intron of kettin (Fig. 1 C).
Kettin as a Protein Consisting of 35 Repeats with Ig Domain Sequences
A previous partial sequence analysis of Kettin (Lakey et al. 1993) showed that repeats of a 130–amino acid-long sequence, made up of 95 amino acids homologous to Ig domain and a less conserved linker sequence, are included in Kettin. In the following, linker is referred to as spacer since the region appears necessary not only for linking Ig domains, but also possibly for exactly spacing them to fit the size of bound actin molecules (see Discussion).
As shown in Fig. 2A and Fig. B, 35 repeating units occupy the entire region of Drosophila Kettin, and each includes an Ig domain and a spacer sequence. Except for the NH2-terminal region, the protein may be divided into four regions according to spacer length and sequence. Region 1 contains four Ig domains (Ig1-4) and spacers (S1-4) of variable length. Ig domains (Ig5-20) in region 2 are separated by spacers almost constant in length; the average spacer length except for a slightly longer S6 was estimated at 40.6 ± 0.7 amino acids. Spacers in region 3 were slightly variable in length except for a very short one (S23) and contained 40–50 amino acids. Fig. 2 B shows that most, if not all, spacers in regions 2 and 3 share in common conserved amino acid sequences, IXXXQH(P/E) (Kettin spacer motif I) and IQXLE (Kettin spacer motif II; X, an arbitrary amino acid). Kettin spacer motifs I and II in region 2 are separated by five amino acids, but those in region 3, by five to nine amino acids. Region 4 is separated from other regions by a long spacer (S31) and possesses four Ig domains (Ig32-35) and relatively short spacers.
Figure 2.
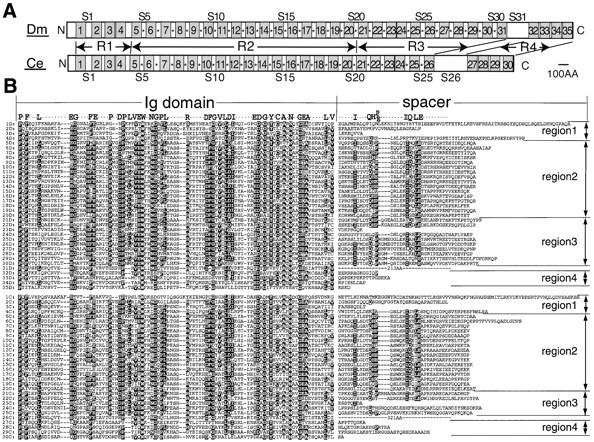
(A) Highly repetitive structures of Drosophila melanogaster (Dm) and C. elegans (Ce) Kettin proteins are schematically shown. Drosophila and C. elegans Kettins contain numerous repeating units consisting of an Ig domain and a spacer sequence. In both Drosophila and C. elegans, Kettin can be divided into four regions, R1-4, except for the NH2-terminal region and a long spacer (S31 and S26, respectively, in Drosophila and C. elegans). Stippled boxes with numbers show Ig domains. S1–S31 indicate spacer numbers. Spacers with conserved Kettin spacer motifs are marked with asterisks. (B) Comparison of Ig domain amino acid sequences of Drosophila and worm Kettins. iD (i = 1–35) indicates the Ig domain and spacer sequences of the ith repeating unit of Drosophila Kettin, while iC (i = 1–30), those of the ith repeating unit of worm Kettin. Ig domains consist of ∼95 amino acids. Highly conserved amino acids are labeled by white letters in black. Note that spacers in regions 2 and 3 share in common two conserved Kettin spacer motifs, I (IXXXQH(P/E)) and II (IQXLE).
Titin family members such as vertebrate titin, Drosophila Projectin, and C. elegans Twitchin are characterized by the presence of not only Ig domains, but also a kinase domain and fibronectin type III repeats (Labeit and Kolmerer 1995; Benian et al. 1999). The PEVK domain, rich in proline, glutamic acid, valine, and lysine, and possibly involved in elasticity, is specific to titin (Kolmerer et al. 1999). Thus, we searched for these sequences in Kettin, but no homology was found. We conclude that, unlike other titin family members, Drosophila Kettin has neither kinase, fibronectin type III, nor PEVK domains.
Colocalization of RNA and Protein Products of kettin in Muscle Attachments in Developing Embryonic Muscles
Although Kettin was initially found to be present in flight muscle Z-discs (Lakey et al. 1993), 132-LacZ expression indicated that kettin is also expressed in embryonic muscle cells (Fig. 3I and Fig. J). To clarify this point, examination was made of temporal and spatial expression of kettin RNA and protein in embryos using whole mount in situ hybridization and anti–Kettin antibody staining (Fig. 3, A–H).
kettin RNA began to be expressed in the mesoderm at stage 11 several hours before myoblast fusion (Fig. 3 A) and persisted in most, if not all, developing embryonic muscle cells until the third instar. At stage 14, strong kettin signals were detected in nearly all somatic muscle cells (Fig. 3 B) along with pharyngeal and visceral muscle cells (Fig. 3 C). At stages 14 and 15, myoblast fusion is complete in most somatic muscles (Bate 1990, Bate 1993). At the end of embryogenesis, somatic muscle tubes become connected to epidermal cells either directly or indirectly (Prokop et al. 1998; Tepass and Hartenstein 1994). Longitudinal muscles/epidermis attachments formed at segment borders are of indirect type and muscle tips, and epidermal cells are linked via tendon matrices (see Fig. 5 G). In contrast, transverse muscles/epidermis attachments are formed directly and involve hemiadherens junctions (see Fig. 5 E). Fig. 3D, shows kettin RNA to be localized for the most part along edges in nascent transverse and longitudinal muscles at stage 16. In contrast, 132-lacZ or kettin-lacZ chimeric RNA expression was not restricted to muscle tips (Fig. 3J and Fig. Q). Thus, kettin RNA is apparently almost uniformly produced from all embryonic muscle cell nuclei, but is transported to and apt to accumulate in the vicinity of muscle tips.
Figure 5.
