Abstract
Polyphosphoinositides are among the most highly charged molecules in the cell membrane, and the most common polyphosphoinositide, phosphatidylinositol-4,5-bisphosphate (PIP2), is involved in many mechanical and biochemical processes in the cell membrane. Divalent cations such as calcium can cause clustering of the polyanionic PIP2, but the origin and strength of the effective attractions leading to clustering has been unclear. In addition, the question of whether the ion-mediated attractions could be strong enough to alter the mechanical properties of the membrane, to our knowledge, has not been addressed. We study phase separation in mixed monolayers of neutral and highly negatively charged lipids, induced by the addition of divalent positively charged counterions, both experimentally and numerically. We find good agreement between experiments on mixtures of PIP2 and 1-stearoyl-2-oleoyl phosphatidylcholine and simulations of a simplified model in which only the essential electrostatic interactions are retained. In addition, we find numerically that under certain conditions the effective attractions can rigidify the resulting clusters. Our results support an interpretation of PIP2 clustering as governed primarily by electrostatic interactions. At physiological pH, the simulations suggest that the effective attractions are strong enough to give nearly pure clusters of PIP2 even at small overall concentrations of PIP2.
Introduction
The concentration of the lipid phosphatidylinositol-4,5-bisphosphate (PIP2) in the cell membrane is only of ∼1%, yet it plays an outsized role in many critical processes, including cell division (1), endocytosis and exocytosis (2), and cell motility (3). Evidence exists that PIP2 forms clusters (4) at the submicron scale in vitro, and it has been speculated that similar domains might form under roughly physiological conditions. It has been conjectured that this clustering is crucial to its function at such low concentration (5,6).
Various mechanisms for the clustering have been proposed, including PIP2-protein interactions (7,8), exclusion from cholesterol-enriched ordered domains (4,9), and hydrogen bonds (10,11). However, recent experiments showed that PIP2 clusters can also be induced simply by adding calcium or other divalent ions (4,12). This raises the question of whether a purely electrostatic, ion-mediated mechanism could cause PIP2 clustering.
A counterion-mediated mechanism would seem unlikely because such attractions are typically quite weak. Biomolecules such as DNA (13) and actin (14) aggregate into large bundles in the presence of multivalent ions, but they each carry a net charge of (∼−102–103e) while PIP2 lipids carry a much smaller net charge (∼3e). Moreover, divalent cations are not sufficient to induce aggregation in bulk aqueous DNA or actin solutions, and the estimated attraction mediated by trivalent or tetravalent species is at most of ∼0.1 kBT per basepair (15). This small magnitude is not surprising because counterion-mediated attractions vanish in the mean-field approximation (16) and are the collective result of a near-cancellation of repulsive and attractive interactions between like and unlike charges, respectively. We note that the near-cancellation implies that the geometry of the charge configuration is likely to be important so that attractions mediated by small ions such as calcium can behave very differently from those mediated by extended cationic molecules such as spermidine (17).
In this article, we show that ion-mediated attractions in low-charged objects such as lipids are surprisingly strong, so that phase separation not only occurs but can be nearly complete at physiological values of the PIP2 charge. We conduct simulations on a model designed to retain only the most critical features of the electrostatics and compare the results to experiments on Langmuir monolayers of a mixture of PIP2 with neutral lipids with added divalent salts. We find semiquantitative agreement between simulations and experiments, suggesting that divalent-ion-mediated attractions are responsible for the observed clustering. The strength of these interactions depends strongly on the net charge of the lipid, which in turn has been shown to depend sensitively on ionic strength and on pH within a physiological range (18).
In addition, the simulations provide, to our knowledge, new insight into the mechanical properties of ion-mediated clusters: at moderate PIP2 charge, they are like two-dimensional liquids in which lipids can diffuse around as usual, but at sufficiently high PIP2 charge they form rigid, gel-like clusters upon exposure to divalent ions.
Materials and Methods
Experiments
We look for phase separation using visual analysis of both epifluorescence micrographs and atomic force micrographs of mixed lipid monolayers prepared in a Langmuir trough (Kibron, Helsinki, Finland). L-α-phosphatidylinositol-4,5-bisphosphate (PIP2) and 1-stearoyl-2-oleoyl phosphatidylcholine (SOPC) were purchased from Avanti Polar Lipids (Alabaster, AL). In the epifluorescence studies, part of the PIP2 (equal to 0.5 mol % of the total lipid content) is replaced by a fluorescently labeled analog (BODIPY FL-PIP2), purchased from Echelon (Salt Lake City, UT).
