Abstract
Cancer patients often have subclinical vitamin A deficiencies and low vitamin A lung levels. Previous studies showed that subclinical vitamin A deficiency increased the severity of pneumonitis induced by whole-lung irradiation in rats. Many studies have shown that lung irradiation increases the number of lung tumors developing from intravenously injected tumor cells in mice. We examined the impact of vitamin A deficiency on the development of lung metastases from a highly metastatic syngeneic rat rhabdomyosarcoma in normal rats and rats receiving prior lung irradiation. Weanling female WAGrijY rats were randomized to receive either a diet lacking both vitamin A and beta-carotene or a control diet. After five weeks, the deficient diet significantly decreased levels of retinol in the lung and liver but not in the serum, modeling the tissue and blood levels seen in prior studies of patients with subclinical vitamin A inadequacy. The vitamin A-deficient diet did not alter the number of lung tumors developing from intravenously injected tumor cells in unirradiated rats. Whole-lung irradiation produced dose-dependent increases in the number of lung tumors developing from tumor cells injected intravenously one or 29 d after irradiation. Vitamin A deficiency did not alter these dose–response curves, indicating that the more intense radiation-induced pneumonitis seen previously in vitamin A-deficient rats did not alter the enhancement of metastases produced by whole-lung irradiation. Moreover, inadequate vitamin A intake did not influence the growth of tumors implanted subcutaneously or increase the number or size of the spontaneous lung metastases developing from these subcutaneous tumors. Thus, although low vitamin A status influences the development of lung injury and is considered a possible modifiable risk factor increasing risk of primary cancer, it did not affect the growth of subcutaneous tumors or increase the development of artificial or spontaneous lung metastases in this rat model.
Keywords: vitamin A, metastases, radiation
Introduction
Approximately 65% of all cancer patients receive radiation therapy with curative or palliative intent. As with all cancer therapies, the intensity of radiotherapy is limited by the toxicity of the treatment to normal tissues. For patients with malignancies in or on the thorax or chest, such as breast cancers, lung cancers, carcinomas of the upper gastrointestinal tract or Hodgkin’s disease, some lung tissue will be irradiated and injury to the lung can be the dose-limiting side-effect of radiotherapy.1–3 Radiation injury to lung occurs in two phases: a phase of inflammatory radiation pneumonitis, which occurs a few weeks to months after irradiation, and a later progressive fibrotic process that can develop months to years after either clinical or subclinical radiation pneumonitis. In addition, irradiation of the lung causes rapid changes in the vasculature within the lung, leading to changes in blood flow and circulation, as well as rapid changes in the immune components within the lung.4–6 These can increase the number of tumor nodules developing from intravenously injected tumor cells in mice and rats. The number of lung tumors developing from injection of a constant number of intravenously injected tumor cells increases with the radiation dose given previously to the lungs, and also varies with the time between irradiation and injection of the tumor cells.4–12 These data have raised concerns that irradiation of lung tissue in cancer patients, who may have circulating tumor cells, may increase the probability that these circulating tumor cells will lodge in the lungs and grow to produce macroscopic metastases.
Vitamin A and its analogs, the retinoids, are potent regulators of epithelial proliferation and differentiation.13 – 15 In rodents, vitamin A deficiency increases susceptibility to lung infection and increases lung toxicity from agents such as ozone.16,17 In humans, low vitamin A intake and/ or reduced serum retinol levels, common in developing countries, are associated with increased risk of infection, reduced night vision, anemia, reduced lung function and risk of injury from environmental agents such as asbestos.16,18 – 20 The prevalence of the milder, subclinical vitamin A nutritional deficits seen in developed countries is unclear, as serum retinol levels are not a good predictor of tissue stores. A number of studies suggest that subclinical vitamin A deficiency or suboptimal vitamin A nutritional status may be common, especially in certain patient populations, including patients with chronic liver disease, obesity, postbariatric surgery, the elderly and those who avoid liver products and vitamin A-supplemented foods.21 – 28 Redlich and her collaborators29 found surprisingly low levels of vitamin A in lung tissues from patients undergoing thoracotomy and lung resection.
These data suggested that altered vitamin A status could modify radiation-induced lung injury. To test that hypothesis, we undertook a series of studies examining the effect of altered vitamin A intake on radiation pneumonitis in RAG/rijY rats. In those studies, reported previously,30 we found that a normal diet for laboratory rats, which is rich in vitamin A, decreased the severity of radiation-induced pneumonitis after thoracic irradiation over that seen in rats fed a deficient diet that produced a subclinical vitamin A deficiency and resulted in low retinol lung levels and liver stores. Those studies suggested that a diet adequate in vitamin A, or supplementation of the diets of those patients with inadequate vitamin A intake, might be of value in reducing lung injury from radiation therapy.
The studies presented here were undertaken to follow-up on our past studies of radiation injury to the lung30–33 and to further assess their implications for patients receiving radiotherapy, by assessing whether inadequate levels of vitamin A would alter the ability of tumor cells to colonize and grow in the irradiated or unirradiated lungs of syngeneic rats. We examined the effect of vitamin A intake on the number and size of the lung tumors developing after intravenous injection of tumor cells and on the increase in lung metastases induced by prior whole-lung irradiation. We also examined the effect of dietary vitamin A intake on the formation of spontaneous lung metastases from primary tumors implanted subcutaneously on the flanks of rats.
