Abstract
Redox-based regulatory systems are essential for many cellular activities. Chlamydomonas reinhardtii exhibits alterations in motile behavior in response to different light conditions (photokinesis). We hypothesized that photokinesis is signaled by variations in cytoplasmic redox poise resulting from changes in chloroplast activity. We found that this effect requires photosystem I, which generates reduced NADPH. We also observed that photokinetic changes in beat frequency and duration of the photophobic response could be obtained by altering oxidative/reductive stress. Analysis of reactivated cell models revealed that this redox poise effect is mediated through the outer dynein arms (ODAs). Although the global redox state of the thioredoxin-related ODA light chains LC3 and LC5 and the redox-sensitive Ca2+-binding subunit of the docking complex DC3 did not change upon light/dark transitions, we did observe significant alterations in their interactions with other flagellar components via mixed disulfides. These data indicate that redox poise directly affects ODAs and suggest that it may act in the control of flagellar motility.
Introduction
Alterations in redox poise are important in many cellular processes, such as transcription factor activation, photosynthesis, defense against oxidative stress, proliferation, and apoptosis (Finkel and Holbrook, 2000). Cell cytoplasm is normally kept reduced by the thioredoxin and glutathione systems, but cytoplasmic redox potential can become more oxidized after metabolic changes or as a consequence of reactive oxygen species (ROS) generated inside the cell or from the surrounding environment. These changes affect the redox state of several cytoskeletal proteins; e.g., oxidative stress leads to the increased stability of actin filaments in yeast (Haarer and Amberg, 2004), and tubulin dimers become cross-linked through disulfide bonds in the brain tissue of Alzheimer's disease patients (Aksenov et al., 2001). In human sperm, a redox-regulated tyrosine phosphorylation cascade plays a key role in capacitation (Baker and Aitken, 2004).
Outer dynein arms (ODAs) and inner arm dyneins are attached to the outer doublet microtubules of the eukaryotic flagellar axoneme and generate the power for flagellar beating. In Chlamydomonas reinhardtii, the ODA is an ∼2-MD complex, which comprises three heavy chain (HC) motor units, two intermediate chains (ICs), and at least 10 distinct light chains (LCs; DiBella and King, 2001), two of which (LC3 and LC5) are closely related to thioredoxin (Patel-King et al., 1996). LC3 binds the NH2-terminal region of the β HC (Sakakibara et al., 1993) and has one complete redox-active thioredoxin motif (36WCGPCK41). Furthermore, molecular modeling suggests that LC3 may have a second potentially redox-sensitive vicinal dithiol (65VCAEKCN71; Patel-King et al., 1996; Harrison et al., 2002). LC5 has one complete thioredoxin motif (33WCGPCK38) and binds the NH2-terminal domain of the α HC (Harrison et al., 2002). The presence of thioredoxins in dynein is not specific to C. reinhardtii, as similar modules have been found in the IC1 protein of sperm flagellar ODAs from the sea urchin Anthocidaris crassispina (Ogawa et al., 1996) and the ascidian Ciona intestinalis (Padma et al., 2001). In addition, two IC1 homologues (nm23-H8 and nm23-H9) are highly expressed in human testis, suggesting that they are also sperm components (Padma et al., 2001; Sadek et al., 2001, 2003). Thus, although thioredoxins have been evolutionarily conserved in axonemal dyneins, the role that these proteins play in dynein function remains unresolved.
In C. reinhardtii, there are two ODA components whose function can be regulated by modulating redox poise in vitro. First, ATPase activity of the γ HC is greatly increased after thiol oxidation (Harrison et al., 2002). Similar increases in enzymatic activity after thiol modification have been observed in both sea urchin sperm and Tetrahymena thermophila dyneins (Ogawa and Mohri, 1972; Shimizu and Kimura, 1974; Gibbons and Fronk, 1979). Second, the ODA docking complex (ODA-DC), which localizes at the base of the ODAs and mediates their binding to specific sites on the outer doublet microtubules (Takada and Kamiya, 1994), contains a redox-sensitive component (Casey et al., 2003b). The ODA-DC is composed of the following three subunits: DC1 (83 kD; Koutoulis et al., 1997), DC2 (62 kD; Takada et al., 2002), and DC3 (21 kD; Casey et al., 2003a). DC3 is an EF-hand protein, and its Ca2+-binding loop contains a vicinal dithiol (65DCDGCI70). Recombinant DC3 binds Ca2+ in vitro only when it is reduced (Casey et al., 2003b).
Identification of these redox-active proteins has raised the possibility that some aspects of ODA function might be regulated by alterations in flagellar redox poise (Ogawa et al., 1996; Patel-King et al., 1996; King, 2000; Casey et al., 2003b). Alternatively, these proteins may not be involved in redox regulation, per se, but, rather, may be required for the structural stability of these protein complexes. For example, in T7 DNA polymerase, thioredoxin derived from the Escherichia coli host is used as a structural component necessary for both fidelity and high processivity of the enzyme (Tabor et al., 1987; Kunkel et al., 1994).
C. reinhardtii exhibits several different light-induced behavioral responses (Witman, 1993; Hegemann, 1997). These include phototaxis (cells swim toward or away from a light source), the photophobic response (PPR; cells stop and/or change swimming direction after a sudden change in light intensity), and photokinesis (alteration of swimming speed in response to changes in light conditions; Pazour et al., 1995; Moss and Morgan, 1999; Casey et al., 2003b). Phototactic steering involves the differential control of the beat frequency of the cis- and transflagella, whereas during the PPR the two flagella transiently switch from an asymmetric to a symmetric waveform. Both of these behaviors are regulated by alterations in intraflagellar Ca2+ (Bessen et al., 1980; Kamiya and Witman, 1984). However, the mechanism by which photokinesis is achieved remains unclear, although interestingly, Moss and Morgan (1999) demonstrated that this response does not occur in mutants lacking ODAs.
In C. reinhardtii, the intracellular NADP/NADPH ratio changes in response to different light conditions (Forti et al., 2003), and consequently may affect flagellar redox poise either directly, or via the malate/oxaloacetate shuttle (Raghavendra and Padmasree, 2003), as flagella contain both NAD- and NADP-dependent malate dehydrogenases (Pazour et al., 2005). Therefore, if redox-active ODA components are actually involved in motor regulation their redox state might change, and/or they might interact, at least transiently, with other molecules via intermolecular disulfide bonds, in response to alterations in light conditions. In this study, we have investigated this hypothesis; our data suggest that a redox-regulatory pathway impinges on ODA function and influences flagellar motility.
Results
Light-modulated alterations of C. reinhardtii flagella motility
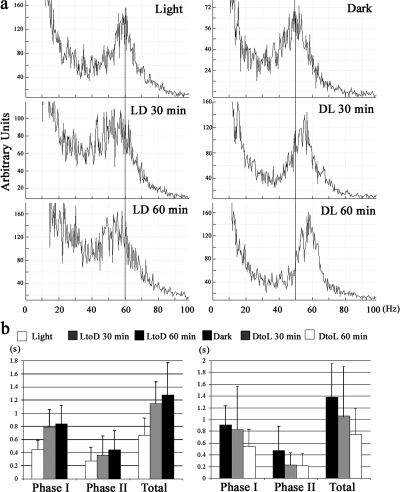
The identification of redox-active components within both C. reinhardtii and sea urchin sperm ODAs (Ogawa et al., 1996; Patel-King et al., 1996) raised the possibility that some general aspect of dynein activity might be altered in response to changes in flagellar redox poise. This suggestion has recently been bolstered by the observation that the intracellular NADP/NADPH ratio in C. reinhardtii changes significantly depending on whether cells are in the dark or light (Forti et al., 2003). Several studies have suggested that C. reinhardtii exhibits photokinesis, where swimming parameters change depending on the light/dark conditions (Pazour et al., 1995; Moss and Morgan, 1999; Casey et al., 2003b). To test whether C. reinhardtii shows photokinesis under our experimental conditions, we measured the flagellar beat frequency of cells adapted to different light/dark conditions (Fig. 1 a). For cells adapted to the light, beat frequency was ∼60 Hz, whereas it was ∼50 Hz for those in the dark (Fig. 2 a). When cells were transferred from light to dark, beat frequency was reduced to ∼50 Hz within 1 h. Conversely, when dark-adapted cells were transferred to the light, beat frequency quickly began to increase and returned to ∼60 Hz within 1 h. We also observed that the duration of both phase I and II of the PPR (Fig. 1 b) changed in a light-dependent manner (Fig. 2 b) and essentially doubled 1 h after the transition from light to dark. The differences observed 1 h after the transition were significant, based on a two-tailed t test; P < 0.05. Control cells kept under the initial light conditions for the duration of the experiment did not show significant alterations in swimming behavior. Thus, the changes observed were not the result of circadian alterations in swimming parameters.
