Abstract
Specific genetic causes for children's interstitial lung disease (chILD) have been identified within the past decade. These include deletions of or mutations in genes encoding proteins important in surfactant production and function (SP-B, SP-C, and ABCA3), surfactant catabolism (GM-CSF receptor), as well as transcription factors important for surfactant production (TTF1) or lung development (Fox F1), with heterozygous deletions or loss-of-function mutations of the latter resulting in alveolar capillary dysplasia (ACD) with misalignment of the pulmonary veins. Familial pulmonary fibrosis in adults may result from mutations in genes encoding components of telomerase and SP-A2. While not yet reported in children, the expression of these genes in alveolar type II epithelial cells supports a key role for the disruption of normal homeostasis in this cell type in the pathogenesis of interstitial lung disease. The identification of specific genetic causes for chILD now allows for the possibility of non-invasive diagnosis, and provides insight into basic cellular mechanisms that may allow the development of novel therapies.
Introduction
Significant advances have been made in the past decade in understanding the underlying causes for children's interstitial lung disease (chILD). The observations that lung disease often had its onset in early infancy and was progressive despite maximal medical treatment and that chILD was often familial suggested that genetic mechanisms were likely to be important in causing chILD.1,2 While historically this heterogeneous group of disorders was classified using schema modeled after adult disorders and based upon the appearance of the lung pathology, specific molecular causes have been identified such that these disorders are often no longer idiopathic in nature. The recognition that specific genetic mechanisms cause some forms of chILD can allow for specific noninvasive diagnostic testing, counseling families concerning recurrence risks, prediction of natural history, and for a classification based upon underlying mechanisms of disease. These disorders also provide insight into normal lung metabolism, and the underlying mechanisms have implications for the pathogenesis of some forms of adult ILD and pulmonary fibrosis. The majority of single gene disorders identified to date encode proteins important in the function and metabolism of pulmonary surfactant, but it seems likely that the number of direct genetic causes or contributors to chILD will continue to expand and involve other pathways.
Overview of Pulmonary Surfactant Components and Metabolism
Pulmonary surfactant is the mixture of lipids and proteins needed to reduce alveolar surface tension and prevent end-expiratory atelectasis.3 Inadequate production of pulmonary surfactant is the main cause of the respiratory distress syndrome (RDS) in prematurely born infants.4 Genetic mechanisms disrupting surfactant production and function can cause diffuse lung disease in full-term infants that clinically and radiographically resembles RDS in premature infants, although it does not resolve or respond to exogenous surfactant replacement.
Surfactant is produced in alveolar type II cells (AEC2s), where it is packaged into lysosomally derived organelles called lamellar bodies, which are secreted by exocytosis.5 The secreted surfactant complex must adsorb to the air–liquid interface and then spread efficiently in order to effectively reduce surface tension. The principal lipid in surfactant responsible for its surface tension lowering properties is disaturated phosphatidylcholine (DSPC). DSPC, however, adsorbs very slowly to an air–liquid interface, and the presence of 2 extremely hydrophobic proteins, surfactant proteins B (SP-B) and C (SP-C), confers important properties upon surfactant lipids to allow for proper surface tension lowering.6 Pulmonary surfactant also contains 2 other more hydrophilic, structurally related proteins, SP-A and SP-D, which are part of the collectin family and have important roles in innate immunity.7,8 Surfactant is both recycled back into type II cells by incompletely characterized mechanisms, as well as catabolized by alveolar macrophages. Maturation of the alveolar macrophages is dependent upon signaling by granulocyte–macrophage colony-stimulating factor (GM-CSF) through binding to a specific receptor on the surface of the macrophages.9,10 Reduction in the functional amount of GM-CSF due to autoantibodies results in defective macrophage clearance of surfactant components from the airspaces and the syndrome of alveolar proteinosis in older children and adults.11–13
Surfactant Metabolic Dysfunction Disorders
Mutations in genes encoding 3 different proteins with important roles in surfactant function and metabolism, SP-B, SP-C, and member A3 of the ATP-binding cassette family of transporters (ABCA3), result in lung disease with overlapping clinical, radiographic, and lung histopathological features. SP-B is a 79-amino acid extremely hydrophobic protein that is encoded by a single gene on chromosome 2 (SFTPB) that directs the production of a larger proprotein from which the mature SP-B peptide found in the airspaces is generated by post-translational proteolytic processing at both the N- and C-termini. SP-C is a 35-amino acid extremely hydrophobic protein that is encoded by a small gene on chromosome 8 (SFTPC). Like SP-B, mature SP-C is generated by post-translational proteolytic processing at both the N- and C-termini of a larger precursor protein (proSP-C).14 Both SP-B and SP-C are found in mammalian-derived surfactants used for replacement therapy in newborns with RDS, and are critical for the effectiveness of these products.15 ABCA3 is a member of a family of transporters that hydrolyze ATP to move substances across biological membranes.16 The 1,704-amino acid protein contains 2 membrane-spanning and 2 nucleotide-binding domains, and is encoded by a large gene on chromosome 16 (ABCA3).17,18 ABCA3 is expressed in a number of tissues, but most highly in the lung, where it is localized to the limiting membrane of lamellar bodies within the alveolar type II cells.19–21 Other members of the ABCA subfamily transport lipids, and given its localization, it is likely that ABCA3 facilitates the transport of lipids essential for surfactant function, in particular DSPC into lamellar bodies, a hypothesis that is supported by data derived from observations in humans and experimental animals.22,23 Reduced surface tension-lowering ability and amounts of surfactant phospholipids, particularly PC, DSPC, and phosphatidylglycerol (PG), were demonstrated in lung fluid obtained from ABCA3-deficient infants.24 AEC2s of ABCA3-deficient infants and mice contain small organelles with densely packed membranes and eccentrically placed electron-dense cores instead of normally formed lamellar bodies, consistent with a role for ABCA3 in lamellar body biogenesis.25–27
Human lung disease due to an inability to produce SP-B was the first recognized genetic cause of surfactant dysfunction.28 Affected infants are generally full-term and develop symptoms and signs of lung disease within hours of birth, and radiographically have diffuse lung disease that resembles RDS in prematurely born infants.29–31 The lung disease is usually relentlessly progressive, and the majority of affected infants die within 3 months of birth despite maximal medical therapy, including surfactant replacement and extracorporeal membrane oxygenation. The disease is inherited in an autosomal recessive fashion, and the diagnosis can be established by the identification of disease-causing mutations on both alleles. A frameshift mutation resulting in a net insertion of 2 bases into codon 121 and termed 121ins2 is the most frequently found SFTPB mutation, has accounted for about two-third of the mutant alleles identified to date, and its occurrence in unrelated subjects is due to a common ancestral origin.29,32,33 Currently, lung transplantation is the only effective therapy for severely affected infants completely unable to produce SP-B.34,35 Rare infants have been reported who have exhibited a relatively milder course, and survived for months to years, and who usually have mutations that allow for some SP-B production.36,37
