Abstract
Parkinson’s disease affects over one million people in the United States. Although there have been remarkable advances in uncovering the pathogenesis of this disabling disorder, the etiology is speculative. Medical treatment and operative procedures provide symptomatic relief only. Compression of the cerebral peduncle of the midbrain by the posterior cerebral artery in a patient with Parkinson’s Disease (Parkinson’s Disease) was noted on magnetic resonance imaging (MRI) scan and at operation in a patient with trigeminal neuralgia. Following the vascular decompression of the trigeminal nerve, the midbrain was decompressed by mobilizing and repositioning the posterior cerebral artery The patient's Parkinson's signs disappeared over a 48-hour period. They returned 18 months later with contralateral peduncle compression. A blinded evaluation of MRI scans of Parkinson's patients and controls was performed. MRI scans in 20 Parkinson's patients and 20 age and sex matched controls were evaluated in blinded fashion looking for the presence and degree of arterial compression of the cerebral peduncle. The MRI study showed that 73.7 percent of Parkinson's Disease patients had visible arterial compression of the cerebral peduncle. This was seen in only 10 percent of control patients (two patients, one of whom subsequently developed Parkinson’s Disease); thus 5 percent. Vascular compression of the cerebral peduncle by the posterior cerebral artery may be associated with Parkinson’s Disease in some patients. Microva-scular decompression of that artery away from the peduncle may be considered for treatment of Parkinson’s Disease in some patients.
Key words: Parkinson's disease, microvascular decompression, contralateral lateral peduncle, vascular compression.
Introduction
The pathogenesis of Parkinson’s Disease is unclear.1,2 The loss of pigmented neurons in substantia nigra, locus ceruleus, and brainstem nuclei is found in most forms of Parkinson’s Disease. Depletion of the neuro-transmitter substance Dopamine has been demonstrated in experimental animals and in humans. Etiologic mechanisms have been attributed to MPTP exposure, environmental neurotoxins, trauma, endogenous neurotoxins, genetic predisposition and genetic associations with a variety of mutations, oxidative stress and apoptosis. By clinical observation, one of us (PJ) noted magnetic resonance imaging (MRI) compression of the cerebral peduncle by the posterior cerebral artery on MRI scan in a patient with Parkinson’s Disease and treated it. This encouraged us to perform a blinded study of MRI scans in age and sex matched Parkinson disease cases and control patients.
Materials and Methods
A 60-year-old woman had intractable left sided trigeminal neuralgia. She also had well-documented Parkinson’s Disease. After microvascular decompression (MVD) of the left trigeminal nerve, MVD of the left anterolateral midbrain was performed. Based upon the MRI and intraoperative findings in the above patient, a blinded MRI study of 40 MRI scans, 20 with known Parkinson’s Disease and 20 controls, was performed under the purview of an IRB approved protocol. Twenty MRI scans in patients with Parkinson’s Disease and 20 age and sex matched control MRI scans were evaluated in a blinded randomized fashion by two groups of two investigators (PJ/DW and MF/RW). The Parkinson’s Disease patients had been seen and followed in our Parkinson’s Disease clinic with the diagnosis well established by clinical criteria. The study was approved by our IRB. The T2 study of the midbrain was evaluated in all patients. The T2 axial views of the midbrain were independently evaluated and interviewer discrepancies (only two in number) were aligned. Arterial compression-distortion of the cerebral peduncles was graded as follows: grade 0, no compression; grade 1, compression only; grade 2, compression and distortion of the midbrain. The offending artery was the posterior cerebral artery in every case.
