Abstract
The KcsA channel is a representative potassium channel that is activated by changes in pH. Previous studies suggested that the region that senses pH is entirely within its transmembrane segments. However, we recently revealed that the cytoplasmic domain also has an important role, because its conformation was observed to change dramatically in response to pH changes. Here, to investigate the effects of the cytoplasmic domain on pH-dependent gating, we made a chimera mutant channel consisting of the cytoplasmic domain of the KcsA channel and the transmembrane region of the MthK channel. The chimera showed a pH dependency similar to that of KcsA, indicating that the cytoplasmic domain can act as a pH sensor. To identify how this region detects pH, we substituted certain cytoplasmic domain amino acids that are normally negatively charged at pH 7 for neutral ones in the KcsA channels. These mutants opened independently of pH, suggesting that electrostatic charges have a major role in the cytoplasmic domain's ability to sense and respond to pH.
Introduction
Ion channels are membrane-spanning proteins with a sensor region that senses and conveys environmental changes to the pore region, which passes ions through the membrane accordingly (1). For ion channels with sensor regions in their cytoplasmic domains, it is commonly thought that the cytoplasmic domain rearranges in response to a stimulus and that this rearrangement determines whether the pore opens or closes (2–4). For example, the BK channel, a large conductance calcium-activated potassium channel, is activated when calcium binds to its cytoplasmic domain, resulting in conformational changes of the cytoplasmic domain that open the pore.
The KcsA channel is a proton-activated potassium channel (5,6). It forms a tetramer, with each subunit having two transmembrane regions (TM1 and TM2) and a cytoplasmic domain that consists of 35 amino acids at the C-terminus (7,8). There is a great deal of evidence indicating that its transmembrane regions are responsible for pH sensing (8–10). For example, a cytoplasmic domain deletion KcsA mutant retained pH-sensitive channel activity (8), and a cluster of charged amino acids at the end of TM2 was found to act as a pH sensor (10). Recently, this cluster was investigated in more detail by Cuello et al. (11,12), who determined that the amino acids H25 in TM1 and R117, E118, and E120 at the end of TM2 are mainly responsible for this sensitivity. However, other authors have found that the cytoplasmic domain also has pH sensitivity. Yuchi et al. (13) found that replacing the cytoplasmic domain with an artificial tetramerization domain causes the channel's thermostability to be insensitive to pH, and in a previous study (14) we showed that unlike wild-type KcsA, cytoplasmic domain deletion mutants have very low activation even at pH 4. In addition, in that study we detected significant conformational changes in the cytoplasmic domain that corresponded with pH-dependent gating, and observed that the channel open probability increased greatly when the KcsA cytoplasmic domain was pushed toward the membrane with an atomic force microscope (15). These results further suggest rearrangement of the cytoplasmic domain in response to pH regulates the opening and closing of the KcsA channel.
In this study, to clarify the role of the KcsA cytoplasmic domain, we sought to determine its effects on pH-dependent gating and propose a corresponding mechanism. To that end, we made a chimera mutant channel by combining the KcsA cytoplasmic domain with the transmembrane domain of the MthK channel and measured its activity at different pH values. The chimera showed a pH dependency similar to that of KcsA, indicating that the cytoplasmic domain acts as a pH sensor. In a separate experiment, we substituted specific amino acids in the cytoplasmic domain of the KcsA to identify which amino acids are involved in the regulation of pH-sensitive gating.
Materials and Methods
Constructs and mutants
KcsA cloned into pQE-30 vectors including an N-terminal hexahistidine tag were kindly provided by Dr. Kubo of the National Institute of Advanced Industrial Science and Technology. MthK cloned into pQE-70 vectors including a C-terminal hexahistidine tag was a gift from Dr. Jiang of University of Texas Southwestern Medical Center. We generated MthK TM by inserting the DNA fragment coding for MthK 1-106 into the pQE-70 SphI- Bgl II site. MthK TM–KcsA cyto was made by replacing MthK 107-336 in pQE-70 with KcsA 123-160. We then generated a neutral mutant and eight mutants in which one or more amino acids that were negatively charged at pH 7 were exchanged with neutral ones (mutants 1–8) in wild-type KcsA using the Quick-Change site-directed mutagenesis kit (Stratagene). All included an E71A mutation to prevent gate inactivation (16).