SFTPC mutations were recognized a cause of interstitial lung disease in 2001.38 The age-of-onset and severity of symptoms of individuals with SFTPC mutations vary greatly, from severe RDS in neonates to apparent idiopathic pulmonary fibrosis in the sixth decade, and adults with mutations associated with disease in other family members may be asymptomatic.39–44 Young infants typically present with hypoxemia in room air, failure to thrive, and diffuse infiltrates on chest radiograph. Multiple SFTPC mutations have been identified and one mutation (c.218T>C, p.I73T) has been found in multiple unrelated families and accounted for 25%–35% of the mutant SFTPC alleles identified to date.39–41,43–48 Apparent de novo SFTPC mutations resulting in sporadic lung disease have accounted for about half of reported cases of SFTPC-related lung disease. All mutations identified to date are predicted to alter the amino acid sequence of the SP-C proprotein. Disease is believed to result from a toxic gain-of-function mechanism whereby mutations cause misfolding of proSP-C, protein aggregation, and exposure of hydrophobic epitopes in the endoplasmic reticulum (ER). These events elicit the unfolded protein response and result in ER stress, with eventual alveolar type II cell apoptosis and inflammation.49–55
The optimal therapies of individuals with SFTPC mutations are unknown. Therapeutic lung lavage in infancy, corticosteroids, and hydroxychloroquine have been reported to improve the clinical status in case reports, but the highly variable natural history of the disease makes interpretation of these uncontrolled observations difficult.43,45,56 No randomized, placebo-controlled nor cross-over studies of these treatments have been reported. Lung transplantation has been performed in individuals with progressive deterioration in lung function.57
ABCA3 deficiency is the most recently recognized cause of surfactant dysfunction but may be the most common.25,30,58 The phenotype of the initial population of infants studied was similar to that of SP-B deficiency with severe and generally fatal RDS. However, the clinical course of patients with ABCA3 deficiency is much more variable than that of SP-B deficiency, and prolonged survival is being increasingly recognized.24,30,44,59–68 While many affected infants had symptoms of lung disease in the immediate neonatal period, onset of respiratory symptoms later in childhood has been recognized.59,64,66 There is extensive allelic heterogeneity, with mutations throughout the gene having been identified. Several mutations have been studied in in vitro systems, and a classification proposed based upon mutations that either preclude ABCA3 production or intracellular transport (type I), or impair the ability of protein to bind and/or hydrolyze ATP or transport phospholipids across membranes (type II).69–72 One specific mutation, the substitution of valine for glutamic acid in codon 292 (p.E292V or c.875A>T) has been identified in multiple unrelated children with generally milder disease and the phenotype of chILD. In vitro studies indicate that this mutation results in less impairment in ABCA3 function than other type II mutations.72 These findings support the hypotheses that retained function may attenuate disease severity, and that genotype may thus be able to predict phenotype to some extent and even a small boost in ABCA3 production or function could improve the clinical status of such patients. The finding that corticosteroids increased ABCA3 expression in vitro provide a rationale for such treatment, although clinical data beyond anecdotal reports supporting the efficacy of steroids (or other treatments) for individuals with proven ABCA3 deficiency are lacking.73
Thyroid Transcription Factor 1 Haploinsufficiency
Thyroid transcription factor 1 (TTF1), also known as Nkx2.1 or TITF1, is a member of the homeobox family of transcription factors that is critically important for the expression of multiple genes important in surfactant production and function, including those for SP-A, SP-B, SP-C, and ABCA3. The gene is located on the long arm of chromosome 14 (14q13.3), and TTF1 is also expressed in the thyroid gland, where it is critical for thyroid development, as well as in the central nervous system, particularly in the basal ganglia.
A role for TTF1 in human lung disease was initially recognized in full- or near-term neonates with RDS and hypothyroidism who had complete deletions of one copy of the Nkx2.1 locus.74,75 Subsequently, complete loss-of-function mutations on one allele (haploinsufficiency) were recognized in individuals with a phenotype of hypothyroidism, neurological manifestations, particularly choreoathetoid movements, and pulmonary disease ranging from neonatal RDS to chronic respiratory symptoms in childhood.75–77 TTF1 mutations were also reported as the cause of benign familial chorea, in which the affected individuals were not recognized to have pulmonary symptoms.78–80 The term “brain–thyroid–lung” syndrome has been used to describe the phenotype, although the extent of symptoms related to each organ involvement is highly variable, such that patients may have normal or borderline thyroid function and normal pulmonary function by history, although many of the patients have not been formally evaluated for lung disease.81–84 Fatal lung disease has been reported, and reported lung histopathology findings are consistent with surfactant dysfunction. Whether TTF1 mutations can result in a phenotype with only pulmonary manifestations is unknown. However as thyroid function may be normal, and the initial neurological symptoms may be non-specific (hypotonia, developmental delay) with chorea developing later, it is possible that this mechanism is not considered in young infants with chILD. The mechanisms for lung disease due to TTF1 haploinsufficiency presumably relate to decreased production of surfactant components, in particular SP-B, SP-C, and ABCA3, but this has not been rigorously examined.
Alveolar Capillary Dysplasia with Misalignment of the Pulmonary Veins
ACD is a disorder of lung development involving inadequate development of the pulmonary capillary bed and with pulmonary veins found in the same bronchovascular bundles as pulmonary arteries rather than associated with pulmonary lymphatics.85–87 Affected infants typically present with severe pulmonary hypertension in the neonatal period that is unresponsive to medical management and ultimately fatal. Rarely, somewhat milder cases with later-onset presentation and more prolonged survival have been reported.88–92 The diagnosis is made primarily through histological examination of lung tissue, although cardiac catheterization may also be helpful. The incidence is unknown, but ACD accounted for the majority of cases of lung developmental disorders as determined by biopsy in one series93 and for 5 of 9 cases of fatal neonatal lung disease in a series from the UK.94 Extrapulmonary-associated anomalies have been observed in 50%–75% of cases, and the occurrence of familial cases supports a genetic mechanism.95
Recently microdeletions in 16q24.1 were found in a group of children with lung pathology findings of ACD along with other anomalies, including cardiac, gastrointestinal, and genitourinary anomalies.96 This region includes the genes for several members of the forkhead box (Fox) family of transcription factors, and sequence analysis revealed heterozygous loss-of-function FoxF1 mutations in 4 of 18 patients with ACD examined, supporting the role of this transcription factor in the pathogenesis of the pulmonary phenotype, although the mechanism remains unknown.
The FoxF1 mutations and 16q24.1 microdeletions were apparent de novo events, and did not account for all of the cases of ACD examined. An autosomal recessive pattern of inheritance has been implicated in some familial cases of ACD, and thus there are almost certainly other genes that can result in this phenotype.95 However, these observations provide the means for a non-invasive diagnosis in some cases, and confirm one genetic basis for this disorder.