Materials and methods
Animals and animal care
All experiments were performed with female WAG/rijY rats, which were bred and raised to weaning in our breeding colony at Yale under specific pathogen free conditions. WAG/rijY rats were selected for these studies because previous studies from our laboratories had delineated the time course, characteristics and dose–response curve for the development of radiation pneumonitis in RAG/rijY rats and had demonstrated that low vitamin A intake increased the severity of radiation pneumonitis after wholelung irradiation.30 – 32 Female rats were used throughout the experiments presented here. Male WAG/rij rats grow more rapidly than females and would have been much larger at the time of irradiation, resulting in differences in radiation dosimetry and irradiation procedures that would have complicated the experiments if rats of both sexes had been used. In addition, the thicker tail skin and the scars on the tails from the bite injuries resulting from the dominance fighting common among group-housed male rats make it more difficult to perform quantitative intravenous injections on male rats than on females. The use of female rats throughout the project therefore reduced the potential sources of experimental variability. The radiation response of the lungs is similar in male and female rodents, and in male and female cancer patients.1–3
All rats were ear tagged at weaning so that they could be monitored individually throughout the experiments. Littermates were assigned to control and vitamin A-deficient groups at weaning and studied simultaneously to minimize experimental variability. Littermates on the deficient and control diets were then assigned to different unirradiated or irradiated groups and treated as described below. Because the age of the rats at the start of the treatment was critical, the data within each set of studies represent a compilation of data for sets of littermates on the vitamin A-deficient and control diets, entered into the experiment at weaning (3 weeks of age) at different times over a period of 3–5 months.
Before weaning, mother rats and their weanlings had access to standard autoclaved breeder chow containing 80,000 unit/kg vitamin A (Prolab R-M-H 35000, Agway, Syracuse, NY, USA). At the time of weaning (three weeks of age), the young rats were randomly assigned to receive one of two experimental diets from ICN Biomedical (Aurora, OH, USA): either a control diet that contained 80,000 units of vitamin A/kg diet and 0.0054 gm beta-carotene/ kg diet or a deficient diet lacking vitamin A and betacarotene (Table 1). Rats remained on the assigned diet for the duration of the experiment (5–13 weeks).
Table 1.
Composition of control and deficient diets used in these studies
| Component | g/kg |
|---|---|
| Vitamin free casein | 240 |
| dl methionine | 3 |
| Whole wheat | 610 |
| AIN mineral mix | 35 |
| Choline chloride | 2 |
| Alphacel non-nutritive bulk | 50 |
| Corn oil | 50 |
| Menadione | 0.050 |
| Thiamine HCL | 0.10 |
| d-calcium pantothenate | 0.0792 |
| Folic acid | 0.0072 |
| Pyridoxine | HCL 0.0264 |
| Biotin | 0.0006 |
| Vitamin B12 (0.1% Trir) | 0.040 |
| Niacin | 0.100 |
| Riboflavin | 0.022 |
| Vitamin D2 (850,000 U/GM) | 0.0062 |
| dl alpha tocopherol powder (250 U/G) | 0.4 |
| Beta-carotene | 0.0054 |
| Vitamin A acetate (500,000 U/GM) | 0.16 |
Amounts are shown in g/kg. Beta-carotene and vitamin A acetate (in bold) were present only in the control diet. Autoclaving reduces vitamin levels. Spot measurement of retinol levels in the autoclaved control chow showed levels of 50–60 mg retinol/kg diet. Retinol was not detected in deficient chow
All experimental protocols were reviewed and approved by the Yale Institutional Animal Care and Use Committee and all experiments were performed in full compliance with institutional and governmental policies and regulations. Animals were cared for by the husbandry and veterinary care staff of the Yale Animal Resource Center, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats were monitored closely for signs of overt vitamin A deficiency (e.g. porphyrin staining around the eyes, coat changes, failure to groom) and were weighed weekly; there were no clinical signs of vitamin A deficiency. The rats on the experimental and control diets grew at the same rate and were similar in appearance and behavior. Subclinical vitamin A deficiency was confirmed by measurements of liver, lung and serum retinol levels as described below.
Measurement of tissue retinol levels
Retinol levels were measured in tissues from weanling rats and from rats that had been fed the deficient or control diets for five and nine weeks. Rats were anesthetized intramuscularly with acetylpromazine/ketamine and blood was collected from the ascending aorta in a heparinized syringe and held on ice until centrifuged. Serum was collected and stored at −70°C. Liver and lung samples were then obtained at necropsy, rinsed in saline to remove excess blood, weighed, then frozen and stored at −70°C. Samples were handled in subdued lighting and protected against light throughout this process. Frozen samples were couriered to Dr Elizabeth Johnson in the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University (Boston, MA, USA) where extracts were prepared and assayed for retinol by gradient reverse-phase high-performance liquid chromatography as described in publications from that group.34 Lung levels of retinol (18 ± 7 ng/mg in weanling rats) remained essentially unchanged in rats on the complete diet 5–9 weeks later (24 ± 10 ng/mg), but fell to 4.9 ± 1.6 ng/mg during this period for rats on the deficient diet. Liver retinol levels increased from 77 ± 21 ng/mg in weanling rats to 467 ± 76 for rats on the complete diet, but decreased to 7.7 ± 4.6 for rats on the deficient diet. Serum retinol levels were not significantly different at 12 ± 4, 9 ± 2 and 11 ± 6 µg/dL in weanling rats, rats on the complete diet and rats on the deficient diet, respectively. Overall, after 5–9 weeks on the deficient diet, lung levels of retinol were 20% of the levels found in the rats on the control diet and liver retinol levels were 2% of the levels in control rats, but serum retinol levels remained unchanged. These changes in tissue and serum retinol levels are similar to those found in our previous studies30 and confirm that this deficient diet produced low levels of retinol in the lung and greatly decreased stored vitamin A (which is stored primarily in liver), but did not produce clinical symptoms of vitamin A deficiency and did not decrease serum levels of vitamin A. The subclinical deficiency in these rats is therefore analogous to that observed by Redlich et al.29 in patients undergoing lung biopsy.