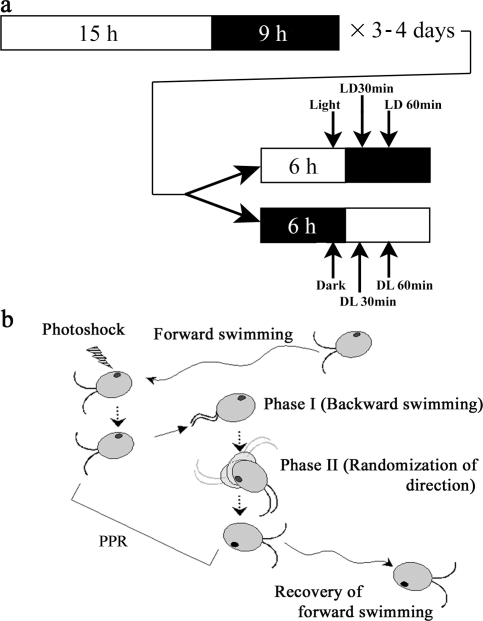
Figure 1.
Light/dark sampling regime and analysis of the photophobic response. (a) Light/dark conditions. A C. reinhardtii preculture was grown under a 15/9 h light/dark cycle for 2–3 d. This culture was then inoculated into several bottles and grown for 1–2 d more (total 3–4 d). On the day before the experiment, half of the bottles were kept under the regular illumination regime, and the other half were wrapped with aluminum foil and kept completely in the dark. Experiments were initiated ∼6 h after the beginning of the light phase. We prepared samples and/or observed cell behavior for each treatment group, and then switched the light conditions; i.e., light-adapted cells were placed in total darkness and vice-versa. Samples were taken 30 and 60 min after the light switch (arrows); for assessment of the in vivo redox state (Fig. 6), additional samples were taken at 1 and 120 min. For chloroplast mutants, precultures were grown in complete darkness for 6–7 d. 1 h before the experiment, half of the culture was transferred to the light. (b) The PPR. When C. reinhardtii cells perceive a sudden change in light intensity, they exhibit the PPR. Cells alter their flagellar waveform from an asymmetric (or ciliary) beat to a symmetric (or flagellar) wave (Phase I) and swim backward or simply stop. After a short period, the cell body rotates to randomize swimming direction (Phase II), and, subsequently, the cell returns to forward swimming. We measured the duration of Phase I, II, and total PPR of single cells by video microscopy.
Figure 2.
Light/dark transitions alter C. reinhardtii swimming behavior. (a) Beat frequency (Hz) of cells adapted to different light conditions. (left) Transition of light-adapted cells to the dark. (right) Transition of dark-adapted cells to the light. Peaks in the FFT power spectra represent the mean beat frequency of 30–50 cells. Reference lines are added at 60 (left) and 50 Hz (right). (b) Duration of the PPR of cells under different light conditions. Phase I represents the duration of backward swimming, and phase II the duration of rotation before recovery of forward swimming. Data represent the mean ± SD for 30 cells. In each graph, data indicated by white and black bars are significantly different. P < 0.05; two-tailed t test.
The photokinetic effect may be mimicked by modulation of redox poise
We hypothesized that these light-dependent changes in flagellar behavior are a response to alterations in flagellar redox poise. To test this idea, we treated cells with low concentrations of the oxidizing reagent H2O2 and two antioxidant ROS scavengers, dimethylthiourea (DMTU) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL), and then measured flagellar beat frequency and the duration of PPR (Fig. 3, a and b). When light-adapted cells were treated with low levels of H2O2, flagellar beat frequency decreased and the duration of PPR increased in a reversible, dose-dependent manner. Conversely, treatment of dark-adapted cells with TEMPOL led to an increase in flagellar beat frequency and a dose-dependent decrease in the duration of PPR. Although DMTU treatment only increased overall beat frequency slightly, it did result in a sharper peak in the power spectrum and a dose-dependent decrease in the duration of PPR phase I. Thus, the effects of light↔dark transitions on flagellar behavior could be mimicked by the addition of exogenous reagents that alter oxidant stress.
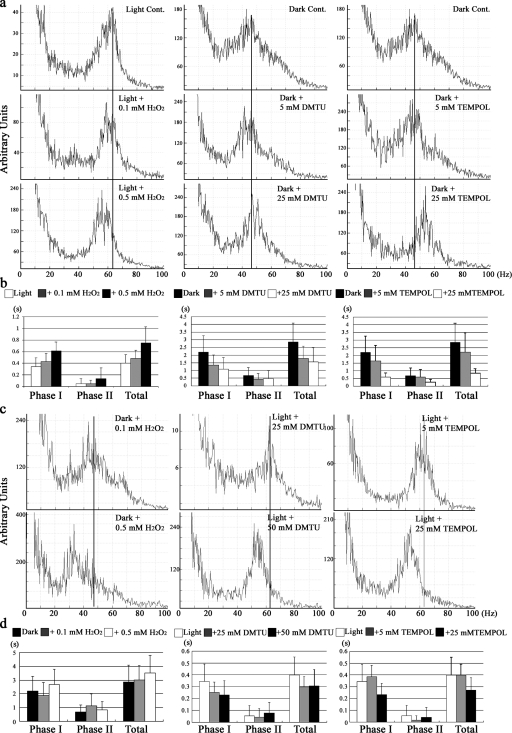
Figure 3.
Exogenous oxidants/antioxidants modify C. reinhardtii photobehavior. (a) Beat frequency of light-adapted cells before and after treatment with low levels of hydrogen peroxide (left), and of dark-adapted cells before and after treatment with DMTU (middle) and TEMPOL (right) is shown. Peaks in the FFT power spectra represent the mean flagellar beat frequency of 30–50 cells. Reference lines are added at the peak value of the untreated samples shown in the top plots. (b) Duration of the PPR of cells treated as in a. In each histogram, data indicated by black and white bars are significantly different (P < 0.05; two-tailed t test), except for phase II of the DMTU treatment series. (c) Beat frequency power spectra for dark-adapted cells treated with H2O2 (left) and light-adapted cells treated with DMTU (middle) and TEMPOL (right). The solid line in each plot represents the peak beat frequency obtained in the absence of oxidizing/reducing agents; see power spectra in top row of a. (d) PPR duration of cells treated as in c. Data represent the mean ± SD for 30 cells.
When light-adapted cells were treated with 25 mM DMTU or 5 mM TEMPOL, beat frequency remained at ∼60 Hz (Fig. 3 c). Higher levels of these reagents (50 mM DMTU or 25 mM TEMPOL) resulted in a modest decrease in beat frequency to ∼55 Hz (Fig. 3 c) and a reduction in the duration of PPR (Fig. 3 d). However, when dark-adapted cells were treated with 0.5 mM H2O2, beat frequency decreased from ∼45 to 35–40 Hz (Fig. 3 c), and the length of PPR showed a modest increase to more than ∼3 s (Fig. 3 d), suggesting that making redox poise more oxidizing than in untreated dark-adapted cells can further enhance the photokinetic effect.