Protein expression and purification
MthK TM, MthK TM–KcsA cyto, and neutral mutants were expressed in Escherichia coli XL1-Blue and purified as previously described (14). Briefly, they were overexpressed in 0.5 mM isopropyl β-D-thiogalactopyranoside (IPTG). Expressed channels were extracted from membrane fractions in 10 mM n-dodecyl β-D-maltoside (Dojin) and purified with the use of Co2+ affinity gel beads (TALON metal affinity resins; Clontech).
Reconstitution into liposomes
Purified channels were reconstituted into liposomes as previously described (14). The liposomes consisted of a phospholipid mixture (POPE/POPG = 3:1).
Channel current recordings
Channel currents were measured by the planar bilayer method (17,18). Bilayers were made by painting a lipid solution (POPE/POPG = 3:1 in n-decane) across a small hole on a thin plastic sheet. Currents were recorded in a symmetrical solution containing 200 mM KCl and 10 mM MES (pH 4.0, pH 5.0, and pH 6.0), 200 mM KCl and 10 mM Tris-Hepes (pH 7.0 and pH 8.0), or 200 mM KCl and 10 mM Tris-HCl (pH 9.0). The bath solution was held at virtual ground such that voltage at the upper solution was connected to a patch-clamp amplifier by an Ag-AgCl electrode-defined membrane potential.
Results
The KcsA cytoplasmic domain acts as a pH sensor
To clarify whether the KcsA cytoplasmic domain is involved in pH-dependent gating, we made a chimera mutant (MthK TM–KcsA cyto) consisting of the cytoplasmic domain of the KcsA channel and the transmembrane region of the MthK channel (Fig. 1 A). Fig. 1 B shows typical single-channel records measured by the planar bilayer method at pH 4 and pH 7 for KcsA (KcsA (E71A)), the transmembrane region of MthK (MthK TM), and our chimera (MthK TM–KcsA cyto). Fig. 1 C shows their corresponding open probabilities from pH 4 to pH 9. KcsA (E71A) includes an E71A mutation that completely abolishes the long closures of the channel and makes it constitutively open at low pH (16,19). KcsA (E71A) activity was seen to be pH-dependent, as previously reported (16). It always opened at pH 4 (0.90 ± 0.14), but had low open probabilities at neutral (0.13 ± 0.2, pH 7) and alkaline (0.06 ± 0.12, pH 9) pH. On the other hand, the open probabilities of MthK-TM were high for all observed pH values, despite the full-length MthK channels being inactive at pH < 6.5 (20,21). MthK TM–KcsA cyto showed a pH dependency similar to that of KcsA (E71A). The open probability was nearly 1.0 (0.97 ± 0.03) at pH 4, but it was only 0.27 (0.27 ± 0.43) at pH 7 and 0.19 (0.19 ± 0.24) at pH 9, which more resembles the behavior of KcsA (E71A) and therefore suggests that the cytoplasmic domain acts as a pH sensor.
Figure 1.