The lipid mixture, consisting of SOPC with a total molar PIP2 fraction of ϕPIP is dissolved in a 2:1 chloroform/methanol mixture. A lipid monolayer is formed on a buffered subphase (10 mM HEPES, 100 μM EDTA, 5 mM DTT) by addition of the lipid solution to the air-water interface. The surface pressure is kept at 20 mN/m, corresponding to an initial area per lipid of ∼90 Å2.
In the fluorescence studies the monolayer is imaged on an inverted epifluorescence microscope, using the 10× objective. We verify that there are at most two bright spots, likely due to nonspecific insoluble aggregates or contaminants, in a field of view at this stage. The divalent salts CaCl2 or MgCl2 are then added at 1 mM to the subphase, followed by gentle mixing to avoid disrupting the monolayer. We allow up to 2 h for domains to coarsen before imaging again.
For the AFM studies, sample preparation is identical with the exception that the buffer in this case contains only 1 μM EDTA. Monolayer samples are transferred from the trough onto a glass coverslip, both before and after addition of divalent salt. These films are imaged on a NanoScope III atomic force microscope (Digital Instruments, Tonawanda, NY) in tapping mode.
We perform these procedures for a range of ϕPIP-values and several pH values: 3, 4.5, 6, 7.4, and 9. At these values of the pH, qPIP is roughly −1.5, −2.7, −3.2, −4.2, and −5.0, respectively, based on acid dissociation constants from Levental et al. (18). However, the ionization state of PIP2 may be influenced by various geometric and chemical factors (18,19), so we do not assume that these qPIP values are exact. We collect the results in a phase diagram for each experiment: The fluorescence measurements are the most direct visualization of domains, but might miss the smallest domains because of limited resolution, while the AFM images provide a more detailed picture at smaller length scales.
Simulations
We retain only the competition between electrostatic interactions and excluded volume repulsions by adopting a model in which both lipids and small ions are represented as charged spheres (radius Ri) with an excluded volume interaction given by the purely repulsive (truncated at its minimum and shifted) Lennard-Jones potential (the WCA potential (20)). Parameterized by an energy scale ε = kBT ≡ 1 (our unit of energy) and length scale σij = Ri + Rj, this potential is given by
for rij < 21/6 σij, and V(rij) = 0 otherwise, where rij is the center-to-center distance. Note that σij is the distance at which the potential equals kBT. N = 1600 lipid particles are confined to the z = 0 plane, to mimic the effect of the hydrophobic interaction that keeps them at the air-water interface. We use Ri = RL = 3 Å for the lipids and Ri = RCI = 2 Å for the small cations that can explore the entire simulation box. In a study of the dependence of the clustering on cation size, we vary it between 0.5 Å ≤ RCI ≤ 2.5 Å. The box is periodic in x- and y-directions (size Lx = 320 Å × Ly = 320 Å and has hard walls at z = 0 and z = Lz = 200 Å. The typical distance between lipids in the monolayer at z = 0 is therefore 8 Å.
In addition, the charged spheres interact via the Coulomb interaction, VC,ij = qiqjlB/rij, where we measure charges q in units of the proton charge. In room temperature water the Bjerrum length lB ≈ 7 Å.
We run molecular-dynamics simulations using LAMMPS (21), with a Nosé-Hoover thermostat (22) and PPPM for the long-range Coulomb interactions (23).
The strong Coulomb attraction between the anionic lipids and the small cations allows them to bind at a distance of roughly σij. The essence of ion-mediated attractions is that these bonds are strong and long-lived enough so that one or two counterions can draw together two lipids and be bound to both simultaneously (24,25). Due to its coarse-grained nature, our model underestimates the binding energy of such bonds. The main source of this effect is that the distance between the lipid particle and the Ca2+ in our model is much larger than the distance between a real phosphate group and a Ca2+ ion in real PIP2. This common side-effect of coarse-graining is typically compensated by adjusting the dielectric constant (see, e.g., Marrink et al. (26)). To find the required correction, we compare the PIP2 charge required for clustering as calculated from the numerical model and as measured in our experiments, at PIP2 fraction ϕPIP = 0.25. Experimentally we find the threshold pH at this PIP2 fraction to be between 3 and 4.5, which corresponds to a charge of roughly qPIP ≈ −2 (18). The dielectric constant required in our model to match this threshold is ∼27 (a factor-three lower than that of water). We then use this value of the dielectric constant to obtain the rest of the phase diagram.