Differential effects of photosystem I and II mutations
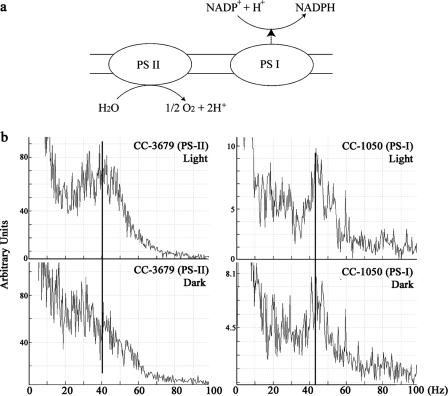
Within the chloroplast, photosystems I and II are coupled to drive electron transport from water to NADPH (Fig. 4 a). To further assess whether changes in redox poise altered flagellar activity, we examined the beat frequency of acetate-requiring mutant strains defective for either photosystem I (ac9) or II (ac115) in the light and the dark (Fig. 4 b). In the light, both mutant strains had a beat frequency of 40–45 Hz, and this did not change in dark-adapted cells lacking photosystem I; i.e., the photokinetic effect was absent. In contrast, dark-adapted cells lacking photosystem II showed a reduction in flagellar beat frequency. These observations suggest that the generation of NADPH by photosystem I is linked to the modulation of flagellar behavior.
Figure 4.
Photosystem I is required for the photokinetic effect. (a) Simplified diagram illustrating the chloroplast light reactions. Electrons are obtained from water by photosystem II (PS-II), passed to photosystem I (PS-I), and ultimately used to reduce NADP+ to NADPH. Consequently, the cellular NADP/NADPH ratio changes in response to light/dark conditions (Forti et al., 2003). Mutants defective in either of these systems require acetate for growth. (b) Beat frequency analysis of light- and dark-adapted mutants defective for photosystem II (left) and I (right). Both mutants have a beat frequency of 40–45 Hz in the light. However, only the photosystem II mutant exhibits a decrease in flagellar beat frequency upon dark adaption, suggesting that a functional photosystem I is required for this response.
Modulation of beat frequency in vitro by redox poise
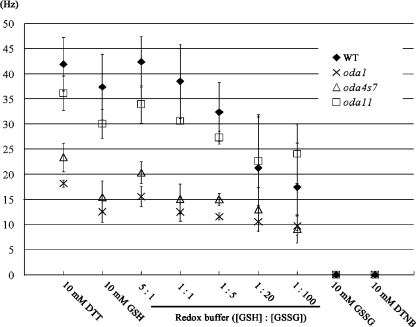
Mutants lacking only the LC3 or LC5 thioredoxins do not exist. Therefore, to determine whether redox poise impinges directly on ODA function, we examined the beat frequency of reactivated detergent-extracted models derived from wild-type cells and from mutants lacking the α HC and LC5 (oda11), the β HC motor domain (oda4-s7), or the entire ODA and associated DC (oda1; Fig. 5). Wild-type cell models had a beat frequency of ∼40 Hz (this value was consistently higher in the presence of 10 mM dithiothreitol [DTT] compared with reduced glutathione [GSH]), and this decreased to ∼18 Hz as the GSH/oxidized glutathione [GSSG] ratio in the redox buffer was increased in favor of GSSG. Under highly oxidizing conditions (10 mM GSSG or 5,5′-dithiobis (2-nitrobenzoic acid) [DTNB]), the axonemes always detached from the cell body and did not reactivate. Oda1 models lack the entire ODA and had a beat frequency of ∼15 Hz under reducing conditions; this value decreased slightly in response to increased levels of GSSG. These data indicate that redox poise affects ODA motor activity and has a more modest effect on the inner arm system. Removal of the ODA α HC and LC5 by the oda11 mutation resulted in a beat frequency reduction of 8–10 Hz under reducing conditions (5:1 GSH/GSSG). Intriguingly, this difference between wild type and oda11 disappeared as the buffer was made more oxidizing (1:20 GSH/GSSG), and beat frequency of oda11 cell models was consistently greater than wild type at 1:100 GSH/GSSG. Lack of the β HC motor domain has a profound effect on the motility of intact oda4-s7 cells (Sakakibara et al., 1993); this was reflected in the reactivated cell models, which had a beat frequency of only 20 Hz at 5:1 GSH/GSSG (∼5 Hz greater than strains lacking the entire structure). Even so, as conditions were made more oxidizing the oda4-s7 cell model beat frequency decreased to ∼10 Hz, suggesting that the remaining ODA motor function in this mutant retains sensitivity to alterations in redox poise.
Figure 5.
Redox poise modulates flagellar beat frequency in reactivated cell models. Detergent-extracted cell models were prepared from wild-type cells and mutants lacking the entire ODA (oda1), the ODA α HC and LC5 (oda11), and the β HC motor domain (oda4-s7). Models were reactivated by addition of ATP and an ATP-regenerating system under fully reducing (10 mM DTT and GSH) and oxidizing (10 mM DTNB and GSSG) conditions, and in the presence of various redox buffers. Flagellar beat frequency was determined, and the data plotted as the mean ± SEM for three independent experiments. Note that in the presence of 10 mM DTNB or GSSG, all cell models lost their flagella and were therefore immotile; visual inspection determined that these detached axonemal structures did not reactivate in the presence of ATP.
Determination of the redox state of ODA/ODA-DC subunits
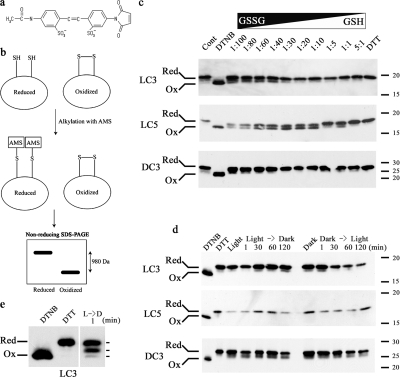
We first tested the 4-acetamido-4-maleimidylstilbene-2,2- disulfonic acid (AMS) alkylation method in vitro to determine whether it could be reliably used to define the redox state of LC3, LC5, and DC3. Isolated flagellar axonemes were incubated with a series of redox buffers (5:1 to 1:100 GSH/GSSG), which were fixed with TCA, and free thiol groups alkylated with AMS. Fully reduced and oxidized control samples were prepared by incubating axonemes with 10 mM DTT or DTNB, respectively, before modification with AMS. Samples were separated by SDS-PAGE in the absence of reducing agents, and LC3, LC5, and DC3 were detected by immunoblotting (Fig. 6). As only reduced sulfhydryl groups can be modified by AMS, reduced proteins with a single vicinal dithiol are 980 D larger than the oxidized form and, consequently, migrate more slowly during electrophoresis. Differences in redox state were detected for both the dynein thioredoxin-like LCs and for DC3 (Fig. 6 c), indicating that the AMS method is useful for monitoring the redox state of dynein components.
Figure 6.
Assessment of the in vivo redox state of LC3, LC5, and DC3. (a) Structure of AMS. (b) Scheme detailing the AMS method to distinguish oxidized and reduced dithiols. The maleimide group of AMS reacts only with reduced thiols and, thus, a protein with a reduced vicinal dithiol will incorporate two AMS moieties (980 D). In contrast, oxidized dithiols are protected from modification. Consequently, reduced proteins may be readily separated from the oxidized forms by nonreducing SDS-PAGE. (c) Axonemes were incubated with redox buffers containing different GSH/GSSG ratios, alkylated with AMS, electrophoresed in the absence of reducing agents, and probed with anti-LC3, -LC5, and -DC3 antibodies. Axonemes alkylated after incubation with 10 mM DTNB or DTT were used as fully oxidized and reduced controls, respectively. The lane marked Cont was only treated with HMEK buffer before AMS modification. The redox state of the three dynein/ODA-DC proteins is altered depending on the GSH/GSSG ratio. The location of the Mr markers (× 103) is at right. (d) Flagella from cells grown under different light conditions (Fig. 1) were fixed with TCA immediately after deflagellation and treated with AMS. Samples were separated in a 15% acrylamide gel without reducing reagents, transferred to nitrocellulose membranes and probed with anti-LC3, -LC5, and -DC3 antibodies. Although LC5 is essentially always reduced, small amounts of LC3 (∼10%) and DC3 (∼30%) are present in the oxidized form. (e) Long exposure of a flagella sample obtained 1 min after a light → dark transition and probed for LC3. Three distinct bands are evident, suggesting that this protein contains two redox-sensitive vicinal dithiols. The fully oxidized and reduced markers are at left.