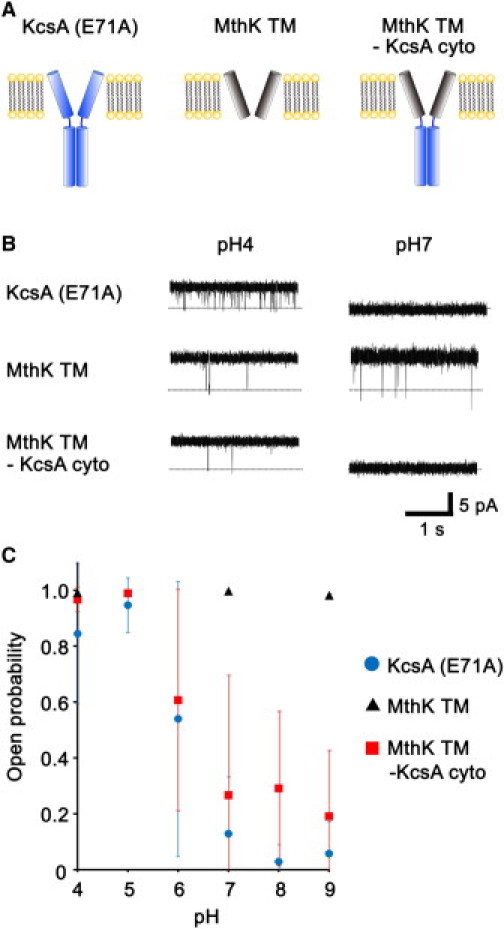
Effect of the KcsA cytoplasmic domain. Single-channel currents were recorded in a symmetrical solution containing 200 mM KCl. (A) Schematic representation of KcsA, MthK TM, and MthK TM–KcsA cyto channels. (B) Representative current records of each at pH 4 and pH 7 at 40 mV. (C) Open probabilities (ordinate) at 40 mV versus pH (abscissa; n = 4–14).
Charged amino acids in the KcsA cytoplasmic domain play an important role in pH-dependent gating
How does the KcsA cytoplasmic domain sense pH to regulate channel opening and closing? To answer this question, we focused on its charged amino acids, which make up about half of its 35 amino acids. For all analyses, we assumed that the pKa values of these residues were those measured in bulk water, and that they were insensitive to the microenvironment. Because the pKa values of aspartic acid and glutamic acid are ∼4.0 and 4.4, respectively, they are neutral at acidic pH but negatively charged at neutral pH. In contrast, a pKa of 6.5 makes histidine positively charged at acidic pH but neutral at neutral pH. The high pKa values of lysine and arginine (∼10.2 and 12.0, respectively) make them positively charged at all tested pH conditions. Fig. 2 shows the amino acids in the cytoplasmic domain and the charged states at pH 4 and pH 7. At pH 4, approximately one-third of the residues are positively charged, making for a positively charged cytoplasmic domain, whereas at pH 7 the numbers of positively and negatively charged amino acids are almost the same, making the cytoplasmic domain effectively neutral.
Figure 2.

Charged states in the KcsA cytoplasmic domain at pH 4 and 7. Amino acid sequence of the KcsA cytoplasmic domain (uniprotkb: P0A334). Blue and red letters, respectively, indicate positively and negatively charged amino acids.
To investigate the effect of the charged state on pH-dependent gating, we made a neutral mutant (Fig. 3). Specifically, eight amino acids (E130, E134, E135, E146, D149, E152, D156, and D157) that were negatively charged at pH 7 were replaced with neutral amino acids such as glutamine and asparagine (Fig. 3 A). The charged state of this mutant at pH 7 was almost the same as that at pH 4. The mutant also had the E71A mutation for the reasons described above. Fig. 3 B shows typical single-channel records for KcsA (E71A) and the neutral mutant at pH 4, 7, and 9 at 40 mV. As summarized in Table 1, the open probabilities of KcsA (E71A) at pH 7 and 9 were low, whereas it was almost 1.0 at pH 4. For the neutral mutant, however, the open probabilities were nearly 1.0 independently of pH.
Figure 3.
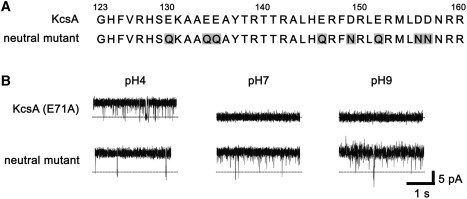
Effects of charged amino acids in the KcsA cytoplasmic domain on gating. A neutral mutant was made by replacing negatively charged amino acids in the cytoplasmic domain with neutral ones. The activity was then measured. (A) Amino acid sequences of the cytoplasmic domain and the neutral mutant. Shaded letters indicate replaced amino acids. (B) Representative current records at pH 4, 7, and 9, and 40 mV.