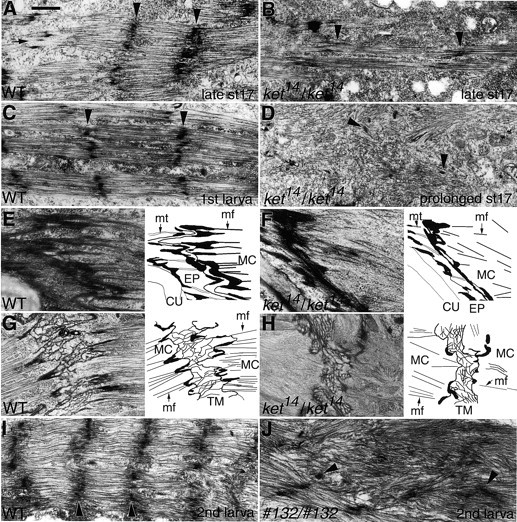
Defects of body wall muscles in kettin mutants. Arrowheads indicate electron dense materials of Z-discs or their presumptive derivatives. Only longitudinal muscle sections are shown. (A) Wild type at late stage 17. (B) ket14 homozygotes at late stage 17 or 20–24 h AEL. Z-discs are reported to be formed at stage 17 (Bernstein et al. 1993). Consistent with this, in wild type, Z-disc formation appeared almost completed by late stage 17 (A, arrowheads); immature Z-discs were observed only occasionally (A, arrow). In some mutant muscles (B), the formation of myofilament bundles was apparent but, even in such a case, accumulation of electron dense Z-discs appeared quite incomplete (arrowheads), suggesting that only unstable sarcomere structures are formed in ket14 homozygotes, lacking virtually all kettin activity. C and D, respectively, show wild-type first-instar larvae muscles and their counterparts in ket14 homozygotes at prolonged stage 17; ket14 homozygotes cannot hatch. Mutants almost completely lost normal regular sarcomere structures and scattered electron dense materials, putative remnants of Z-discs, were frequently observed (D, arrowheads). E and F and G and H, respectively, show tendon morphologies at direct and indirect muscle attachments. (E and G) Wild type; (F and H) ket14 homozygotes. As schematically shown in the right of each panel, in both direct and indirect muscle attachments, complicated folding of the muscle cell membrane largely disappeared in mutants. In addition, the size of the tendon matrix (TM), connecting muscle cells (MC) to the epidermis (EP) at indirect muscle attachment sites, reduced in ket14 homozygotes. CU, cuticle; mf, myofilaments; and mt, microtubles. (I and J) Somatic muscles of wild type (I) and 132 homozygotes (J) in second instar. Regularly arranged Z-discs in wild-type muscles appear disorganized in mutants. Bar: (A–D and G–J) 1 μm; (E and F) 0.5 μm.
The protein product of kettin showed expression quite like that of kettin RNA, although the protein appeared somewhat late compared with RNA (Fig. 3, E–H). Although less effective than RNA, Kettin protein was also concentrated at the edges of longitudinal and transverse somatic muscles at stage 16 (Fig. 3 H) as demonstrated by confocal images of double staining with anti–Kettin and anti–MHC antibodies (Fig. 3, R–T). These findings may suggest that, as schematically shown in Fig. 3 O, most Kettin molecules are translated from mRNA localized in muscle tips, and serve as anchors for fixing muscle fibers in the vicinity of direct and indirect muscle attachments, at least in embryonic muscles. No, or only a little, Kettin expression could be detected in cardiac muscle cells (Fig. 3M and Fig. N).
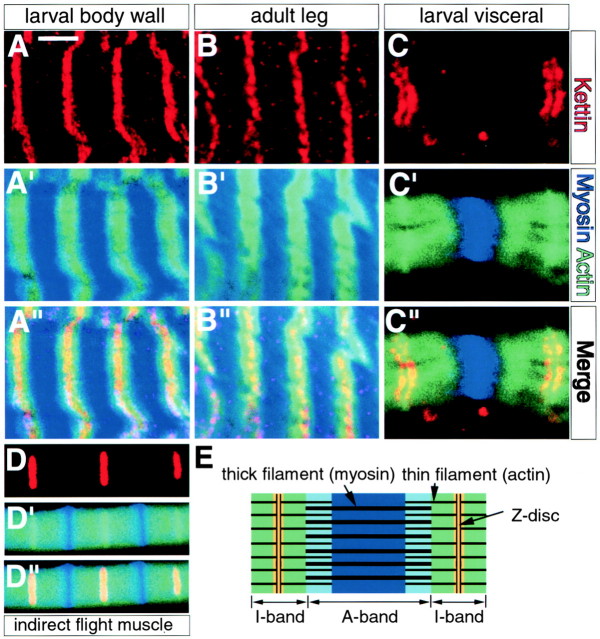
As Z-discs begin to be formed at stage 17, the last stage of embryogenesis (Bernstein et al. 1993), we investigated the spatial distribution of Kettin in mature muscles. Larval body wall, visceral, and adult leg muscles along with IFMs were stained for Kettin, actin, and myosin. As shown in Fig. 4, repetitive Kettin signals were observed in all muscles examined, and Kettin signals were always situated in the center of actin expression regions or putative Z-disc location. Thus, we consider that Kettin is located at or near Z-discs in many types of Drosophila muscles and may have common functions at Z-discs in larval and adult muscles.
Figure 4.
Z-disc expression of Kettin in several muscle types. (A, A′, and A′′) Larval body wall muscles. (B, B′, and B′′) Adult leg muscles. (C, C′, and C′′) Larval visceral muscles. (D, D′, and D′′) IFMs. Kettin (red) expression images (A–D) and anti-MHC (blue)/FITC-phalloidin (green) double staining pictures (A′–D′) are merged in (A′′–D′′). FITC-phalloidin staining shows actin expression regions. (E) Schematically shows the relationship between muscle gene expression and sarcomere structures. In all cases examined here, Kettin is expressed exclusively in the region colored in yellow in E, or the center of actin-expressing regions, indicating Kettin in Z-discs and their immediate neighbors. By unknown reasons, the region positive to antimyosin antibody staining was much narrower than expected, in particular, in larval visceral muscles and IFMs. Bar: (A and B) 5 μm; (C) 4 μm; (D) 3 μm.
kettin, A Gene Essential for Fly Viability
Flies homozygous for 132 were not viable, but this lethality was completely eliminated by removing the 132-P element, indicating that lethality is due to 132-P insertion in kettin. By imprecise P excision and subsequent lethality screening, we also obtained two additional kettin mutants, ket5 and ket14.
