Abstract
Previous studies demonstrate that in developing Drosophila bristles, two cross-linking proteins are required sequentially to bundle the actin filaments that support elongating bristle cells. The forked protein initiates the process and facilitates subsequent cross-linking by fascin. Using cross-linker–specific antibodies, mutants, and drugs we show that fascin and actin are present in excessive amounts throughout bundle elongation. In contrast, the forked cross-linker is limited throughout bundle formation, and accordingly, regulates bundle size and shape. We also show that regulation of cross-linking by phosphorylation can affect bundle size. Specifically, inhibition of phosphorylation by staurosporine results in a failure to form large bundles if added during bundle formation, and leads to a loss of cross-linking by fascin if added after the bundles form. Interestingly, inhibition of dephosphorylation by okadaic acid results in the separation of the actin bundles from the plasma membrane. We further show by thin section electron microscopy analysis of mutant and wild-type bristles that the amount of material that connects the actin bundles to the plasma membrane is also limited throughout bristle elongation. Therefore, overall bundle shape is determined by the number of actin filaments assembled onto the limited area provided by the connector material. We conclude that assembly of actin bundles in Drosophila bristles is controlled in part by the controlled availability of a single cross-linking protein, forked, and in part by controlled phosphorylation of cross-links and membrane actin connector proteins.
Keywords: actin bundle assembly, fascin, forked, phosphorylation, actin–membrane interaction
Introduction
Bundles of actin filaments are common features of eukaryotic cells. Obvious examples include microvilli, the acrosomal process of invertebrate sperm, stereocilia, stress fibers, filopodia, and the bristles and hairs of Drosophila. We have been trying to understand what determines where in the cell the bundles appear, what controls their length and diameter, and what proteins regulate the packing of the filaments in the bundles. It has become increasingly clear that compact well-ordered bundles require more than one cross-link. To determine what each cross-link does, we have studied the elongation of Drosophila sensory bristle cells as a model system because there exists a large number of mutants which have malformed bristles. Thus, by using genetics and molecular and cell biological techniques, we can determine the function of each cross-link.
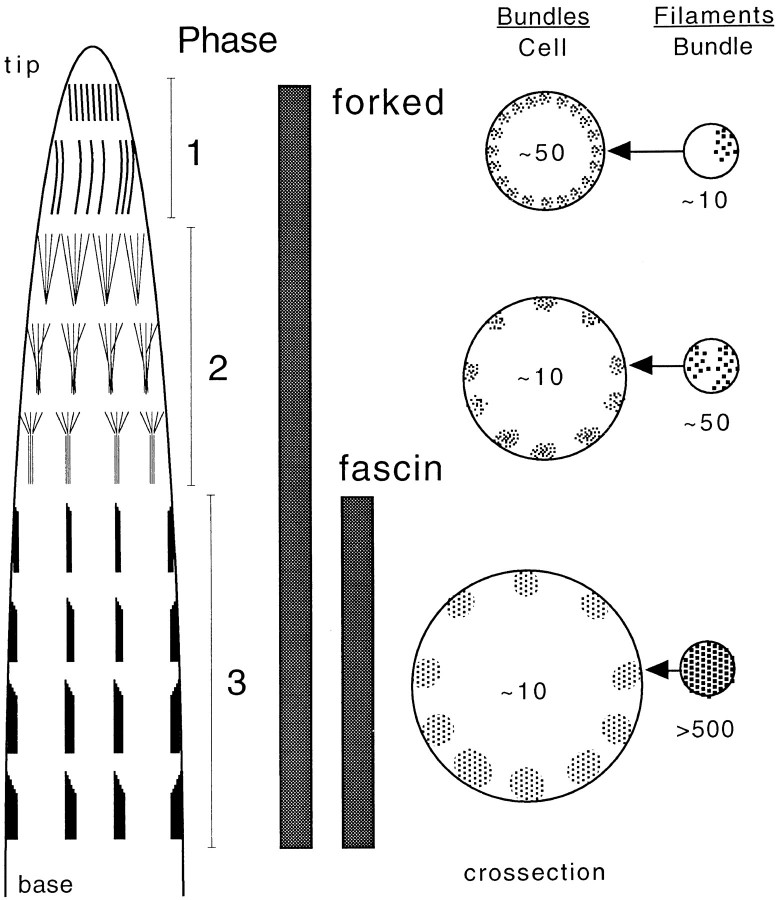
Drosophila bristles are single cells with very long extensions initially supported by actin bundles. Each bundle is composed of repeating units or modules attached end to end. As the bristle elongates, actin filaments are continually formed at the tip of the bristle and then progressively gathered together in a three-stage process to form new modules composed of maximally cross-linked filaments (Fig. 1). First, tiny cortical bundles of actin filaments appear; second, these tiny bundles aggregate into larger bundles; and third, the filaments in the larger bundles become maximally cross-linked together. For steps 1 and 2, the cross-linker forked is used, and in step 3 forked facilitates the entry and cross-linking by a second cross-link, fascin (Tilney et al. 1998). Since there is a continuum of maturing modules in a single cytoplasm, it is unlikely that transcriptional and translational controls are responsible for the sequential appearance of forked, then fascin, in each module. Rather, the sequential incorporation of cross-links in each bundle must be controlled during the assembly process itself.
Figure 1.
Model for the stages of actin filament bundling and module formation during Drosophila bristle development. Phase 1: (left to right) actin filaments are initiated on the cytoplasmic side of the plasma membrane at the bristle tip and form tiny bundles (thin vertical lines). The barbed end of all filaments is oriented toward the tip of the bristle. Thin cross sections show that ∼50 tiny bundles of ∼10 filaments/bundle are uniformly distributed around the circumference of the bristle. At this stage filaments exhibit liquid order packing. The forked protein is responsible for this packing. Phase 2: (left to right) as development proceeds, the tiny bundles aggregate into ∼10 larger bundles each containing ∼50 filaments. These aggregated bundles exhibit liquid order packing with noticeable gaps. The forked protein is also required for this phase. Phase 3: (left to right) as the bundles mature by the addition of fascin, additional actin filaments are added to each bundle, which now consist of ≥500 filaments/bundle. These filaments now show hexagonal packing.
In keeping with this idea we find that fascin, the cross-link that operates exclusively in stage 3, is present in excessive amounts throughout bristle elongation, even at the earliest stages. Even more puzzling, we find fascin dispersed throughout the bristle cytoplasm, not just in regions where cross-linking is taking place. Furthermore, analysis of mutants suggests that this soluble fascin is competent to cross-link actin filaments. How then is the sequential use of cross-links controlled?
One idea is that another component, not fascin, is limiting at all stages in bundle initiation and elongation and that this limited component regulates the process (Tilney et al. 1998; Wulfkuhle et al. 1998). It is also possible, of course, that this component and/or other components are further regulated posttranslationally by phosphorylation, for example. Our experiments reported here using mutants, specific antibodies to the cross-links, and drugs that affect actin assembly and/or phosphorylation help us understand more about the control of bundle formation in vivo. Once we know what happens in a cell, we can begin to dissect how individual components are regulated, a regulation that ultimately involves cascades orchestrated by cdc42 (Eaton et al. 1996) and Ras (Harden et al. 1995; Eaton et al. 1996), Rho (Strutt et al. 1997; Hacker and Perrimon 1998), Raf (Tsuda et al. 1993), and Ral (Sawamoto et al. 1999). In addition, our results give us clues as to what regulates other features of the actin bundles, such as their shape.
Materials and Methods
Drosophila Stocks
The Oregon-R strain of Drosophila melanogaster was used as the wild-type in these studies. The singed stock (sn3) and the forked stock (f36a) were obtained from the Drosophila Stock Center (Indiana University) and maintained as viable homozygotes. A viable and fertile stock containing two copies of the wild-type forked + gene and four transgenic copies (for a total of six copies per diploid female genome) was generously supplied by Nancy Petersen (University of Wyoming, Laramie, WY) (Petersen et al. 1994). Female larvae containing 50% of the normal forked protein concentration were f36a/+ heterozygotes. Female larvae containing 50% of the normal singed protein concentration were sn 3/+ heterozygotes. Flies were maintained on standard cornmeal-molasses-yeast food at 25°C, 60–70% relative humidity, with a 12 h/12 h day/night cycle. Complete descriptions of genes and symbols can be found in Lindsley and Zimm 1992 and on FlyBase (FlyBase Consortium 1998).
Developmental Staging
All animals were staged from the point of puparium formation, an easily recognizable and brief stage lasting 30 min at the beginning of metamorphosis (Bainbridge and Bownes 1981). White prepupae were collected and placed on double-sided scotch tape in a petri dish that was put back into the 25°C incubator. At the appropriate time of incubation, the petri dish was removed and the pupae dissected.