To assess the in vivo redox state of LC3, LC5, and DC3 and the effects of light/dark transitions, a culture of wild-type C. reinhardtii, which was grown on the regular light/dark cycle for 3-4 d, was split into two. One sample was kept in the light, and the other was kept in the dark. After we prepared initial flagellar samples from these cultures, the light conditions were switched and additional samples were prepared (scheme in Fig. 1 a). Immunoblot analysis revealed that LC5 was almost completely reduced under all conditions tested (Fig. 6 d). Small quantities of both LC3 (∼10%) and DC3 (∼30%) were found to be oxidized, but, again, in multiple experiments the levels did not change significantly under differing light/dark conditions (Fig. 6 d). In several experiments, we observed a third LC3 band, suggesting that this protein contains two vicinal dithiols that may both be modified by AMS (Fig. 6 e). These results imply either that the redox state of these dynein polypeptides does not change or that they cyclically interact with and reduce other proteins (becoming oxidized themselves) and are constantly being rereduced by other flagellar components.
Interaction of flagellar proteins via intermolecular disulfide bonds in vivo
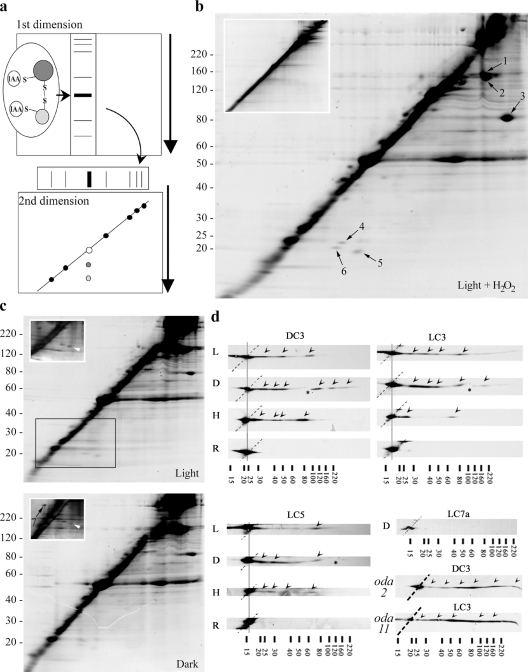
If LC3, LC5, and DC3 are involved in a redox-based flagellar regulatory system, they must interact with other proteins via transient intermolecular disulfide bonds. To test this hypothesis, we used a 2D SDS-PAGE procedure (redox 2D gel), in which samples were first electrophoresed in the absence of reducing agents, whereas the second dimension used standard reducing SDS-PAGE. Any protein not involved in disulfide bond formation will run at the same position in both dimensions, and, consequently, most flagellar proteins align as a diagonal stripe. Proteins joined by intermolecular disulfides migrate more slowly in the first dimension, and after reduction are visible as discrete spots below the main diagonal (Fig. 7 a); proteins that form intramolecular disulfides that alter migration properties significantly are observed above the diagonal. Note that all proteins align on a diagonal when both dimensions use reducing conditions (Fig. 7 b, inset).
Figure 7.
Identification of redox-sensitive flagellar components. (a) Schematic of the redox 2D gel method. Samples were initially alkylated with IAA in the absence of reducing reagents and electrophoresed in the first dimension (intermolecular disulfides are stable under these conditions). The gel lane was excised, reduced, and alkylated, and then electrophoresed again. Any proteins initially cross-linked by intermolecular disulfide bonds focus under the diagonal. (b) Redox 2D gel analysis of C. reinhardtii flagella from light-adapted cells treated with 0.5 mM H2O2 and stained with SYPRO Ruby; multiple spots are observed below the diagonal. The inset shows a similar 2D gel that used reducing reagents in both dimensions; note that no spots were detected below the diagonal. The arrows indicate protein spots identified by mass spectrometry (Table I). (c) Redox 2D gel analysis of flagella from light- and dark-adapted cells stained with SYPRO Ruby. The insets show the boxed region of similar gels stained with silver. Arrowheads indicate a spot (corresponds to spot 5 in b) that is more prominent in light-adapted cells. (d) Immunoblots of redox-2D gels probed with antibodies against LC3, LC5, and DC3. Flagellar samples were obtained from cells adapted to the light (L) and the dark (D), and cells treated with 0.5 mM H2O2 in the light (H). R represents the control reducing–reducing 2D gel. (bottom right) An immunoblot probed with antibody against LC7a, which does not contain any Cys residues. In each immunoblot, off-diagonal spots which derive from binding other proteins are indicated with arrowheads. Note that additional spots appear in the dark versus light for all three redox-active proteins. H2O2 treatment also gave a distinct pattern for LC3, suggesting that enhanced oxidative stress results in the preferential formation of certain mixed disulfides containing this protein. Samples lacking the entire ODA (including LC3 and LC5), but retaining the ODA-DC (from oda2) and missing only the α HC and LC5 (from oda11) were probed for DC3 and LC3, respectively (bottom right). Asterisks indicate minor cracks in the original gel and dotted lines mark the diagonal on which most proteins align. The location of Mr markers are indicated (× 103).
When flagellar proteins from cells kept in the light were separated using this method, we observed multiple spots (including tubulin) after staining with SYPRO Ruby (Fig. 7, b and c). Many of these spots became more prominent when light-adapted cells were placed in a more oxidizing environment (Fig. 7 b). A similar sample from cells incubated in the dark did not reveal obvious changes when stained with SYPRO Ruby (Fig. 7 c, bottom). Some apparent changes in the intensity of spot 5 (Fig. 7 c, insets, arrowheads) were observed when similar gels were silver stained. However, clear differences between these samples were observed only after immunoblot analysis (Fig. 7 d).
The seven most abundant spots (excluding tubulin) that consistently moved from the diagonal on redox 2D gels (indicated in Fig. 7, b and c) were identified by mass spectrometry (Table I). Two spots (Fig. 7 b, spots 4 and 6) are thioredoxin peroxidases (peroxiredoxins) that are known to form mixed disulfides. One of these proteins (Fig. 7 b, spot 6; C_20138) has been identified within the flagellar proteome (Pazour et al., 2005), whereas the second (Fig. 7 b, spot 4; C_200197) has been assigned as a chloroplast precursor based on phylogenetic analysis and its similarity to known chloroplast components of higher plants (Goyer et al., 2002). This C. reinhardtii protein exhibits ∼70% sequence identity with the pea (CAC48323) and tobacco (CAC84143) chloroplast 2-Cys peroxiredoxins and also shares 61% identity with the human cytoplasmic enzyme (P32119). Sequence analysis of C_200197 using TargetP (Nielsen et al., 1997) predicts the presence of a chloroplast transit peptide. However, a nearly identical high score is obtained for the presence of a mitochondrial-targeting peptide, and, consequently, the calculated reliability of the analysis is low.
Table I. Identification of redox-active flagellar proteins.
| Spot | Proteina | Numberb | Mass | Predicted locationc |
|---|---|---|---|---|
| D | ||||
| 1 and 2 | FAP102 | C_330108 | 159,978 | Fl-Ax |
| 3 | FAP41 | N/A | 50,102 | Fl-Ax |
| 4 | Thioredoxin peroxidase (2-Cys peroxiredoxin) | C_200197 | 25,945 | Chd |
| 5 | Hypothetical protein | C_800054 | 45,460 | N/A |
| 6 | Similar to peroxiredoxin 4 | C_20138 | 21,628 | Fl-MM |
| 7 | Mr35,000 COOH-terminal region of flagellar membrane glycoprotein 1a/b |
C_730054/ C_730051 |
383,380/ 219,985 |
Fl-MM |
Ax, axoneme; Ch, chloroplast; FAP, flagellar-associated protein; Fl, flagella; MM, membrane and matrix.