In ket14, a genomic sequence ranging from 0 to 4 kb downstream of the P insertion site, which includes the third to fifth exons of kettin, is deleted (Fig. 1 C). Embryos homozygous for ket14 produced protein products that could be hardly detected (Fig. 3 L), indicating ket14 to be a strong loss-of-function or null allele of kettin. Embryos homozygous for ket14 died as prolonged stage 17 embryos without hatching (Table ).
Table 1.
Phenotypes of kettin Mutants
| Genotype | Lethal stage | Mutant rank | Flight ability | Sarcomere length‡ | Thin/thick filament ratio‡ |
|---|---|---|---|---|---|
| μm | |||||
| ket 14/ket 14 | embryo | 6 | − | − | − |
| 132/132 | larva | 5 | − | − | − |
| ket 5/ket 5 | pupa | 4 | flightless§ | 2.4 ± 0.2 (n = 30) | 445/1263 (0.35) |
| ket 14/+ | viable | 3 | flightless | 2.3 ± 0.2 (n = 30) | 486/1392 (0.35) |
| 132/+ | viable | 2 | normal | ND∥ | ND |
| +/+ | viable | 0 | normal | 2.3 ± 0.2 (n = 47) | 559/1559 (0.36) |
In contrast to ket14, ket5 is a weak hypomorphic allele. In ket5, the structure of the kettin gene is identical to that in 132 (Fig. 1 C) except for a deletion within the P element. Almost all ket5 homozygous embryos hatched and grew into pupae and some became pharate adults dying without eclosion (Table ). So far, we have found five escapers, one of which was female and fertile when crossed with 132 heterozygotes.
132 homozygotes died at various times from the very end of embryogenesis to pupal stages. 132 homozygotes appeared to be intermediates in the phenotype between ket5 and ket14. Although only low level Kettin signals were detected in 132 embryos (Fig. 3 K), there was no or little abnormality in anti-MHC staining at stage 16 (data not shown) and most, if not all, 132 embryos normally hatched. Unhatched eggs contained fully formed larvae, capable of head movement but never emerged and eventually died. Hatched larvae showed normal behavior just after hatching, but movement slowly diminished. Many individuals that died at larval stages had double cuticles judging from the number of mouth hooks, suggesting muscular power was too weak to shed old skin. Larvae that grew to the third instar became incapable of movement by the end of this stage, could not climb the vial wall and died. However, many became pupae when transferred onto wet paper.
Virtually all flies heterozygous for ket14 were viable and became adults (Table ). But, as shown below, they had flight defects. Taken together, these results lead us to the conclusion that kettin is an essential muscle gene in Drosophila.
Requirement of Kettin for Formation and Maintenance of Normal Sarcomere Structure in Larval Muscles
In wild-type embryos, somatic muscle Z-discs first become detectable at stage 17 (Bernstein et al. 1993). Z-discs could be detected much more clearly in late stage 17 embryos (Fig. 5 A) or in the first instar larvae fixed at 24–36 h AEL (Fig. 5 C). In contrast, sarcomere structure was highly disorganized in any prolonged stage 17 embryos (24–36 h AEL embryos); thick and thin filaments appeared randomly scattered and no regularly arranged Z-disc–like structures could be detected (Fig. 5 D). These findings may indicate that kettin is essential for either the formation or maintenance of normal Z-discs or both. To determine which is the case, sarcomere structure of ket14 homozygotes at earlier stages was examined. As shown in Fig. 5 B, in late stage 17 embryos, we occasionally found sarcomere-like bundles of thick and thin filaments associated with incomplete Z-disc–like structures, which is not so dense but almost regularly spaced. Since ∼24 h AEL ket14 embryos appeared to possess muscle contraction ability to some extent, we tentatively conclude that kettin possesses important roles in both the formation and maintenance of Z-discs and sarcomere structures formed in the absence of Kettin is quite unstable and destroyed immediately with a low level of muscle contraction.
A study was also undertaken to determine the effects of the ket14 mutation on muscle attachments, where kettin RNA and protein are localized at embryonic stage 16. As shown in Fig. 5 F, the ends of muscles homozygous for ket14 were abnormally flat, whereas those of wild-type muscles were zigzag (Fig. 5 E; see also Tepass and Hartenstein 1994). Similar effects were also detected in indirect muscle attachment sites, where muscle tips are connected to epidermal cells via tendon matrices (Fig. 5, compare G with H; Prokop et al. 1998). These results suggest that kettin is also essential for the formation and/or maintenance of normal muscle attachment sites.
To determine whether the reduced activity of 132 larvae is also due to possible defects in the contractile apparatus of the musculature, wild-type and mutant larval muscles were examined. With the growth of larvae, the number of sarcomeres per muscle and muscle mass increased for the wild type; in the second instar larvae, all somatic and visceral muscles exhibited well-ordered Z-disc–like structures frequently associated with membrane invagination (Fig. 5 I, and data not shown). In most first instar larvae homozygous for 132, Z-disc–like structures were normal for the most part (data not shown). However, striated muscle patterns of 132 larvae were heavily disrupted in second instar larvae (Fig. 5 J). Thus, it would appear that Kettin must be continuously available for the maintenance of normal Z-disc structures in larvae.
Flightlessness of kettin Mutants
Drosophila Kettin was initially identified as a component of Z-discs in flight muscle myofibrils (Lakey et al. 1993), while our results described above show that continuous Kettin expression may be important for normal muscle development, suggesting the involvement of kettin in flying. Thus, examination was made as to whether flies heterozygous for kettin are capable of flying, using the cylinder method (Koana and Hotta 1978). Flies, 2–3 d after eclosion, were dropped through a funnel into a cylinder coated with liquid paraffin so that only those capable of flying would stick to the wall (Fig. 6). As shown in Fig. 6, nearly all wild-type flies stuck to the wall, whereas most, if not all, lacking wings simply dropped to the bottom. Whereas flies heterozygous for 132 or ket5 showed normal flight activity (data not shown), most of those heterozygous for ket14 dropped to the bottom. Five escapers of ket5 homozygotes obtained to date also could not fly at all (data not shown). 132 and ket5 are hypomorphic alleles, whereas ket14 is a strong loss-of-function or null allele. Moreover, flight defects in ket14heterozygotes were eliminated by introducing Dp(3;Y)B21 with a wild-type copy of kettin (Fig. 6). Thus, the flightless phenotype of ket14/+ flies appears to be due to haploinsufficiency of kettin.