Dissection of Pupae and Culturing the Dorsal Thoracic Epithelium
After removing the pupal case, we filleted the pupae as outlined in detail in Tilney et al. 1998. We carried this out under Grace's insect cell culture medium (GIBCO BRL), preheated to 25°C rather than under phosphate-buffered saline. Each fillet, which consists of the dorsal surface of the thorax, was placed on its back and very delicately the large tracheoles and fat bodies were removed with fine forceps. It is important not to clean the preparation too thoroughly (e.g., by removing all the muscles and all the fat body), as it is easy to damage the underlying epidermis containing the bristles. These isolated thoracic fillets were then placed in 60 × 15 mm petri plates with 5 ml of Grace's medium along with 5–10 μm of inhibitor. These compounds were diluted into medium from concentrated stocks (made in DMSO or ethanol). Control incubations using DMSO or ethanol alone (typically 0.1–0.2%) were performed in parallel. After swirling the medium in the petri dishes to dilute the compounds, we added the isolated thoraces and the plates were returned to the 25°C incubator for 5–7 h. At the end of this period, thoraces were removed and fixed for light or EM. The volume of medium used was just sufficient to cover the tissue, allowing for good oxygen exchange.
Reagents
Jasplakinolide was obtained from Molecular Probes, Inc. A 1-mM stock solution in DMSO was prepared and aliquots stored at −20°C. Okadaic acid and staurosporine were obtained from Calbiochem. Okadaic acid was prepared as a 1-mM stock solution in DMSO, whereas staurosporine was made up as a 500-μM stock in ethanol. Aliquots were stored at −20°C, and each reagent diluted to the required final concentration in Grace's medium.
Fixation and Processing for Light Microscopy
The thoraces were fixed by immersion in 2% formaldehyde in PBS for 5 min, washed three times in 0.1% Triton X-100 in PBS for 5 min each, and then placed in 0.1% Triton X-100 containing 10−6 M phalloidin conjugated to rhodamine (Sigma Chemical Co.) at 4°C overnight in the dark. The next morning the sample was placed on a slide, with the ventral side downward, the excess fluid removed with filter paper, and the thoraces mounted in Citifluor glycerol (Ted Pella, Inc.). A coverslip was applied, excess Citifluor glycerol removed, and the preparation sealed with nail polish. Slides were examined with either a Leica model TCS 4D confocal microscope or an Olympus fluoview model BX50 confocal microscope. Bristles were visualized by the fluorescence of the phalloidin-stained actin bundles. Bristle length measurements were made from confocal images using NIH Image. For each procedure, at least 40 bristles were measured.
To establish the presence or absence of fascin or the forked proteins, we took the thoraces and fixed them for 5 min in 4% formaldehyde in PBS. These thoraces were then blocked by PBS/1% BSA, and washed three times in PBS. The specimens were incubated in the primary antibody, diluted in PBS containing 1% BSA, for 3 h or overnight at 4°C. The thoraces were then washed three times in PBS/1% BSA for 30 min each time and incubated in secondary antibodies conjugated with fluorescein or Alexa 488 for 3 h. The specimens were then washed and mounted as described above. Confocal images were examined and printed using Adobe Photoshop (Adobe Systems, Inc.).
The primary antibodies to the forked proteins were obtained courtesy of Nancy Petersen (University of Wyoming) and the anti–Drosophila fascin antibodies courtesy of Lynn Cooley (Yale University, New Haven, CT). They were used at concentrations of 1:500 and 1:10, respectively. Secondary antibodies were conjugated with fluorescein (Sigma Chemical Co.) or Alexa 488 (Molecular Probes, Inc.).
Fixation and Methods for Transmission EM
The methods we used are detailed in Tilney et al. 1998.
Quantitation of the Percentage of Cytoplasm Occupied by Actin Filaments
To quantify the percentage of cytoplasm occupied by bundles of actin filaments, we cut transverse thin sections of microchaetes. Since the bristle tapers, the diameter of the cross section through each bristle tells us approximately where the section through the bristle was cut, e.g., near the tip the diameter must be very small, at the base it must be maximal. We then traced each transverse section on mylar film and cut out the tracing and weighted it. We then cut out the region occupied by the 7–11 actin bundles in that bristle and weighed them. The ratio of area or weight occupied by the bundles relative to the cytoplasmic area or weight of the bristle in that section was calculated for transverse sections of bristle with different diameters. This was done for mature bristles, e.g., 48 h or bristles that were fixed at stages in their elongation phase for both mutant and wild-type bristles.
Results
Timed Appearance of Cross-links Does Not Regulate Bundle Formation
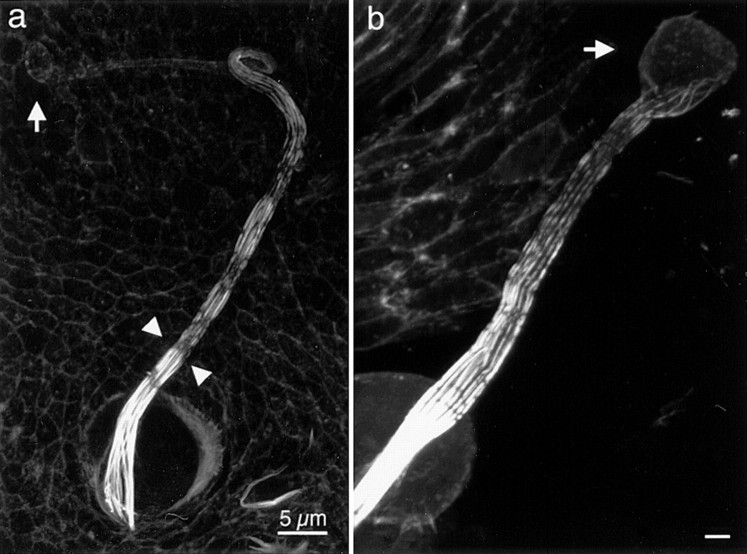
Bristles first sprout from the thorax 32 h after puparium formation and gradually increase in length, reaching their maximum length by ∼48 h. The tiny sprouting bristle present in 32-h pupae contains an excess of the forked proteins when assayed by immunofluorescence microscopy (Wulfkuhle et al. 1998). By thin section EM, each sprout consists of ∼25 tiny cortical bundles of 9–12 filaments (Tilney et al. 1996). Because each tiny bundle is so close to others, we could not distinguish individual bundles by either rhodamine-phalloidin staining or by staining with antibodies to the forked protein (Fig. 2, a and b). The intense forked staining at the bristle tip is likely to represent forked proteins associated with actin filaments plus unbound forked protein within the cytoplasm. It should be emphasized that diffuse staining for forked protein only occurs at the tip where filament elongation and initial bundling occurs. As the bristle elongates, the tip still stained intensely, as did the individual bundles that formed below the tip (Fig. 2, a and b). There was little or no diffuse cytoplasmic staining in regions where there were large discrete bundles. Here the staining was confined exclusively to the bundles. Thus, the localization of the forked proteins at the tip of the bristle correlates precisely with the region in which actin filament polymerization and initial bundle formation occurs.
Figure 2.
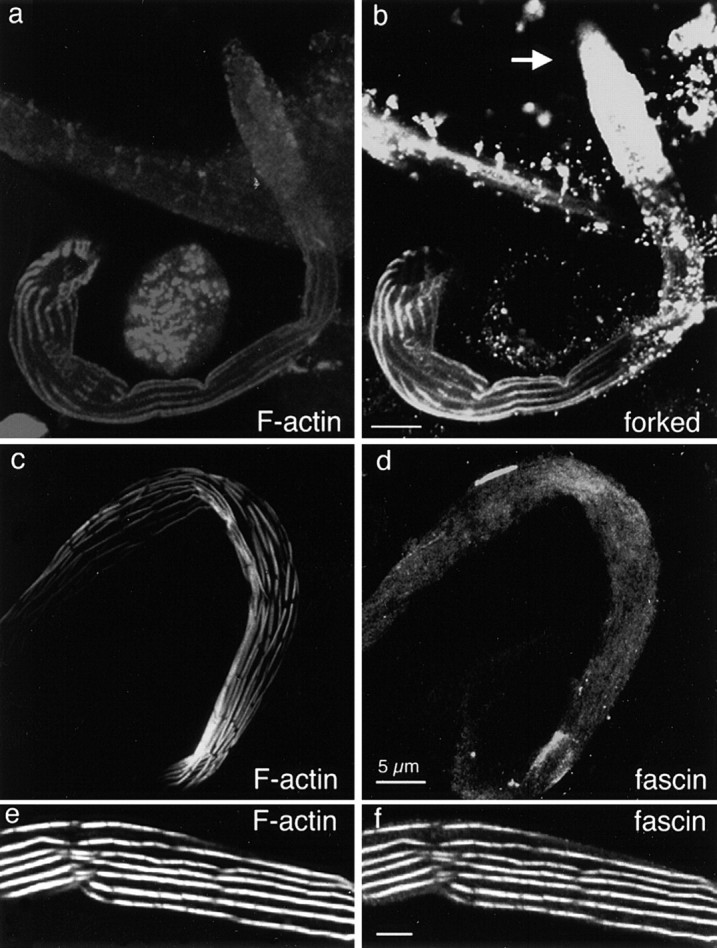
Confocal analysis of cross-linking proteins in developing bristles. A macrochaete from a 36-h pupae was imaged after staining with rhodamine-phalloidin to illustrate F-actin (a), and with an antibody against the forked proteins to show the forked protein distribution (b). Of interest is that except for a diffuse staining at the tip of the bristle, the phalloidin staining is confined to the actin bundles. The forked proteins are present in large amounts at the tip of the bristle (arrow). Along the rest of the bristle shaft, the forked proteins are confined to the actin bundles. A growing macrochaete was imaged after rhodamine-phalloidin staining (c) and staining with antibodies against fascin (d). Of interest is that the fascin which seems to be present in large amounts is present throughout the bristle cytoplasm, not confined to or concentrated in the actin bundles. A mature macrochaete (48 h) was imaged after rhodamine-phalloidin staining (e) and after staining with antibody against fascin (f). Here fascin is localized primarily to mature actin bundles. In addition, some cytoplasmic fascin is still apparent. Bars, 5 μm.