Case Report
A 60-year-old woman was seen in the Jannetta Cranial Nerve Center, Department of Neurosurgery, Allegheny General Hospital, with the chief complaint of left facial pain of 11 years duration, which was lancinating, intermittent, and located in the left V3 distribution. This pain was exacerbated by the usual factors. The diagnosis of trigeminal neuralgia was made. She had taken increasing doses of phenytoin, baclofen, gabapentin, and carba-mazepine, all of which had to be discontinued because of a lack of relief or side effects. Past medical history showed type 2 diabetes, and one transient ischemic attack. The diagnosis of Parkinson’s Disease had been established 2 years prior to admission based on progressively severe resting tremor of the upper extremities, mask-like facies, postural instability, and cog-wheel rigidity. The family history was non-contributory. Her medications included metformin for the DM2, carbamazepine for facial pain and amantadine for parkinsonism in a dose of 100 mg. b.i.d. She had been on carbidopa-levodopa to which she became intolerant. She was seen in consultation regarding operative treatment of her intractable trigeminal neuralgia. Physical examination showed a 60-year-old woman who had a prominent stare, mask-like facies, cog-wheel rigidity of the upper extremities and an intermittent pill rolling type of tremor of the upper extremities. Her posture was stooped and gait showed small, uncertain steps with a tendency for festination. Neurological examination was otherwise normal. Specifically, the corneal reflexes, both upper and lower, were brisk and equal. Sensory and motor examination of the trigeminal nerve was normal. The diagnoses included: i) Intractable trigeminal neuralgia in the left V3 distribution; ii) Parkinson’s Disease treated with amantadine; iii) Type 2 diabetes mellitus treated with metformin.
Special studies
Magnetic resonance imaging of the brain was reported as normal. One of us (PJ) noted that the left posterior cerebral artery was compressing and distorting the left cerebral peduncle (Figure 1). After discussion with the patient and her husband, it was decided to evaluate and treat the compression of the midbrain, if feasible, after microvascular decompression of the trigeminal nerve.
Figure 1.
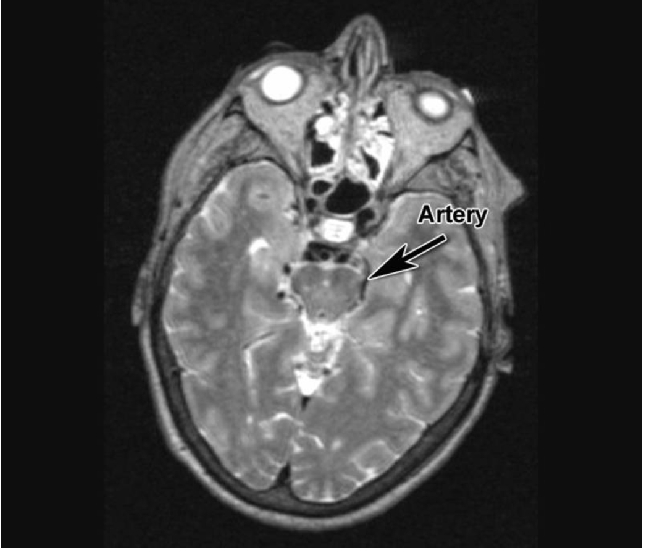
Magnetic resonance imaging scan T2 axial view at level of midbrain. Arrow points to area of compression of left cerebral peduncle by posterior cerebral artery. Clear space filled with cerebral fluid is seen on the right.
Operation
With the patient under general endotracheal anesthesia in the right lateral decubitus position a left retromastoid craniectomy and microvascular decompression of the trigeminal nerve was performed using techniques previously described.3 The anterolateral midbrain was then exposed by angling the microscope rostrally. The posterior cerebral artery grooving the midbrain (Figure 2) was mobilized and held away with an implant of soft shredded Teflon felt (Figure 3). The wound was then closed in the usual manner.
Figure 2.
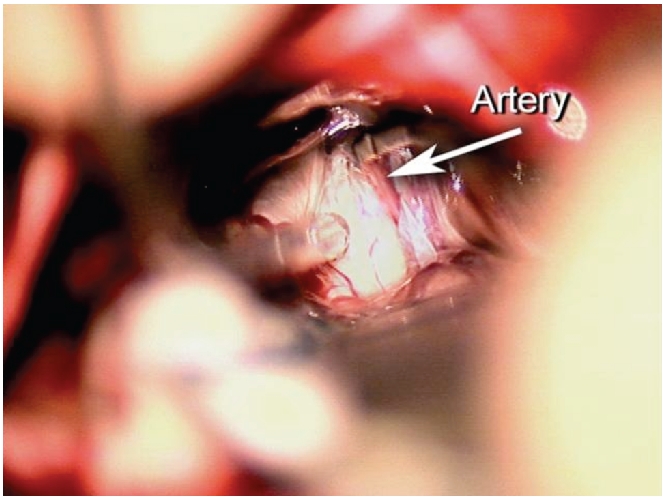
View through microscope of left cerebral peduncle in patient with Parkinson’s Disease. Arrow points to PCA partly buried in the midbrain.