ket14/+ IFMs were examined by electron microscopy (Fig. 7) and were found associated with severe myofibrillar aberrations. Transverse sections of IFMs indicated that, for wild type, IFM thick filaments are tightly packed (Fig. 7, A–C), whereas in mutants, they are loosely packed or untied, particularly along the periphery (Fig. 7, F–H). Longitudinal sections showed occasional myofibril branching and branched myofibrils to be connected to and integrated into neighboring ones (Fig. 7, compare D with I). Incomplete and apparently broken Z-discs were also frequently observed (Fig. 7 I, arrows). Since IFMs of late pupae (pharate adults) heterozygous for ket14 were virtually normal in morphology and indistinguishable from those of wild type (Fig. 8, compare A and B with C and D) and there was no apparent difference in sarcomere length and thin/thick filament ratio between wild type and the mutant (Table ), IFM disorganization found in ket14/+ adults may be due to muscle degeneration most probably caused by muscle contraction.
Figure 7.
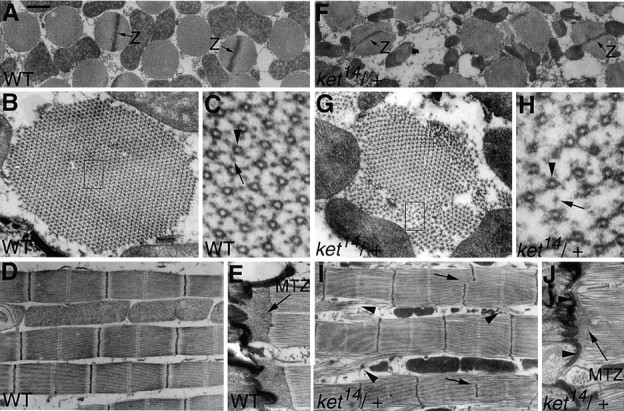
Electron micrographs of IFMs of wild-type (A–E) and ket14/+ (F–J) adult flies. (A–C and F–H) Transverse sections of muscles. (D, E, I and J) Longitudinal sections of muscles. (A and F) Arrows labeled Z indicate Z-discs. Note that one of two Z-discs in the mutant (F) does not reach the muscle bundle periphery. (B and G) Enlarged muscle bundles. Peripheral regions of bundles heterozygous for ket14 (G) are considerably disorganized. Regions enclosed by boxes are enlarged in (C and H). Arrowhead, myosin or thick filament. Arrow, actin or thin filament. The hexagonal lattice of normally packed filaments is deformed in the periphery of mutant muscle fiber bundles. (D and I) Z-disc defects and myofibril aberrations such as branching and breakage frequently found in ket14/+ IFMs are shown by arrows and arrowheads, respectively. (E and J) Myofilaments are connected to muscle cell membrane via modified terminal Z-discs (MTZ). Note that, in ket14/+ heterozygotes, modified terminal Z-discs are diminished in size and some myofilaments appear to contact to muscle cell membrane directly as shown by the arrowhead in J. Bar: (A, D, F, and I) 0.8 μm; (B and G) 0.16 μm; (E and J) 0.5 μm.
Figure 8.
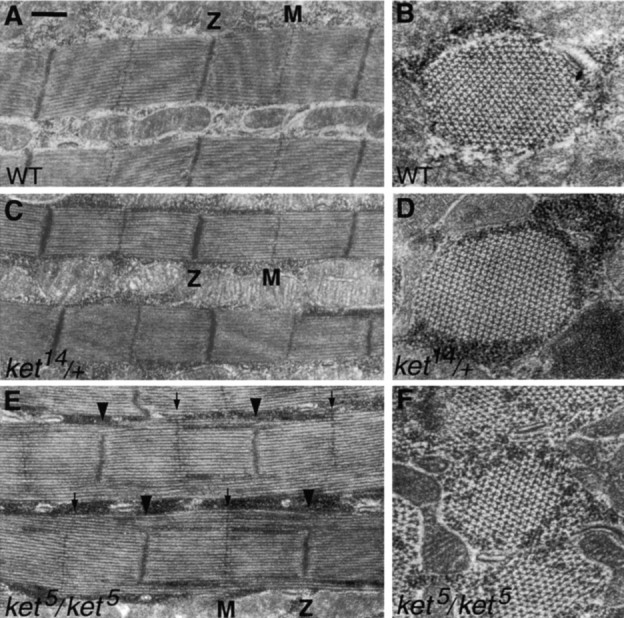
Electron micrographs of IFMs derived from wild-type and kettin mutant pharate adults (late pupae). A, C, and E are longitudinal sections, whereas B, D, and F are transverse sections. A and B, wild type; C and D, ket14/+; and E and F, ket5/ket5. IFM contraction is considered to be initiated after eclosion. ket14/+ IFMs are virtually normal in appearance (compare C and D with A and B), but destroyed after eclosion (Fig. 7 I), indicating that kettin is essential for the maintenance of IFMs. On the other hand, ket5/ket5 IFMs possessed morphological defects before eclosion; myofilaments were normally packed to generate the typical hexagonal lattice in the center, but the lattice structure appeared loose in the periphery near Z-discs (E and F, arrowheads); as shown by the arrows in E, M-lines are normally formed. These findings may suggest that kettin is also involved in normal IFM formation most probably through Z-disc formation. Bar: (A, C, and E) 0.5 μm; (B, D, and F) 0.25 μm.
However, the above finding may not exclude the possibility that Kettin is involved in IFM sarcomere formation. Indeed, we found morphological defects in late pupal IFMs homozygous for ket5 (Fig. 8E and Fig. F), in which Kettin expression may be lower than that in ket14/+ flies (Table ). As shown in Fig. 8 E, M-lines normally reached the myofibril periphery, whereas most Z-discs did not. Sarcomere length and thin/thick filament ratio appeared normal (Table ). Since IFM contraction is not yet initiated at late pupae, it may follow that Kettin is also required for IFM Z-disc formation.
All myofibrils in IFMs were anchored to the plasma membrane of muscle cells by modified terminal Z-discs (Fig. 7 E; Reedy and Beall 1993). In ket14/+ flies, the terminal Z-discs showed diminished size and departure from normal shape (Fig. 7, compare J with E), suggesting the role of Kettin in terminal Z-disc formation in IFM.