Table 1.
Open probabilities of the neutral mutant
| pH 4 | pH 7 | pH 9 | |
|---|---|---|---|
| KcsA (E71A) | 0.90 ± 0.14 | 0.13 ± 0.2 | 0.06 ± 0.12 |
| Neutral mutant | 0.99 ± 0.01 | 0.99 ± 0.01 | 0.97 ± 0.03 |
Open probabilities of KcsA (E71A) and the neutral mutant were measured at 40 mV in a symmetrical solution containing 200 mM KCl (mean ± SD, n = 4–14).
E146 and D149 affect pH-dependent gating
To clarify which charged amino acids are involved in pH-dependent gating, we made eight more mutants in which one or more amino acids that were negatively charged at pH 7 were exchanged for neutral ones (mutant 1: E130Q; mutant 2: E130QE134QE135Q; mutant 3: E130QE134QE135QE146QD149N; mutant 4: E146QD149NE152QD156ND157N; mutant 5: E146QD149N; mutant 6: D156ND157N; mutant 7: E146QD149NE152Q; and mutant 8: E152QD156ND157N; Fig. 4 A). These mutants also had the E71A mutation. Fig. 4 B shows the open probabilities at pH 7 and 40 mV. The open probabilities for mutants 1 and 2 were similar to that of KcsA (E71A). On the other hand, mutants 3–5 had much higher open probabilities (0.89 ± 0.14, 0.89 ± 0.18, and 0.80 ± 0.26, respectively). These three shared two mutations, E146Q and D149N, which were the only mutated amino acids in mutant 5. Fig. 4 C shows typical single-channel records for mutant 5 at pH 4, 7, and 9. This mutant was activated independently of pH even though KcsA (E71A) was nearly closed at pH 7 and 9 (Fig. 3 B), suggesting that E146 and D149 have an important role in pH-dependent gating. In addition, mutant 6, which has the mutations D156N and D157N, showed a higher open probability at pH 7 than KcsA (E71A) (although not to the same degree), suggesting that these amino acids also have a role in pH-dependent gating. Mutants 7 and 8 were discarded from the analysis because they closed within several seconds and never opened after they were reconstituted into a bilayer membrane even at pH 4.
Figure 4.
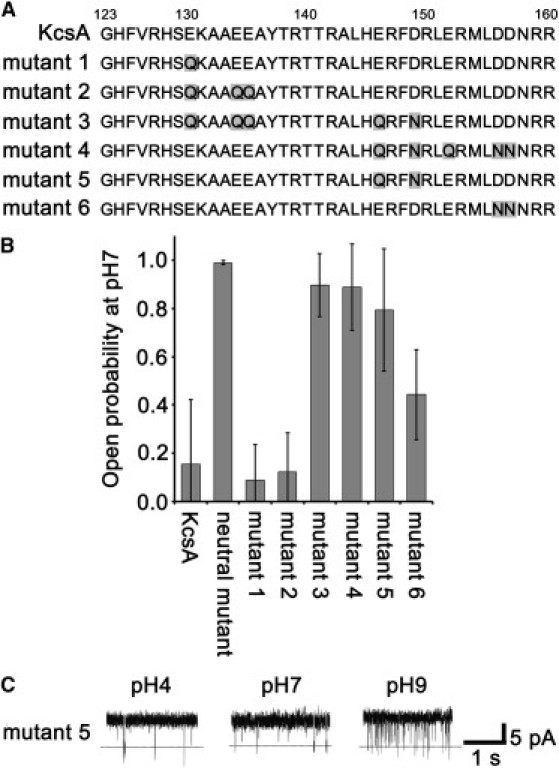
Effects of E146 and D149 on gating. Six mutants with different mutations in the cytoplasmic domain had their activities measured. (A) Amino acid sequences of the cytoplasmic domain for KcsA and the mutants. Shaded letters indicate differences from the KcsA sequence. (B) Open probabilities at pH 7 and 40 mV (n = 4–14). (C) Representative current records of mutant 5 at pH 4, 7, and 9, and 40 mV.