Figure 3.
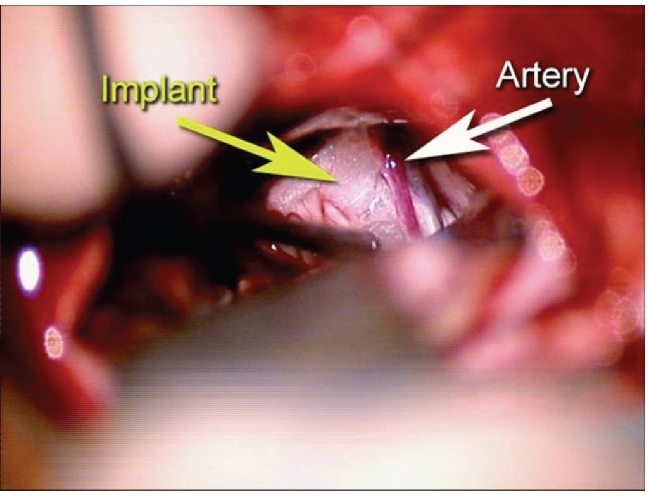
Same view as Figure 2. Artery has been mobilized away from the cerebral peduncle and decompressed using shredded PTFE felt.
Results
The postoperative course was benign. The patient awoke with the face pain relieved and without the mask-like facies. The cogwheeling rigidity was gone by the first postoperative day. There was no tremor at any time. She was discharged from the hospital on the second postoperative day. When seen on the fifth postoperative day, she was pain free and without signs of parkinsonism. She remained well for 18 months and then suffered recurrence. MRI scan now showed compression of the contralateral cerebral peduncle by the PCA (Figure 4).
Figure 4.
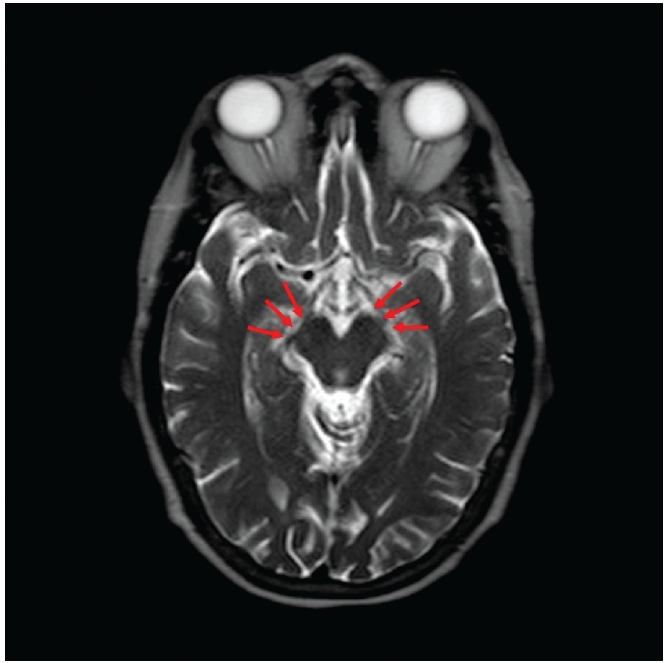
Magnetic resonance imaging T2 axial view two years postoperatively. Recurrence began about 18 months postoperatively. Left caudal peduncle shows implant separating PCA (three arrows). Right cerebral peduncle now compressed by PCA (two arrows).
Magnetic resonance imaging study
The MRI study was deemed inadequate in one Parkinson’s Disease patient. Of the 19 Parkinson’s Disease patients, 13 had clear compression-distortion of one or both of the cerebral peduncles by the posterior cerebral artery (78%). Four were bilateral. Seven were Grade 2. Of the 20 control patients, only 2 had compression of the cerebral peduncle, each with a Grade 1. One of these two patients was later diagnosed with Parkinson’s Disease. Three patients had questionable compression. The other control scans showed no arterial compression-distortion of the peduncle. (Figure 5) These data are collated in Tables 1 and 2. Figure 6 shows bilateral compression in a Parkinson’s Disease patient. Statistical analysis was performed utilizing MedCalc for PC 9.6.4.0. A forward logistic regression was performed with the diagnosis of Parkinson’s Disease as the dependent variable and age, sex and maximum degree of vascular compression on either side as covariates.