Thus, we have a coarse-grained model in which lipids are replaced by spheres of the appropriate charge and simulated with explicit counterions using an adjusted dielectric constant. This simplification enables us to explore a large parameter space with modest computations. Of course, this coarse-graining approach is not quantitatively precise, but neither are calculations using typical approximations such as a uniform dielectric constant of 80 for water surrounding highly charged objects. We note that despite the simplicity of our model, it gives surface pressures of ∼20–50 mN/m, which is of the same order as in the experiments. Both in the experiments and in the simulations, the surface pressure drops by a few percent at low ϕPIP, and between 10% and 30% at ϕPIP = 0.25, upon addition of Ca2+.
These simulations were performed for a range of PIP2 charges qPIP and PIP2 fractions ϕPIP. To study the mobility of lipids within clusters and the cluster rigidity, we performed additional simulations at ϕPIP = 1.
Results
Phase behavior: experiments and simulation
Fig. 1 shows our experimental results: phase diagrams and snapshots of AFM (Fig. 1 a) and fluorescence (Fig. 1 b) studies of calcium-induced domain formation. At high PIP2 charge, cluster formation is readily observed, for example at pH 7.4 in either experiment, where qPIP ≈ −4.2 (Fig. 1, a 2, a 3, and b). Fig. 1 b shows epifluorescence micrographs, taken both before (left) and after (right) transferring the sample to a glass coverslip, at 25% PIP2 and pH 7.4. In these images, bright spots mark regions where PIP2 is concentrated. In the phase diagram, conditions for which these bright spots are seen are marked with solid disks. Cases that did not show signs of clustering are marked with open circles.
Figure 1.
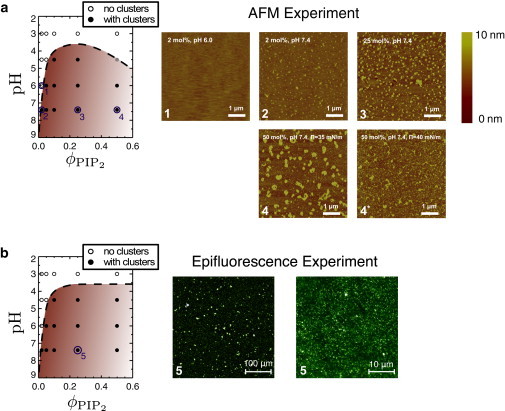
Phase diagrams (pH versus PIP2 fraction) and snapshots of experiments on mixed lipid monolayers (containing SOPC and PIP2) exposed to divalent salt. (a) Phase diagram. (Shaded coexistence region) Where clustering was observed, obtained from AFM studies. (Open disks) Parameter values where no clustering was observed. (Shaded disks are too close to the boundary to determine their behavior with certainty.) The AFM snapshots 1, 2, and 3 represent the conditions indicated by the corresponding points in the diagram: At ϕPIP = 0.02, there is no cluster formation at pH 6 but clusters are clearly present at pH 7.4. Larger domains are obtained for ϕPIP = 0.25. Domains persist when the surface pressure Π is increased to 35 or 40 mN/m (panels 4 and 4∗). (b) A very similar cluster formation phase diagram is obtained using epifluorescence with labeled PIP2. Snapshots are shown for ϕPIP = 0.25. (Left snapshot) Taken directly in the Langmuir trough. (Right snapshot) Taken after transferring the sample to a glass coverslip. We note that the apparent area fraction in the image is <0.25 because many of the PIP2 domains are too small to detect optically.
We note that domains usually appear within minutes, but we allow coarsening for up to 2 h before concluding there is no clustering. Thus we obtain the boundaries of the parameter region that lead to domain formation (shaded in the phase diagram). Fig. 1 a shows AFM images of the transferred samples. These images show a clear distinction between conditions that lead to domain formation (panels a 2 and a 3) and conditions in which the AFM image is flat (panel a 1). Control AFM images of samples without divalent salt did not show any sign of domain formation either. Domains persist when the surface pressure is increased to 35 or 40 mN/m (panels a 4 and a 4∗, respectively).