In contrast, fascin was present in large amounts throughout the cytoplasm at the earliest stages in bristle elongation (Fig. 2 d). This large pool of fascin was not localized specifically at the elongating tip nor was it located exclusively on actin bundles identified by fluorescent phalloidin. Instead, most of the fascin was located diffusely throughout the bristle cytoplasm. As elongation proceeded, more and more fascin was found in the bundles and there was progressively less diffuse cytoplasmic labeling, so that mature bristles exhibited largely bundle-restricted fascin staining (Fig. 2 f). Even so, some cytoplasmic fascin was still detected right up to the end of bristle elongation. These results are similar to those described by Wulfkuhle et al. 1998. In conclusion, we found that the first cross-link (forked) was present only at the site of actin filament elongation, the tip, and in formed bundles. The second cross-link (fascin) was present throughout the bristle early in cell elongation. As the bristle grew longer, more fascin was incorporated into the bundles until in mature length bristles, most of it was present in the bundles.
Because there was diffuse staining with the fascin antibody at early stages in bristle elongation, we were concerned that the fascin antibody, or the secondary antibody, or both, might be precipitating or staining nonspecifically. This does not seem to be the case, because the antifascin antibody shows good specificity (Cant et al. 1994) and the secondary antibody reagents alone yield little or no immunofluorescence in our hands. Furthermore, as the bristle elongated to its final length, the staining of the bundles increased, whereas the diffuse cytoplasmic staining decreased.
The Forked Proteins Are Limited at all Stages in Bristle Elongation, which in turn Regulates the Size of the Actin Bundles
Using standard genetic techniques, we constructed animals with increased or decreased levels of cross-linkers. The forked36a allele behaves as a genetic-null and directs the synthesis of little or no forked protein (Petersen et al. 1994). Thus, f36a/f36a homozygotes lack forked protein (designated here as 0× forked) and f36a/+ heterozygotes, we presume, contain ∼50% of wild-type levels of the forked protein (designated 1× forked). In this nomenclature, wild-type animals contain two normal copies of the forked gene (2× forked). It is also possible to examine 6× forked animals containing two copies of the wild-type forked + gene plus four transgenic copies (Petersen et al. 1994). Examination of bristle cells from all these mutant animals is described below.
No Forked Protein (0× forked).
We know that bristles formed in the absence of forked protein are shorter than wild-type bristles (Tilney et al. 1995). More germane to our discussion here is the observation that the actin bundles in the forked-less bristles are tiny relative to the wild-type. The largest contains only 50 filaments compared with 500–700 filaments per bundle in the wild-type. We tried to quantify this difference between wild-type and mutant bristles. Because the bristle tapers, and thus cross-sectional area decreases as one approaches the bristle tip, we measured the cross-sectional area occupied by the bundles and normalized this to the cross-sectional area of the bristle section. This normalization is justified, because the number of filaments per bundle is related to the taper, or to be more accurate, is related quantitatively to the cross-sectional area of that part of the bristle (Tilney, L.G., P.S. Connelly, K.A. Vranich, M.K. Shaw, and G.M. Guild, manuscript submitted for publication). One might wonder why we didn't count the total number of actin filaments in cross sections instead of measuring the cross-sectional area occupied by the bundles, since accurate counts of filament number should be more precise than measuring areas. It turns out that counting the total number of actin filaments throughout an entire cross section is just not possible. This is because the bristle extends in an arc over the thorax with continuous curvature. Thus, even if the bundles on the underside of the bristle are cut perfectly transversely and thus are countable, those on the upper surface will be sufficiently oblique that accurate counts of filament number cannot be carried out. Thus, the total number of filaments across the whole cross section cannot be made. Accordingly, we measured the areas occupied by the filaments, as in most cases the filaments are hexagonally packed, so that area measurements which are directly related to filament number are reliable. In Table we compare filament areas in the forked-less mutants and in wild-type bristles. The values we calculated show that filament bundles in the null mutant occupy only about one third the area occupied by wild-type bundles (2.2% for 0× forked vs. 7.5% for the wild-type). This means that bundles constructed with no forked protein contain ∼29% of the normal number of filaments. It should be remembered that forked-less bristles are ∼56% the length of wild-type bristles (Table ). By making the simplifying assumption that a bristle represents a right circular cone, we can use geometry to estimate that the volume (V = 1/3 πr2h) of a forked-less microchaete is 56% that of the wild-type. By combining the reduction in filament number and the reduction in cell volume, we estimate that the forked-less bristles contain ∼16% (0.29 × 0.56) of the polymerized actin, or a sixfold reduction relative to the wild-type bristles. Since the singed genes (encoding fascin) were not affected in these mutants, the bundles, although tiny, are composed of hexagonally packed filaments. In longitudinal section they display the 12-nm period attributable to fascin (Tilney et al. 1995, Tilney et al. 1998).
Table 1.
Actin Bundle Size in Wild-type and Mutant Bristles
| Gene copy no. | Time | Relative bundle area | No. observed | ||
|---|---|---|---|---|---|
| forked | singed | ||||
| h | % | ||||
| Wild-type | 2 | 2 | 40 | 6.3 | 7 |
| Wild-type | 2 | 2 | 48 | 7.5 | 11 |
| 0× forked | 0 | 2 | 48 | 2.2 | 3 |
| 1× forked | 1 | 2 | 46 | 5.0 | 4 |
| 6× forked | 6 | 2 | 48 | 15.3 | 2 |
| 0× fascin | 2 | 0 | 46 | 4.8 | 9 |
| 1× fascin | 2 | 1 | 48 | 10.0 | 5 |
Table 2.
Wild-type and Mutant Bristle Lengths
| Gene copy no. | Time | Length | No. observed | ||
|---|---|---|---|---|---|
| Forked | Singed | ||||
| h | |||||
| Wild-type | 2 | 2 | 48 | 63.1 μm ± 1.99 | 40 |
| 6× forked | 6 | 2 | 48 | 60.26 μm ± 0.9 | 84 |
| 1× forked | 1 | 2 | 48 | 69.7 μm ± 1.7 | 43 |
| 0× forked | 0 | 2 | 48 | 35.5 μm ± 0.63 | 28 |
| 0× fascin | 2 | 0 | 48 | 56.9 μm ± 1.68 | 37 |
| 1× fascin | 2 | 1 | 48 | 67.6 μm ± 1.3 | 46 |
| 0× fascin, 0× forked | 0 | 0 | 48 | 42.9 μm ± 0.71 | 47 |
1× forked.
Wild-type females were mated with f36a males to yield f36a/+ female pupae. These animals should exhibit an ∼50% reduction in the amount of forked protein, but contain wild-type levels of fascin. The bundles in these mutant bristles are thinner than in the wild-type and are often wavy in profile (Tilney et al. 1998). Thin sections through the bundles reveal that even though there were patches in each bundle where the filaments were hexagonally packed, most of the filaments in the bundles displayed a more diffuse packing. As with the other mutants, we compared the normalized cross-sectional areas occupied by the filament bundles. We found that this area was ∼2/3 that of the wild-type (5% in 1× forked animals vs. 7.5% in the wild-type). Since the filaments were not hexagonally packed (a situation that maximizes the number of filaments per unit area) this means that the number of filaments present in comparable regions of the poorly packed bundle was reduced significantly more than is indicated by our area measurements. From actual counts of the number of filaments present in four maximally cross-linked wild-type bundles and in three liquid order 1× forked bundles in cross-section, we found that the density of filaments is 25% less in liquid order bundles than in maximally cross-linked bundles. Taking into account both differences in density due to liquid ordering and in area, we calculate that there is an overall reduction in the number of filaments by 46%, or twofold. This reduction must be present throughout the bristle because bristles in the 1× forked animals and the wild-type are similar in overall length (Table ).