Proteins were identified by mass spectrometry.
Number refers to records in the C. reinhardtii protein database (http://genome.jgi-psf.org/chlre2/chlre2.home.html).
Localization is predicted from the genome database entry annotation and/or the experimentally derived C. reinhardtii flagellar proteome (Pazour et al., 2005).
The data are unclear as to whether this protein is in the chloroplast.
Other proteins identified include a hypothetical protein (C_80054) that contains a scavenger receptor cysteine-rich domain and two axonemal proteins, FAP102 and FAP41, that contain a FAS1 domain and a predicted transmembrane region, respectively. One protein (Fig. 7 b, spot 7) was consistently observed to migrate above the diagonal with Mr∼35,000 under reducing conditions. Mass spectrometry revealed that this polypeptide derived from the COOH-terminal region of flagellar glycoprotein 1a/b (AA025117), suggesting that it either represents an alternate form of this membrane protein or possibly derives by proteolysis from a larger precursor.
LC3, LC5, and DC3 form mixed disulfides with flagellar components in vivo
To assess whether the redox-active dynein/ODA-DC components form mixed disulfides with other flagellar components, flagella samples were separated using the redox 2D SDS-PAGE system and probed with antibodies against LC3, LC5, and DC3 (Fig. 7 d). All three proteins were present in multiple spots that focused below the diagonal, indicating the formation of transient disulfide bonds with several other proteins. We also observed that the number of spots obtained increased in flagella from dark-adapted cells compared with those in the light. Interestingly, H2O2 treatment resulted in a reduction in the number of spots observed to contain LC3, suggesting that certain mixed disulfides preferentially form when the intraflagellar redox state becomes more oxidizing. When reducing conditions were used in both dimensions as a control, LC3, LC5, and DC3 were found exclusively at the diagonal, as expected. As a further negative control, we also probed a redox 2D gel for LC7a, which is an ODA LC that does not contain any Cys residues, and found that this protein was also focused only at the diagonal (Fig. 7 d); a similar result was obtained for LC2, which contains three nonvicinal Cys residues (not depicted). These data suggest that the redox state of the flagellar cytoplasm is more oxidized when cells are in the dark.
As the amounts of LC3, LC5, and DC3 captured in the mixed disulfide form are low, it has not been feasible to identify the substrate polypeptides directly. One obvious possibility, though, is that these proteins act upon each other in an intradynein redox cascade. To test this, we used the redox 2D system to examine flagella from dark-adapted mutant strains oda2 (lacks the ODA but retains the ODA-DC) and oda11 (lacks the α HC and LC5). Immunoblot analysis using antibodies against LC3 and DC3 revealed multiple spots for both proteins (Fig. 7) even though both samples were consistently found to spread out rather than focus tightly. Although these data do not rule out the possibility of intradynein redox interactions, they do suggest that most (if not all) of the off-diagonal spots detected by these antibodies reflect disulfide bond formation with flagellar components that are not ODA subunits.
Discussion
In this study, we have examined the role of redox poise in modulating flagellar motility in C. reinhardtii. We found that a photokinetic effect on beat frequency and alterations in the PPR duration occurred upon light–dark transitions and could be mimicked by altering oxidant stress. Furthermore, these alterations in flagellar beat frequency were observed in reactivated cell models exposed to a series of redox buffers, and were greatly reduced in mutants lacking functional ODAs. We also found that redox-active components of the ODA and ODA-DC formed mixed disulfides with other flagellar proteins in vivo, and that several of these complexes also changed in response to alterations in illumination conditions. Together, these data suggest that redox poise plays an important role in the control of flagellar behavior.
Regulation of photokinetic behavior
Beat frequency is mainly regulated by the ODA (Kamiya, 1995), and mutants lacking ODAs do not exhibit photokinesis (Moss and Morgan, 1999). As beat frequency depends directly on ATP concentration in vitro (Kamiya and Okamoto, 1985), one possible explanation for the photokinetic effect is that intraflagellar ATP levels decrease in the dark. However, direct measurement of the intracellular ATP concentration in C. reinhardtii revealed that significant changes do not occur in a light-dependent manner (Forti et al., 2003). In addition, Mitchell et al. (2005) described an ATP synthesis system within C. reinhardtii flagella involving phosphoglycerate mutase, enolase, and pyruvate kinase, which provides for at least part of the flagellar energy budget. This system presumably depends on diffusion or active transport of 3-phosphoglycerate. However, significant amounts of the ATP necessary for flagellar assembly and beating must derive from the cell body, as an enolase-deficient mutant exhibits ∼64% of wild-type beat frequency (Zhang and Mitchell, 2004).
An alternative possibility is that photokinesis is controlled by redox poise. Forti et al. (2003) demonstrated light-dependent changes in the intracellular NADP/NADPH ratio in C. reinhardtii, which would lead to alteration of cytoplasmic redox poise. Furthermore, redox-active proteins have been identified within the ODAs of a wide variety of organisms (Ogawa et al., 1996; Padma et al., 2001; Patel-King et al., 1996; Sadek et al., 2001, 2003), and alterations in redox state affect dynein enzymatic activity in vitro (Ogawa and Mohri, 1972; Shimizu and Kimura, 1974; Gibbons and Fronk, 1979; Harrison et al., 2002).
Oxidant/antioxidant addition mimics the photokinetic response
We found that the alterations in beat frequency and PPR duration that were observed after light/dark transitions could be recapitulated by the addition of an exogenous oxidant to light-adapted cells and the addition of an antioxidant to dark-adapted cells. The PPR is initiated by an increase in intraflagellar Ca2+ from <10−6 to 10−4 M (Bessen et al., 1980; Kamiya and Witman, 1984), and the duration of this response is presumably dependent on the rate at which Ca2+ is pumped out of the flagellum. Thus, it is possible that C. reinhardtii flagellar Ca2+ channels respond directly to alterations in redox state, as has been suggested for the Ca2+ channels involved in the acrosome reaction of mammalian sperm (Aitken et al., 1998). In addition, a Ca2+ channel (inositol 1,4,5-trisphosphate receptor type 1) on the endoplasmic reticulum is regulated by redox poise via a thioredoxin family protein (Higo et al., 2005). Recently, Josef et al. (2005) hypothesized that a diffusive signal emanating from the cell body serves to optimize flagellar behavior in C. reinhardtii. Our analysis of mutants defective for photosystems I and II revealed that photosystem I (which reduces NADP+ to NADPH) was necessary for the photokinetic response, supporting the hypothesis that alteration in redox state caused by changes in the concentration of chloroplast-derived reducing equivalents might act as such a signal.
Interestingly, after treatment of light-adapted cells with high concentrations of DMTU or TEMPOL, most cells did not show backward swimming, per se, but merely stopped very briefly upon receiving a photoshock. One possibility is that these reagents place the cell under reductive stress and prevent the transient formation of mixed disulfides or the oxidation of specific flagellar components.
Redox poise affects ODA function in reactivated cell models
To examine whether alterations in redox state act directly on ODA components, we examined the motility of detergent-extracted reactivated cell models. Wild-type cells showed a clear redox-dependent alteration in beat frequency (from >40 to <20 Hz) as conditions became more oxidizing. In contrast, a strain lacking the ODA and DC exhibited a minor alteration in motility, suggesting that redox sensitivity is a property of the ODA. Intriguingly, we found that mutant cell models lacking the α HC and LC5 thioredoxin had a higher beat frequency than wild type under oxidizing conditions, but a lower frequency in more reducing buffers, indicating that the lack of these two components alters the axonemal response to redox poise. The α HC/LC5 has been proposed to modulate ATPase activity of the γ HC (Nakamura et al., 1997; Sakato and King, 2003). Furthermore, γ HC ATPase is specifically increased by treatment with low concentrations of DTNB (Harrison et al., 2002). Our data support the idea that the γ HC is activated by oxidation in the absence of the α HC/LC5. We also observed that cell models reactivated under highly oxidizing conditions (10 mM DTNB or GSSG) lost their flagella. Thus, some components of the deflagellation machinery may be redox sensitive.