Although the two experimental approaches probe the system on different length scales, both of them give the same phase diagram. The exception is one data point, at pH 4.5 and qPIP = 0.5, which showed clustering in the fluorescence experiments, but which were not as clearly clustered as the other data points in the AFM experiments (marked with a shaded dot in the phase diagram). We note that, in general, the AFM images are less noisy and therefore lead to a more clear-cut distinction between clustering and nonclustering conditions.
The simulation snapshot in Fig. 2 c, obtained after simulating for 3.5 ns using 25% PIP2 with charge qPIP = −4, shows still-growing clusters at a scale of ∼10 nm. As expected, the positions of the condensed calcium ions (red disks in Fig. 2 c) clearly indicate their role in binding the charged lipids (green disks) together. To map out the phase diagram in the simulations, we follow the coarsening dynamics by keeping track of the static structure factor of the charged lipids,
where N is the number of PIP2 particles. As a function of k ≡ |k|, a maximum in this function at k = kpeak indicates that the PIP2 positions are developing structure at a length scale 2π/kpeak. For the more pronounced cases of cluster formation (deep in the phase-separated regime), we followed this peak as a function of time and verified that it scales with time as kpeak ∼ t−1/3, consistent with the general theory of coarsening of a binary fluid mixture (27). Thus, even though the counterion-mediated origin of phase separation yields irregularly shaped clusters instead of circular ones, this does not seem to affect the kinetics of coarsening. In the phase diagram in Fig. 2 a, all parameter values (ϕPIP, qPIP) for which an appreciable peak appears that approaches kpeak = 0 in S(k) for long times were marked as cluster-forming (within the coexistence region). Both in the experiment and simulation, we found that divalent cations cause phase separation when the lipid charge is high enough (pH 4.5 or higher in experiment, qPIP ≤ −2 in simulation). Monovalent cations were never seen to induce clusters.
Figure 2.
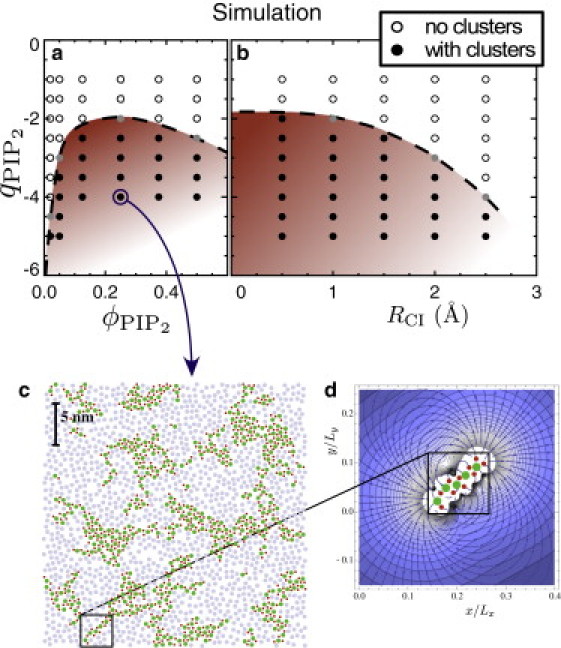
Phase diagram (charge versus PIP2-fraction) and snapshots from simulation of charged-neutral mixed lipid monolayers exposed to divalent salt. (a) Phase diagram obtained using a divalent ion radius RCI = 2 Å. (Solid disks in the shaded coexistence region) Where clustering was observed. (Open circles) Mixed samples. (Gray disks are too close to the boundary to determine their behavior with certainty.) (b) Larger divalent ions require a higher lipid charge to induce clustering (shown for ϕPIP = 0.05). (c) The simulation (PIP2 charge qPIP = −4, PIP2 fraction ϕPIP = 0.25, and divalent ion radius RCI = 2 Å) after 3.5 ns of coarsening. Charged and neutral lipids are dark green and light gray discs, respectively, and divalent ions that are close to the lipid monolayer are indicated with smaller dark red dots. (d) Strength (shaded contours) and direction (streamlines) of the electric field around a stringlike domain taken from the simulation, illustrating that further growth of the domain is likely to occur at the end.