Figure 5.
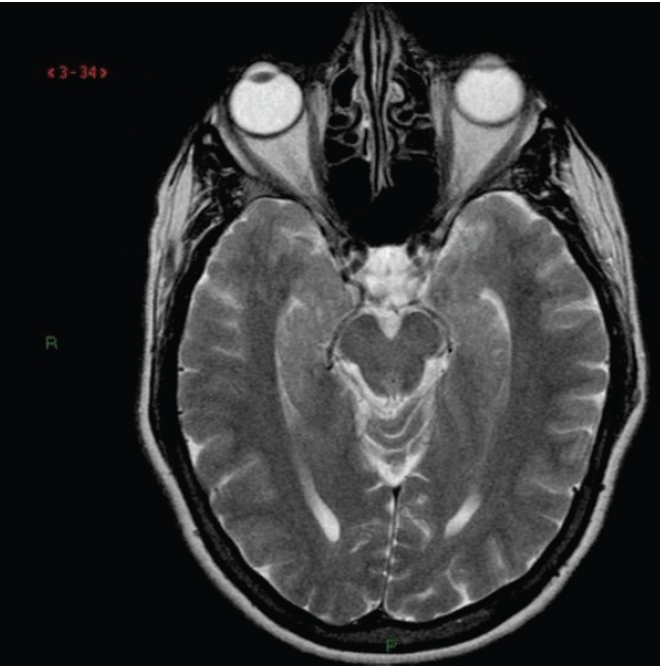
Magnetic resonance imaging T2 axial view at the level of midbrain in control patient without Parkinson Disease. Note that posterior cerebral arteries do not impinge upon cerebral peduncle. A clear space filled with cerebrospinal fluid is seen bilaterally.
Table 1. Parkinson disease: compression of cerebral peduncle by posterior cerebral artery.
| Parkinson Disease (Parkinson’s disease) Group n=19 | Arterial compression | Control group n=20 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID # | Right | Left | Sex | Age | Grading Scale | ID # | Right | Left | Sex | Age |
| 1 | 1 ?2 | 2 | F | 64 | 0 No Contact | 2 | 0 | 0 | M | 56 |
| 4 | 0 | 2 | F | 67 | 1 Contact | 3 | 0 | 0 | M | 58 |
| 6 | ? | 1 | M | 57 | 2 Distortion | 5 | 0 | 0 | M | 58 |
| 7 | 0 | 0 | F | 69 | ? Possible contact | 8 | 0 | 0 | M | 59 |
| 9 | 0 | 0 | M | 59 | 11 | 0 | 0 | M | 61 | |
| 10 | ? | 0 | M | 71 | Parkinson’s disease female mean age | 12 | ? | 0 | M | 62 |
| 13 | 0 | 0 | M | 62 | 66.625 | 14 | 0 | 0 | M | 63 |
| 16 | 1 | 0 | F | 82 | 15 | 0 | 0 | M | 63 | |
| 17 | 0 | 0 | M | 72 | Parkinson’s disease male mean age | 19 | 0 | 0 | M | 64 |
| 18 | 0 | 1 | M | 62 | 61.727 | 20 | 0 | 0 | M | 70 |
| 21 | 1 | 0 | F | 81 | 22 | ? | 0 | F | 57 | |
| 23 | 0 | 1 | M | 59 | Control female mean age | 24 | 0 | 0 | F | 57 |
| 26 | 2 | 0 | M | 68 | 62.6 | 25 | 0 | 0 | F | 57 |
| 27 | 2 | 0 | F | 67 | 29 | 1 | 0 | F | 59 | |
| 28 | 1 | 2 | F | 50 | Control male mean age | 30 | 0 | 0 | F | 59 |
| 31 | ? | 1 | M | 50 | 61.4 | 33 | ? | 0 | F | 63 |
| 32 | 1 | 2 | F | 53 | 34 | 0 | 0 | F | 66 | |
| 35 | 2 | 1 | M | 59 | Parkinson’s disease & controls mean age | 36 | 0 | 0 | F | 67 |
| 39 | 0 | 0 | M | 60 | 62.871 | 38 | 0 | ? | F | 70 |
| 40 | 1 | 1 | F | 71 | ||||||
Parkinson’s disease patients vs. controls. Side, frequency, and degree (Grade) of arterial compression. 0 = No contact of artery on cerebral peduncle. 1 = Artery is touching cerebral peduncle. 2 = Artery is compressing cerebral peduncle to cause distortion. ? = Scan not clear enough to determine if artery touching cerebral peduncle or not.