6× forked.
We also examined bristles from the stock with a total of six copies of the forked gene constructed by Petersen et al. 1994. What is significant for our discussion here is that the actin bundles in these bristles were very large and often triangular in shape. In addition, smaller bundles of filaments were also found in the cytoplasm and some of these were connected on one of their surfaces to the large triangular-shaped peripheral bundles (Fig. 3). The filaments in these bundles were hexagonally packed and in longitudinal section showed the 12-nm period, indicating that fascin is present (see Tilney et al. 1998). We compared the relative cross-sectional areas of these bundles with wild-type bristle bundles (Table ). Of interest is the large increase in area occupied by bundles, e.g., 15% in the 6× forked vs. 7.5% in the wild-type, indicating that additional forked protein will drive the formation of even larger bundles.
Figure 3.
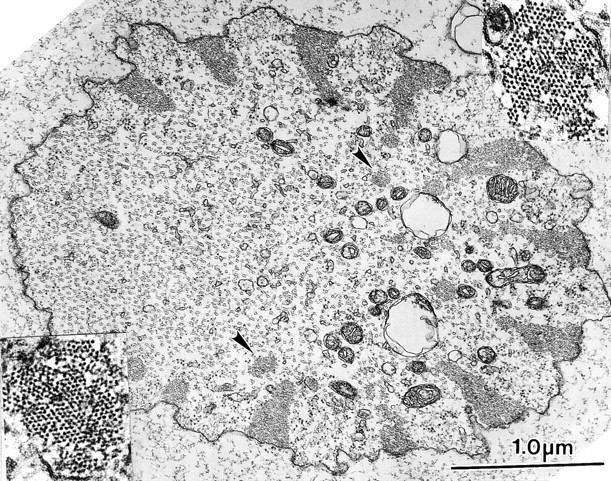
Transverse section through a microchaete from a mutant containing a wild-type (2×) number of fascin genes but an excess (6×) of forked genes. Of interest is that the membrane-associated actin bundles are triangular in shape. Nearby these bundles, arrowheads indicate many small internal bundles. The filaments in all bundles are hexagonally packed (see insets) and show a 12-nm period in longitudinal section (data not shown).
Fascin Is Not Limiting during Bristle Elongation
1× Fascin.
Since by immunofluorescence microscopy fascin was present in large amounts in bristles (Fig. 2 d), we asked if that fascin is competent to fully cross-link actin filaments even if the gene dosage for fascin is reduced. Wild-type females were mated with sn3 males to yield sn/+ female pupae with an ∼50% reduction in fascin, the product of the singed gene. When we compared the actin bundles of the wild-type animals containing two functional copies of the singed gene (2× fascin) with those found in heterozygous singed mutants containing only one functional copy of the singed gene (1× fascin), we found that both bristle types had a comparable number of similarly sized bundles (Fig. 4 a). Note that both bristle types contained two functional copies of the forked gene. Of particular interest here is that the filaments in the bundles were hexagonally packed, and in longitudinal section display the 12-nm period (Fig. 5 b) attributable to fascin (Tilney et al. 1995, Tilney et al. 1996). There were small regions or gaps in the bundles of both the mutant and the wild-type in which filaments were missing (Fig. 5 a, insets). Recall that in phase 2 of bundle formation (Fig. 1) tiny bundles aggregate together. Since these tiny bundles are irregular in shape, gaps will appear in the aggregate if additional filaments are not added.
Figure 4.
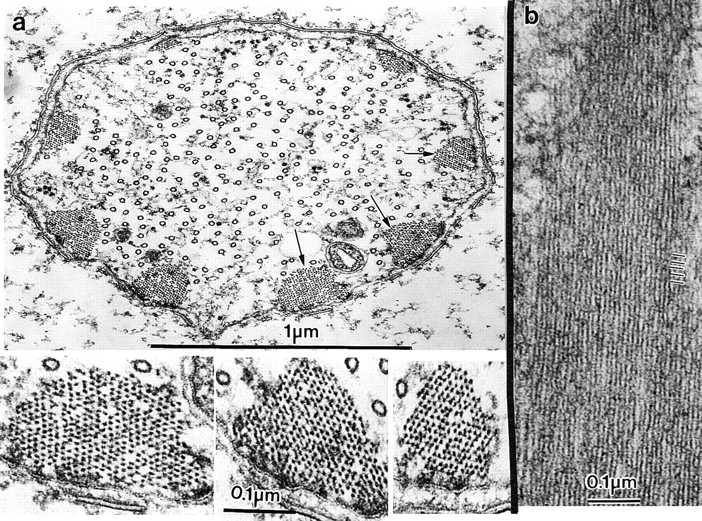
Thin sections through fully elongated bristles of a sn/+ pupae (1× fascin) containing one half the number of fascin genes as the wild-type. (a) Transverse section through a microchaete showing eight cortical actin bundles and a central core of microtubules. Three of the actin bundles indicated by the arrows are shown at higher magnification below. Notice that the dots which are actin filaments cut in cross section are hexagonally packed, albeit with a few gaps in the packing. Similar gaps are seen in the wild-type bristles. (b) Longitudinal section through an actin bundle. The lines at the margin of the micrograph indicate 12-nm striations, which demonstrate the presence of fascin.
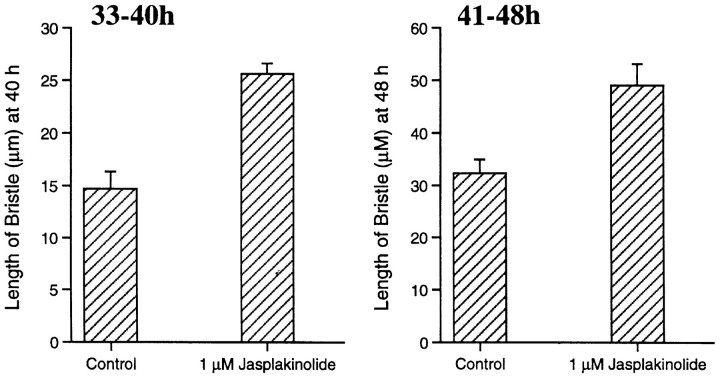
Figure 5.
Bar graphs illustrating that in the presence of jasplakinolide, the bristles elongate faster than in untreated bristles. These thoraces were cultured for 7 h. Half the thoraces were incubated in 1 μM jasplakinolide, then fixed, and the lengths of the bristle compared with the other half cultured at the same time without jasplakinolide.
Quantitatively, the relative cross-sectional areas occupied by the filaments in the wild-type (7.5%) and the 1× fascin bristle cells (10%) are not significantly different (Table ). If anything, the 1× fascin areas were slightly larger. Furthermore, since the bristles in this mutant and the wild-type have the same overall length (Table ), this pattern is constant all along the bristle length. Thus, mutants that contain only one fascin gene still form bundles that are quantitatively and qualitatively similar to the wild-type.
2× Fascin, 6× forked.
We also examined bristles from the stock containing a total of six forked genes. This stock produces normal amounts of fascin. What is significant here is that even though there was an increase in the number and size of bundles (Fig. 3; Table ), all the actin filaments were maximally cross-linked by fascin as indicated by the fact that the filaments are all hexagonally packed and display the 12-nm period in longitudinal section. Therefore, fascin cannot be limiting in these mutants, even though the amount of polymerized actin is twice as much as the wild-type.
Actin Is Not Limiting during Bristle Elongation
Two sets of observations lead us to the conclusion that actin is not limiting during bristle elongation. First, in bristle cells containing an excess of the forked genes (6× forked), there appeared to be a large increase in the number of actin filaments (Table ; Fig. 3), even though the average lengths of bristles in the 6× forked and the wild-type animals were not significantly different (Table ). Accordingly, the amount of actin is either upregulated in this mutant, possibly in response to the extra copies of the forked genes, or there is an excess of monomeric actin in the bristle cell that can be polymerized into filaments and incorporated into bundles.
Second, we carried out a series of experiments in which we incubated isolated thoraces in the sponge toxin, jasplakinolide. From in vitro experiments of Bubb et al. 1994, this toxin, like phalloidin, stabilizes existing filaments by lowering (to zero) the off rate of monomers from filaments. Accordingly, in the presence of this toxin the number of filaments should increase if there is a pool of unassembled actin monomer, because once a filament polymerizes it should be unable to disassemble. Since jasplakinolide, unlike phalloidin, is membrane-permeable we asked what happens to the rate of bristle elongation when the thoraces are cultured in this drug and how is this related to the appearance of bundles and/or free filaments in bristle cytoplasm.