Identification of C. elegans kettin
To determine whether the structure of Kettin is conserved in evolution, the entire genomic DNA of Caenorhabditis elegans, with virtually complete nucleotide sequence (The C. elegans Sequencing Consortium, 1998), was examined. A genomic clone R05D8 encoding a long polypeptide highly similar in amino acid sequence to Kettin was initially identified, and the study of surrounding genomic DNA sequences confirmed the presence of a putative C. elegans kettin gene (Fig. 1 F). The true C. elegans Kettin was presumed to have maximum homology with Drosophila Kettin. The structure of the putative C. elegans kettin gene is outlined in Fig. 1 F. Fig. 2A and Fig. B, shows that C. elegans Kettin is a 468-kD protein comprised of 4,219 amino acids, containing 30 Ig domains and highly similar in overall structure to D. melanogaster Kettin. As with Drosophila Kettin, regions 2 and 3 contain spacer sequences of nearly constant length with two conserved Kettin spacer motifs found in Drosophila (Fig. 2 B). The entire Kettin structure is concluded to be highly conserved in evolution.
Kettin, as a Protein Distinct from D-Titin
D-titin is a hypothetical gene proposed by Machado et al. 1998 based on circumstantial evidence such as in situ hybridization to polytene chromosome and antibody staining. Although the sequence of D-titin was represented by those of four short DNA fragments, KZ, NB, LG, and JT, there is no solid evidence that they are derivatives of an identical gene (Machado et al. 1998). After the nucleotide sequence of the kettin coding region had been determined, we found that KZ is completely identical to the 5′ terminal sequence of kettin cDNA, coding for the NH2-terminal region of Kettin (Fig. 1 C). D-titin and kettin RNA may, thus, be alternative spliced products to each other and if so, Kettin and D-Titin protein may be considered to possess an NH2-terminal sequence in common.
To clarify this point further, we carried out Northern blotting and in situ hybridization using an NB probe (NB2). As shown in Fig. 1 D, lane 2, no or little signals were detected when RNA extracted from wild-type embryos or larvae was subjected to northern hybridization. In contrast, apparent hybridization signals were detected when kettin probes were used (see lane 1). Fig. 3 U shows a part of the results of whole mount in situ hybridization to embryos. Again, unlike kettin probe, D-titin probe, NB, gave no or little signals throughout embryonic development. We interpret these results as strongly suggesting that either D-titin RNA represents a minor transcriptional unit at the kettin locus or D-titin is a gene completely different from kettin. That there are no or little NB signals in embryos also indicates that, unlike Kettin, putative D-Titin protein may not be involved in embryonic muscle development and/or function.
According to Machado et al. 1998, D-Titin is a chromosomal protein, and anti–KZ antibody can visualize chromosomes in early Drosophila embryos, presumably recognizing the NH2-terminal region of Kettin. Thus, we stained Drosophila embryonic nuclei by our anti–Kettin antibody. As shown in Fig. 3V-W, no or little chromosomal signals of Kettin were detected, possibly suggesting that Kettin is not a chromosomal protein.
Discussion
Kettin, a Giant Muscle Protein with Overall Structure Highly Conserved in Evolution and Multiple Actin Binding Sites
Based on genomic DNA and cDNA sequences, the entire amino acid sequence of the major isoform of Drosophila Kettin was deduced (Fig. 2). Drosophila Kettin is highly repetitive in sequence, and each repeating unit is comprised of an Ig domain ∼95 amino acids long and a spacer sequence. Unlike other titin family members (Benian et al. 1999), Kettin possesses neither protein kinase, fibronectin type III, nor PEVK domains (Fig. 2 A). Kettin structure appears highly conserved in evolution, since the predicted C. elegans Kettin is very similar in overall structure to the Drosophila counterpart (see Fig. 2).
The central 25 spacers in Drosophila Kettin may have unique properties since these spacers share in common two Kettin spacer motifs (I and II; Fig. 2 B). All spacers in region 2 other than S6 show little variation in length. Kettin is thought to bind to actin (Lakey et al. 1993). Plots of binding data showed that 28 mol of actin monomer can bind to 1 mol of the 500-kD Lethocerus leg Kettin at maximum (Straaten et al. 1999), suggesting that the 540-kD Drosophila Kettin (the major isoform) possesses a similar number of actin binding sites. Ig domain itself has no actin binding activity, whereas an Ig domain flanked with spacers is capable of binding to actin (Lakey et al. 1993), indicating the importance of spacer sequences in actin binding. The number of spacers with conserved motifs I and II is 25, this being essentially identical to that predicted for actin binding sites. Thus, it might be speculated that region 2 and region 3 spacers with conserved Kettin motifs provide actin binding sites, and the major isoform of Drosophila Kettin possesses 25 actin binding sites in all. In vertebrates, single nebulin molecules span each thin actin filament and are presumed to serve as protein rulers for thin filament formation (Trinick et al., 1994). However, Kettin may not be involved in such a process since the actin filament distal to the Z-disc is occupied by tropomyosin, a muscle protein competing with Kettin for actin (Straaten et al. 1999). It is also quite feasible that, unlike titin (for review see Gautel et al. 1999), Kettin is not a mechanical integrator of thick and thin filaments during contraction because it lacks myosin binding activity (Lakey et al. 1993).
Kettin may also interact with α-actinin, since α-actinin is released from Z-discs of IFMs through the proteolysis of Kettin with calpain (Lakey et al. 1993). Consistent with this, a Kettin Ig domain associated with spacer sequences has been shown to be capable of binding to α-actinin (Lakey et al. 1993). Immunoelectron microscopy showed, in IFM, Kettin to be oriented with the NH2 terminus in the Z-disc and the COOH terminus outside (Straaten et al. 1999). Thus, Ig repeats in region 1 and the NH2-terminal nonconserved sequences might be involved in possible head-to-head interactions of Kettin molecules and/or interactions between Kettin and other Z-disc proteins including α-actinin.