GM-CSF Receptor Deficiency
One of the histological features of surfactant dysfunction is an accumulation of granular, eosinophilic material in the distal airspaces, a finding that resembles what is seen in pulmonary alveolar proteinosis (PAP) in adults. The onset of symptoms in PAP is usually more insidious and slowly progressive, and while the airspaces are filled with proteinaceous material the underlying lung architecture is generally well preserved without the AEC2 hyperplasia, mesenchymal thickening, and fibrosis observed with surfactant dysfunction disorders. Moreover, the molecular basis for PAP is due to the presence of neutralizing antibodies to GM-CSF, leading to impairment of alveolar macrophage development and failure of the macrophages to properly catabolize surfactant.97 PAP is thus a distinct entity clinically, pathologically, and mechanistically, and the term congenital alveolar proteinosis to describe newborns with surfactant dysfunction is best avoided.
GM-CSF acts through binding to a specific receptor that has 2 components, a specific α chain and a β chain that is also shared by the receptors for IL-3 and IL-5. Ablation of the β chain in mice resulted in the phenotype of PAP in homozygous null animals.98,99 Functional deficiency of β chain was reported in 1997 in children with infantile onset of PAP, but the early phenotype of these children was not consistent with that of PAP, and no convincing defect in the gene encoding the β chain (CSF2RB) was identified or has yet been reported.100 Clear genetic defects in the gene encoding the α chain (CSF2RA), which is located in the pseudoautosomal region of the X chromosome, were recently reported as a cause for PAP in children.101,102 These reports convincingly establish that genetic mechanisms can disrupt GM-CSF signaling and result in PAP in childhood. The incidence and prevalence of this disorder are unknown, as are the extent of variability in the age of onset due to mutations in this pathway. While there may be clinical overlap with chILD, the lung pathology is likely to remain distinct from that of surfactant dysfunction and other forms of chILD.
Lung disease with features of PAP can also be seen in children with lysinuric protein intolerance (LPI), a disorder of cationic amino acid transport caused by mutations in the solute carrier gene, SLC7A7.103,104 Children affected by this autosomal recessive disorder may have episodes of hyperammonemia, recurrent vomiting, and failure to thrive, but can present with pulmonary symptoms in infancy.105–107 While the basic defect for LPI has been elucidated, the mechanisms for PAP resulting from this disorder are unknown. The recurrence of pulmonary disease in an infant following heart–lung transplantation suggests that correcting the metabolic defect in pulmonary epithelial cells did not resolve the underlying pathophysiology.108
Surfactant Protein A2 and Telomerase Mutations in Adults with Familial Pulmonary Fibrosis
Mutations in the genes encoding the components of telomerase (TERT, TERC) have been reported as a cause of familial pulmonary fibrosis in adults, and recently mutations in one of the genes encoding surfactant protein A, SFTPA2, have been reported in association with the phenotype of familial pulmonary fibrosis and pulmonary adenocarcinoma.109–112 In addition to synthesizing surfactant components including SP-A, AEC2s are the progenitor cells for type I cells following lung cell injury, and telomerase is necessary for the maintenance of a dividing cell population. While mutations in these genes have not yet been reported as a cause of chILD, these observations further support a key role for injury to the AEC2 in the pathogenesis of ILD and pulmonary fibrosis.
Pathology
The lung pathology findings associated with surfactant dysfunction are discussed elsewhere in this issue. The findings at the level of light microscopy are not specific for a given disorder, although electron microscopy can be helpful in distinguishing SP-B and ABCA3 deficiencies from other causes of surfactant dysfunction. Genetic testing, which is now available for many of these disorders in clinical diagnostic labs, is needed to provide a specific diagnosis, although there are children with lung pathology findings of surfactant dysfunction in whom testing for mutations in all known genes has proved negative. These observations suggest that either genetic variants in regions of the genes not examined, such as untranslated regions, or mutations in other genes not yet established as having a role in surfactant metabolism and dysfunction are also a cause of this phenotype.
Genetic Approach to Diagnosis
Specific features of each chILD disorder with known genetic etiologies are summarized in Table 1. The identification of specific genetic causes of chILD provides a means for establishing a diagnosis non-invasively. Timely diagnosis will allow for accurate counseling regarding prognosis, avoiding unnecessary therapies, and referral for specific therapies such as lung transplantation if indicated. However, clinical genetic testing is expensive, not all disorders have a known genetic cause, and not all mutations in a gene are detected by current approaches. Interpretation of results of genetic testing may not be straightforward. Determination of whether novel missense mutations or ones close to splice junctions cause lung pathology or are simply rare yet benign polymorphisms may not be possible. There is also no easy way to distinguish whether a child heterozygous for only one SFTPB or ABCA3 disease-causing mutation is affected or simply a carrier with a different underlying mechanism for disease. Rapidly progressive disease may preclude waiting for the results of genetic testing. Lung biopsy thus remains important for diagnosis in some patients.
Table 1.
Known Genetic Causes of ChILD Syndrome
| Alveolar capillary dysplasia | SP-B deficiency | ABCA3 deficiency | SP-C dysfunction | Brain–thyroid–lung syndrome | GM-CSF receptor deficiency, α chain | Lysinuric protein intolerance | |
|---|---|---|---|---|---|---|---|
| OMIM | #265380 | #265120 SMDP1 | #610921 SMDP3 | #610913 SMDP2 | #610978 | #300770 SMDP4 | #222700 |
| Locus | FoxFl | SFTPB | ABCA3 | SFTPC | TTF1 (Nkx2.1) | CSF2RA | SLC7A7 |
| Chromosomal location | 16q24.1 | 2p12-p11.2 | 16p13.3 | 8p21 | 14q13.3 | Xp22.32, Yp11.3 | 14q11.2 |
| Inheritance | AD, sporadic | AR | AR | AD, sporadic | AD, sporadic | AR | AR |
| Mechanism | Haplo insufficiency | Loss of function | Loss of function | Toxic gain of function | Haplo insufficiency | Loss of function | Loss of function |
| Onset of pulmonary symptoms | Neonate | Neonate | Neonate, infancy, childhood | Neonate < infancy to adult | Newborn, infancy | Childhood | Infancy to childhood |
| Principal histology | ACD/MPV | SDM | SDM | SDM | SDM | PAP | PAP |
| Other findings | Cardiac, GI, or GU malformations | Hypothyroidism Neurological | Hyperammonemia Vomiting Failure to thrive |
||||
| Course | Severe, fatal | Severe, fatal | Variable | Variable | Variable | Progressive |
Abbreviations: OMIM, Online Mendelian Inheritance in Man; SMDP, surfactant metabolism dysfunction, pulmonary; AD, autosomal dominant; AR, autosomal recessive; ACD/MPV, alveolar capillary dysplasia with misalignment of the pulmonary veins; SDM, surfactant dysfunction, metabolic; PAP, pulmonary alveolar proteinosis; GI, gastrointestinal; GU, genitourinary; GM-CSF, granulocyte–macrophage colony-stimulating factor; SP-B, surfactant protein B; SP-C, surfactant protein C; ABCA3, surfactant protein ABCA3; TTF1, thyroid transcription factor 1.