Before describing our results we should mention that in order to study the effect of jasplakinolide on bristle elongation, it was necessary to isolate the thorax from the whole animal and culture it in vitro. This was necessary because the dorsal surface of the pupae is covered with a thick exoskeleton of chitin, and thus is impermeable to drugs like jasplakinolide. However, after isolation the basal or internal surface of the thoracic epithelium is permeable to drugs like jasplakinolide. Details of our culture system and characterization of bristle elongation in vitro will be given in another report (Tilney, L.G., P.S. Connelly, K.A. Vranich, M.K. Shaw, and G.M. Guild, manuscript submitted for publication). Suffice it to say that elongation in vitro occurs at rates identical to undissected pupae for at least 7 h at 25°C, and the fine structure of the actin bundles is identical to that already described in situ.
When we examined bristle elongation in the presence of jasplakinolide, we were surprised to discover that the rate of elongation of the bristles was 50–75% faster than in control thoraces (Fig. 5). This was true for two different time periods, one where thoraces from pupae 33 h of age were incubated for 7 h, and where pupae 41 h of age were incubated for 7 h.
Examination of thin sections through bristles treated with jasplakinolide for 5 h (36–41-h pupae) or 2 h (41–43-h pupae) and then fixed revealed that besides the 7–11 cortical actin bundles, there were numerous internal bundles, many of which were composed of 25 filaments or less. Interestingly, the filaments in these small bundles were hexagonally packed, indicating that fascin must be present (Fig. 6, insets). Sometimes these small bundles were perpendicular to the long axis of the bristle. Accordingly, these bundles must be short, minimally no longer than the diameter of the bristle. In addition to the small bundles, there were snarls of randomly oriented filaments (Fig. 6, arrows). Thus, the more rapid elongation in jasplakinolide is correlated with increased assembly of small bundles of cross-linked filaments as well as snarls of individual filaments, all of which increases the rate of bristle elongation. Unfortunately we have no way of quantifying the amount of polymerized actin in each bristle after treatment with jasplakinolide, since quantification of the amount of actin in the tiny bundles and in the snarls is impossible. However, our impression is that actin monomers are not limiting during bristle elongation.
Figure 6.
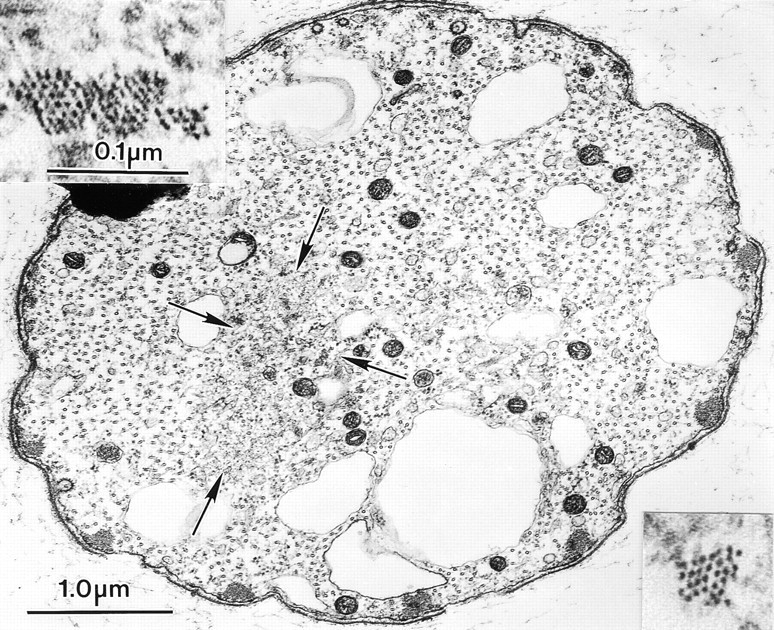
Transverse thin section through a thoracic bristle from a 43-h pupae that had been cultured for 2 h in medium containing 1 μM jasplakinolide. Of interest is that besides the 11 small peripheral actin bundles, there is a central region containing a snarl of randomly oriented actin filaments (arrows). Within this snarl are tiny bundles of actin filaments (insets) that are hexagonally packed.
The Amount of the Protein Connecting the Bundles to the Plasma Membrane Is Fixed
Using a planimeter, we measured from individual thin sections the total length of the plasma membrane surrounding the bristle and the length of this perimeter connected to the actin bundles. Because the bristle tapers, and thus cross-sectional areas decrease as one approaches the bristle tip, we calculated the fraction of the perimeter connected to the actin bundles (Table ). Although there was individual variation from section to section, the mean values of 11 measurements show that 30% of the bristle perimeter had bundles connected to it in mature wild-type bristles irrespective of bristle diameter (Table ). The simplest explanation for these observations is that the amount of material that attaches the bundles to the plasma membrane is limiting and dependent upon cytoplasmic volume. Interestingly, in mutant bristles where the size of actin bundles is reduced or enhanced due to a reduction in fascin expression (0× fascin or 1× fascin) or in forked overexpression (6× forked), the fraction of the perimeter attached to the bundles remained remarkably constant (Table ). However, if bundle size is below a minimum value, one might expect a lower percentage of membrane occupation by bundles. This was the case for the 0× and 1× forked mutants (Table ).
Table 3.
Percentage of Plasma Membrane Connected to Actin Bundles in Wild-type and Mutant Bristles
| Gene copy no. | Membrane occupied | |||
|---|---|---|---|---|
| forked | singed | No. observed | ||
| % | ||||
| Wild-type | 2 | 2 | 31 | 11 |
| 0× forked | 0 | 2 | 21 | 3 |
| 1× forked | 1 | 2 | 22 | 4 |
| 6× forked | 6 | 2 | 29 | 7 |
| 0× fascin | 2 | 0 | 29 | 10 |
| 1× fascin | 2 | 1 | 34 | 5 |
Next we wondered if the perimeter fraction connected to actin bundles was constant during elongation. Within experimental error, the values for all stages in development were similar to the mature bristle, except for the 36-h 6× forked mutant where the value of 20 vs. 30% seemed somewhat lower (Table ). With this exception, it appears as if the actin membrane–connecting material becomes available at the same rate during the formation of bundles.
Table 4.
Developmental Comparison of the Percentage of Plasma Membrane Connected to Actin Bundles
| Pupal age | Gene copy no. | Membrane occupied | No. observed | ||
|---|---|---|---|---|---|
| forked | singed | ||||
| h | % | ||||
| 36 | Wild-type | 2 | 2 | 36 | 8 |
| 40 | Wild-type | 2 | 2 | 27 | 9 |
| 48 | Wild-type | 2 | 2 | 30.6 | 11 |
| 36 | 6× forked | 6 | 2 | 19.5 | 17 |
| 40 | 6× forked | 6 | 2 | 36.6 | 2 |
| 48 | 6× forked | 6 | 2 | 29 | 7 |
| 40 | 0× fascin | 2 | 0 | 27.6 | 7 |
| 46 | 0× fascin | 2 | 0 | 28.8 | 10 |
The Amount of Cross-linkers Determines the Number of Filaments and thereby the Shape of the Bundles
By comparing wild-type and mutant bristles in thin sections, it is obvious that the shape of the 7–11 membrane-associated actin bundles varies depending on the mutant. To illustrate this, we made tracings of representative cross sections cut through wild-type and mutant bristles (Fig. 7). In the wild-type, the actin bundles in mature bristles were semicircular in shape. In both the singed mutant (0× fascin), a mutant that fails to express fascin, and in the 0× forked mutant, the number of actin filaments was reduced (see Tilney et al. 1995, Tilney et al. 1998) and the bundles are ribbon-like with the flat surfaces of the ribbon attached to the limiting membrane. In contrast, in mutants that contain a large number of filaments such as 6× forked, the bundles are triangular in shape with the small side of the triangle attached to the limiting membrane. In the singed-forked double mutant, a mutant lacking both fascin and forked (0× forked, 0× fascin), there was an extreme reduction in the number of actin filaments. Only a monolayer of actin filaments is seen attached to the limiting membrane (see Tilney et al. 1995). From these examples, it seems likely that bundle shape is related to the number of actin filaments present, provided there is a fixed amount of material connecting the bundle to the membrane.
Figure 7.