In insect IFMs, the size of a half Z-disc is ∼50 nm (Straaten et al. 1999). A single repeating unit (Ig + Spacer) of Kettin was shown to cover ∼3 nm of the filament (Straaten et al. 1999) and, thus, the ∼50 nm may roughly correspond to 16 units, the region spanning repeating units 5–20 (region 2). In region 2, both spacer length and the distance between spacer motifs I and II are almost strictly conserved not only in Drosophila, but also in C. elegans (Fig. 2 A). Thus, the region 2/region 3 boundary of Kettin might serve as a signal to separate Kettin into two regions: one capable of interacting with Z-disc components, and the other not. Precise spacing of actin binding sites on Kettin may guarantee tight binding of Kettin to thin filament, in which actin monomers may form a helical structure with a constant pitch, to generate stable sarcomere end structures.
Kettin Functions and Possible Functional Relationships among Kettin, Titin, and Projectin
Our structural analysis showed that Drosophila Kettin and vertebrate titin share in common Ig domain repeats, but neither protein kinase nor fibronectin type III domains are included in the former, indicating that Kettin is not a Drosophila counterpart of titin.
Projectin is another titin family member found in Drosophila, and its nearly complete amino acid sequence has been determined (Daley et al. 1998). Strangely, the location of Projectin within the sarcomere varies depending on muscle types (Vigoreaux et al. 1991). As schematically shown in Fig. 9, Projectin is situated mainly in the A-band region in synchronous muscles, which includes larval somatic muscles, whereas, in asynchronous muscles such as IFM, its location is within the I-band region. The A-band region of titin is constructed in a modular fashion and includes intermingled repeats of Ig and fibronectin type III domains, which together provide binding sites for thick filaments. The A-band region of titin also includes a protein kinase domain. Projectin is quite similar in modular structure to the A-band region of titin, although its size is much smaller than that of titin (Labeit and Kolmerer, 1995a; Daley et al. 1998). A protein kinase domain similar in sequence to that of the A-band region of titin is also included in Projectin (Ayme-Southgate et al. 1995). Thus, at least in synchronous muscles, Projectin might be considered to be a functional substitute for the A-band region of titin (for review see Benian et al. 1999). Consistent with this, as with titin (for review see Kolmerer et al. 1999), Projectin is necessary for the contraction of larval somatic muscles (Benian et al. 1999).
Figure 9.
Comparison of structures and intra-sarcomere localizations of Drosophila Kettin, Drosophila Projectin and vertebrate titin (modified from Benian et al. 1999; Kolmerer et al. 1999). Drosophila muscle sarcomeres are boxed. In human muscles, the NH2- and COOH-terminal regions of titin are embedded in Z-disc and M-line, respectively, and hence, extend across the half sarcomere (Labeit and Kolmerer 1995). As with the A-band region of titin, Projectin possesses not only Ig domains but also fibronectin type III and kinase domains (for review see Gautel et al. 1999). In Drosophila synchronous and asynchronous muscles, Projectin is situated in the A-band and I-band regions, respectively (Vigoreaux et al. 1991). In both types of muscles, Kettin is a Z-disc protein. Almost all parts of Kettin consist of repeat units of an Ig domain and a spacer sequence and lack fibronectin type III, kinase, and PVEK domains. X and Y, respectively, are hypothetical Drosophila muscle proteins corresponding to the PEVK domain and the A-band region of titin.
Kettin is located in the vicinity of the Z-disc in all types of Drosophila muscles so far examined (Fig. 4), suggesting its general roles at the sarcomere end. The corresponding region of titin consists of Ig domains, specific motifs called Z-repeats, phosphorylation sites, and flanking sequences (Young et al. 1998). The Z-disc region of titin is capable of binding to α-actinin directly and thin filaments indirectly. Although there is little similarity in the amino acid sequence between Kettin and the Z-disc region of titin except for Ig domains, Kettin is also capable of binding to α-actinin along with actin, most probably through its repeating units (Lakey et al. 1993). In vertebrates, titin and nebulin may together form a scaffold for Z-disc assembly (Gautel et al. 1999). Our results may indicate that Kettin is also involved in a similar process in Drosophila. Indeed, Fig. 5B and Fig. D, shows that Z-disc–like structures formed in synchronous somatic muscles mutant for kettin are very unstable (Fig. 5B and Fig. D). Similarly, premature Z-discs were formed in IFM homozygous for ket5(see Fig. 8E and Fig. F). In IFM heterozygous for ket14, Z-discs virtually normal in appearance were formed at pupal stages (Fig. 8C and Fig. D) but frequently were destroyed in adults (Fig. 7, F–J), leading to the flightless phenotype (Fig. 6). Consistent with these findings, Kettin promoted antiparallel assembly of actin filaments in vitro in the absence of α-actinin (Straaten et al. 1999). Our results (Fig. 3 A) indicated that kettin expression in muscle cells is initiated at the early stages of muscle cell development when no muscle fiber is yet formed; similar early expression is also known in the case of titin (for review see Fulton 1999).
The I-band region of titin contains tandem Ig domain modules and the PEVK domain (Labeit and Kolmerer 1995). Tandem Ig domains elongate with little increase in passive tension, while unfolding of the PEVK domain requires substantial force. These domains may, thus, account for the elasticity of titin (for review see Horowits 1999). Projectin, situated in the I-band region of IFM sarcomeres is supposed to be elastic, though there is no PEVK sequence (Benian et al. 1999). D-Titin (the protein product of a hypothetical gene, D-titin) was reported to contain a PEVK-like region (Machado et al. 1998), but Fig. 3 U shows that there is no or little expression of D-titin at least in embryonic somatic muscles. The function of Ig domains themselves is not yet clear. Since Ig domains are involved in protein–protein interactions (Williams and Barclay 1988), Kettin Ig domains might interact with themselves or other Ig domain-containing proteins such as Projectin. As with Ig repeats and the PEVK domain of titin (Labeit and Kolmerer 1995), Kettin Ig repeats might be flexible in structure and involved in regulation of possible breakage/reunion of thin filaments during muscle contraction/relaxation. A recent structural analysis of the Twitchin protein kinase domain indicates Ig domain to be capable of binding to the protein kinase domain (Kobe et al. 1996). Thus, Kettin Ig domains might interact with protein kinases possibly involved in Z-disc assembly to regulate their activity.
kettin Is a Gene Unrelated to D-titin
D-titin is a Drosophila gene, predicted as the counterpart of vertebrate titin by Machado et al. 1998. A short genomic DNA fragment of D-titin, LG, was initially isolated from a Drosophila genomic expression library using the human autoimmune scleroderma serum. Then, three additional short pieces of cDNA, KZ, NB, and JT, were isolated. In particular, KZ was isolated using P insertion, V(3)ET1, presumed to be located at 62C1-2, the putative D-titin locus, but there is no solid evidence that KZ and other D-titin fragments are derivatives of an identical gene. Our results show that KZ is identical in sequence to the 5′-terminal region of kettin (Fig. 1 C), but kettin possesses no homology to other D-titin fragments, suggesting that D-titin RNA is one of possible alternative splicing products from the kettin locus. This hypothesis was first tested by Northern hybridization and whole mount in situ hybridization to embryos using an NB probe, but no signals were detected in both cases (see Fig. 1 D, lane 2, and Fig. 3 U). Thus, D-titin might be a very minor transcriptional unit at the kettin locus, if any.