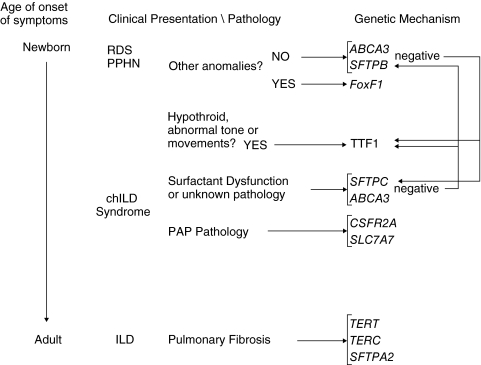
Newborns with hypoxemic respiratory failure and diffuse disease radiographically due to surfactant dysfunction or ACD are not distinguishable on clinical criteria from those with reversible causes of lung disease. A positive family history of lung disease, lack of clinical risk factors associated with severe lung disease in full-term infants, and failure to improve in the expected timeframe should prompt consideration of one of these disorders. Extrapulmonary organ involvement, including cardiac, gastrointestinal, or genitourinary tract anomalies suggests the possibility of ACD and FoxF1 haploinsufficiency, and hypothyroidism or CNS abnormalities suggests TTF1 haploinsufficiency. As deletions in the regions involving the loci responsible for both conditions have been reported, a comparative genomic hybridization assay should be considered, along with targeted mutational testing for FoxF1 and TTF1, respectively. If the disease solely involves the lungs, mutational analysis for ABCA3 and SP-B deficiencies should be considered. SFTPC testing in critically ill neonates should be performed if prior testing for ABCA3 and SP-B is negative and a strong index of suspicion for surfactant dysfunction persists (Fig. 1). Lung biopsy still may be needed in some cases.
FIG. 1.
Approach to genetic diagnosis of children's interstitial lung disease (chILD). Potential genetic mechanisms based upon age of the patient (neonatal to adult, from top to bottom) and phenotypic characteristics (middle) are listed on the right. Arrows point to primary genes to be analyzed; if results of initial studies are negative, arrows on right indicate secondary genetic studies to be considered. Abbreviations: RDS, Respiratory distress syndrome; PPHN, persistent pulmonary hypertension of the newborn; PAP, pulmonary alveolar proteinosis. See text and Table 1 for details on genetic loci.
As prolonged survival is unusual in children with SP-B deficiency or ACD, testing for SFTPB mutations or FoxF1 mutations and deletions is likely to have very low yield in older children with diffuse lung disease. Genetic testing for SFTPC and ABCA3 mutations should be considered in older children who present with hypoxemia, failure-to-thrive, and/or diffuse lung disease by imaging studies when no clear diagnosis has been established. The onset of symptoms in the neonatal period favors ABCA3 deficiency as the mechanism, whereas later onset of symptoms is more typical of SFTPC mutations, but there is sufficient overlap in the age-of-onset of symptoms that analyzing both genes is often necessary, and heterozygosity for an ABCA3 mutation may influence the course of patients with SFTPC mutations. Neurological symptoms, particularly choreoathetoid movements, and/or chemical hypothyroidism and elevated levels of thyroid-stimulating hormone should prompt evaluation for deletions and mutations in TTF1. Children whose clinical and bronchoalveolar lavage or lung biopsy findings are more consistent with PAP should be evaluated for CSF2RA defects and the diagnosis of LPI considered. The figure outlines which genes should be considered for analysis based upon age of the patient and phenotype.
Acknowledgments
The author wishes to thank Drs. Susan Wert, Jeffrey Whitsett, Timothy Weaver, Aaron Hamvas, and F. Sessions Cole for their collaboration and support. This work was supported through a grant from the National Institutes of Health, HL 54703, and the author also receives support from the Eudowood Board.
References
- 1.Dinwiddie R. Sharief N. Crawford O. Idiopathic interstitial pneumonitis in children: a national survey in the United Kingdom and Ireland. Pediatr Pulmonol. 2002;34:23–29. doi: 10.1002/ppul.10125. [DOI] [PubMed] [Google Scholar]
- 2.Fan LL. Langston C. Chronic interstitial lung disease in children. Pediatr Pulmonol. 1993;16:184–196. doi: 10.1002/ppul.1950160309. [DOI] [PubMed] [Google Scholar]
- 3.Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim Biophys Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 4.Farrell PM. Avery ME. Hyaline membrane disease. Am Rev Respir Dis. 1975;111:657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- 5.Weaver TE. Na CL. Stahlman M. Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin Cell Dev Biol. 2002;13:263–270. doi: 10.1016/s1084952102000551. [DOI] [PubMed] [Google Scholar]
- 6.Weaver TE. Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol. 2001;63:555–578. doi: 10.1146/annurev.physiol.63.1.555. [DOI] [PubMed] [Google Scholar]
- 7.Crouch E. Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 8.Hawgood S. Poulain FR. The pulmonary collectins and surfactant metabolism. Annu Rev Physiol. 2001;63:495–519. doi: 10.1146/annurev.physiol.63.1.495. [DOI] [PubMed] [Google Scholar]
- 9.Trapnell BC. Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 10.Shibata Y. Berclaz PY. Chroneos ZC. Yoshida M. Whitsett JA. Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- 11.Uchida K. Nakata K. Trapnell BC. Terakawa T. Hamano E. Mikami A. Matsushita I. Seymour JF. Oh-Eda M. Ishige I. Eishi Y. Kitamura T. Yamada Y. Hanaoka K. Keicho N. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–1098. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- 12.Bonfield TL. Russell D. Burgess S. Malur A. Kavuru MS. Thomassen MJ. Autoantibodies against granulocyte macrophage colony-stimulating factor are diagnostic for pulmonary alveolar proteinosis. Am J Respir Cell Mol Biol. 2002;27:481–486. doi: 10.1165/rcmb.2002-0023OC. [DOI] [PubMed] [Google Scholar]
- 13.Kitamura T. Tanaka N. Watanabe J. Uchida Kanegasaki S. Yamada Y. Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver TE. Synthesis, processing and secretion of surfactant proteins B and C. Biochim Biophys Acta. 1998;1408:173–179. doi: 10.1016/s0925-4439(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 15.Whitsett JA. Ohning BL. Ross G. Meuth J. Weaver T. Holm BA. Shapiro DL. Notter RH. Hydrophobic surfactant-associated protein in whole lung surfactant and its importance for biophysical activity in lung surfactant extracts used for replacement therapy. Pediatr Res. 1986;20:460–467. doi: 10.1203/00006450-198605000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Dean M. Rzhetsky A. Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 17.Peelman F. Labeur C. Vanloo B. Roosbeek S. Devaud C. Duverger N. Denèfle P. Rosier M. Vandekerckhove J. Rosseneu M. Characterization of the ABCA transporter subfamily: identification of prokaryotic and eukaryotic members, phylogeny and topology. J Mol Biol. 2003;325:259–274. doi: 10.1016/s0022-2836(02)01105-1. [DOI] [PubMed] [Google Scholar]