Redox-dependent interaction of dynein with flagellar components
We monitored the in vivo redox state of LC3, LC5, and DC3 using the AMS alkylation method. Although the relative amounts of oxidized and reduced forms were different for the three proteins, presumably because of variations in the reduction potentials of these specific dithiols, we did not consistently observe significant changes in response to alterations in light conditions. However, using redox 2D gels, we found that these proteins interact with multiple flagellar components through the formation of mixed disulfides, and that several products were observed only in dark-adapted cells. These transient associations suggest that dynein components undergo cyclic redox transitions.
Because recombinant DC3 is able to bind Ca2+ only when it is reduced in vitro (Casey et al., 2003b), ligand binding by DC3 may be redox regulated in vivo, as ∼30% of this protein is present in the oxidized form. However, Casey et al. (2003b) found that a DC3-null mutant (oda14) transformed with a mutant version of DC3 unable to bind Ca2+ in vitro showed normal photokinesis and PPR. Thus, the redox sensitivity of DC3 does not appear to be directly related to these reactions, although it remains possible that interaction with other ODA-DC components alters the Ca2+-binding properties of mutagenized DC3 (Casey et al., 2003b).
Identification of nondynein redox-sensitive flagellar proteins
Using the redox 2D gel method we identified six distinct flagellar proteins (including tubulin) that form intermolecular disulfides in vivo. Two are peroxiredoxins, which as a general class are involved in redox signaling, and three of which (FAP41, FAP102, and C_800054) are of currently unknown function. Furthermore, we found a 35-kD version of flagellar membrane glycoprotein 1a/b that underwent a large redox-dependent conformational change and either represents a novel processed form or a proteolytic fragment. Further use of this 2D electrophoretic method combined with proteomic analysis should allow for a mixed disulfide interaction map of the flagellum to be constructed.
In summary, we have examined the effects of redox poise on C. reinhardtii swimming behavior and the biochemical properties of dynein components in vivo and in vitro. Our data support a role for a redox-based regulatory system that modulates flagellar beat frequency through alterations in ODA activity.
Materials and methods
Strains and culture conditions
C. reinhardtii wild-type strain (CC124), oda2 (CC2230; Kamiya, 1988), oda1 (CC2228; Kamiya, 1988), oda4-s7 (CC2999; Sakakibara et al., 1993), and oda11 (CC2672; Sakakibara et al., 1991) were grown in Tris-acetate-phosphate medium (Gorman and Levine, 1965) and aerated with 15% CO2 and 85% air at 20°C on a 15/9 h light/dark cycle. Mutants that are defective in photosystems I (ac9; strain CC1050) and II (ac115; strain CC3679) require acetate for growth and were cultured in the same medium. As lengthy illumination is lethal for the photosystem mutants, precultures of these strains were grown in complete darkness.
To test for alterations in flagellar motility and the in vivo redox state of flagellar proteins, cells were grown in the light (112 μmol m−2 s−1) or in complete darkness. Precultures were grown on the regular light/dark cycle for 3–4 d, and then inoculated into several flasks. In the dark phase of the day before sample preparation, flasks were split into two groups; one was kept under the regular light/dark cycle, and the other was kept in continuous darkness (wrapped tightly with aluminum foil). Samples were prepared 6 h after the transition to the light phase. For motility analyses, the light conditions were altered after removing initial samples, and additional samples were assayed 30 and 60 min later (Fig. 1 a). Precultures of the photosystem mutants were grown for 6–7 d in the dark and split into two groups, and light-adapted strains were illuminated for 1 h.
Effects of oxidant stress on C. reinhardtii motility in vivo
To assess the effects of oxidant stress on C. reinhardtii behavior, hydrogen peroxide (final concentrations of 0.1 and 0.5 mM), N,N′-DMTU (Sigma-Aldrich), and TEMPOL (Fluka; final concentrations of 5, 25, and 50 mM) were added directly to the culture medium. DMTU is a hydroxyl radical scavenger, whereas TEMPOL is a spin-label reagent and a superoxide dismutase mimetic. These two compounds act as membrane-permeable ROS scavengers. After reagent addition, cells in sample tubes were kept in the light or dark for 30 min before analysis of their swimming behavior (see Video microscopic analysis of the C. reinhardtii PPR).
Beat frequency analysis
C. reinhardtii flagellar beat frequency was determined using the method of Kamiya (2000), with minor modifications (DiBella et al., 2004). A photodetector with a gradient filter was set on top of a dark-field microscope (BX-51; Olympus), and cells were observed with dim red light. Signals derived from cell body vibration were transferred to the computer sound board and fast-Fourier transformed (FFT) using SIGVIEW (SignalLab). Transformed signals were averaged for ∼30 s, and the resulting power spectrum represents the beat frequency distribution of ∼30–50 cells. The beat frequency of reactivated cell models was determined with a similar FFT system, which used an A/D converter (SE-U55X; ONKYO) instead of the sound board, and had a photomultiplier and amplifier (constructed by R. Kamiya, University of Tokyo, Tokyo, Japan) in place of the photodetector.
Video microscopic analysis of the C. reinhardtii PPR
C. reinhardtii swimming was observed using a dark-field microscope (model BX51; Olympus), and images were captured with a video camera (7266MD; World Precision Instruments) at 30 frames/s and recorded using a DVD recorder (RDR-GX7; Sony). After allowing cells to swim under dim red light for several seconds, photoshock was initiated by removing the red filter. To avoid analysis of cells that had become adapted to the photoshock conditions, fresh samples were used for each determination.
Definition of PPR phase
During normal forward swimming, C. reinhardtii flagella have an asymmetric or ciliary beat, and when exposed to a rapid increase in light intensity they exhibit the PPR and swim backward. This response consists of two major stages (Fig. 1 B). First, cells change waveform in a Ca2+-dependent manner and swim backward or stop (phase I). Next, they rotate to randomize swimming direction (phase II) before the recovery of normal forward swimming (Schmidt and Eckert, 1976; Yoshimura et al., 1997). We defined the start of phase I PPR as the point at which cells switched their swimming direction or stopped after the red filter was removed. We defined the start of phase II PPR as the point when cells stopped moving backward and/or started rotating; when cells moved from that position with their flagella forward was used to indicate the start of the recovery of forward swimming. We replayed the video data frame-by-frame, and counted the number of frames to determine the durations of phase I and II PPR.
Reactivation of cell models
Demembranated cell models were reactivated as described in Kamiya and Witman (1984), with modifications. In brief, cells were washed with HES buffer (10 mM Hepes, pH 7.4, 0.5 mM EGTA, and 4% sucrose) and demembranated with 0.2% IGEPAL CA-630 (Sigma-Aldrich; replaces Nonidet P-40) in HMEK (30 mM Hepes, pH 7.4, 5 mM MgSO4, 1 mM EGTA, and 50 mM K-acetate). Cell models were then treated with a series of redox buffers in HMEKP (HMEK + 1% polyethylene glycol; mean molecular mass 20,000 D) containing 70 U/ml creatine kinase and 5 mM phosphocreatine as an ATP-regenerating system. The redox buffers used consisted of 5 mM GSH with 1 mM GSSG; 3 mM GSH and GSSG; 1 mM GSH and 5 mM GSSG; 0.3 mM GSH and 6 mM GSSG; 0.06 mM GSH and 6 mM GSSG; 10 mM DTT or GSH were added as reducing controls; and 10 mM 5,5′-dithio-bis(2-nitrobenzoic acid; DTNB) or GSSG as oxidizing controls. After incubation on ice for 1 h, cell models were reactivated by addition of ATP at a final concentration of 1 mM. Beat frequency was determined using the same method as for live cells.