Tumors
The experiments reported here used a metastatic subline of the BA1112 rat rhabdomyosarcoma (designated BMB). The BA1112 tumor has been used extensively in radiation biology and experimental cancer therapy studies in our laboratory and elsewhere.35 – 38 The BMB subline arose as a spontaneous variant of the BA1112, and was first noted during a tumor therapy experiment, when rats bearing the normally non-metastatic BA1112 tumors began developing large numbers of lung metastases while being followed for long-term tumor control (cure). The growth rate and histology of this metastatic subline resemble those described previously for the parental tumor line;35,38 however, the incidence of lung metastases is greatly elevated.
Lung colonies
To study model lung metastases, a tumor cell suspension was prepared from a BMB tumor using standard mincing and trypsinization procedures35 that result in single-cell suspensions having reproducibly high viability. The viable tumor cells were counted under a phase contrast microscope using trypan blue to exclude damaged cells and were then diluted to a concentration of 1.5 × 105 viable tumor cells per mL. Aliquots containing 3 × 104 cells were inoculated into the tail veins and rats were observed for four weeks to allow cells that had lodged in the lungs to grow into macroscopic colonies. Rats remained on the assigned diet throughout this period. Rats were euthanized and the lungs were gently inflated with saline, removed, rinsed in saline and then fixed in Bouins fixative for 24 h. The lungs were then washed with ethanol and stored in ethanol until they could be assayed. Lungs were examined under a dissecting microscope and tumors on the surface of the lungs were counted. The diameters of the lung tumors were estimated using a calibrated eyepiece micrometer. The lungs were coded at fixation, and the observer who counted and measured the lung tumors was blinded to both the diet and the radiation treatment.
To study spontaneous metastases, a tumor cell suspension prepared as described above was diluted to a concentration of 105 cells/mL and 0.05 mL of the suspension was inoculated subcutaneously in the right flank of each anesthetized rat. While this site was selected for convenience, it should be recalled that rhabdomyosarcomas arise from skeletal muscle and can present clinically as subcutaneous masses, noted by palpation or visual observation. Rats were monitored three times a week, the three diameters of the tumors were measured using vernier calipers and the tumor volume was calculated from these diameters.35 When each tumor reached a volume of 1000 mm3, the rat was euthanized and necropsied. The lungs were removed, fixed and coded as described above. The spontaneous lung metastases visible on the surfaces of the lungs under the dissecting microscope were enumerated and the diameters of the lung tumors were measured using an eyepiece micrometer.
Irradiation
To assess the effects of lung irradiation, rats were anesthetized after five weeks on the experimental diets, positioned on their backs and the thoraxes were irradiated with doses ranging from 5 to 15 Gy, using an X-ray machine operating at 250 kV and 15 mA, with 2 mm Al filtration, at a dose rate of 1.23 Gy per min.30 – 33 The head and body of each rat were shielded with lead to reduce the doses to these areas to less than 5% of the lung dose. Unirradiated rats were anesthetized, positioned as if for irradiation, and held in the anteroom to the irradiator to control for any stresses associated with the irradiation procedure. Littermates on each diet were irradiated or sham-irradiated simultaneously.
In one set of experiments studying intravenously injected tumor cells, rats were inoculated to produce artificial lung metastases 24 h after irradiation (the time that had been observed by others to produce the maximum increase in lung tumor formation).4,7 In a second series of experiments, rats were inoculated with tumor cells 29 d after irradiation (the time of the peak of radiation-induced pneumonitis in the rats in our previous studies).30 – 32 For studies of spontaneous metastases, subcutaneous tumors were injected the day after irradiation of the lungs with 15 Gy.