- 18.Kaminski WE. Piehler A. Wenzel JJ. ABC A-subfamily transporters: structure, function and disease. Biochim Biophys Acta. 2006;1762:510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Dean M. Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33:475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 20.Yamano G. Funahashi H. Kawanami O. Zhao LX. Ban N. Uchida Y. Morohoshi T. Ogawa J. Shioda S. Inagaki N. ABCA3 is a lamellar body membrane protein in human lung alveolar type II cells. FEBS Lett. 2001;508:221–225. doi: 10.1016/s0014-5793(01)03056-3. [DOI] [PubMed] [Google Scholar]
- 21.Mulugeta S. Gray JM. Notarfrancesco KL. Gonzales LW. Koval M. Feinstein SI. Ballard PL. Fisher AB. Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- 22.Ban N. Matsumura Y. Sakai H. Takanezawa Y. Sasaki M. Arai H. Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–9634. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald ML. Xavier R. Haley KJ. Welti R. Goss JL. Brown CE. Zhuang DZ. Bell SA. Lu N. McKee M. Seed B. Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–632. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Garmany TH. Moxley MA. White FV. Dean M. Hull WM. Whitsett JA. Nogee LM. Hamvas A. Surfactant composition and function in patients with ABCA3 mutations. Pediatr Res. 2006;59:801–805. doi: 10.1203/01.pdr.0000219311.14291.df. [DOI] [PubMed] [Google Scholar]
- 25.Shulenin S. Nogee LM. Annilo T. Wert SE. Whitsett JA. Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 26.Edwards V. Cutz E. Viero S. Moore AM. Nogee L. Ultrastructure of lamellar bodies in congenital surfactant deficiency. Ultrastruct Pathol. 2005;29:503–509. doi: 10.1080/01913120500323480. [DOI] [PubMed] [Google Scholar]
- 27.Tryka AF. Wert SE. Mazursky JE. Arrington RW. Nogee LM. Absence of lamellar bodies with accumulation of dense bodies characterizes a novel form of congenital surfactant defect. Pediatr Dev Pathol. 2000;3:335–345. doi: 10.1007/s100249910048. [DOI] [PubMed] [Google Scholar]
- 28.Nogee LM. de Mello DE. Dehner LP. Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 29.Nogee LM. Wert SE. Proffit SA. Hull WM. Whitsett JA. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161:973–981. doi: 10.1164/ajrccm.161.3.9903153. [DOI] [PubMed] [Google Scholar]
- 30.Somaschini M. Nogee LM. Sassi I. Danhaive O. Presi S. Boldrini R. Montrasio C. Ferrari M. Wert SE. Carrera P. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J Pediatr. 2007;150:649–53. doi: 10.1016/j.jpeds.2007.03.008. 653.e1. [DOI] [PubMed] [Google Scholar]
- 31.Tredano M. Griese M. de Blic J. Lorant T. Houdayer C. Schumacher S. Cartault F. Capron F. Boccon-Gibod L. Lacaze-Masmonteil T. Renolleau S. Delaisi B. Elion J. Couderc R. Bahuau M. Analysis of 40 sporadic or familial neonatal and pediatric cases with severe unexplained respiratory distress: relationship to SFTPB. Am J Med Genet A. 2003;119A:324–339. doi: 10.1002/ajmg.a.20058. [DOI] [PubMed] [Google Scholar]
- 32.Nogee LM. Garnier G. Dietz HC. Singer L. Murphy AM. deMello DE. Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest. 1994;93:1860–1863. doi: 10.1172/JCI117173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tredano M. Cooper DN. Stuhrmann M. Christodoulou J. Chuzhanova NA. Roudot-Thoraval F. Boëlle PY. Elion J. Jeanpierre M. Feingold J. Couderc R. Bahuau M. Origin of the prevalent SFTPB indel g.1549C > GAA (121ins2) mutation causing surfactant protein B (SP-B) deficiency. Am J Med Genet A. 2006;140:62–69. doi: 10.1002/ajmg.a.31050. [DOI] [PubMed] [Google Scholar]
- 34.Hamvas A. Nogee LM. Mallory GB., Jr Spray TL. Huddleston CB. August A. Dehner LP. deMello DE. Moxley M. Nelson R. Cole FS. Colten HR. Lung transplantation for treatment of infants with surfactant protein B deficiency. J Pediatr. 1997;130:231–239. doi: 10.1016/s0022-3476(97)70348-2. [DOI] [PubMed] [Google Scholar]
- 35.Palomar LM. Nogee LM. Sweet SC. Huddleston CB. Cole FS. Hamvas A. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J Pediatr. 2006;149:548–553. doi: 10.1016/j.jpeds.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Ballard PL. Nogee LM. Beers MF. Ballard RA. Planer BC. Polk L. deMello DE. Moxley MA. Longmore WJ. Partial deficiency of surfactant protein B in an infant with chronic lung disease. Pediatrics. 1995;96:1046–1052. [PubMed] [Google Scholar]
- 37.Dunbar AE., 3rd Wert SE. Ikegami M. Whitsett JA. Hamvas A. White FV. Piedboeuf B. Jobin C. Guttentag S. Nogee LM. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48:275–282. doi: 10.1203/00006450-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Nogee LM. Dunbar AE., 3rd Wert SE. Askin F. Hamvas A. Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 39.Soraisham AS. Tierney AJ. Amin HJ. Neonatal respiratory failure associated with mutation in the surfactant protein C gene. J Perinatol. 2006;26:67–70. doi: 10.1038/sj.jp.7211417. [DOI] [PubMed] [Google Scholar]
- 40.Cameron HS. Somaschini M. Carrera P. Hamvas A. Whitsett JA. Wert SE. Deutsch G. Nogee LM. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr. 2005;146:370–375. doi: 10.1016/j.jpeds.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Nogee LM. Dunbar AE., III Wert S. Askin F. Hamvas A. Whitsett JA. Mutations in the surfactant protein C gene associated with interstitial lung disease. Chest. 2002;121(3 Suppl):20S–21S. doi: 10.1378/chest.121.3_suppl.20s. [DOI] [PubMed] [Google Scholar]
- 42.Thomas AQ. Lane K. Phillips J., III Prince M. Markin C. Speer M. Schwartz DA. Gaddipati R. Marney A. Johnson J. Roberts R. Haines J. Stahlman M. Loyd JE. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 43.Abou Taam R. Jaubert F. Emond S. Le Bourgeois M. Epaud R. Karila C. Feldmann D. Scheinmann P. de Blic J. Familial interstitial disease with I73T mutation: A mid- and long-term study. Pediatr Pulmonol. 2009;44:167–175. doi: 10.1002/ppul.20970. [DOI] [PubMed] [Google Scholar]