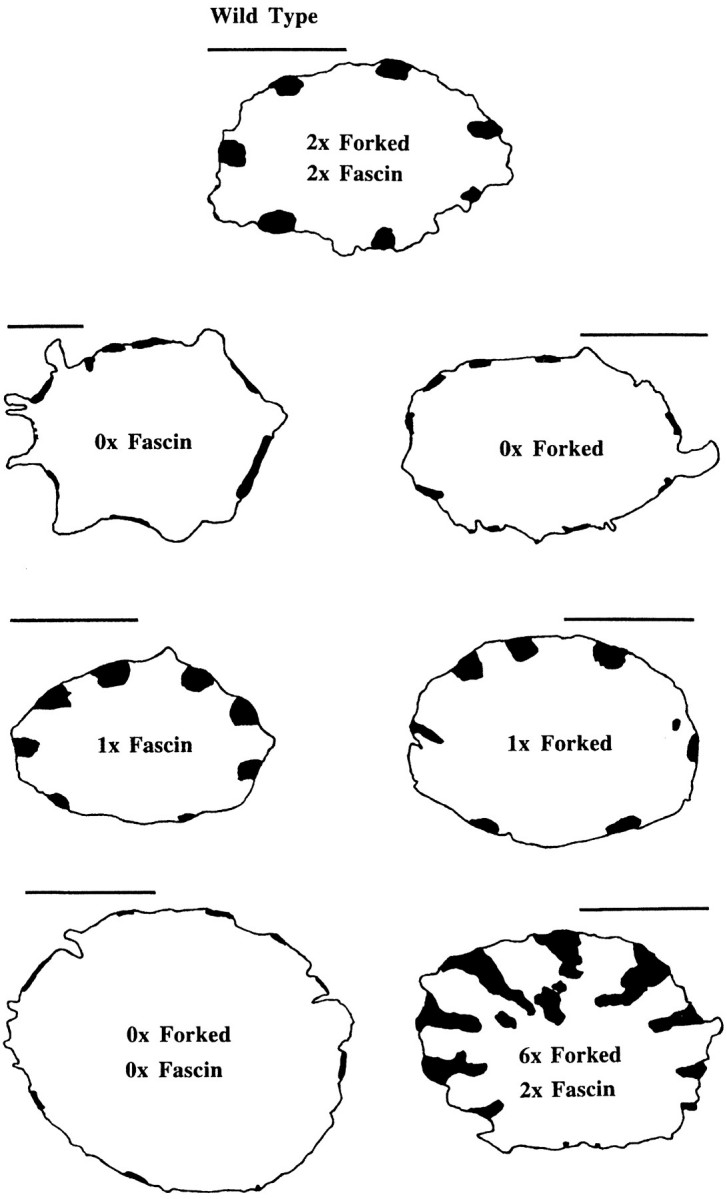
Tracings of transverse sections of bristles from a wild-type and six different mutant pupae. The actin bundles present in these micrographs are in black. Notice the difference in size and shape of the bundles. Bars, 1 μm.
Inhibitors of Phosphorylation and Phosphatase Activity Affect Bundle Formation
From our studies and those of Wulfkuhle et al. 1998 using antifascin antibodies and our studies on mutants, we conclude that fascin is present in excess in the cytoplasm from the initial stages of bristle elongation all the way to maturity. Because this fascin seems to be functional based upon our observations on the 1× fascin, the 6× forked animals, and the results of incubation with jasplakinolide, one wonders how a sequential usage of cross-links (forked then fascin) can be controlled. An obvious possibility is the posttranslational modification of the cross-links either by phosphorylation or dephosphorylation, or coupling to other as-yet unknown components. To address the first possibility, we added drugs that affect phosphorylation (staurosporine) or dephosphorylation (okadaic acid).
Blocking Phosphorylation.
Staurosporine is a potent, cell-permeable broad spectrum inhibitor of protein kinases (Tamaoki et al. 1986; Matsumoto and Sasaki 1989). We found that although this drug does not affect the rate of bristle elongation (Fig. 8), abnormalities in the actin bundles formed during treatment as well as damage to bundles that had formed before treatment occured. These changes could be seen by studying the fluorescence of phalloidin-stained 41-h bristles that had been cultured in staurosporine from 36–41 h. In the apical portion of the bristle, a region where new bundles were formed in the presence of staurosporine, instead of large bundles we found weakly fluorescent bundles with large gaps between modules (Fig. 9, a and b). Although the gaps in adjacent bundles were generally in transverse register as in untreated bristles, the modules were very short in length. At the extreme tip of the bristle we failed to find modules or they were so weakly fluorescent as to be invisible; in some cases the tip of the bristle was bulbous (Fig. 9 b).
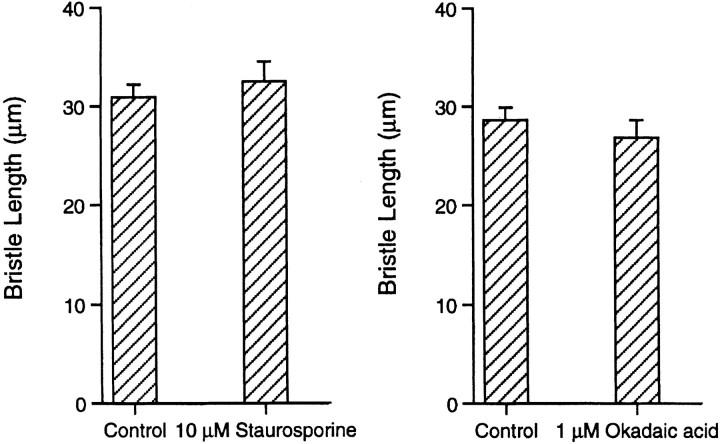
Figure 8.
Bar graph depicting the elongation of bristles from isolated thoraces of 36–41-h pupae incubated in the presence of staurosporine or okadaic acid. The rate of elongation in these drugs do not vary from untreated thoraces incubated at the same time.
Figure 9.
Confocal micrographs of the two bristles from isolated thoraces which have been incubated in staurosporine for 5 h (36–41-h pupae), then stained with fluorescent phalloidin. Weakly fluorescent bundles are found in apical region formed in the presence of staurosporine (arrowheads in a). Both bristles exhibit bulbous tips (arrows). Bars, 5 μm.
In thin transverse sections cut through the basal end of bristles and thus through bundles that had formed before staurosporine treatment, we found large bundles as expected, but the packing of the filaments in these bundles was less ordered (Fig. 10 a, inset). There were regions of hexagonal packing, but also regions where the filaments, although closely packed, were not hexagonal. In longitudinal sections we found regions (presumably the same regions where the filaments are hexagonally packed) with the 12-nm period (Fig. 10 b) attributable to fascin. There were also regions with no 12-nm periodicity, which presumably correspond to the regions in which the filaments are not hexagonally packed.
Figure 10.
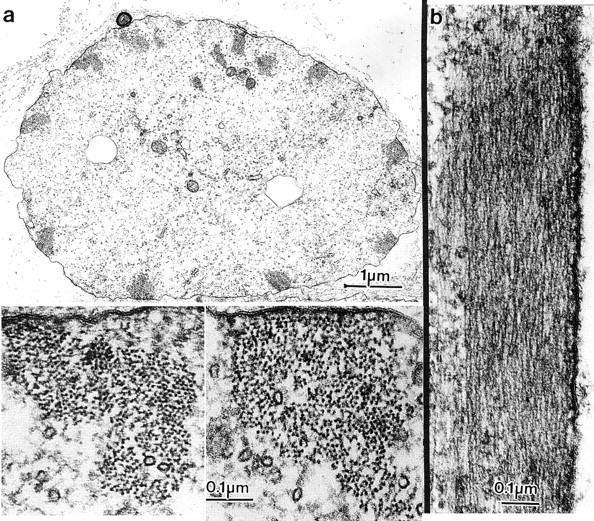
Thin sections through the basal portion of bristles from thoraces that had been incubated with staurosporine for 5 h (36–41-h pupae). (a) Transverse section. Around the periphery of this bristle are 11 actin bundles and a central population of microtubules. Two of the bundles are depicted at higher magnification in the insets. Notice that although there are regions where the packing is hexagonal, in many places the packing lacks hexagonality and is disordered. (b) Longitudinal section through an actin bundle. If this figure is turned 90° so one can look across the bundle, one sees some 12-nm striations due to the fascin cross-bridge, but these striations are not present throughout the bundle, indicating a reduction of the fascin cross-bridge.
In regions midway between the base and the tip where bundles were in the process of being formed when the drug was applied, we found very small bundles of filaments attached to the limiting membrane, e.g., maximally containing only ∼40 filaments (Fig. 11). These very small bundles must represent the weakly fluorescent bundles we saw by phalloidin labeling. In these regions the filaments do not appear hexagonally packed.
Figure 11.
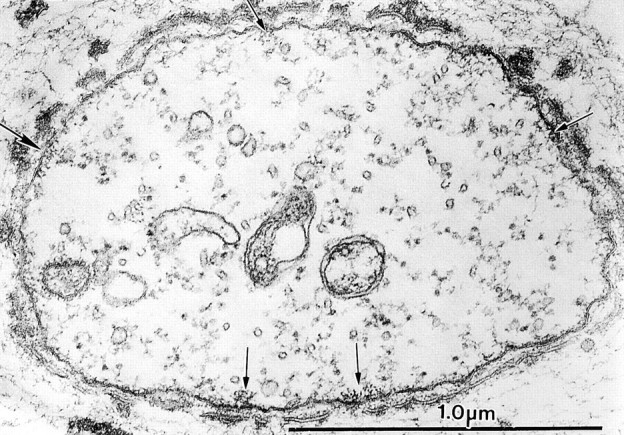
Thin transverse section near the tip of a bristle from a thorax cultured in the presence of staurosporine for 5 h (36–41-h pupae). Of interest is the lack of large actin bundles attached to the plasma membrane. Instead, we see tiny clusters of actin filaments (arrows).