Results
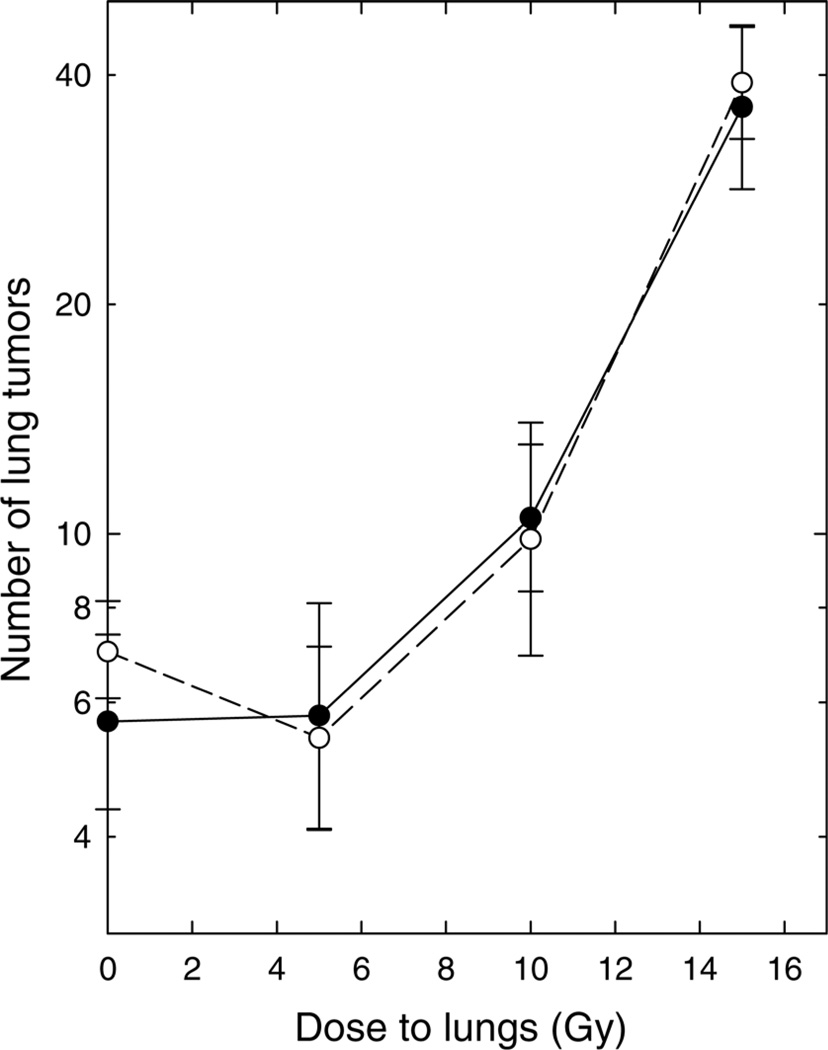
In the experiments shown in Figure 1, weanling rats were assigned to one of the two experimental diets (vitamin A deficient or control) and were fed that diet for five weeks. The lungs were then irradiated and tumor cells were inoculated intravenously one day after irradiation. Rats were continued on the assigned diet for the additional four weeks of the experiment; they were then necropsied and the lungs removed, fixed and examined under a dissecting microscope to determine the number and size of the lung tumors. Irradiation of the lungs increased lung tumor formation in a dose-dependent manner: a dose of 5 Gy did not produce a significant change from the control value, but the number of metastases increased progressively in rats that had been irradiated 24 h previously with 10 or 15 Gy of whole-lung irradiation. This is consistent with past data from several laboratories, including our own, in studies using experimental mice.4–12 The numbers of lung tumors were indistinguishable in rats fed the vitamin A-deficient and control diets, both in sham-irradiated control rats (0 Gy) and at all radiation doses examined.
Figure 1.
Number of tumors developing in the lungs of rats after intravenous inoculation of 30,000 tumor cells one day after irradiation. ●: Rats fed the control diet. ○: Rats fed the diet deficient in vitamin A. Points are means ± standard errors; 8–12 rats per group
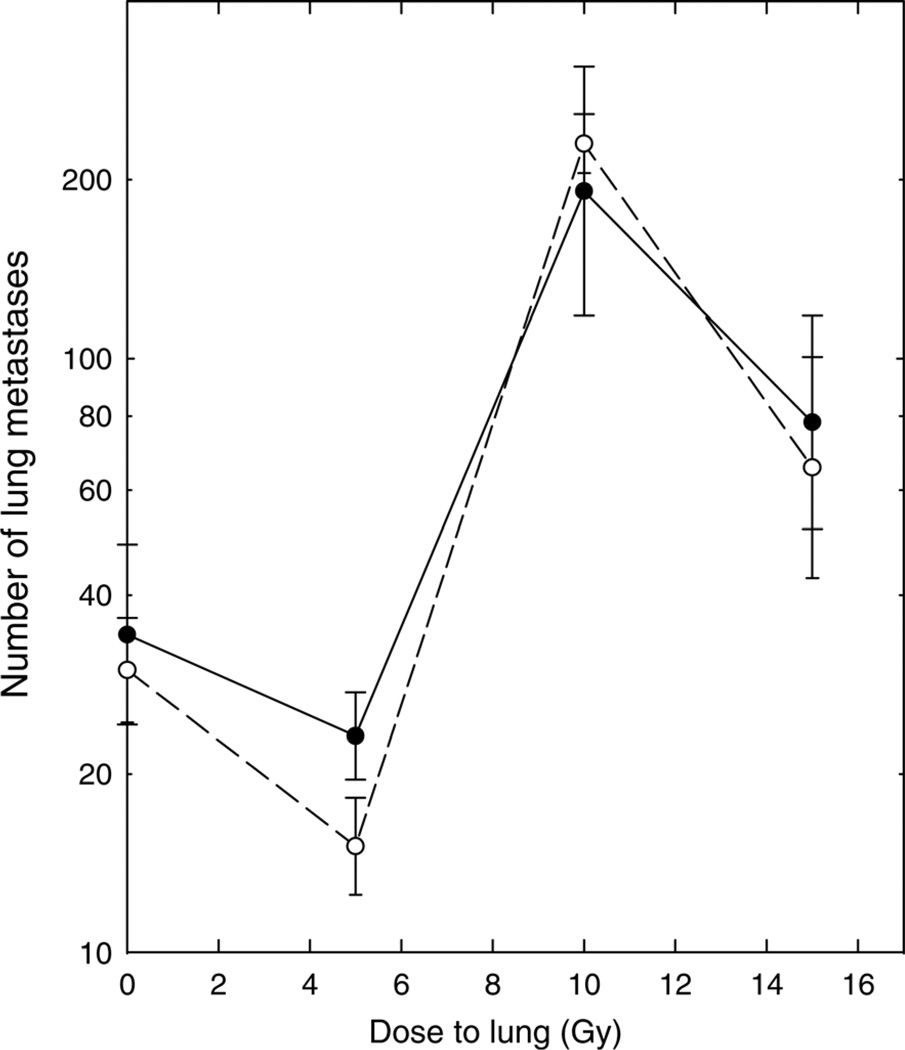
In the experiments shown in Figure 2, weanling rats were likewise assigned to a vitamin A-deficient diet or control diet for five weeks before irradiation and remained on the assigned diet throughout the remainder of the experiment. Tumor cells were inoculated intravenously 29 d after irradiation; this is the time of the peak radiation-induced pneumonitis in the rats.30 – 32 Irradiation of the lungs increased tumor formation in a dose-dependent manner. A dose of 5 Gy did not produce a significant change in the number of lung metastases, but the numbers of metastases were significantly greater in rats that had received 10 or 15 Gy of whole-lung irradiation than in sham-irradiated rats. The numbers of lung tumors were not significantly different in rats fed the vitamin A-deficient and control diets, either in sham-irradiated rats (0 Gy) or at all radiation doses examined (Mann–Whitney U test, P > 0.1).