- 44.Bullard JE. Nogee LM. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr Res. 2007;62:176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- 45.Brasch F. Griese M. Tredano M. Johnen G. Ochs M. Rieger C. Mulugeta S. Müller KM. Bahuau M. Beers MF. Interstitial lung disease in a baby with a de novo mutation in the SFTPC gene. Eur Respir J. 2004;24:30–39. doi: 10.1183/09031936.04.00000104. [DOI] [PubMed] [Google Scholar]
- 46.Percopo S. Cameron HS. Nogee LM. Pettinato G. Montella S. Santamaria F. Variable phenotype associated with SP-C gene mutations: fatal case with the I73T mutation. Eur Respir J. 2004;24:1072–1073. doi: 10.1183/09031936.04.00092304. [DOI] [PubMed] [Google Scholar]
- 47.Stevens PA. Pettenazzo A. Brasch F. Mulugeta S. Baritussio A. Ochs M. Morrison L. Russo SJ. Beers MF. Nonspecific interstitial pneumonia, alveolar proteinosis, and abnormal proprotein trafficking resulting from a spontaneous mutation in the surfactant protein C gene. Pediatr Res. 2005;57:89–98. doi: 10.1203/01.PDR.0000147567.02473.5A. [DOI] [PubMed] [Google Scholar]
- 48.Guillot L. Epaud R. Thouvenin G. Jonard L. Mohsni A. Couderc R. Counil F. de Blic J. Taam RA. Le Bourgeois M. Reix P. Flamein F. Clement A. Feldmann D. New surfactant protein C gene mutations associated with diffuse lung disease. J Med Genet. 2009;46:490–494. doi: 10.1136/jmg.2009.066829. [DOI] [PubMed] [Google Scholar]
- 49.Whitsett JA. Wert SE. Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 50.Nerelius C. Martin E. Peng S. Gustafsson M. Nordling K. Weaver T. Johansson J. Mutations linked to interstitial lung disease can abrogate anti-amyloid function of prosurfactant protein C. Biochem J. 2008;416:201–209. doi: 10.1042/BJ20080981. [DOI] [PubMed] [Google Scholar]
- 51.Bridges JP. Xu Y. Na CL. Wong HR. Weaver TE. Adaptation and increased susceptibility to infection associated with constitutive expression of misfolded SP-C. J Cell Biol. 2006;172:395–407. doi: 10.1083/jcb.200508016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bridges JP. Wert SE. Nogee LM. Weaver TE. Expression of a human surfactant protein C mutation associated with interstitial lung disease disrupts lung development in transgenic mice. J Biol Chem. 2003;278:52739–52746. doi: 10.1074/jbc.M309599200. [DOI] [PubMed] [Google Scholar]
- 53.Mulugeta S. Nguyen V. Russo SJ. Muniswamy M. Beers MF. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32:521–530. doi: 10.1165/rcmb.2005-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beers MF. Mulugeta S. Surfactant protein C biosynthesis and its emerging role in conformational lung disease. Annu Rev Physiol. 2005;67:663–696. doi: 10.1146/annurev.physiol.67.040403.101937. [DOI] [PubMed] [Google Scholar]
- 55.Mulugeta S. Maguire JA. Newitt JL. Russo SJ. Kotorashvili A. Beers MF. Misfolded BRICHOS SP-C mutant proteins induce apoptosis via caspase-4- and cytochrome c-related mechanisms. Am J Physiol Lung Cell Mol Physiol. 2007;293:L720–L729. doi: 10.1152/ajplung.00025.2007. [DOI] [PubMed] [Google Scholar]
- 56.Rosen DM. Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med. 2005;352:207–208. doi: 10.1056/NEJM200501133520223. [DOI] [PubMed] [Google Scholar]
- 57.Hamvas A. Nogee LM. White FV. Schuler P. Hackett BP. Huddleston CB. Mendeloff EN. Hsu FF. Wert SE. Gonzales LW. Beers MF. Ballard PL. Progressive lung disease and surfactant dysfunction with a deletion in surfactant protein C gene. Am J Respir Cell Mol Biol. 2004;30:771–776. doi: 10.1165/rcmb.2003-0323OC. [DOI] [PubMed] [Google Scholar]
- 58.Garmany TH. Wambach JA. Heins HB. Watkins-Torry JM. Wegner DJ. Bennet K. An P. Land G. Saugstad OD. Henderson H. Nogee LM. Cole FS. Hamvas A. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63:645–649. doi: 10.1203/PDR.0b013e31816fdbeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bullard JE. Wert SE. Whitsett JA. Dean M. Nogee LM. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172:1026–1031. doi: 10.1164/rccm.200503-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brasch F. Schimanski S. Mühlfeld C. Barlage S. Langmann T. Aslanidis C. Boettcher A. Dada A. Schroten H. Mildenberger E. Prueter E. Ballmann M. Ochs M. Johnen G. Griese M. Schmitz G. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med. 2006;174:571–580. doi: 10.1164/rccm.200509-1535OC. [DOI] [PubMed] [Google Scholar]
- 61.Bruder E. Hofmeister J. Aslanidis C. Hammer J. Bubendorf L. Schmitz G. Rufle A. Bührer C. Ultrastructural and molecular analysis in fatal neonatal interstitial pneumonia caused by a novel ABCA3 mutation. Mod Pathol. 2007;20:1009–1018. doi: 10.1038/modpathol.3800928. [DOI] [PubMed] [Google Scholar]
- 62.Kunig AM. Parker TA. Nogee LM. Abman SH. Kinsella JP. ABCA3 deficiency presenting as persistent pulmonary hypertension of the newborn. J Pediatr. 2007;151:322–324. doi: 10.1016/j.jpeds.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 63.Saugstad OD. Hansen TW. Rønnestad A. Nakstad B. Tølløfsrud PA. Reinholt F. Hamvas A. Coles FS. Dean M. Wert SE. Whitsett JA. Nogee LM. Novel mutations in the gene encoding ATP binding cassette protein member A3 (ABCA3) resulting in fatal neonatal lung disease. Acta Paediatr. 2007;96:185–190. doi: 10.1111/j.1651-2227.2007.00016.x. [DOI] [PubMed] [Google Scholar]
- 64.Doan ML. Guillerman RP. Dishop MK. Nogee LM. Langston C. Mallory GB. Sockrider MM. Fan LL. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 65.Yokota T. Matsumura Y. Ban N. Matsubayashi T. Inagaki N. Heterozygous ABCA3 mutation associated with non-fatal evolution of respiratory distress. Eur J Pediatr. 2008;167:691–693. doi: 10.1007/s00431-007-0542-8. [DOI] [PubMed] [Google Scholar]
- 66.Young LR. Nogee LM. Barnett B. Panos RJ. Colby TV. Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 67.Anandarajan M. Paulraj S. Tubman R. ABCA3 Deficiency: an unusual cause of respiratory distress in the newborn. Ulster Med J. 2009;78:51–52. [PMC free article] [PubMed] [Google Scholar]