Discussion
Ion channels have a sensor region that detects and conveys environmental information to the pore region, thereby regulating whether the pore opens or closes. Some groups have observed that the region that is responsible for sensing pH in the KcsA channel is a cluster of charged amino acids at the end of TM2 and H25 in TM1 (8–10). These amino acids are located at the boundary between the membrane and cytoplasm. Recently, Cuello et al. (11,12) confirmed this charge cluster acts as a pH sensor, and proposed a pH-dependent gating mechanism whereby electrostatic repulsions mainly between R117, E118, E120, and H25 open the channel at acidic pH. At neutral pH, this repulsion vanishes so that the channel takes the closed conformation. Consequently, there is a general consensus that the transmembrane region is the only region that functions as a pH sensor in the KcsA channel.
However, our previous study raised the possibility that the cytoplasmic domain also acts as a pH sensor, based on observations that the KcsA cytoplasmic domain dramatically rearranged in a manner that correlated with changes in pH (14). Here, we confirmed this result by mutating the cytoplasmic domain to identify which amino acids are most likely responsible for this effect. Our chimera mutant, MthK-TM–KcsA-cyto, showed a sensitivity similar to that of KcsA (E71A) but not MthK (Fig. 1, B and C), whereas MthK-TM, which lacks the MthK cytoplasmic domains, showed no pH dependency in its gating (Fig. 1, B and C). This is consistent with another study (21), although the open probabilities differ. Although the transmembrane domains of KcsA and MthK channels are homologous (22), MthK has fewer charged amino acids at the region that corresponds to the cluster region of charged amino acids in KcsA. Therefore, the KcsA cytoplasmic domain was responsible for pH sensing.
To investigate the mechanism for this pH sensing, we attempted to identify which amino acids in the cytoplasmic domain regulate pH-dependent gating. Exchanging amino acids that are negatively charged in a neutral environment for neutral ones resulted in an open probability at pH 7 equal to that of KcsA (E71A) at pH 4, and indicated that amino acids E146, D149, D156, and D157 at the C-terminus of the cytoplasmic domain are especially important for pH sensing. According to a crystal structural study on the closed structure of KcsA (20), D149 forms a salt bridge with the R147 from an adjacent domain to form a bundle (PDB ID: 3EFF, 3EFD). Although that report did not specifically mention E146, D156, or D157, these three amino acids appear to be located between the adjacent helices or on the surface of the bundle formed by the four cytoplasmic domains, but not inside the bundle. This would expose them to the intracellular environment and thus make them potentially sensitive to changes in pH.
If the KcsA cytoplasmic domain is indeed sensitive to pH, how does it regulate the pore? Uysal et al. (23) revealed that the C-terminals of the cytoplasmic domain (Y137-N158) form a bundle in the closed state that does not dissociate during gating. We also did not detect any conformational change in this region (14), suggesting that Y137-N158 is robust to pH. However, we observed that the membrane-proximal region (Q119-A136) undergoes a major conformational change during gating, and others have found E118-E135 to be flexible and mobile (8,23). It is therefore likely that Y137-N158 makes at least some slight rearrangement in response to pH changes. It may be that these modest changes in the C-terminus of the cytoplasmic domain are amplified to create adjacent large conformational changes in the membrane-proximal region, leading to pore regulation. We have also proposed a mechanism that builds on our previous one (14,15) to explain how the cytoplasmic domain significantly changes its conformation in response to changes in pH (Fig. 5). At neutral pH, the amino acids in the cluster region at the boundary between the membrane and cytoplasmic are charged. This charge causes the amino acids to interact with each other in a manner that stabilizes the region around the cluster. A similar interaction in the cytoplasmic domain between the approximately equal number of positive and negative amino acids occurs, causing the four cytoplasmic domains to assemble. These two interaction states can stabilize KcsA to take the closed conformation. At acidic pH, the cluster region and cytoplasmic domains become positively charged. This causes the four subunits of the KcsA channel to repulse, resulting in a rearrangement that opens the pore.