Figure 2.
Number of tumors developing in the lungs of rats after intravenous inoculation of 30,000 tumor cells at the peak of the development of radiation pneumonitis, 29 d after irradiation. ●: Rats fed the control diet. ○: Rats fed the diet deficient in vitamin A. Points are means and standard errors; 8–12 rats per group
The effect of subclinical vitamin A deficiency on the development of spontaneous lung metastases was also examined. In these experiments, rats were assigned at weaning to the vitamin A-deficient or control diet and were fed that diet for five weeks. At that time, the lungs of some rats were irradiated with 15 Gy, while other rats were sham-irradiated. Twenty-four hours after irradiation, tumors were implanted in the subcutaneous tissue of the flanks of the rats and the growth of these tumors was followed by serial measurements of the tumors. There were no statistically significant differences in the growth rates of the primary subcutaneous tumors in the four different groups. As shown in Table 2, the time needed for tumors to grow to a volume of 500 mm3 was the same in rats on the control and vitamin A-deficient diets. It was also the same in unirradiated rats and rats receiving lung irradiation, for rats on each of the diets. Thus, neither the diet nor the development of radiation pneumonitis influenced the growth of primary tumors implanted subcutaneously on the flanks.
Table 2.
Time (days) needed for subcutaneous BA1112-BMB tumors to grow to 500 mm3 as a function of diet and whole-lung irradiation
| Days to grow to 500 mm3 | ||
|---|---|---|
| Diet | Unirradiated | Lung irradiation |
| Control diet | 21.9 ± 1.0 | 21.5 ± 0.8 |
| Deficient diet | 22.1 ± 1.6 | 21.2 ± 0.8 |
Values are means ± SEM for 9–11 rats. None of the differences are statistically significant
Each tumor was allowed to grow until its volume reached 1000 mm3, at which time the rat was euthanized and necropsied to assay the number and size of the spontaneous lung metastases. Table 3 summarizes our data on the development of spontaneous lung metastases in these rats. The vitamin A-deficient diet did not alter the number of lung metastases in unirradiated rats (Mann–Whitney U test, P = 0.30). The number of lung metastases was significantly smaller in rats that had received whole-lung irradiation than in unirradiated rats, both for rats on the control diet and for rats on the vitamin A-deficient diet (P < 0.01). In rats receiving lung irradiation, the number of lung metastases was significantly smaller in rats on the vitamin A-deficient diet than in rats fed the control diet (P = 0.04). The sizes of the lung tumors were similar in all four groups.
Table 3.
Number and size of metastases developing in rats bearing spontaneous tumors as a function of diet and lung irradiation
| Group | Number of lung metastases |
Mean volume of metastases |
|---|---|---|
| Control diet, no radiation | 48.1 ± 7.1 | 1.16 ± 0.07 |
| Vitamin A-deficient diet, no radiation | 37.3 ± 3.9 | 1.28 ± 0.09 |
| Control diet plus radiation (15 Gy) | 22.9 ± 2.7 | 1.17 ± 0.08 |
| Vitamin A-deficient diet plus radiation (15 Gy) | 15.4 ± 2.4 | 1.33 ± 0.15 |
Values are means ± SEM for 12–22 rats. The vitamin A-deficient diet did not alter the number of lung metastases in unirradiated rats (Mann–Whitney U test, P = 0.30). The number of lung metastases was decreased significantly in rats receiving whole-lung irradiation, both for rats on the control diet (P < 0.01) and for rats on the vitamin A-deficient diet (P > 0.01). The difference between the number of tumors in rats on the vitamin A-deficient and control diets was significant in rats receiving lung irradiation (P = 0.04)
Discussion
Clinical and subclinical vitamin A deficiency remains common and many cancers are associated with factors such as high alcohol intake, smoking, obesity and older age that can be associated with low vitamin A intake. Suboptimal vitamin A intake and low vitamin A tissue levels have been reported in cancer patients.29 The experiments reported here were performed as a follow-up to our previous studies showing that subclinical vitamin A deficiency increased the susceptibility of rats to the development of radiation pneumonitis. These past studies raised concerns that the observed subclinical vitamin A deficiencies in cancer patients receiving radiotherapy to the lung could increase the risk of early radiation pneumonitis and of late radiation-induced lung fibrosis in cancer patients.