KZ is presumed to encode the NH2-terminal region of D-Titin (Machado et al. 1998). Machado et al. 1998 showed that anti–KZ antibody is capable of staining the chromosomes of HEp-2 cells (human cells), and maintained that D-Titin serves as a scaffold not only for the sarcomere but for the chromosome as well. However, their experiments using antibodies raising against the NH2-terminal region of human titin showed the contrary (Machado et al. 1998). No chromosomal titin signals could be detected in HEp-2 cells when antibodies for the NH2-terminal region of human titin were used. To account for this apparent discrepancy, they postulated that there are two forms of titin (or D-Titin), chromosomal and muscle types, and the NH2-terminal sequences of these two types are different from each other (Machado et al. 1998). However, this notion may not be applicable to D-Titin since our results showed that the KZ region, the presumptive NH2-terminal region of chromosomal type D-Titin, is essential for muscle function. Indeed, all kettin mutants examined in this study have defects in the KZ region, and, in particular, in ket14 mutants, the KZ region is almost completely deleted (Fig. 1 C). As shown in Fig. 5, homozygotes of these kettin mutations possess muscle defects at late developmental stages, but are almost normal in early embryonic stages when muscles are not yet formed (Hakeda, S., unpublished data), indicating that KZ is essential for muscle development but not for chromosomal scaffolding. Consistent with this, no chromosomal signals were detected by anti–Kettin antibody raising against the central region of Kettin (see Fig. 3V and Fig. W). Thus, chromosomal KZ signals detected in HEp-2 cells and nuclei in early Drosophila embryos by Machado et al. 1998 are quite likely to be due to possible cross-reaction of their anti–KZ antibody to a completely different protein.
An additional problem of D-titin is that LG, isolated originally as a fragment encoding the putative PEVK domain of D-Titin is a fortuitous composite of fragments derived from different chromosomal loci. Indeed, by examining the Drosophila genomic sequence, we found that a genomic DNA sequence at 62A10-62B5 (accession number, AC005557) is identical to the sequence of a 3′ half of LG, while no homology to the remaining half, which encodes the PEVK domain, could be found not only in the sequence of 62A10-62B5, but also in other Drosophila genomic regions whose sequences are available. Taken together, these considerations may lead us to the conclusion that D-titin is a putative gene unrelated to kettin at all.
Acknowledgments
We are most grateful to Dr. B. Bullard (European Molecular Biology Laboratory, Heidelberg, Germany) and colleagues for communicating their results on Drosophila kettin sequence to us before publication. We thank again B. Bullard, D. Kiehart (Duke University Medical Center, North Carolina, USA), T. Suzuki, T. Kojima (University of Tokyo, Tokyo, Japan), and Bloomington Drosophila stock center for antibodies, fly stocks, and/or discussion. We also thank Berkeley Drosophila Genome Project, and The C. elegans Sequencing Consortium for sequence data and/or cDNA.
This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan to K. Saiko.
Footnotes
Abbreviations used in this paper: AEL, after egg laying; EST, expressed sequence tag; IFM, indirect flight muscle; MHC, myosin heavy chain; ORF, open reading frame; RT, reverse transcriptase.
References
- Ayme-Southgate A., Southgate R., Saide J., Benian G.M., Pardue M.L. Both synchronous and asynchronous muscle isoforms of Projectin (the Drosophila bent locus product) contain functional kinase domains. J. Cell Biol. 1995;128:393–403. doi: 10.1083/jcb.128.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate M. The embryonic development of larval muscles in Drosophila . Development. 1990;110:791–804. doi: 10.1242/dev.110.3.791. [DOI] [PubMed] [Google Scholar]
- Bate M. The mesoderm and its derivatives. In: Bate M., Arias A.M., editors. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1993. pp. 1013–1090. [Google Scholar]
- Benian G.M., Ayme-Southgate A., Tinley T.L. The genetics and molecular biology of the titin/connectin-like proteins of invertebrates. Rev. Physiol. Biochem. Pharmacol. 1999;138:235–268. doi: 10.1007/BFb0119629. [DOI] [PubMed] [Google Scholar]
- Bernstein S.I., O'Donnell P.T., Cripps R.M. Molecular genetic analysis of muscle development, structure, and function in Drosophila . Int. Rev. Cytol. 1993;143:63–152. doi: 10.1016/s0074-7696(08)61874-4. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J.A., Hartenstein V. The Embryonic Development of Drosophila melanogaster 1985. Springer-Verlag; Berlin: pp. 227 [Google Scholar]
- Crossley A.C. The morphology and development of the Drosophila muscular system. In: Ashburner M., Wright T.R.F., editors. The Genetics and Biology of Drosophila 2b. Academic Press, Inc; London/New York/San Francisco: 1978. pp. 499–560. [Google Scholar]
- Daley J., Southgate R., Ayme-Southgate A. Structure of the Drosophila Projectin proteinisoforms and implication for Projectin filament assembly. J. Mol. Biol. 1998;279:201–210. doi: 10.1006/jmbi.1998.1756. [DOI] [PubMed] [Google Scholar]
- Fridell Y.W., Searles L.L. vermilion as a small selectable marker gene for Drosophila transformation. Nucleic Acids Res. 1991;19:5082. doi: 10.1093/nar/19.18.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A.B. The elastic filament system in myogenesis. Rev. Physiol. Biochem. Pharmacol. 1999;138:139–161. doi: 10.1007/BFb0119626. [DOI] [PubMed] [Google Scholar]
- Gautel M., Mues A., Young P. Control of sarcomeric assemblythe flow of information on titin. Rev. Physiol. Biochem. Pharmacol. 1999;138:97–137. doi: 10.1007/BFb0119625. [DOI] [PubMed] [Google Scholar]
