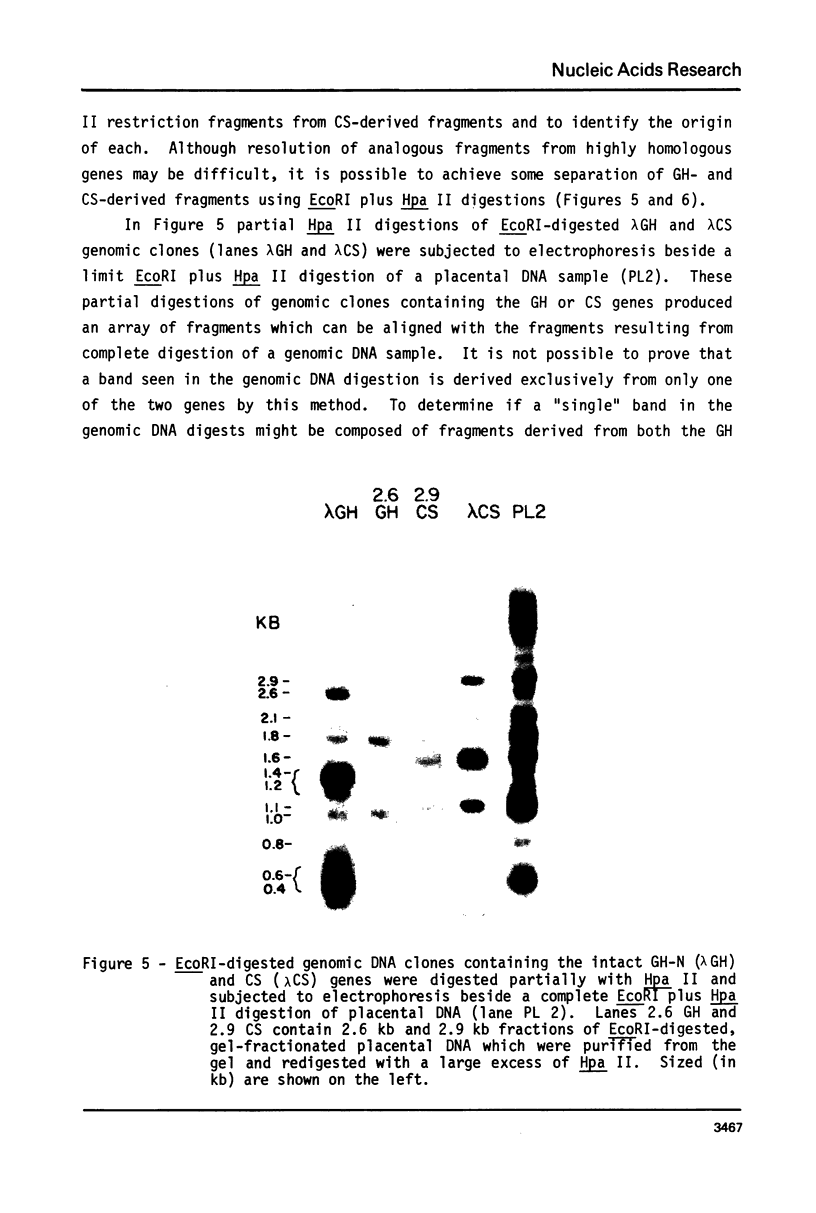
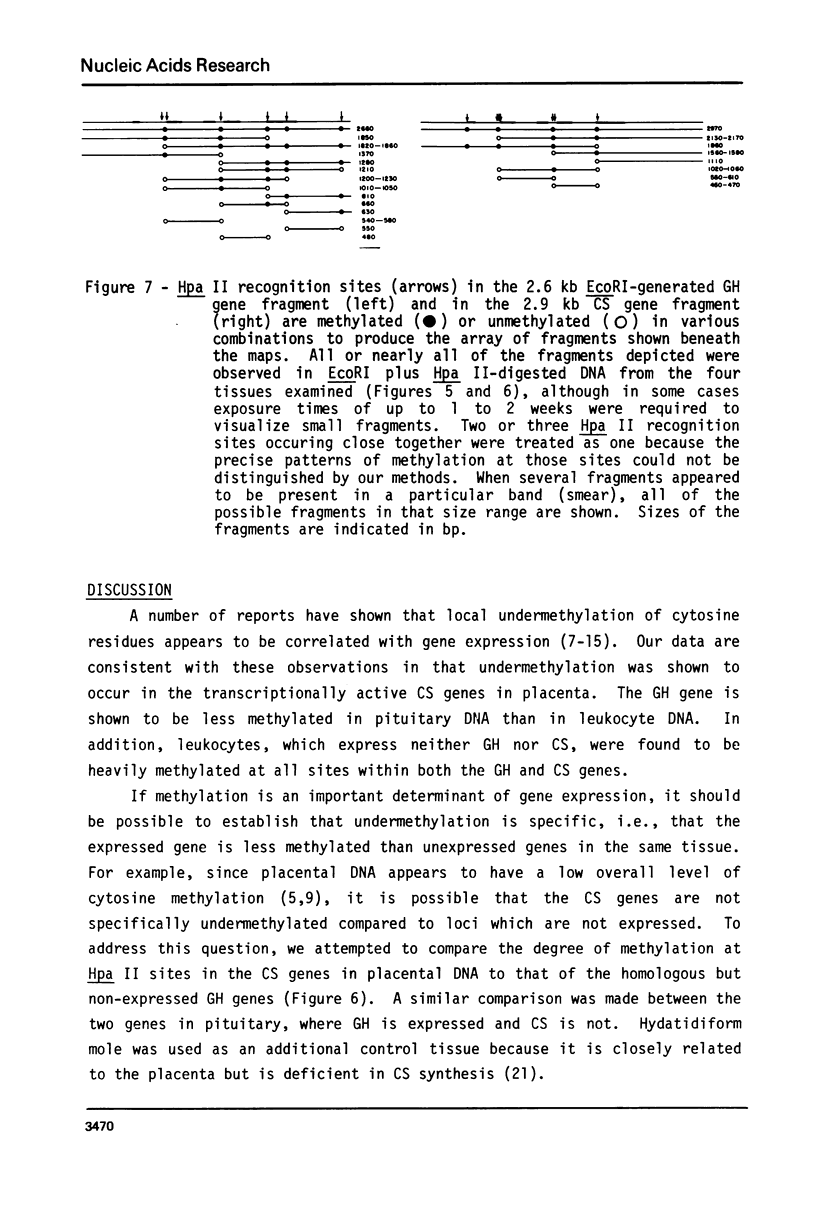
Abstract
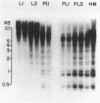
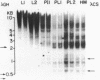
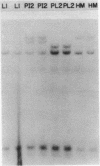
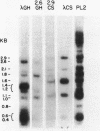
It has been shown that the extent of methylation of cytosine in vertebrate DNA is inversely correlated with gene expression. We studied cytosine methylation in and around the homologous human growth hormone (GH) and chorionic somatomammotropin (CS) genes to determine if these genes are undermethylated in DNA from tissues in which they are expressed (pituitary and placenta, respectively) compared to other tissues. Hpa II and Hha I (which cleave only unmethylated 5' CCGG 3' and 5' GCGC 3' respectively) and Msp I (which cleaves CCGG and CmeCGG) were used to digest DNA samples followed by gel electrophoresis, Southern transfer and hybridization with a GH cDNA probe. The extent of methylation of Hpa II and Hha I sites in the GH and CS genes was leukocyte much greater than pituitary greater than placenta = hydatidiform mole. Taken as a whole, our data support the hypothesis that undermethylation is a necessary but not sufficient condition for gene expression since placental and pituitary DNAs are less methylated than leukocyte DNA in this region. However, the correlation between gene expression and undermethylation is imperfect since (1) hydatiform mole DNA has a very similar methylation pattern compared to placental DNA even though moles make little or no CS and (2) the level of methylation of the GH gene compared to the CS gene does not vary in a tissue-specific manner.