The implications of prior lung irradiation for the development of lung metastases have been examined in several experimental rodent model systems.4–12 Irradiation of the lungs before the intravenous injection of a large number of tumor cells increases the formation of lung tumors in many transplantable mouse tumor models. The number of lung tumors increases with increasing radiation dose. The dose–response curves are somewhat different for different rodent tumor models, probably reflecting the many variables between the different studies, which include differences in radiation conditions and radiation dosimetry, the host strain, the microbiology of the hosts, and the tumor lines used in the studies. In addition, many of the published studies were performed decades ago, before the microbiology and husbandry conditions in animal facilities were as rigorous and consistent as those in use today. Within a laboratory, when a constant number of cells are injected, the number of lung tumors developing varies with the time between irradiation and injection, generally increasing to a maximum at approximately 24 h after irradiation. Our data showing marked, dose-dependent enhancement for tumor cells injected 24 h after irradiation are consistent with this previous literature.
In our experiments, we also examined the development of lung tumors in rats that had received lung irradiation 29 d previously. This interval between irradiation and injection of the tumor cells was chosen because the peak level of radiation pneumonitis observed after whole-lung irradiation of WAG/rij rats occurred over the period of 25–35 d.30 – 32 This reaction was characterized by a marked increase in inflammatory cell infiltration, as seen both by bronchoalveolar lavage and by histological analysis, a reduced alveolar cellularity, and a marked protein leak. We found a marked enhancement in the number of metastases in rats that had been injected with tumor cells at this time after 10 or 15 Gy of whole-thorax irradiation. No significant increase in metastasis was seen in rats irradiated at a low dose of 5 Gy, which produced no significant pneumonitis in our previous studies30 – 33 and no increase in the development of lung tumors in rats injected with tumor cells one day after irradiation (Figure 1). Few studies have examined the effects of radiation on the development of lung tumors injected during this period; those which have provide mixed findings, with increased numbers of metastases seen in some studies, but not in others.
Subclinical vitamin A deficiency did not alter the number of lung tumors developing from intravenously injected tumor cells either in unirradiated rats or in rats irradiated one or 29 d previously. For each point on the dose–response curves shown in Figures 1 and 2, the numbers of tumors developing in rats on the complete and vitamin A-deficient diets are very similar, and none of the differences are statistically significant. It should be recalled that the design of these experiments was chosen to maximize the ability of the studies to detect a difference between the rats on the two diets: each comparison resulted from a pair of littermates that were randomly allocated to one diet or the other, and that were irradiated or sham-irradiated simultaneously and injected with aliquots from the same tumor cell suspension. The experimental data for different radiation doses (0–15 Gy) were accumulated over a period of several months, as matched littermate rats were being allocated to different radiation treatments. The error limits on the graphs reflect the larger errors inherent in the use of different cohorts of rats and different inocula.
Subclinical vitamin A deficiency did not enhance either the growth of subcutaneously injected tumors or the numbers of lung metastases developing from these tumors either in unirradiated rats or in rats receiving prior wholelung irradiation (Table 3). Whole-lung irradiation with 15 Gy given one day before inoculation of the tumor cells likewise did not alter the growth of the subcutaneously inoculated tumors, which were injected into a location that was outside the irradiated field. Thus, the systemic effect of the lung irradiation, which was given at a dose high enough to allow the development of significant lung injury during the period of tumor growth, was not sufficient to perturb the growth of these subcutaneous tumors.
However, there were differences in the patterns of spontaneous lung metastases in rats on the control and deficient diet, and in sham-irradiated and irradiated rats. Irradiated rats developed fewer lung metastases than sham-irradiated rats. This effect contrasts to the increase in lung tumors developing from intravenously injected cells in the irradiated rats. The reason for this difference is unclear, but in both cases the effects were marked, reproducible and statistically significant. This suggests that other factors, not modeled by the intravenous injections, are playing a role in the development of the spontaneous metastases.39 These might include, for example, abscopal effects or immunological effects of the whole-lung irradiation that allow cells to enter the vasculature or other effects of lung irradiation that alter early steps39 in the metastatic process. Vitamin A intake did not have a statistically significant effect on the development of spontaneous lung metastases in unirradiated rats, but did have a significant effect on the development of metastases in irradiated rats. The number of lung metastases was unexpectedly lower in rats on the vitamin A-deficient diet than in rats on the control diet.
Taken together, our data provide no evidence that the vitamin A-deficient diet used in these studies altered the growth of tumors implanted subcutaneously, increased the number or size of the lung tumors developing from intravenously injected tumors cells, or increased the number or size of the spontaneous lung metastases developing from subcutaneously implanted tumors. This was true both in unirradiated rats and in rats that had been treated previously with whole-lung irradiation at doses known to produce significant radiation injury to the lung. Whether a longer period of low vitamin A intake that produced frank symptoms of vitamin A deficiency would have a different effect remains unknown.
ACKNOWLEDGEMENTS
This project was supported by grant 99B043 from the American Institute for Cancer Research and used core facilities that were supported in part by the Yale Cancer Center through NCI grant CA16359. The authors thank Marianne Kelly for her assistance throughout the development and performance of this study and dedicate this paper to her memory. The authors also thank Jacqueline Mendes for her assistance with the performance of the experiments and Bettina Harris for her assistance with the preparation and submission of the manuscript.