Larger divalent ions than Ca2+ should mediate weaker attractions, because larger binding distances imply lower Coulomb energies. This effect should manifest itself in a higher charge on the PIP2 needed to obtain cluster formation with larger ions. We verified this in experiments at qPIP = 0.25 using Mg2+, which has a larger hydrated radius than Ca2+, although the precise values are uncertain (note that the reported hydrated radii vary, mainly due to different methods to determine them, but Mg2+ is consistently larger (3–7 Å) than Ca2+ (2.6–6.3 Å) (28–30)). We find that Mg2+ only induces clusters if while Ca2+ already does it at pH 4.5. In agreement with this observation, the ability of divalent cations to drive cluster formation in our simulations also decreases with increasing ion size (Fig. 2 b).
Cluster morphology
The morphology observed in the early stages of coarsening in the simulations illustrates some particular features of ion-mediated attractions. The PIP2-rich clusters (see Fig. 2 c) are often irregularly shaped, and even stringlike. This occurs because the attraction, of the order of a few kBT, is the net result of strong attractions (PIP2-Ca2+) and strong repulsions (PIP2-PIP2 and Ca2+-Ca2+) that can each be several tens of kBT.
In the earliest stages of coarsening, most domains are stringlike, because for very small clusters such linear arrangements have the lowest Coulomb energy. As the domains grow, compact shapes become energetically favorable but are difficult to reach for two kinetic reasons: First, once there is a stringlike cluster, the electric field in its neighborhood is focused toward the end of the string (see Fig. 2 d), making it more likely for the next lipid to bind at the end, thus extending the string. Second, any rearrangement of the lipids requires the nearby counterions to move aside, which involves energy barriers of the order of the bare (tens of kBT) interactions. As a result, the evolution toward more compact shapes is severely hindered kinetically, and irregularly shaped domains, which have also been seen experimentally (4,7), can persist even in the later stages of coarsening (Fig. 2 c). This observation also strongly suggests that irregularly shaped clusters are gel-like because diffusion of lipids within the cluster should be hindered by the same energy barriers.
Cluster rigidity
For those PIP2 charges at which cluster formation was observed, additional simulations at qPIP = 1 provide information on cluster rigidity or gelation. As shown in Fig. 3 a, we find from the mean-square displacement that at qPIP ≤ −3.5, the PIP2 do not diffuse over the course of the simulation (corresponding to 3.5 ns), indicating that clusters are mechanically rigid on that timescale. At qPIP ≥ −2.5, on the other hand, the lipids diffuse around freely, indicating that the clusters are fluid. These curves are averaged over five runs with identical parameters but different initial random conditions. At qPIP = 3, the system appears to be marginally rigid on the timescale of our runs; the lipids diffuse in some runs but not in others.
Figure 3.
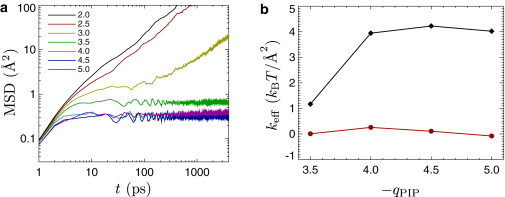
Diffusion and rigidity of lipids within PIP2 domains at ϕPIP = 1 and RCI = 1 Å. (a) Mean-square displacement for lipids in a PIP2-domain as a function of time, for various PIP2 charges, as shown in the legend. For sufficiently negative PIP2 charge, the domains are solid. (b) The stiffness of the effective harmonic interaction between neighboring PIP2 molecules in the PIP2 domain, obtained by displacement correlation analysis. (Black diamonds) Stiffness corresponding to normal (central) effective interactions. (Red disks) The (negligibly small) effective tangential stiffness.
Within a rigid cluster, each lipid has a well-defined average position about which it fluctuates thermally. What keeps them in place can be described as an effective interaction between nearby PIP2 molecules, mediated by the divalent counterions. The strength of this effective interaction is obtained from the matrix of displacement correlations U, defined via
| (1) |
where ui(t) is the deviation of coordinate i from its average value at time t. Hence, U is 2N × 2N for our two-dimensional system. When these deviations are small they explore the effective potential energy Veff around its minimum, so we can describe it by a second-order Taylor expansion. This allows extraction of the dynamical matrix K of the system as the inverse of the correlation matrix,
| (2) |
which can be obtained directly from the partition function (31). The elements of the dynamical matrix then provide the stiffness of the effective spring that acts between two neighboring PIP2. The result is shown in Fig. 3 b: The tangential stiffness of the effective interaction between neighboring PIP2 is negligible, indicating that the effective interaction does not prevent particles from sliding past each other, while the normal effective stiffness is ∼4 kBT/Å2 when qPIP ≤ −4.