Full text
PDF



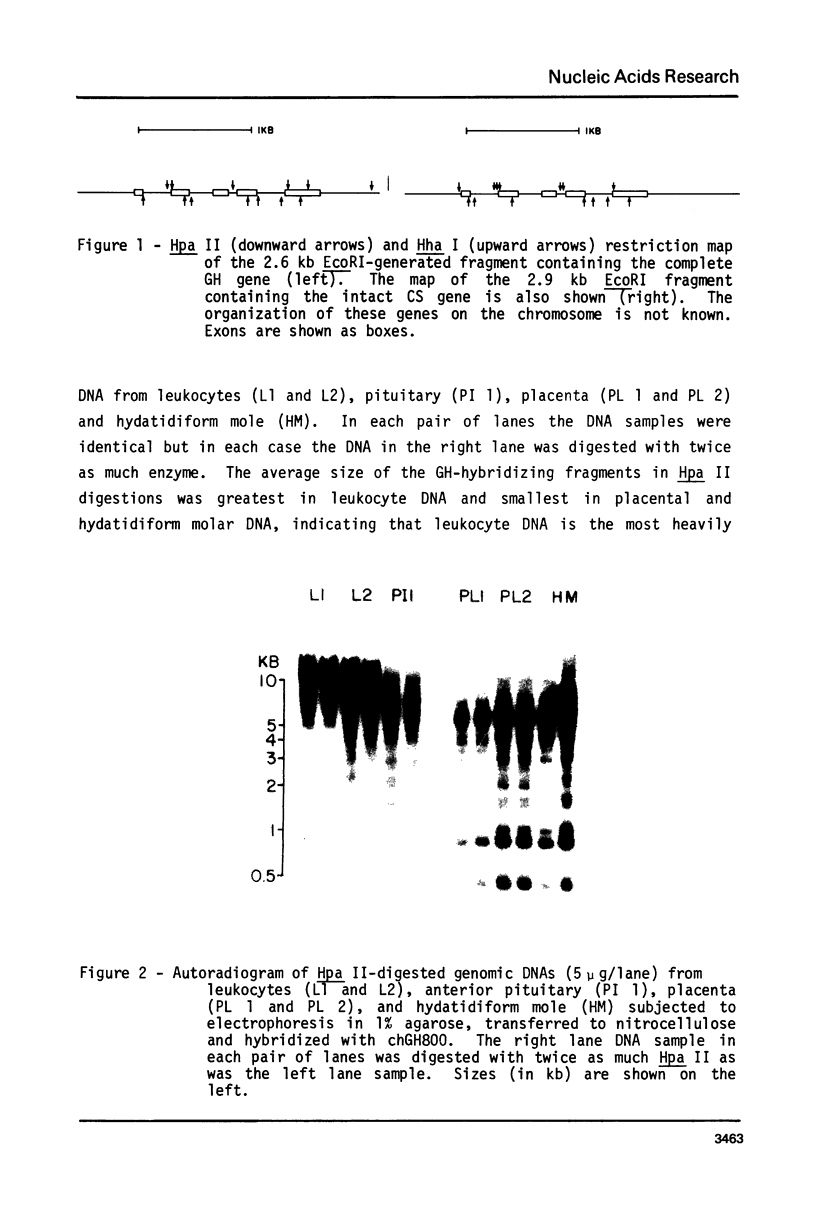
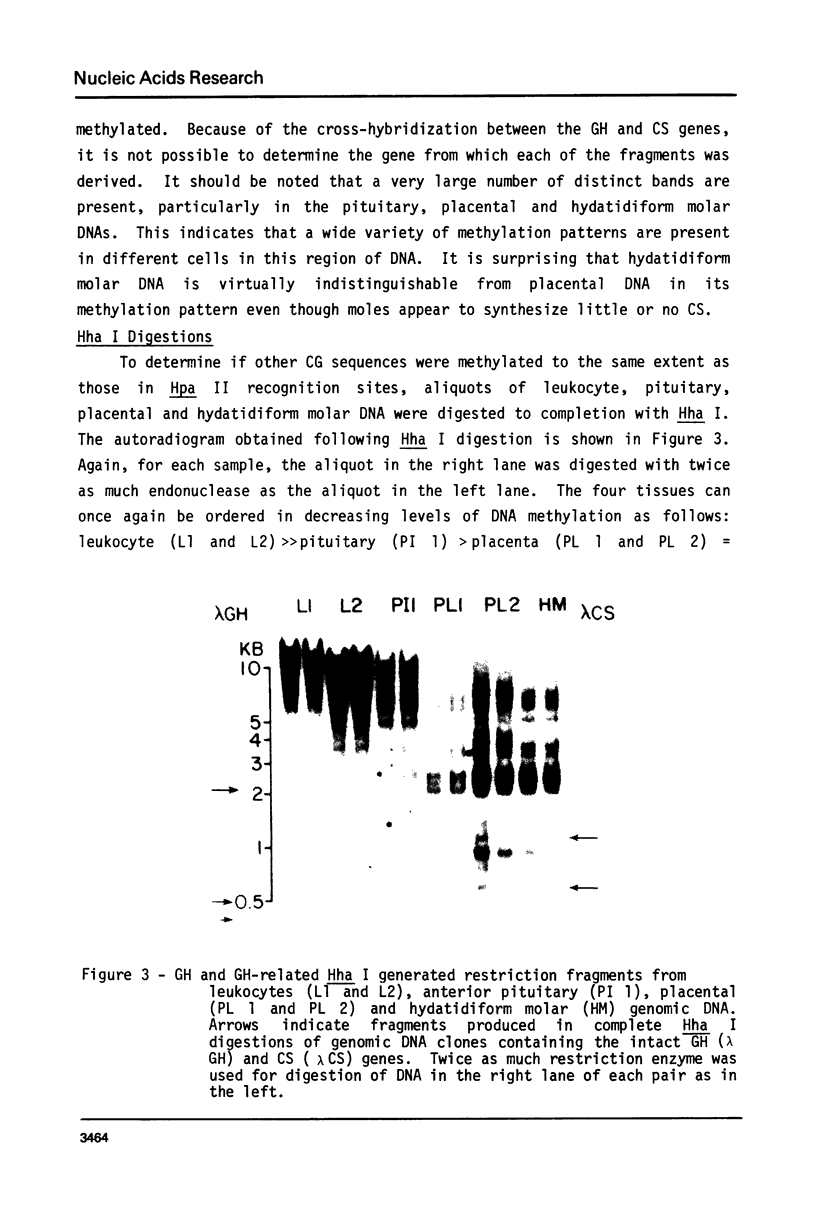

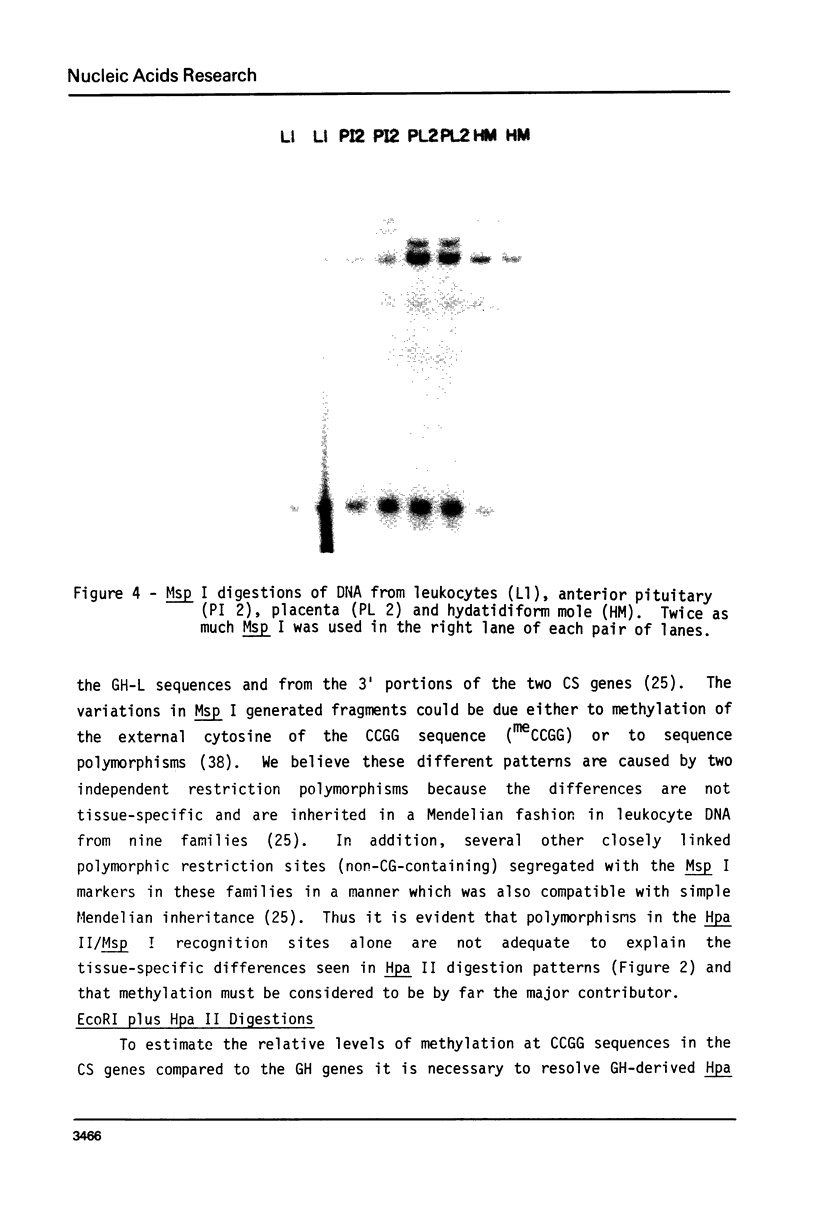






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Compere S. J., Palmiter R. D. DNA methylation controls the inducibility of the mouse metallothionein-I gene lymphoid cells. Cell. 1981 Jul;25(1):233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- DOSKOCIL J., SORM F. Distribution of 5-methylcytosine in pyrimidine sequences of deoxyribonucleic acids. Biochim Biophys Acta. 1962 Jun 11;55:953–959. doi: 10.1016/0006-3002(62)90909-5. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Wang R. Y. 5-Methylcytosine in eukaryotic DNA. Science. 1981 Jun 19;212(4501):1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Seeburg P. H., DeNoto F. M., Hallewell R. A., Baxter J. D., Goodman H. M. Structure of genes for human growth hormone and chorionic somatomammotropin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4294–4298. doi: 10.1073/pnas.76.9.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Phillips J. A., 3rd, Francke U., Seeburg P. H. The genes for growth hormone and chorionic somatomammotropin are on the long arm of human chromosome 17 in region q21 to qter. Hum Genet. 1981;57(2):138–141. doi: 10.1007/BF00282009. [DOI] [PubMed] [Google Scholar]