Nonreducing–reducing (redox) 2D SDS-PAGE
200-ml cultures of C. reinhardtii (∼4 × 106 cells/ml) were deflagellated by the addition of acetic acid (final concentration 25 mM), and TCA (final concentration 5% vol/vol) was immediately added to freeze the in vivo redox state of flagellar proteins. After elimination of cell bodies by differential centrifugation, flagella were pelleted and washed with acetone to remove TCA. Dried flagellar samples were treated with an alkylation solution (0.1 M Tris-HCl, pH 7.4, 1% SDS, 0.1 M iodoacetamide (IAA; MP Biomedicals) for 30 min at room temperature, mixed with 2× SDS sample buffer without reducing reagents, and incubated at 37°C for 30 min.
Initially samples were electrophoresed in a 5–15% gradient polyacrylamide gel. The gel lanes were excised, and then soaked in 1× SDS sample buffer containing 0.1 M DTT for 30 min and, subsequently, in 1× SDS sample buffer containing 0.1 M DTT and 0.1 M IAA for 30 min. After washing with 1× SDS sample buffer without DTT and IAA for 30 min to reduce streaking artifacts, gel strips were placed onto the second dimension gel (another 5–15% polyacrylamide gradient) and sealed with 1% agarose in 50 mM Tris-HCl, pH 6.8. After electrophoresis, gels were either stained with SYPRO Ruby (Invitrogen) or silver (Sambrook and Russell, 2001) or blotted to nitrocellulose and probed with affinity-purified anti-LC3 (R4930) and -LC5 (R4929) antibodies (Patel-King et al., 1996) and anti-DC3 antiserum (Casey et al., 2003a), which was provided by G. Witman and D. Casey (University of Massachusetts Medical School, Worcester, MA). In addition, blot-purified antibody R7178 against LC7a, which is an ODA LC that does not contain any Cys residues (Bowman et al., 1999), was used as a negative control. The primary rabbit antibodies were detected using a peroxidase-conjugated secondary antibody and an enhanced chemiluminescent detection system (GE Healthcare). Data were digitized using a scanner (Perfection 1250; Epson).
To identify redox-sensitive proteins, individual spots from the redox 2D gels were excised after staining with SYPRO Ruby and treated with trypsin, and the resulting fragments were subjected to mass spectrometric analysis in the Proteomic Mass Spectrometry Laboratory (University of Massachusetts Medical School, Worcester, MA).
Assessment of the in vivo redox state of dynein components
A 50-ml culture of C. reinhardtii (∼3 × 106 cells/ml) was deflagellated and treated with TCA, as described in the previous section, to freeze the in vivo redox state of flagellar proteins. After elimination of cell bodies by differential centrifugation, flagella were pelleted and washed with acetone to remove TCA. Samples were then alkylated with 20 mM AMS (Invitrogen) in 0.1 M Tris-HCl, pH 7.4, and 1% SDS, incubated for 30 min at room temperature, and subjected to nonreducing 1D SDS-PAGE. Treatment with AMS increases the mass of a protein by 490 D per modified thiol. Fully oxidized and reduced standards were obtained by treating isolated axonemes (∼6 μg/μl) in HMEK with 10 mM DTNB and DTT in HMEK, respectively, at 37°C for 30 min, followed by TCA fixation and AMS treatment. All samples were mixed with an equal volume of 2× SDS sample buffer without any reducing reagents (0.1 M Tris-HCl, pH 6.8., 4% SDS, 20% glycerol, and 0.01% Bromophenol blue) and incubated for 30 min before electrophoresis. This methodology has been adapted from that described by Kobayashi et al. (1997).
In vitro modulation of axonemal redox state
Redox buffers were prepared with GSH and GSSG in HMEK buffer. The final GSH/GSSG concentrations were 2.5:250, 2.5:200, 2.5:150, 2.5:100, 2.5:75, 2.5:50, 5:50, 5:25, 25:25, and 25:5 (mM:mM). Purified axonemes were resuspended at a concentration of ∼6 μg/μl in the redox buffer series, incubated at room temperature for 30 min, fixed with TCA (final 5% vol/vol), and then processed as described above.
Acknowledgments
We thank Drs. George Witman and Diane Casey for the anti-DC3 antibody, Dr. John Leszyk for mass spectrometry analysis, Dr Elizabeth Harris for guidance on growing mutants with defective chloroplasts, and Dr. Vladimir Rodionov, Panteleimon Rompolas, and Dr. Toshiki Yagi for helpful advice on constructing the video microscope system.
This study was supported by a Lalor Foundation postdoctoral fellowship (to K. Wakabayashi) and grant GM51293 from the National Institutes of Health (to S.M. King). S.M. King is an investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Abbreviations used in this paper: AMS, 4-acetamido-4-maleimidylstilbene-2,2-disulfonic acid; DC, docking complex; DMTU, dimethylthiourea; DTNB, 5,5′-dithiobis (2-nitrobenzoic acid); DTT, dithiothreitol; FFT, fast-Fourier transformed; HC, heavy chain; IAA, iodoacetamide; IC, intermediate chain; LC, light chain; ODA, outer dynein arm; PPR, photophobic response; ROS, reactive oxygen species; TEMPOL, 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl.
References
- Aitken, R.J., D. Harkiss, W. Knox, M. Paterson, and S. Irvine. 1998. On the cellular mechanisms by which the bicarbonate ion mediates the extragenomic action of progesterone on human spermatozoa. Biol. Reprod. 58:186–196. [DOI] [PubMed] [Google Scholar]
- Aksenov, M.Y., M.V. Aksenova, D.A. Butterfield, J.W. Geddes, and W.R. Markesbery. 2001. Protein oxidation in the brain in Alzheimer's disease. Neurosci. Lett. 103:373–383. [DOI] [PubMed] [Google Scholar]
- Baker, M.A., and R.J. Aitken. 2004. The importance of redox regulated pathways in sperm cell biology. Mol. Cell. Endocrinol. 216:47–54. [DOI] [PubMed] [Google Scholar]
- Bessen, M., R.B. Fay, and G.B. Witman. 1980. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 86:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, A.B., R.S. Patel-King, S.E. Benashski, J.M. McCaffery, L.S. Goldstein, and S.M. King. 1999. Drosophila roadblock and Chlamydomonas LC7: a conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 146:165–180. [PMC free article] [PubMed] [Google Scholar]
- Casey, D., K. Inaba, G. Pazour, S. Takada, K. Wakabayashi, C. Wilkerson, R. Kamiya, and G. Witman. 2003. a. DC3, the 21-kD subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell. 14:3650–3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, D., T. Yagi, R. Kamiya, and G. Witman. 2003. b. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J. Biol. Chem. 278:42652–42659. [DOI] [PubMed] [Google Scholar]
- DiBella, L.M., and S.M. King. 2001. Dynein motors of the Chlamydomonas flagellum. Int. Rev. Cytol. 210:227–268. [DOI] [PubMed] [Google Scholar]
- DiBella, L.M., E.F. Smith, R.S. Patel-King, K. Wakabayashi, and S.M. King. 2004. A novel Tctex2-related light chain is required for stability of inner dynein arm I1 and motor function in the Chlamydomonas flagellum. J. Biol. Chem. 279:21666–21676. [DOI] [PubMed] [Google Scholar]
- Finkel, T., and N.J. Holbrook. 2000. Oxidants, oxidative stress and the biology of ageing. Nature. 408:239–247. [DOI] [PubMed] [Google Scholar]
- Forti, G., A. Furia, P. Bombelli, and G. Finazzi. 2003. In vivo changes of the oxidation-reduction state of NADP and of the ATP/ADP cellular ratio linked to the photosynthetic activity in Chlamydomonas reinhardtii. Plant Physiol. 132:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons, I.R., and E. Fronk. 1979. A latent adenosine triphosphatase form of dynein 1 from sea urchin sperm flagella. J. Biol. Chem. 254:187–196. [PubMed] [Google Scholar]