Figure 5.
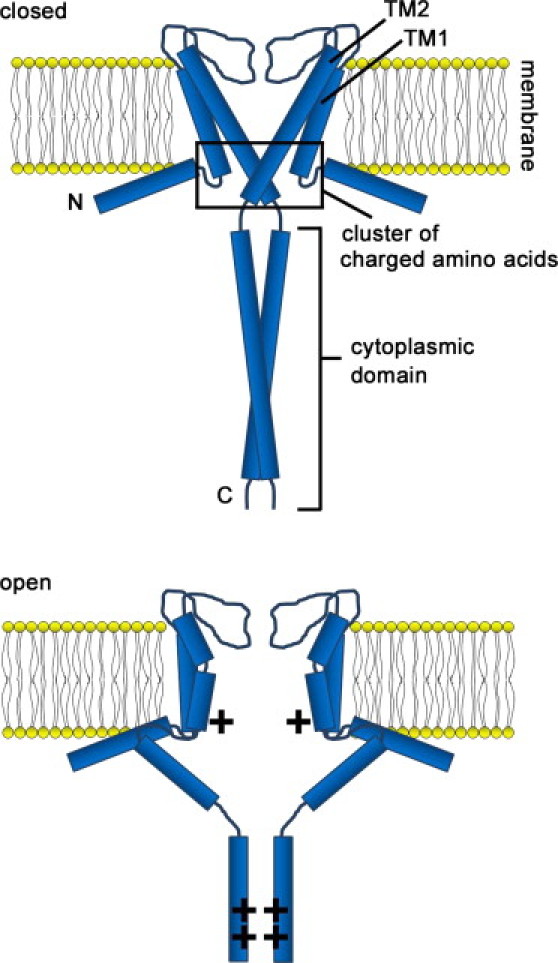
Model for cytoplasmic domain regulation of pH gating. Two of four subunits are shown. At pH 7, the cytoplasmic domains and the cluster region formed by the charged amino acids at the boundary between the membrane and cytoplasm are neutral, resulting in an oligomer that stabilizes the closed state. At pH 4, the cytoplasmic domains and the cluster region are positively charged. This causes them to repel one another, resulting in a rearrangement that opens the pore.
As described above, the KcsA channel has two pH-sensing regions: 1), the charge cluster region at the boundary between the membrane and cytoplasm; and 2), the cytoplasmic domain. Our results suggest, however, that the cytoplasmic domain plays the dominant role in pH sensing, for several reasons. For example, the MthK-TM channel, which is normally not pH-sensitive, showed sensitivity when it was made into a chimera with the cytoplasmic domain of the KcsA channel (Fig. 1). Furthermore, as shown in Fig. 3, exchanging negatively charged amino acids for neutral ones made the KcsA channel insensitive to pH even though there were no amino-acid changes at the transmembrane region.
In this study, we found that the KcsA pH sensor is in the channel's cytoplasmic domain. In previous studies, we showed that large conformational changes in the cytoplasmic domain are accompanied by the channel gating (14). The results presented here and in our previous single-channel manipulation study (15) strongly suggest that these conformational changes lead to the opening and closing of the channel. However, in these studies, we did not directly see any conformation change; instead, we relied on static information while measuring channel function with single-channel recordings at very high temporal resolution. To better clarify the structure-function relationship, conformational changes of a single-channel protein at temporal resolutions on a par with the single-channel current recording are needed.