Discussion
The two experiments yield nearly identical phase diagrams, showing clustering of PIP2 for pH ≥ 4.5 at PIP2 fractions at ∼25%, a threshold which approaches pH 7.4 at PIP2 fractions as low as 2%.
The phase diagram of our numerical model compares surprisingly well with the experiments. The only parameter we introduce is the dielectric correction factor, a usual necessity in coarse-grained simulations. It is fixed by comparing clustering at one packing fraction (ϕPIP = 0.25), after which the rest of the phase diagram is reproduced without any free parameters.
It should be noted that, although hydrogen bonds between the PIP2 molecules exist and may play a role when the charges are small (10), our work strongly suggests that they do not play a dominant role in multivalent ion-induced clustering—if they did, having a higher PIP2 charge would make it harder to form clusters, rather than easier, as we report in Figs. 1 a and 2 a.
One might ask how relevant our results are to biological membranes. Most of our measurements are taken at a relatively low surface pressure of 20 mN/m to prevent barrier leakage of the lipids. However, the formation of domains persists when surface pressure is increased up to 35 or 40 mN/m (see Fig. 1 a 4), and between 20 and 35 mN/m the typical domain size even grows with surface pressure. This is a characteristic signature of domain formation driven by electrostatic correlations, because a denser aggregate containing charged lipids will attract more divalent ions. We also observed domains by AFM in monolayers containing 1% PIP2 at 35 mN/m over subphases containing 150 mM KCl, pH 7.4, suggesting that even at roughly physiological conditions, Ca2+-induced clustering can be relevant (Y.-H. Wang and P. A. Janmey, unpublished).
As for the use of monolayers instead of real membranes, we first note that PIP2 in the cell membrane only resides on the inner leaflet. In addition, the use of monolayers will not significantly affect the electrostatics because distances between opposite charges are much smaller than the thickness of the low-dielectric layer of a membrane. However, an important limitation of monolayers in both experiment and simulation is that membrane curvature is not allowed. There might be changes in the exact concentrations or charges at which domains first form when membrane curvature is allowed, and indeed the cation-driven changes in surface pressure we measure on the PIP2-containing leaflet might be enough to trigger local curvature in a bilayer.
Because the interactions in our model have been stripped down to the bare minimum of electrostatics and steric repulsion, the only attractive interaction in the simulations is the Coulomb attraction between PIP2 and Ca2+. Therefore, the observed phase separation must be due to counterion-mediated attractions. In both DNA solutions and in PIP2, the negative charges come from phosphate groups and are typically several Å apart. For PIP2, however, the net binding energy per lipid in 30-lipid clusters with Ca2+ is 6 kBT for qPIP ≈ −3, which is much stronger than in DNA (15). (This binding energy is calculated with respect to a reference state of 15 lipid dimers, neutralized with Ca2+, so that the cluster is charge-neutral and monopole terms do not dominate the result.) This large difference must originate from rather subtle differences in the packing geometry of charges in the two cases. Chain connectivity of DNA prevents the charges from organizing in the low-energy configurations that our lipids take (see Fig. 2 c), but instead forces both negative and positive charges into roughly linear arrangements (33), increasing repulsive contributions to the electrostatic energy and thereby weakening the effective attraction.
Although the binding energy between lipids in a cluster is a collective effect and can only be estimated with respect to a chosen reference state, the linearized effective interaction between neighboring PIP2 is always well defined. One can think of this as the potential of mean force between PIP2 that is left after integrating out the positions of the calcium ions, expanded around the average distance between the PIP2 molecules involved. We determined the stiffness of the effective calcium-mediated bond between PIP2 molecules to be ∼4 kBT/Å2 for the case of gel-like clusters of highly charged PIP2 (qPIP ≤ −4). This is approximately an order-of-magnitude lower than the stiffness with which a single Ca2+ is bound to a PIP2 in our simulations, consistent with the notion that ion-mediated attractions are the result of near-cancellation of much stronger attractive and repulsive interactions. Yet at qPIP ≤ −4 the ion-mediated attractions are still strong enough to lead not only to phase separation, but also to mechanical rigidity in PIP2-rich domains.