Table 2. Parkinson’s disease. data collated – parkinson’s disease patients and control patients.
| Grade | 0 | % | 1 or 2 | % | ? | % |
|---|---|---|---|---|---|---|
| PD n=19 | 5 | 26.3 | 13 | 68.4 | 1 | 5.3 |
| C n=20 | 14 | 70 | 2 | 10 | 4 | 20 |
Parkinson disease: arterial compression of Midbrain. Grading scale: 0 No Contact; 1 Contact; 2 Distortion; ? Possible Contact/Distortion. Note: one of 2 patients with compression subsequently developed Parkinson’s disease.
Figure 6.
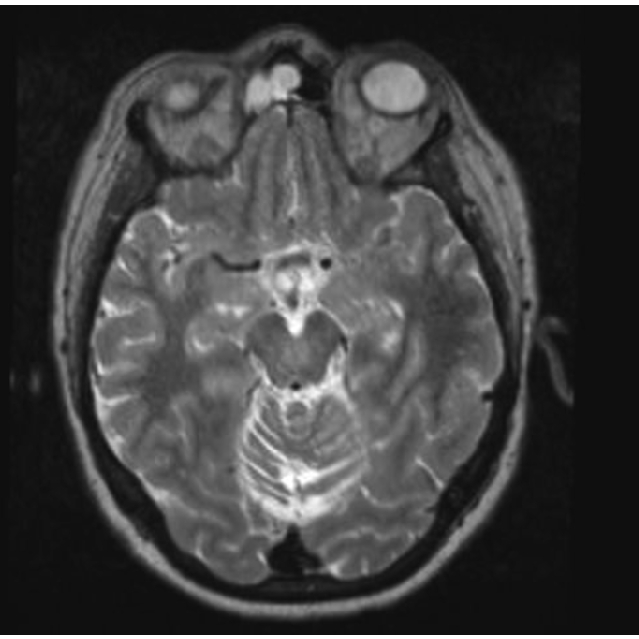
Magnetic resonance imaging T2 Axial view. Bilateral compression in patients with Parkinson Disease.
There was a significant relationship between a diagnosis of Parkinson’s Disease and maximal degree of radiographic vascular compression (P=.006), OR 9.0 (95% CI 1.9–43.1), with 78% of cases correctly classified. Although the overall distribution of extent of compression was nearly identical when comparing each side (Chi-square 1.63, P=.65) the correlation of degree of compression in each individual was low (kappa = .2).
Discussion
Beginning in 1966, a growing number of cranial nerve and brainstem syndromes have been reported to be caused by pulsatile vascular compression, arterial or venous or both. These include such apparently disparate problems as trigeminal neuralgia3–5 (both typical and atypical), hemifacial spasm,6,7 Meniere’s disease,8 disabling positional vertigo,9 glossopharyngeal neuralgia,10–12 Bell’s palsy,13 essential hypertension14–17 and type 2 diabetes.18,19 This knowledge of the above entities, although known and accepted in the neurosurgical community, have not penetrated into the general medicine literature. Beginning in the same year (1966), treatment of the pulsatile compression (microvascular decompression) was developed. These findings and treatments were possible because of the use of then new technology, and the magnification, lighting and recording capabilities of the surgical binocular microscope.13–19
It is estimated that over one million people in the United States have Parkinsonism from various causes. The incidence increases with advanced age, although it is known that young people may develop a rapidly advancing form of Parkinson’s Disease. The true etiology of Parkinson’s Disease has been unclear, but it is well recognized that loss of pigmented neurons, especially the substantia nigra, locus ceruleus, and brainstem nuclei is found in virtually all forms. Many careful investigators have stated that they do not know the etiology and have called Parkinson’s Disease idiopathic, i.e., the true primary etiology is unknown. The loss of pigmented neurons is associated with the presence of Lewy bodies in the basal ganglia and upper brainstem. Pathogenic mechanisms have been related to MPTP exposure, environmental neurotoxins, trauma, the enigmatic neuroprotective effect of smoking, endogenous neurotoxins, and genetic factors.