- Horowits R. The physiological role of titin in striated muscle. Rev. Physiol. Biochem. Pharmacol. 1999;138:57–96. doi: 10.1007/BFb0119624. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W.J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22 . Cell. 1986;44:429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Ingolia T.D., Craig E.A., McCarthy B.J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980;21:669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Kiehart D.P., Feghali R. Cytoplasmic myosin from Drosophila melanogaster . J. Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koana T., Hotta Y. Isolation and characterization of flightless mutants in Drosophila melanogaster . J. Embryol. Exp. Morphol. 1978;45:123–143. [PubMed] [Google Scholar]
- Kobe B., Heierhorst J., Feil S.C., Parker M.W., Benian G.M., Weiss K.R., Kemp B.E. Giant protein kinasesdomain interactions and structural basis of autoregulation. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:6810–6821. [PMC free article] [PubMed] [Google Scholar]
- Kolmerer B., Witt C.C., Freiburg A., Millevoi S., Stier G., Sorimachi H., Pelin K., Carrier L., Schwartz K., Labeit D., Gregorio C.C., Linke W.A., Labeit S. The titin cDNA sequence and partial genomic sequencesinsights into the molecular genetics, cell biology and physiology of the titin filament system. Rev. Physiol. Biochem. Pharmacol. 1999;138:19–55. doi: 10.1007/BFb0119623. [DOI] [PubMed] [Google Scholar]
- Kushida H. A new polyester embedding method for ultrathin sectioning. J. Electron Microsc. 1960;9:113–116. [Google Scholar]
- Labeit S., Kolmerer B. Titinsgiant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270:293–296. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- Lakey A., Ferguson C., Labeit S., Reedy M., Larkins A., Butcher G., Leonard K., Bullard B. Identification and localization of high molecular weight proteins in insect flight and leg muscle. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:3459–3467. doi: 10.1002/j.1460-2075.1990.tb07554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey A., Labeit S., Gautel M., Ferguson C., Barlow D.P., Leonard K., Bullard B. Kettin, a large modular protein in the Z-disc of insect muscles. EMBO (Eur. Mol. Biol. Organ.) J. 1993;12:2863–2871. doi: 10.1002/j.1460-2075.1993.tb05948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C., Sunkel C.E., Andrew D.J. Human autoantibodies reveal titin as a chromosomal protein. J. Cell Biol. 1998;141:321–333. doi: 10.1083/jcb.141.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki S., Ohtani Y., Kimura S., Maruyama K. Isolation and characterization of a kettin-like protein from crayfish claw muscle. J. Muscle Res. Cell Motil. 1995;16:579–585. doi: 10.1007/BF00130239. [DOI] [PubMed] [Google Scholar]
- Mohler J., Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of Drosophila . Development. 1992;115:957–971. doi: 10.1242/dev.115.4.957. [DOI] [PubMed] [Google Scholar]
- Poole S.J., Kauvar L.M., Drees B., Kornberg T. The engrailed locus of Drosophilastructural analysis of an embryonic transcript. Cell. 1985;40:37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Prokop A., Martin-Bermudo M.D., Bate M., Brown N.H. Absence of PS integrins or laminin A affects extracellular adhesion, but not intracellular assembly, of hemiadherens and neuromuscular junctions in Drosophila embryos. Dev. Biol. 1998;196:58–76. doi: 10.1006/dbio.1997.8830. [DOI] [PubMed] [Google Scholar]
- Reedy M.C., Beall C. Ultrastructure of developing flight muscle in Drosophila. I. Assembly of myofibrils. Dev. Biol. 1993;160:466–479. doi: 10.1006/dbio.1993.1321. [DOI] [PubMed] [Google Scholar]
- Robertson H.M., Preston C.R., Phillis R.W., Johnson-Schlitz D.M., Benz W.K., Engels W.R. A stable genomic source of P element transposase in Drosophila melanogaster . Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. Molecular CloningA Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 1989. [Google Scholar]
- Shishido E., Ono N., Kojima T., Saigo K. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–2128. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Spradling A.C., Rubin G.M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Straaten M., Gouldling D., Kolmerer B., Labeit S., Clayton J., Leonard K., Bullard B. Association of Kettin with actin in the Z-disc of insect flight muscle. J. Mol. Biol. 1999;285:1549–1562. doi: 10.1006/jmbi.1998.2386. [DOI] [PubMed] [Google Scholar]
- Suzuki M.G., Shimada T., Kobayashi M. Bm kettin, homologue of the Drosophila kettin gene, is located on the Z chromosome in Bombyx mori and is not dosage compensated. Heredity. 1999;82:170–179. doi: 10.1038/sj.hdy.6884570. [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev. Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- The C. elegans Sequencing Consortium Genome sequence of the nematode C. elegansa platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- Trinick J. Titin and nebulinprotein rulers in muscle? Trends Biochem. Sci. 1994;19:405–409. doi: 10.1016/0968-0004(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Vigoreaux J.O. The muscle Z bandlessons in stress management. J. Muscle Res. Cell Motil. 1994;15:237–255. doi: 10.1007/BF00123477. [DOI] [PubMed] [Google Scholar]
- Vigoreaux J.O., Saide J.D., Pardue M.L. Structurally different Drosophila striated muscles utilize distinct variants of Z-band-associated proteins. J. Muscle Res. Cell Motil. 1991;12:340–354. doi: 10.1007/BF01738589. [DOI] [PubMed] [Google Scholar]
- Waterston R.H., Thomson J.N., Brenner S. Mutants with altered muscle structure of Caenorhabditis elegans . Dev. Biol. 1980;77:271–302. doi: 10.1016/0012-1606(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Williams A.F., Barclay A.N. The immunoglobulin superfamily—domains for cell surface recognition. Annu. Rev. Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Young P., Ferguson C., Banuelos S., Gautel M. Molecular structure of the sarcomeric Z-disktwo types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO (Eur. Mol. Biol. Organ.) J. 1998;17:1614–1624. doi: 10.1093/emboj/17.6.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]