In transverse sections, through what we think are the bulbous tips based upon the diameter of the bristle and the presence of a large population of microtubules cut in transverse section, we are hard pressed to find any cortical bundles of actin (data not shown). However, tiny internal bundles are present.
In summary, staurosporine inhibits the formation of new clusters of actin filaments by forked and fascin as well as partially breaking down the cross-linking of fully formed, hexagonally packed bundles. Accordingly, we presume that phosphorylation of fascin, a cross-linker that stabilizes mature hexagonally packed bundles, must be regulated at least in part by phosphorylation. In contrast, tiny or small bundles that are not hexagonally packed remain and/or are formed in the presence of staurosporine during bristle extension. Such nonhexagonal packing that occurs must be attributable to the forked cross-links (see Tilney et al. 1995, Tilney et al. 1998), a fact that suggests that the activity of forked is not influenced by phosphorylation.
Blocking Dephosphorylation.
Okadaic acid is a potent inhibitor of protein phosphatases 1 and 2A, but not of tyrosine phosphatases or alkaline or acid phosphatases (Cohen et al. 1990). As with staurosporine, if the dorsal thorax is cultured in okadaic acid in pupae 36–41 h of age, the rate of bristle elongation was identical to thoraces cultured without okadaic acid (Fig. 8). However, thin sections of bristles from 41-h pupae fixed after okadaic acid treatment for 5 h revealed a striking change from the control. Although the filament bundles were hexagonally packed and display in longitudinal section the 12-nm period attributable to fascin, in most cases the bundles were not attached to the limiting plasma membrane but were instead located in the cytoplasm proper (Fig. 12). Near the base of the bristle, the bundles tended to cluster together, but elsewhere along the bristle they were located at any position in the cytoplasm. In those rare instances in which the bundles were connected to the plasma membrane, only a few filaments on the membrane-facing surface of the bundle are connected to the membrane. Often, the nonconnected filaments adjacent to the membrane lost their hexagonal packing and were loosely packed.
Figure 12.
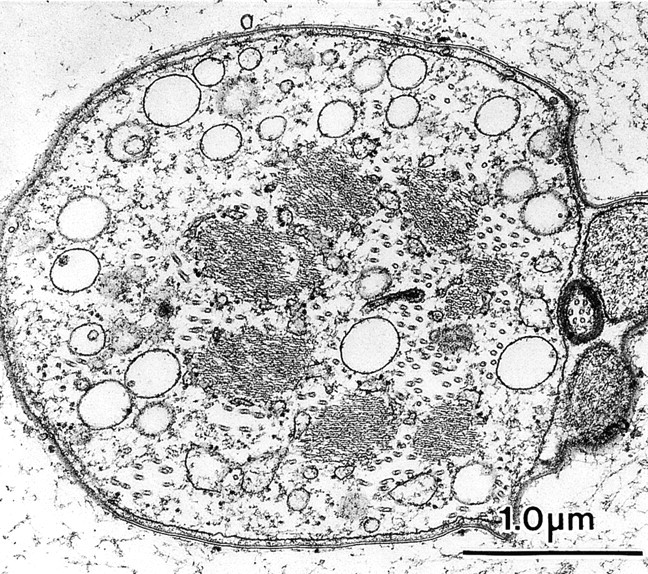
Thin transverse section through a bristle from a thorax treated with okadaic acid for 5 h (36–41-h pupae) then fixed. Of interest is that the seven or eight actin bundles are not attached to the plasma membrane but are instead found within the central cytoplasm of the bristle shaft along with microtubules.
We noted two additional features of okadaic acid–treated bristles. First, there were frequently gaps in the large bundles as if the individual subbundles fail to adhere to each other. Thus, small bundles were present either adjacent to larger bundles or scattered throughout the cytoplasm, albeit still oriented parallel to the longitudinal axis of the bristle. In some cases, we have transverse sections through what we assume is the tip of the bristle, in which there is a large population of microtubules, but no actin bundles are visible. Second, okadaic acid affects cuticle deposition. In thin sections through bristles we found that the cuticle balloons away from the plasma membrane as if cuticular material was being exocytosed from the bristle cytoplasm at a higher rate than normal.
To summarize, blocking phosphatases does not affect cross-bridging or filament number, but instead affects the attachment of the bundles to the plasma membrane and individual subbundles to each other. Cuticle disposition is also affected but exactly why remains unclear.
Discussion
The Forked Cross-linker Controls Bundle Assembly
We showed previously that the formation of actin bundles in Drosophila bristles requires the sequential action of two cross-linking proteins, forked and fascin (Tilney et al. 1995, Tilney et al. 1998). The forked protein acts first to aggregate tiny bundles into the 7–11 larger membrane-associated bundles. It then facilitates fascin binding that results in straight rigid bundles (Fig. 1). These data suggest a sequential appearance of crossbridges. Surprisingly we show here, as did Wulfkuhle et al. 1998, using antibodies to fascin, that fascin is present in excess from bristle initiation throughout elongation. Similar conclusions were reached using mutants. In contrast, forked is localized to the tip of elongating bristles and to the formed actin bundles and is limiting at all stages in elongation. We conclude that a major control of actin bundle assembly in Drosophila bristles is the amount and/or availability at each step in elongation of only a single component, the forked protein. Recently, Grieshaber and Petersen 1999 showed that overexpression of forked in cultured vertebrate cells also drives the formation of actin bundles, again indicating forked's pivotol role in actin bundling.
We also showed that forked must be limiting because attempts to stimulate bundle assembly with jasplakinolide, although resulting in increased rates of elongation, did not increase bundle size. Rather, many tiny bundles and snarls of actin developed, and both of these require no additional amounts of the forked protein.
There Must Be a Fixed Amount of Material Connecting the Bundles to the Membrane
If the percentage of the inner surface of the plasma membrane to which the actin bundles bind is constant irrespective of the number of filaments in the bundle, then proteins that connect the outermost filaments of a bundle to the plasma membrane must be made in limited amounts. What is interesting and important is that this membrane-connecting substance(s) must be synthesized or activated gradually throughout bristle elongation, just like actin and forked. If these membrane-connecting substances were present in excess or activated all at once, enormous numbers of actin filaments would be connected to the limiting membrane at all stages in bristle elongation, something we do not see. Furthermore, since module formation begins with the appearance of tiny membrane-associated bundles, this unknown connector must be present as bristles sprout. Significantly, if one compares the amount of membrane surface occupied by actin filaments relative to the entire perimeter at the earliest stage (Fig. 1, phase 2) to the amount seen in the later stages when the bundles become maximally cross-linked by fascin, the percentage of the surface connected to actin seems roughly constant (Table ).
Bundle Shape Is Controlled by Actin Membrane Interactions and Filament Number
From our data (e.g., Table ), we find that the fraction of membrane surface involved in binding the bundles is constant in most mutants, even though for varying reasons the number of actin filaments in the bristles can vary over an order of magnitude. Nevertheless, assuming that all the actin filaments are in membrane-associated bundles, it seems reasonable to conclude that bundle shape must be a product of the number of actin filaments, provided that the bundle–plasma membrane connector is constant, which observationally it is. Thus, if the number of filaments is low (e.g., in 0× fascin mutants), a situation where there is a sixfold reduction in bundle size yet the amount of membrane connector substance is constant, the bundles must be flat or ribbonlike. Indeed, they are. Alternatively, if there is an excess of actin filaments per bristle (e.g., in 6× forked) and the membrane connector is constant, the bundle should appear triangular in shape with the small side of the triangle attached to the plasma membrane. Again this is true. What is important to recognize is that bundle shape may in turn determine the overall characteristic of the bristle. Thus, if the bundle is thin or ribbonlike, the bristle may be bent or twisted and lack rigidity like those found in singed and forked mutants. On the other hand if the bundles are large and semicircular or triangular in shape, the bristle may be very stiff and straight as it is in Stubble mutants (our unpublished observations) and 6× forked (Tilney et al. 1996). Thus, bundle shape, and accordingly, bundle rigidity seem to be a product of a constant amount of membrane actin connector and the number of filaments per bundle, assuming of course that the filaments in the bundles are cross-linked.