Footnotes
Author contributions: All authors participated in the design of the project, the analyses of the data and the interpretation of the results. SR and YL conducted the experiments.
REFERENCES
- 1.Marks LB, Yu X, Vujaskovic Z, Small W, Jr, Folz R, Anscher MS. Radiation-induced lung injury. Semin Radiat Oncol. 2003;13:333–345. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 2.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Physics. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 3.Roberts KB, Rockwell S. Radiation pneumonitis. In: Fishman AP, editor. Fishman’s Pulmonary Diseases & Disorders. 4th edn. New York: McGraw-Hill; 2008. pp. 1173–1191. [Google Scholar]
- 4.Milas L, Peters LJ. Conditioning of tissues for metastasis formation by radiation and cytotoxic drugs. In: Nicolson GL, Milas L, editors. Cancer Invasion and Metastasis: Biologic and Therapeutic Aspects. New York: Raven Press; 1984. pp. 321–336. [PubMed] [Google Scholar]
- 5.Withers HR, Milas L. Influence of preirradiation of lung on development of artificial pulmonary metastases of fibrosarcoma in mice. Cancer Res. 1973;33:1931–1936. [PubMed] [Google Scholar]
- 6.Peters LJ, Mason K, McBride WH, Patt YZ. Enhancement of lung colony-forming efficiency by local thoracic irradiation: interpretation of labeled cell studies. Radiology. 1978;126:499–505. doi: 10.1148/126.2.499. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM. The effect of lung irradiation on the incidence of pulmonary metastases in mice. Br J Radiol. 1973;46:613–618. doi: 10.1259/0007-1285-46-548-613. [DOI] [PubMed] [Google Scholar]
- 8.Thompson SC. Tumour colony growth in the irradiated mouse lung. Br J Cancer. 1974;30:337–341. doi: 10.1038/bjc.1974.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill RP, Bush RS. A lung-colony assay to determine the radiosensitivity of the cells of a solid tumour. Int J Radiat Biol. 1969;15:435–444. doi: 10.1080/09553006914550721. [DOI] [PubMed] [Google Scholar]
- 10.Rockwell S. Effect of host age on the transplantation, growth, and radiation response of EMT6 tumors. Cancer Res. 1981;41:527–531. [PubMed] [Google Scholar]
- 11.Rockwell S, Nierenburg M, Irvin CG. Enhancement of artificial lung metastases by misonidazole. Int J Radiat Oncol Biol Phys. 1984;10:1395–1398. doi: 10.1016/0360-3016(84)90356-0. [DOI] [PubMed] [Google Scholar]
- 12.Rockwell S, Kelley M. Radiation enhancement of lung nodule formation in mice is not potentiated by treatment with a perfluorochemical emulsion and carbogen. Radiother Oncol. 1989;14:49–53. doi: 10.1016/0167-8140(89)90008-x. [DOI] [PubMed] [Google Scholar]
- 13.Lotan R. Roles of retinoids and their nuclear receptors in the development and prevention of upper aerodigestive tract cancers. Environ Health Perspect. 1997;105:985–988. doi: 10.1289/ehp.97105s4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biesalski HK, Nohr D. Importance of vitamin-A for lung function and development. Mol Aspects Med. 2003;24:431–440. doi: 10.1016/s0098-2997(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 15.Russell RM. The enigma of β-carotene in carcinogenesis: what can be learned for animal studies. J Nutr. 2004;143:262S–268S. doi: 10.1093/jn/134.1.262S. [DOI] [PubMed] [Google Scholar]
- 16.Chyrtil F. The lungs and vitamin A. Am J Physiol. 1992;262:L517–L527. doi: 10.1152/ajplung.1992.262.5.L517. [DOI] [PubMed] [Google Scholar]
- 17.Paquette NC, Zhang LY, Ellis WA, Scott AL, Kleeberger SR. Vitamin A deficiency enhances ozone-induced lung injury. Am J Physiol. 1996;270:L475–L482. doi: 10.1152/ajplung.1996.270.3.L475. [DOI] [PubMed] [Google Scholar]
- 18.Semba RD, Bloem MW. The anemia of vitamin A deficiency: epidemiology and pathogenesis. Eur J Clin Nutr. 2002;56:271–281. doi: 10.1038/sj.ejcn.1601320. [DOI] [PubMed] [Google Scholar]
- 19.Paiva SA, Godoy I, Vannucchi H, Favaro RM, Geraldo RR, Campana AO. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am J Clin Nutr. 1996;64:928–934. doi: 10.1093/ajcn/64.6.928. [DOI] [PubMed] [Google Scholar]
- 20.Mayne ST, Redlich CA, Cullen MR. Dietary vitamin A and prevalence of bronchial metaplasia in asbestos-exposed workers. Am J Clin Nutr. 1998;68:630–635. doi: 10.1093/ajcn/68.3.630. [DOI] [PubMed] [Google Scholar]
- 21.van den Berg H. Vitamin A intake and status. Eur J Clin Nutr. 1996;50 Suppl 3:S7–S12. [PubMed] [Google Scholar]