- Gorman, D.S., and R.P. Levine. 1965. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 54:1665–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyer, A., C. Haslekas, M. Miginiac-Maslow, U. Klein, P. Le Marechal, J.P. Jacquot, and P. Decottignies. 2002. Isolation and characterization of a thioredoxin-dependent peroxidase from Chlamydomonas reinhardtii. Eur. J. Biochem. 269:272–282. [DOI] [PubMed] [Google Scholar]
- Haarer, B.K., and D.C. Amberg. 2004. Old yellow enzyme protects the actin cytoskeleton from oxidative stress. Mol. Biol. Cell. 15:4522–4531. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Harrison, A., M. Sakato, H.W. Tedford, S.E. Benashski, R.S. Patel-King, and S.M. King. 2002. Redox-based control of the γ heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil. Cytoskeleton. 52:131–143. [DOI] [PubMed] [Google Scholar]
- Hegemann, P. 1997. Vision in microalgae. Planta. 203:265–274. [DOI] [PubMed] [Google Scholar]
- Higo, T., M. Hattori, T. Nakamura, T. Natsume, T. Michikawa, and K. Mikoshiba. 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell. 120:85–98. [DOI] [PubMed] [Google Scholar]
- Josef, K., J. Saranak, and K.W. Foster. 2005. Ciliary behavior of a negatively phototactic Chlamydomonas reinhardtii. Cell Motil. Cytoskeleton. 61:97–111. [DOI] [PubMed] [Google Scholar]
- Kamiya, R. 1988. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J. Cell Biol. 107:2253–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya, R. 1995. Exploring the function of inner and outer dynein arms with Chlamydomonas mutants. Cell Motil. Cytoskeleton. 32:98–102. [DOI] [PubMed] [Google Scholar]
- Kamiya, R. 2000. Analysis of cell vibration for assessing axonemal motility in Chlamydomonas. Methods. 22:383–387. [DOI] [PubMed] [Google Scholar]
- Kamiya, R., and M. Okamoto. 1985. A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J. Cell Sci. 74:181–191. [DOI] [PubMed] [Google Scholar]
- Kamiya, R., and G.B. Witman. 1984. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, S.M. 2000. The dynein microtubule motor. Biochim. Biophys. Acta. 1496:60–75. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., S. Kishigami, M. Sone, H. Inokuchi, T. Mogi, and K. Ito. 1997. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc. Natl. Acad. Sci. USA. 94:11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutoulis, A., G.J. Pazour, C.G. Wilkerson, K. Inaba, H. Sheng, S. Takada, and G.B. Witman. 1997. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J. Cell Biol. 137:1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, T.A., S.M. Patel, and K.A. Johnson. 1994. Error-prone replication of repeated DNA sequences by T7 DNA polymerase in the absence of its processivity subunit. Proc. Natl. Acad. Sci. USA. 91:6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, B.F., L.B. Pedersen, M. Feely, J.L. Rosenbaum, and D.R. Mitchell. 2005. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol. Biol. Cell. 16:4509–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, A.G., and D.D. Morgan. 1999. Rapid analysis of Chlamydomonas reinhardtii flagellar beat activity with a LSCM. Microscopy and Analysis. 34:7–9. [Google Scholar]
- Nakamura, K., C.G. Wilkerson, and G.B. Witman. 1997. Functional interaction between Chlamydomonas outer arm dynein subunits: the γ subunit suppresses the ATPase activity of the αβ dimer. Cell Motil. Cytoskeleton. 37:338–345. [DOI] [PubMed] [Google Scholar]
- Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1–6. [DOI] [PubMed] [Google Scholar]
- Ogawa, K., and H. Mohri. 1972. Studies on the flagellar ATPase from sea urchin spermatozoa. I. Purification and some properties of the enzyme. Biochim. Biophys. Acta. 256:142–155. [DOI] [PubMed] [Google Scholar]
- Ogawa, K., H. Takai, A. Ogiwara, E. Yokota, T. Shimizu, K. Inaba, and H. Mohri. 1996. Is outer arm dynein intermediate chain 1 multifunctional? Mol. Biol. Cell. 7:1895–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma, P., A. Hozumi, K. Ogawa, and K. Inaba. 2001. Molecular cloning and characterization of a thioredoxin/nucleoside diphosphate kinase related dynein intermediate chain from the ascidian, Ciona intestinalis. Gene. 275:177–183. [DOI] [PubMed] [Google Scholar]
- Patel-King, R.S., S.E. Benashki, A. Harrison, and S.M. King. 1996. Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonas outer dynein arm. J. Biol. Chem. 271:6283–6291. [DOI] [PubMed] [Google Scholar]
- Pazour, G., O. Sineschekov, and G. Witman. 1995. Mutational analysis of the phototransduction pathway of Chlamydomonas reinhardtii. J. Cell Biol. 131:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour, G., N. Agrin, J. Leszyk, and G. Witman. 2005. Proteomic analysis of a eukaryotic flagellum. J. Cell Biol. 170:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra, A.S., and K. Padmasree. 2003. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 8:546–553. [DOI] [PubMed] [Google Scholar]
- Sadek, C.M., A.E. Damdimopoulos, M. Pelto-Huikko, J.A. Gustafsson, G. Spyrou, and A. Miranda-Vizuete. 2001. Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells. 6:1077–1090. [DOI] [PubMed] [Google Scholar]
- Sadek, C., A. Jimenez, A. Damdimopoulos, T. Kieselbach, M. Nord, J.-A. Gustafsson, G. Spyrou, E. Davis, R. Oko, F. van der Hoorn, and A. Mirande-Vizuete. 2003. Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelia and spermatid manchette and axoneme. J. Biol. Chem. 278:13133–13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara, H., D.R. Mitchell, and R. Kamiya. 1991. A Chlamydomonas outer arm dynein mutant missing the α heavy chain. J. Cell Biol. 113:615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara, H., S. Takada, S.M. King, G.B. Witman, and R. Kamiya. 1993. A Chlamydomonas outer arm dynein mutant with a truncated β heavy chain. J. Cell Biol. 122:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato, M., and S. King. 2003. Calcium regulates ATP-sensitive microtubule binding by Chlamydomonas outer arm dynein. J. Biol. Chem. 278:43571–43579. [DOI] [PubMed] [Google Scholar]
- Sambrook, J. and Russell, D.W. 2001. Molecular Cloning. A Laboratory Manual. Third edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schmidt, J.A., and R. Eckert. 1976. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 262:713–715. [DOI] [PubMed] [Google Scholar]
- Shimizu, T., and I. Kimura. 1974. Effects of N-ethylmaleimide on dynein adenosine triphosphatase activity and its recombining ability with outer fibers. J. Biochem. (Tokyo). 76:1001–1008. [PubMed] [Google Scholar]
- Tabor, S., H.E. Huber, and C.C. Richardson. 1987. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J. Biol. Chem. 262:16212–16232. [PubMed] [Google Scholar]
- Takada, S., and R. Kamiya. 1994. Functional reconstitution of Chlamydomonas outer dynein arms from α–β and γ subunits: requirement of a third factor. J. Cell Biol. 126:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., C.G. Wilkerson, K. Wakabayashi, R. Kamiya, and G.B. Witman. 2002. The outer dynein arm-docking complex: composition and characterization of a subunit (Oda1) necessary for outer arm assembly. Mol. Biol. Cell. 13:1015–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman, G.B. 1993. Chlamydomonas phototaxis. Trends Cell Biol. 3:403–408. [DOI] [PubMed] [Google Scholar]
- Yoshimura, K., C. Shingyoji, and K. Takahashi. 1997. Conversion of beating mode in Chlamydomonas flagella induced by electric stimulation. Cell Motil. Cytoskeleton. 36:236–245. [DOI] [PubMed] [Google Scholar]
- Zhang, H., and D.R. Mitchell. 2004. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J. Cell Sci. 117:4179–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]