- 68.Hamvas A. Nogee LM. Wegner DJ. Depass K. Christodoulou J. Bennetts B. McQuade LR. Gray PH. Deterding RR. Carroll TR. Kammesheidt A. Kasch LM. Kulkarni S. Cole FS. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily A, member 3 genes. J Pediatr. 2009;155:854–859.e1. doi: 10.1016/j.jpeds.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheong N. Madesh M. Gonzales LW. Zhao M. Yu K. Ballard PL. Shuman H. Functional and trafficking defects in ABCA3 mutants associated with respiratory distress syndrome. J Biol Chem. 2006;281:9791–9800. doi: 10.1074/jbc.M507515200. [DOI] [PubMed] [Google Scholar]
- 70.Matsumura Y. Ban N. Ueda K. Inagaki N. Characterization and classification of ATP-binding cassette transporter ABCA3 mutants in fatal surfactant deficiency. J Biol Chem. 2006;281:34503–34514. doi: 10.1074/jbc.M600071200. [DOI] [PubMed] [Google Scholar]
- 71.Matsumura Y. Sakai H. Sasaki M. Ban N. Inagaki N. ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 2007;581:3139–3144. doi: 10.1016/j.febslet.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 72.Matsumura Y. Ban N. Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:L698–L707. doi: 10.1152/ajplung.90352.2008. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida I. Ban N. Inagaki N. Expression of ABCA3, a causative gene for fatal surfactant deficiency, is up-regulated by glucocorticoids in lung alveolar type II cells. Biochem Biophys Res Commun. 2004;323:547–555. doi: 10.1016/j.bbrc.2004.08.133. [DOI] [PubMed] [Google Scholar]
- 74.Devriendt K. Vanhole C. Matthijs G. de Zegher F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med. 1998;338:1317–1318. doi: 10.1056/NEJM199804303381817. [DOI] [PubMed] [Google Scholar]
- 75.Iwatani N. Mabe H. Devriendt K. Kodama M. Miike T. Deletion of NKX2.1 gene encoding thyroid transcription factor-1 in two siblings with hypothyroidism and respiratory failure. J Pediatr. 2000;137:272–276. doi: 10.1067/mpd.2000.107111. [DOI] [PubMed] [Google Scholar]
- 76.Pohlenz J. Dumitrescu A. Zundel D. Martiné U. Schönberger W. Koo E. Weiss RE. Cohen RN. Kimura S. Refetoff S. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. J Clin Invest. 2002;109:469–473. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krude H. Schütz B. Biebermann H. von Moers A. Schnabel D. Neitzel H. Tönnies H. Weise D. Lafferty A. Schwarz S. DeFelice M. von Deimling A. van Landeghem F. DiLauro R. Grüters A. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human NKX2-1 haploinsufficiency. J Clin Invest. 2002;109:475–480. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Devos D. Vuillaume I. de Becdelievre A. de Martinville B. Dhaenens CM. Cuvellier JC. Cuisset JM. Vallée L. Lemaitre MP. Bourteel H. Hachulla E. Wallaert B. Destée A. Defebvre L. Sablonnière B. New syndromic form of benign hereditary chorea is associated with a deletion of TITF-1 and PAX-9 contiguous genes. Mov Disord. 2006;21:2237–2240. doi: 10.1002/mds.21135. [DOI] [PubMed] [Google Scholar]
- 79.Breedveld GJ. van Dongen JW. Danesino C. Guala A. Percy AK. Dure LS. Harper P. Lazarou LP. van der Linde H. Joosse M. Grüters A. MacDonald ME. de Vries BB. Arts WF. Oostra BA. Krude H. Heutink P. Mutations in TITF-1 are associated with benign hereditary chorea. Hum Mol Genet. 2002;11:971–979. doi: 10.1093/hmg/11.8.971. [DOI] [PubMed] [Google Scholar]
- 80.do Carmo Costa M. Costa C. Silva AP. Evangelista P. Santos L. Ferro A. Sequeiros J. Maciel P. Nonsense mutation in TITF1 in a Portuguese family with benign hereditary chorea. Neurogenetics. 2005;6:209–215. doi: 10.1007/s10048-005-0013-1. [DOI] [PubMed] [Google Scholar]
- 81.Willemsen MA. Breedveld GJ. Wouda S. Otten BJ. Yntema JL. Lammens M. de Vries BB. Brain-Thyroid-Lung syndrome: a patient with a severe multi-system disorder due to a de novo mutation in the thyroid transcription factor 1 gene. Eur J Pediatr. 2005;164:28–30. doi: 10.1007/s00431-004-1559-x. [DOI] [PubMed] [Google Scholar]
- 82.Carré A. Szinnai G. Castanet M. Sura-Trueba S. Tron E. Broutin-L'Hermite I. Barat P. Goizet C. Lacombe D. Moutard ML. Raybaud C. Raynaud-Ravni C. Romana S. Ythier H. Léger J. Polak M. Five new TTF1/NKX2.1 mutations in brain-lung-thyroid syndrome: rescue by PAX8 synergism in one case. Hum Mol Genet. 2009;18:2266–2276. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 83.Moya CM. Perez de Nanclares G. Castaño L. Potau N. Bilbao JR. Carrascosa A. Bargadá M. Coya R. Martul P. Vicens-Calvet E. Santisteban P. Functional study of a novel single deletion in the TITF1/NKX2.1 homeobox gene that produces congenital hypothyroidism and benign chorea but not pulmonary distress. J Clin Endocrinol Metab. 2006;91:1832–1841. doi: 10.1210/jc.2005-1497. [DOI] [PubMed] [Google Scholar]
- 84.Ferrara AM. De Michele G. Salvatore E. Di Maio L. Zampella E. Capuano S. Del Prete G. Rossi G. Fenzi G. Filla A. Macchia PE. A novel NKX2.1 mutation in a family with hypothyroidism and benign hereditary chorea. Thyroid. 2008;18:1005–1009. doi: 10.1089/thy.2008.0085. [DOI] [PubMed] [Google Scholar]
- 85.Langston C. Misalignment of pulmonary veins and alveolar capillary dysplasia. Pediatr Pathol. 1991;11:163–170. doi: 10.3109/15513819109064753. [DOI] [PubMed] [Google Scholar]
- 86.Oldenburg J. Van Der Pal HJ. Schrevel LS. Blok AP. Wagenvoort CA. Misalignment of lung vessels and alveolar capillary dysplasia. Histopathology. 1995;27:192–194. doi: 10.1111/j.1365-2559.1995.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 87.Thibeault DW. Garola RE. Kilbride HW. Alveolar capillary dysplasia: an emerging syndrome. J Pediatr. 1999;134:661–662. doi: 10.1016/s0022-3476(99)70264-7. [DOI] [PubMed] [Google Scholar]
- 88.Abdallah HI. Karmazin N. Marks LA. Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit Care Med. 1993;21:628–630. doi: 10.1097/00003246-199304000-00026. [DOI] [PubMed] [Google Scholar]
- 89.Boggs S. Harris MC. Hoffman DJ. Goel R. McDonald-McGinn D. Langston C. Zackai E. Ruchelli E. Misalignment of pulmonary veins with alveolar capillary dysplasia: affected siblings and variable phenotypic expression. J Pediatr. 1994;124:125–128. doi: 10.1016/s0022-3476(94)70267-5. [DOI] [PubMed] [Google Scholar]