- 22.Kraemer K, Waelti M, de Pee S, Moench-Pfanner R, Hathcock JN, Bloem MW, Semba RD. Are low tolerable upper intake levels for vitamin A undermining effective food fortification efforts? Nutr Rev. 2008;66:517–525. doi: 10.1111/j.1753-4887.2008.00084.x. [DOI] [PubMed] [Google Scholar]
- 23.de Paula TP, Ramalho A, Braulio VB. The effectiveness of relative dose response to retinol intake as an evaluation of vitamin A status of cirrhotic patients. J Hum Nutr Diet. 2010;23:583–589. doi: 10.1111/j.1365-277X.2010.01072.x. [DOI] [PubMed] [Google Scholar]
- 24.Chiplonkar SA, Agte VV, Mengale SS, Tarwadi KV. Are lifestyle factors good predictors of retinol and vitamin C deficiency in apparently healthy adults? Eur J Clin Nutr. 2002;56:96–104. doi: 10.1038/sj.ejcn.1601291. [DOI] [PubMed] [Google Scholar]
- 25.Strobel M, Tinz J, Biesalski HK. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur J Nutr. 2007;46 Suppl. 1:11–20. doi: 10.1007/s00394-007-1001-z. [DOI] [PubMed] [Google Scholar]
- 26.Chau N, Tebi A, Creton C, Belbraouet S, Debry G. Relationship between plasma retinol and infectious diseases in the elderly. A case-control study. Ann Nutr Metab. 2000;44:256–262. doi: 10.1159/000046693. [DOI] [PubMed] [Google Scholar]
- 27.Pereira S, Saboya C, Chaves G, Ramalho A. Class III obesity and its relationship with the nutritional status of vitamin A in pre- and postoperative gastric bypass. Obes Surg. 2009;19:738–744. doi: 10.1007/s11695-008-9478-y. [DOI] [PubMed] [Google Scholar]
- 28.Leotsinidis M, Alexopoulos A, Schinas V, Kardara M, Kondakis X. Plasma retinol and tocopherol levels in greek elderly population from an urban and a rural area: associations with the dietary habits. Eur J Epidemiol. 2000;16:1009–1016. doi: 10.1023/a:1010895227352. [DOI] [PubMed] [Google Scholar]
- 29.Redlich CA, Grauer JN, Van Bennekum AM, Clever SL, Ponn RB, Blaner WS. Characterization of carotenoid, vitamin A, and alpha-tocopherol levels in human lung tissue and pulmonary macrophages. Am J Respir Crit Care Med. 1996;154:1436–1443. doi: 10.1164/ajrccm.154.5.8912761. [DOI] [PubMed] [Google Scholar]
- 30.Redlich CA, Rockwell S, Chung JS, Sikora AG, Kelley M, Mayne ST. Vitamin A inhibits radiation-induced pneumonitis in rats. J Nutr. 1998;128:1661–1664. doi: 10.1093/jn/128.10.1661. [DOI] [PubMed] [Google Scholar]
- 31.Rosiello RN, Merrill WW, Rockwell S, Carter D, Cooper JADJ, Care S, Amento EP. Radiation pneumonitis. Brohchoalveolar lavage assessment and induction by a recombinant cytokine. Am Rev Respir Dis. 1993;148:1671–1676. doi: 10.1164/ajrccm/148.6_Pt_1.1671. [DOI] [PubMed] [Google Scholar]
- 32.Rockwell S, Kelley M, Rosiello RA, Merrill WW, Carter D. Administration of a perfluorochemical emulsion plus carbogen breathing does not alter radiation pneumonitis. Proc Soc Exper Biol Med. 1995;208:288–293. doi: 10.3181/00379727-208-43858. [DOI] [PubMed] [Google Scholar]
- 33.Redlich CA, Gao X, Rockwell S, Kelley M, Elias JA. IL-11 enhances survival and decreases TNF production after radiation-induced thoracic injury. J Immunol. 1996;157:1705–1710. [PubMed] [Google Scholar]
- 34.Liu C, Chung J, Seitz HK, Russell RM, Wang X-D. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1703–1709. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 35.Martin DF, Rockwell S, Fischer JJ. Development of an in vitro assay for the survival of cells suspended from BA1112 rat sarcomas. Eur J Cancer Clin Oncol. 1983;19:791–797. doi: 10.1016/0277-5379(83)90011-1. [DOI] [PubMed] [Google Scholar]
- 36.Suit HD, Rockwell S, Zietman A, Silobrcic V, Porter EA, Ramsay J, Sedlacek R. Quantitative transplantation assays of the rat rhabdomyosarcoma BA1112 isografts into the WAG/Rij Y rat and xenotransplantation into the athymic NCr(nu/nu) nude mouse. Eur J Cancer. 1989;25:1463–1466. doi: 10.1016/0277-5379(89)90105-3. [DOI] [PubMed] [Google Scholar]
- 37.Sostman HD, Rockwell S, Sylvia AL, Madwed D, Cofer G, Charles HG, Negro-Vilar R, Moore D. Evaluation of BA1112 rhabdomyosarcoma oxygenation with microelectrodes, optical spectrophotometry, radiosensitivity, and magnetic resonance spectroscopy. Magn Res Med. 1991;20:253–267. doi: 10.1002/mrm.1910200208. [DOI] [PubMed] [Google Scholar]
- 38.Collingridge D, Rockwell S. Pentoxifylline improves the oxygenation and radiation response of BA1112 rat rhabdomyosarcomas and EMT6 mouse mammary carcinomas. Radiat Oncol Invest. 2000;90:256–264. [PubMed] [Google Scholar]
- 39.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5668. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]