Whether or not this rigidifying effect could be noticeable in living cells is questionable. First, we note that the timescale of our simulations is of the order of nanoseconds; more highly negative values of qPIP would be needed to achieve rigidity at longer timescales relevant to experiments and to biological processes. Second, other effects that were not included in our simulations—such as active processes (e.g., from molecular motors) and increased disorder (because real lipids are not spheres in a plane)—also act to drive the threshold value of qPIP for rigidity beyond the physiological value of qPIP ≈ −4. We note that a similar calcium-induced gelation effect has been observed experimentally in polymer amphiphile systems (34). In that context, gelation is less surprising because the total charge per molecule is much higher for the polymer amphiphiles than for PIP2.
In summary, we have presented experiments and coarse-grained simulations on lipid monolayers that demonstrate the clustering of PIP2 in mixed monolayers via calcium-mediated electrostatic attractions. Furthermore, we detected a transition from fluid to gel domains as the charge on the PIP2 increased, and obtained the conditions for cluster rigidity from the simulations. Between PIP2 charges of −2 and −4, the strength of ion-mediated attractions is highly sensitive to the PIP2 charge; they become strong enough to make long-lived cross-links between lipids when qPIP ≈ −4, as illustrated by the interaction stiffnesses in Fig. 3.
In all, our results suggest that at physiological pH the effective calcium-mediated attraction can drive the formation of fluid clusters of PIP2 even at PIP2 mole fractions of 2% or lower. In the cell, other factors such as the presence of other polycationic ligands, i.e., polyamines and protein domains, can also affect PIP2 distribution; however, the clustering effect of Ca2+ is likely to remain a significant influence on PIP2 distribution.
Acknowledgments
We thank I. Levental and A. Travesset for discussions.
This work was supported by the National Science Foundation through the UPenn Materials Research and Engineering Center under grant No. DMR-0520021, and grant No. DMR-0605044 to A.J.L.; by National Institutes of Health grant No. HL067286 to P.A.J.; and by the Netherlands Organisation for Scientific Research (NWO) through a Veni grant to W.G.E.
Contributor Information
Wouter G. Ellenbroek, Email: w.g.ellenbroek@tue.nl.
Paul A. Janmey, Email: janmey@mail.med.upenn.edu.
Andrea J. Liu, Email: ajliu@physics.upenn.edu.
References
- 1.Saul D., Fabian L., Brill J.A. Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J. Cell Sci. 2004;117:3887–3896. doi: 10.1242/jcs.01236. [DOI] [PubMed] [Google Scholar]
- 2.Martin T.F.J. PI(4,5)P(2) regulation of surface membrane traffic. Curr. Opin. Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 3.Janmey P.A. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu. Rev. Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 4.Levental I., Christian D.A., Janmey P.A. Calcium-dependent lateral organization in phosphatidylinositol 4,5-bisphosphate (PIP2)- and cholesterol-containing monolayers. Biochemistry. 2009;48:8241–8248. doi: 10.1021/bi9007879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin-Belmonte F., Gassama A., Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richer S.M., Stewart N.K., Oakley M.G. High affinity binding to profilin by a covalently constrained, soluble mimic of phosphatidylinositol-4,5-bisphosphate micelles. ACS Chem. Biol. 2009;4:733–739. doi: 10.1021/cb900121r. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin S., Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 8.Laux T., Fukami K., Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levental I., Byfield F.J., Janmey P.A. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem. J. 2009;424:163–167. doi: 10.1042/BJ20091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levental I., Cebers A., Janmey P.A. Combined electrostatics and hydrogen bonding determine intermolecular interactions between polyphosphoinositides. J. Am. Chem. Soc. 2008;130:9025–9030. doi: 10.1021/ja800948c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redfern D.A., Gericke A. pH-dependent domain formation in phosphatidylinositol polyphosphate/phosphatidylcholine mixed vesicles. J. Lipid Res. 2005;46:504–515. doi: 10.1194/jlr.M400367-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho K., Ramos L., Picart C. Giant unilamellar vesicles containing phosphatidylinositol(4,5)bisphosphate: characterization and functionality. Biophys. J. 2008;95:4348–4360. doi: 10.1529/biophysj.107.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guldbrand L., Nilsson L.G., Nordenskiöld L. A Monte Carlo simulation study of electrostatic forces between hexagonally packed DNA double helices. J. Chem. Phys. 1986;85:6686–6698. [Google Scholar]
- 14.Tang J.X., Janmey P.A. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J. Biol. Chem. 1996;271:8556–8563. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 15.Bloomfield V.A. DNA condensation by multivalent cations. Biopolymers. 1997;44:269–282. doi: 10.1002/(SICI)1097-0282(1997)44:3<269::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Levin Y. Electrostatic correlations: from plasma to biology. Rep. Prog. Phys. 2002;65:1577–1632. [Google Scholar]
- 17.Zhang R., Shklovskii B. The pulling force of a single DNA molecule condensed by spermidine. Phys. A. Stat. Mech. Appl. 2005;349:563–570. [Google Scholar]
- 18.Levental I., Janmey P.A., Cēbers A. Electrostatic contribution to the surface pressure of charged monolayers containing polyphosphoinositides. Biophys. J. 2008;95:1199–1205. doi: 10.1529/biophysj.107.126615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travesset A., Vangaveti S. Electrostatic correlations at the Stern layer: physics or chemistry? J. Chem. Phys. 2009;131:185102. doi: 10.1063/1.3257735. [DOI] [PubMed] [Google Scholar]
- 20.Weeks J.D., Chandler D., Andersen H.C. Role of repulsive forces in determining the equilibrium structure of simple liquids. J. Chem. Phys. 1971;54:5237–5247. [Google Scholar]
- 21.Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995;117:1–19. [Google Scholar]
- 22.Hoover W.G. Canonical dynamics: equilibrium phase-space distributions. Phys. Rev. A. 1985;31:1695–1697. doi: 10.1103/physreva.31.1695. [DOI] [PubMed] [Google Scholar]
- 23.Hockney R.W., Eastwood J.W. Taylor and Francis; London, UK: 1988. Computer Simulation Using Particles. [Google Scholar]
- 24.Flanagan L.A., Cunningham C.C., Janmey P.A. The structure of divalent cation-induced aggregates of PIP2 and their alteration by gelsolin and τ. Biophys. J. 1997;73:1440–1447. doi: 10.1016/S0006-3495(97)78176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah D.O., Schulman J.H. Binding of metal ions to monolayers of lecithins, plasmalogen, cardiolipin, and dicetyl phosphate. J. Lipid Res. 1965;6:341–349. [PubMed] [Google Scholar]
- 26.Marrink S.J., de Vries A.H., Mark A.E. Coarse grained model for semiquantitative lipid simulations. J. Phys. Chem. B. 2004;108:750–760. [Google Scholar]
- 27.Lifshitz I., Slyozov V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids. 1961;19:35–50. [Google Scholar]
- 28.Kielland J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937;59:1675–1678. [Google Scholar]
- 29.Volkov A.G., Paula S., Deamer D.W. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem. Bioenerg. 1997;42:153–160. [Google Scholar]
- 30.Kiriukhin M.Y., Collins K.D. Dynamic hydration numbers for biologically important ions. Biophys. Chem. 2002;99:155–168. doi: 10.1016/s0301-4622(02)00153-9. [DOI] [PubMed] [Google Scholar]
- 31.Chen K., Ellenbroek W.G., Yodh A.G. Low-frequency vibrations of soft colloidal glasses. Phys. Rev. Lett. 2010;105:025501. doi: 10.1103/PhysRevLett.105.025501. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted in proof.
- 33.Lee K.-C., Borukhov I., Stevens M.J. Effect of mono- and multivalent salts on angle-dependent attractions between charged rods. Phys. Rev. Lett. 2004;93:128101. doi: 10.1103/PhysRevLett.93.128101. [DOI] [PubMed] [Google Scholar]
- 34.Christian D.A., Tian A., Discher D.E. Spotted vesicles, striped micelles and Janus assemblies induced by ligand binding. Nat. Mater. 2009;8:843–849. doi: 10.1038/nmat2512. [DOI] [PMC free article] [PubMed] [Google Scholar]