- 90.Al-Hathlol K. Phillips S. Seshia MK. Casiro O. Alvaro RE. Rigatto H. Alveolar capillary dysplasia. Report of a case of prolonged life without extracorporeal membrane oxygenation (ECMO) and review of the literature. Early Hum Dev. 2000;57:85–94. doi: 10.1016/s0378-3782(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 91.Shankar V. Haque A. Johnson J. Pietsch J. Late presentation of alveolar capillary dysplasia in an infant. Pediatr Crit Care Med. 2006;7:177–179. doi: 10.1097/01.PCC.0000202570.58016.67. [DOI] [PubMed] [Google Scholar]
- 92.Ahmed S. Ackerman V. Faught P. Langston C. Profound hypoxemia and pulmonary hypertension in a 7-month-old infant: late presentation of alveolar capillary dysplasia. Pediatr Crit Care Med. 2008;9:e43–e46. doi: 10.1097/PCC.0b013e31818e383e. [DOI] [PubMed] [Google Scholar]
- 93.Deutsch GH. Young LR. Deterding RR. Fan LL. Dell SD. Bean JA. Brody AS. Nogee LM. Trapnell BC. Langston C. Albright EA. Askin FB. Baker P. Chou PM. Cool CM. Coventry SC. Cutz E. Davis MM. Dishop MK. Galambos C. Patterson K. Travis WD. Wert SE. White FV Pathology Cooperative Group; chILD Research Co-operative; Pathology Cooperative Group; chILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cassidy J. Smith J. Goldman A. Haynes S. Smith E. Wright C. Haworth S. Davis P. Firmin R. Kasem K. Davis C. The incidence and characteristics of neonatal irreversible lung dysplasia. J Pediatr. 2002;141:426–428. doi: 10.1067/mpd.2002.126602. [DOI] [PubMed] [Google Scholar]
- 95.Sen P. Thakur N. Stockton DW. Langston C. Bejjani BA. Expanding the phenotype of alveolar capillary dysplasia (ACD) J Pediatr. 2004;145:646–651. doi: 10.1016/j.jpeds.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 96.Stankiewicz P. Sen P. Bhatt SS. Storer M. Xia Z. Bejjani BA. Ou Z. Wiszniewska J. Driscoll DJ. Maisenbacher MK. Bolivar J. Bauer M. Zackai EH. McDonald-McGinn D. Nowaczyk MM. Murray M. Hustead V. Mascotti K. Schultz R. Hallam L. McRae D. Nicholson AG. Newbury R. Durham-O'Donnell J. Knight G. Kini U. Shaikh TH. Martin V. Tyreman M. Simonic I. Willatt L. Paterson J. Mehta S. Rajan D. Fitzgerald T. Gribble S. Prigmore E. Patel A. Shaffer LG. Carter NP. Cheung SW. Langston C. Shaw-Smith C. Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet. 2009;84:780–791. doi: 10.1016/j.ajhg.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trapnell BC. Whitsett JA. Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 98.Nishinakamura R. Nakayama N. Hirabayashi Y. Inoue T. Aud D. McNeil T. Azuma S. Yoshida S. Toyoda Y. Arai K. Mice deficient for the IL-3/GM-CSF/IL-5 beta c receptor exhibit lung pathology and impaired immune response, while beta IL3 receptor-deficient mice are normal. Immunity. 1995;2:211–222. doi: 10.1016/1074-7613(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 99.Robb L. Drinkwater CC. Metcalf D. Li R. Köntgen F. Nicola NA. Begley CG. Hematopoietic and lung abnormalities in mice with a null mutation of the common beta subunit of the receptors for granulocyte-macrophage colony-stimulating factor and interleukins 3 and 5. Proc Natl Acad Sci USA. 1995;92:9565–9569. doi: 10.1073/pnas.92.21.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dirksen U. Nishinakamura R. Groneck P. Hattenhorst U. Nogee L. Murray R. Burdach S. Human pulmonary alveolar proteinosis associated with a defect in GM-CSF/IL-3/IL-5 receptor common beta chain expression. J Clin Invest. 1997;100:2211–2217. doi: 10.1172/JCI119758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki T. Sakagami T. Rubin BK. Nogee LM. Wood RE. Zimmerman SL. Smolarek T. Dishop MK. Wert SE. Whitsett JA. Grabowski G. Carey BC. Stevens C. van der Loo JC. Trapnell BC. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med. 2008;205:2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martinez-Moczygemba M. Doan ML. Elidemir O. Fan LL. Cheung SW. Lei JT. Moore JP. Tavana G. Lewis LR. Zhu Y. Muzny DM. Gibbs RA. Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. J Exp Med. 2008;205:2711–2716. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sperandeo MP. Bassi MT. Riboni M. Parenti G. Buoninconti A. Manzoni M. Incerti B. Larocca MR. Di Rocco M. Strisciuglio P. Dianzani I. Parini R. Candito M. Endo F. Ballabio A. Andria G. Sebastio G. Borsani G. Structure of the SLC7A7 gene and mutational analysis of patients affected by lysinuric protein intolerance. Am J Hum Genet. 2000;66:92–99. doi: 10.1086/302700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borsani G. Bassi MT. Sperandeo MP. De Grandi A. Buoninconti A. Riboni M. Manzoni M. Incerti B. Pepe A. Andria G. Ballabio A. Sebastio G. SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat Genet. 1999;21:297–301. doi: 10.1038/6815. [DOI] [PubMed] [Google Scholar]
- 105.Di Rocco M. Interstitial lung disease in lysinuric protein intolerance. J Pediatr. 1994;124:655. doi: 10.1016/s0022-3476(05)83153-1. [DOI] [PubMed] [Google Scholar]
- 106.Kerem E. Elpelg ON. Shalev RS. Rosenman E. Bar Ziv Y. Branski D. Lysinuric protein intolerance with chronic interstitial lung disease and pulmonary cholesterol granulomas at onset. J Pediatr. 1993;123:275–278. doi: 10.1016/s0022-3476(05)81703-2. [DOI] [PubMed] [Google Scholar]
- 107.Parto K. Svedström E. Majurin ML. Härkönen R. Simell O. Pulmonary manifestations in lysinuric protein intolerance. Chest. 1993;104:1176–1182. doi: 10.1378/chest.104.4.1176. [DOI] [PubMed] [Google Scholar]
- 108.Santamaria F. Brancaccio G. Parenti G. Francalanci P. Squitieri C. Sebastio G. Dionisi-Vici C. D'argenio P. Andria G. Parisi F. Recurrent fatal pulmonary alveolar proteinosis after heart-lung transplantation in a child with lysinuric protein intolerance. J Pediatr. 2004;145:268–272. doi: 10.1016/j.jpeds.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 109.Armanios MY. Chen JJ. Cogan JD. Alder JK. Ingersoll RG. Markin C. Lawson WE. Xie M. Vulto I. Phillips JA., 3rd Lansdorp PM. Greider CW. Loyd JE. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y. Kuan PJ. Xing C. Cronkhite JT. Torres F. Rosenblatt RL. DiMaio JM. Kinch LN. Grishin NV. Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cronkhite JT. Xing C. Raghu G. Chin KM. Torres F. Rosenblatt RL. Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsakiri KD. Cronkhite JT. Kuan PJ. Xing C. Raghu G. Weissler JC. Rosenblatt RL. Shay JW. Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]