- Grippo P., Iaccarino M., Parisi E., Scarano E. Methylation of DNA in developing sea urchin embryos. J Mol Biol. 1968 Sep 14;36(2):195–208. doi: 10.1016/0022-2836(68)90375-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Martial J. A., Hallewell R. A., Baxter J. D., Goodman H. M. Human growth hormone: complementary DNA cloning and expression in bacteria. Science. 1979 Aug 10;205(4406):602–607. doi: 10.1126/science.377496. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- McWilliams D., Boime I. Cytological localization of placental lactogen messenger ribonucleic acid in syncytiotrophoblast layers of human placenta. Endocrinology. 1980 Sep;107(3):761–765. doi: 10.1210/endo-107-3-761. [DOI] [PubMed] [Google Scholar]
- McWilliams D., Callahan R. C., Boime I. Human placental lactogen mRNA and its structural genes during pregnancy: quantitation with a complementary DNA. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1024–1027. doi: 10.1073/pnas.74.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Morikawa H., Kawaguchi K., Tojo S. Growth hormone, prolactin and chorionic somatomammotropin in normal and molar pregnancy. J Clin Endocrinol Metab. 1976 Sep;43(3):614–621. doi: 10.1210/jcem-43-3-614. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Hogan M. L., Sauer R., Rosenblum I. Y., Greenwood F. C. Sequences of pituitary and placental lactogenic and growth hormones: evolution from a primordial peptide by gene reduplication. Proc Natl Acad Sci U S A. 1971 Apr;68(4):866–870. doi: 10.1073/pnas.68.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerbach D., Rutter W. J., Martial J. A., Baxter J. D., Shows T. B. Genes for growth hormone, chorionic somatommammotropin, and growth hormones-like gene on chromosome 17 in humans. Science. 1980 Jul 11;209(4453):289–292. doi: 10.1126/science.7384802. [DOI] [PubMed] [Google Scholar]
- Phillips J. A., 3rd, Hjelle B. L., Seeburg P. H., Zachmann M. Molecular basis for familial isolated growth hormone deficiency. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6372–6375. doi: 10.1073/pnas.78.10.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Groffen J., Flavell R. A. A novel type of secondary modification of two CCGG residues in the human gamma delta beta-globin gene locus. Nucleic Acids Res. 1980 Oct 24;8(20):4563–4574. doi: 10.1093/nar/8.20.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]