Acknowledgments
We thank Dr. Tai Kubo (Advanced Industrial Science and Technology, Tukuba, Japan) for providing the plasmid pQE-30/KcsA, and Dr. Youxing Jiang (University of Texas Southwestern Medical Center, Dallas, Texas) for providing the plasmid pQE-70/MthK. We also thank Dr. Peter Karagiannis for carefully revising the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Fluorescence Live Imaging” (No. 23113519) and “Fluctuations toward Biological Functions” (No. 21107518), for Scientific Research B (No. 22370059) and for Young Scientists B (No. 23770190) of The Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- 1.Hille B. Sinauer; Sunderland, MA: 2001. Ion Channels of Excitable Membranes. [Google Scholar]
- 2.Magleby K.L. Gating mechanism of BK (Slo1) channels: so near, yet so far. J. Gen. Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y., Lee A., MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 4.Chakrapani S., Perozo E. How to gate an ion channel: lessons from MthK. Nat. Struct. Mol. Biol. 2007;14:180–182. doi: 10.1038/nsmb0307-180. [DOI] [PubMed] [Google Scholar]
- 5.Cuello L.G., Romero J.G., Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–3236. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- 6.Heginbotham L., LeMasurier M., Miller C. Single streptomyces lividans K(+) channels: functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 1999;114:551–560. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle D.A., Morais Cabral J., MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 8.Cortes D.M., Cuello L.G., Perozo E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 2001;117:165–180. doi: 10.1085/jgp.117.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi K., Takahashi H., Shimada I. Identification and characterization of the slowly exchanging pH-dependent conformational rearrangement in KcsA. J. Biol. Chem. 2007;282:15179–15186. doi: 10.1074/jbc.M608264200. [DOI] [PubMed] [Google Scholar]
- 10.Thompson A.N., Posson D.J., Nimigean C.M. Molecular mechanism of pH sensing in KcsA potassium channels. Proc. Natl. Acad. Sci. USA. 2008;105:6900–6905. doi: 10.1073/pnas.0800873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuello L.G., Cortes D.M., Perozo E. A molecular mechanism for proton-dependent gating in KcsA. FEBS Lett. 2010;584:1126–1132. doi: 10.1016/j.febslet.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuello L.G., Jogini V., Perozo E. Design and characterization of a constitutively open KcsA. FEBS Lett. 2010;584:1133–1138. doi: 10.1016/j.febslet.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuchi Z., Pau V.P., Yang D.S. GCN4 enhances the stability of the pore domain of potassium channel KcsA. FEBS J. 2008;275:6228–6236. doi: 10.1111/j.1742-4658.2008.06747.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirano M., Takeuchi Y., Ide T. Rearrangements in the KcsA cytoplasmic domain underlie its gating. J. Biol. Chem. 2010;285:3777–3783. doi: 10.1074/jbc.M109.084368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitta M., Ide T., Kawai T. Direct manipulation of a single potassium channel gate with an atomic force microscope probe. Small. 2011;7:2379–2383. doi: 10.1002/smll.201002337. [DOI] [PubMed] [Google Scholar]
- 16.Cordero-Morales J.F., Cuello L.G., Perozo E. Molecular determinants of gating at the potassium-channel selectivity filter. Nat. Struct. Mol. Biol. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 17.Ide T., Yanagida T. An artificial lipid bilayer formed on an agarose-coated glass for simultaneous electrical and optical measurement of single ion channels. Biochem. Biophys. Res. Commun. 1999;265:595–599. doi: 10.1006/bbrc.1999.1720. [DOI] [PubMed] [Google Scholar]
- 18.Ide T., Takeuchi Y., Yanagida T. Development of an experimental apparatus for simultaneous observation of optical and electrical signals from single ion channels. Single Molecules. 2002;1:33–42. [Google Scholar]
- 19.Cordero-Morales J.F., Jogini V., Perozo E. Molecular driving forces determining potassium channel slow inactivation. Nat. Struct. Mol. Biol. 2007;14:1062–1069. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 20.Ye S., Li Y., Jiang Y. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 2006;126:1161–1173. doi: 10.1016/j.cell.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Li Y., Berke I., Jiang Y. Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J. Gen. Physiol. 2007;129:109–120. doi: 10.1085/jgp.200609655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y., Lee A., MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 23.Uysal S., Vásquez V., Kossiakoff A. Crystal structure of full-length KcsA in its closed conformation. Proc. Natl. Acad. Sci. USA. 2009;106:6644–6649. doi: 10.1073/pnas.0810663106. [DOI] [PMC free article] [PubMed] [Google Scholar]