Phosphorylation Affects Bundle Size and Cross-linking
Staurosporine is a broad spectrum inhibitor of protein kinases. Of interest is that the filament bundles that formed before incubation in staurosporine remain after treatment, but the filaments making up the bundles were no longer hexagonally packed. Instead, the filaments appear randomly ordered, albeit with tiny regions of hexagonality. In longitudinal section, the 12-nm period attributable to fascin is seen to be largely lost. In the distal third of the bristle, or in regions of the bristle where bundles were formed during incubation with staurosporine, we find only tiny bundles. The filaments in these bundles are closely packed but not hexagonally packed, and again lack the 12-nm period in longitudinal section attributable to fascin. All these data combined suggest that inhibition of phosphorylation inhibits fascin cross-linking. Of course, inhibition of phosphorylation may not affect fascin directly, but rather could involve a cascade that indirectly affects fascin cross-linking. In either case, the net result would be improper cross-linking by fascin. Interestingly, work from Matsumura's laboratory has shown that whereas phosphorylation by kinases can regulate actin–fascin interactions in vitro (Yamakita et al. 1996; Shoichiro et al. 1997), the stoichiometry of in vivo phosphorylation is low, suggesting that other mechanisms control fascin–actin interactions under normal conditions. In addition, since only tiny bundles form during staurosporine treatment as assayed either by our phalloidin staining or our thin sections, the forked cross-link may also be affected either directly or indirectly by this drug.
Phosphorylation Inhibits Bundle–Membrane Attachment
Thus far we do not know the identity of the linker(s) connecting the bundles to the membrane. The mutants we have on hand and/or have generated have not helped us identify these components. Nevertheless, we have managed to learn something about the behavior of this substance by using okadaic acid, a phosphatase inhibitor. We show here that this drug must inactivate this connector, either directly or indirectly, so that the bundles lose their connections to the plasma membrane. Since these internal bundles are found at or near the base of the bristle, this means that the bundles that were connected to the plasma membrane before incubation in okadaic acid became detached from the membrane during incubation.
Several additional facts can be gleaned from studying bristles grown in the presence of okadaic acid. First, since the bristle continues to elongate with okadaic acid present and since there are bundles of actin all the way to the bristle tip, we presume new bundles can form. Second, the 12-nm period attributable to fascin remains after incubation in okadaic acid, indicating that fascin cross-linking is not compromised. Even so, not only does the layer of actin filaments immediately attached to the membrane lose its connection to the membrane, but deeper layers of actin filaments near the former membrane attachment site become uncross-linked from one another and appear randomly oriented. Since forked is responsible for subbundle-to-subbundle assembly, the simplest explanation for the okadaic acid results is that forked is also involved in the membrane–bundle connection. Of course, it could be more complicated than this, as our images are reminiscent of the early stages in actin bundle breakdown, e.g., in 54–56-h pupae (see Tilney et al. 1996).
Okadaic acid also has an effect upon other events that occur in conjunction with the plasma membrane, because cuticle deposition, a process that involves exocytosis, is often disrupted after okadaic acid treatment. What we see is that the cuticle often balloons away from the plasma membrane as if it is not connected properly.
During Bristle Elongation There Has To Be a Coordination between Actin Assembly, Cross-linking, Connections to the Plasma Membrane, and the Overall Length and Width of the Bristle
What is obvious is that even though there are many steps in bristle assembly, all of these must be coordinated. Even so, we should emphasize that bristle elongation is not directly linked to normal bundle growth, because if jasplakinolide is added to elongating bristles they grow faster, but the newly formed bundles at the bristle tip are small and never reach mature size. Likewise, bristles elongate at the same rate in the presence or absence of staurosporine, yet the bundle size in staurosporine is tiny relative to the untreated bristles. Furthermore, reduction in the forked proteins or elimination of fascin allows bristle elongation, but the bristles are shorter than normal and are bent, twisted, or forked. Thus, many components are essential to form a wild-type bristle. Many of these, by changing the characteristics of the actin bundles, affect the elongation process. Others do not, even though the characteristics of the bristle are altered. These cautions notwithstanding, what we have concluded here using mutants and antibodies is that the precise regulation of forked determines the size of the bundles, or to put this another way, the number of actin filaments per bundle. But it is also clear that the amount of membrane actin cross-linker regulates bundle shape and thus the overall characteristics of the bundle. What we have failed to mentioned is how bristle length and bristle width must also be controlled. From earlier work of Lees and Picken 1944, bristle volume is the same in the singed and Stubble bristles, but since both are shorter than the wild-type they are proportionally larger in diameter. How all these factors play out in morphogenesis is our continuing puzzle.
Acknowledgments
Many thanks to Nancy Petersen and Lynn Cooley for their generous gifts of fly strains and antibodies against the forked protein and fascin, respectively. Special thanks go to the reviewers of this manuscript for their helpful comments and particularly one who spent much time improving our English and therefore the readability of this manuscript.
This work was supported by grants from the National Institutes of Health GM-52857 to L.G. Tilney, and from the University of Pennsylvania Research Foundation to G.M. Guild.
References
- Bainbridge S.P., Bownes M. Staging the metamorphosis of Drosophila melanogaster . J. Embryol. Exp. Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Bubb M.R., Senderowicz A.M.J., Sausville E.A., Duncan K.L.K., Korn E.D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- Cant K., Knowles B.A., Mooseker M.S., Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J. Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Holmes C.F.B., Tsukitani Y. Okadaic acida new probe for the study of cellular regulation. Trends Biochem. Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Eaton S., Wept R., Simmons K.S. Roles of Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila . J. Cell Biol. 1996;135:1277–1289. doi: 10.1083/jcb.135.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FlyBase Consortium FlyBasea Drosophila database. Nucleic Acids Res. 1998;26:85–88. doi: 10.1093/nar/26.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshaber S., Petersen N.S. The Drosophila forked protein induces the formation of actin fiber bundles in vertebrate cells. J. Cell Sci. 1999;13:2203–2211. doi: 10.1242/jcs.112.13.2203. [DOI] [PubMed] [Google Scholar]
- Hacker U., Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changed during gastrulation in Drosophila . Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N., Loh H.Y., Chia W., Lim L. A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila . Development. 1995;121:903–914. doi: 10.1242/dev.121.3.903. [DOI] [PubMed] [Google Scholar]
- Lees A.D., Picken L.E.R. Shape in relation to fine structure in the bristles of Drosophila melanogaster . Proc. R. Soc. Lond. Ser. B Biol. Sci. 1944;132:396–423. [Google Scholar]
- Lindsley D.L., Zimm G.G. The Genome of Drosophila melanogaster 1992. Academic Press; San Diego, CA: pp. 1,133 [Google Scholar]
- Matsumoto H., Sasaki Y. Staurosporine, a protein kinase C inhibitor interferes with proliferation of arterial smooth muscle cells. Biochem. Biophys. Res. Commun. 1989;158:105–109. doi: 10.1016/s0006-291x(89)80183-4. [DOI] [PubMed] [Google Scholar]
- Petersen N.S., Lankenau D.-H., Mitchell H.K., Young P., Corces V.G. Forked proteins are components of fiber bundles present in developing bristles of Drosophila melanogaster . Genetics. 1994;136:173–182. doi: 10.1093/genetics/136.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto K., Winge P., Koyoma S., Hireta Y., Yamada C., Miyao S., Yoshikawa S., Jin M.L., Kikuchi A., Okano H. The Drosophila ral GTPase regulated developmental cell shape changes through the Jun NH2-terminal kinase pathway. J. Cell Biol. 1999;146:361–372. doi: 10.1083/jcb.146.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichiro O., Yamakita Y., Yamashiro S., Matsudaira P.T., Gnarra J.R., Obinata T., Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylaton site on human fascin. J. Biol. Chem. 1997;272:2527–2533. doi: 10.1074/jbc.272.4.2527. [DOI] [PubMed] [Google Scholar]
- Strutt D.I., Weber U., Mlodzik M. The role of RhoA in tissue polarity and Frezzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca2+-dependent protein kinase. Biochem. Biophys. Res. Commun. 1986;135:397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Tilney L.G., Tilney M.S., Guild G.M. F-actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J. Cell Biol. 1995;130:629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Connelly P., Smith S., Guild G.M. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J. Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney L.G., Connelly P.S., Vranich K.A., Shaw M.K., Guild G.M. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J. Cell Biol. 1998;143:121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda L., Inoue Y.H., Yoo M., Mizuno M., Hata M., Lim Y., Adachi-Yamada T., Ryo Y., Masamune Y., Nishida Y. A protein kinase similar to MAP kinase activator acts downstream of the Raf kinase in Drosophila . Cell. 1993;72:407–414. doi: 10.1016/0092-8674(93)90117-9. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle J.D., Petersen N.S., Otto J.J. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophilathe role of singed and forked proteins. Cell Motil. Cytoskelet. 1998;40:119–132. doi: 10.1002/(SICI)1097-0169(1998)40:2<119::AID-CM2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Yamakita Y., Ono S., Matsumura F., Yamashiro S. Phosphorylation of human fascin inhibits its actin binding and bundling activities. J. Biol. Chem. 1996;271:12632–12638. doi: 10.1074/jbc.271.21.12632. [DOI] [PubMed] [Google Scholar]