One reason that arterial compression of the cerebral peduncle was never noted may be because of the technique of the standard post mortem examination of the brain. For the post mortem exam, the calvarium is removed with a power saw. The dura mater is removed. The forebrain is then raised up off the frontal-temporal fossa and the region of the midbrain transected using a scalpel or scissors. This, the area of the vascular compression, is unfortunately stretched, distorted and at times transected. Subtle neurovascular relationships are lost. The autopsy technique of removal of the hindbrain also has denied careful study of neurovascular relationships of the pons and medulla even if the brain has been removed in one piece. In any case, arterial relationships have generally been found by pathologists.
The pathophysiology of pulsatile compression of the cerebral peduncles may be the same as vascular compression of the cranial nerves and medulla oblongata. Severe compression of central nervous tissue causes rapid or severe loss of function. This is seen in Bells palsy when a shifting artery, usually the anterior inferior cerebellar artery stretches and compresses the facial (and to a lesser degree) the auditory nerve.13 Fortunately, this physically tough motor nerve usually (80 percent) recovers function in time. Modest and progressive pulsatile compression, on the other hand, causes hyperactive symptoms, a caricature of the normal function of the nerve, (i.e., sensation --- pain, motor function --- twitching and spasm). As it progresses, loss of function occurs, (i.e., numbness, weakness). ultrastructural changes of hypermyelination, hypomyelination, kissing axis cylinders, dead, dying and regenerating axis cylinders are seen spread over a background of normal axons and myelin.20–22 The loss of substantia nigra cells and coloration may be a part of this process. This of course is a later stage of the disease seen at post mortem exam while the ultrastructural changes in the cranial nerves are from operative specimens. These changes reflect a dynamic and progressive process of hyperactivity and the loss of function. Ephaptic transmission and kindling may each play an important role.23,24
Medical and operative treatment consists of dopamine agonists and the replacement of MAO and COMT inhibition and operative intervention with brainstem lesions and stimulation.25–27 In virtually all cases the disease progresses despite therapeutic intervention. Various studies including glial-derived nerve growth factor, and many medications and possible operative intervention are currently in process.
The present data raise questions regarding etiology which may be answered over time: i.e., Does unilateral cerebral peduncle arterial compression cause unilateral disease only? Can it cause bilateral Parkinson’s Disease in time? Can MVD relieve parkinsonism? Is there a form of parkinsonism related to arterial compression of the cerebral peduncle? Is Parkinson’s Disease in young people the same etiologically as in the older population? Are Cognitive changes reversible? Will MVD of the cerebral peduncle be as safe and effective as MVD is for other problems?
A multi center MRI trial has been organized for further evaluation of this concept. Microvascular decompression of the brainstem should be performed only by specially prepared neurosurgeons with a background in microvascular decompression and training by the senior author.
On the basis of these data, some cases of Parkinsonism may be associated with arterial compression of the cerebral peduncle by the posterior cerebral artery which may frequently be seen on MRI studies. Improvement and prolonged relief of Parkinson’s Disease in one patient demonstrates that relief of Parkinson’s Disease may be possible in certain circumstances.
Acknowledgments:
the authors would like to thank the Eden Hall Foundation for its generous financial support, and Ms. Michele Birgelen and Cindy Angle, RN for administrative assistance.
References
- 1.Parkinson J. London: Whittingham and Rowland for Sherwood, Neely, and Jones; 1817. An Essay on the Shaking Palsy. [Google Scholar]
- 2.Yahr M. Parkinsonism. In: Rowland L, editor. Merritt's Texbook of Neurology. 7th ed. Philadelphia, PA: Lea & Febiger; 1984. pp. 526–537. [Google Scholar]
- 3.Jannetta P. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26:159–62. doi: 10.3171/jns.1967.26.1part2.0159. [DOI] [PubMed] [Google Scholar]
- 4.Barker FG, 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–83. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 5.Yonas H, Jannetta PJ. Neurinoma of the trigeminal root and atypical trigeminal neuralgia: their commonality. Neurosurgery. 1980;6:273–7. [PubMed] [Google Scholar]
- 6.Jannetta PJ. Microsurgical exploration and decompression of the facial nerve in hemifacial spasm. Curr. Top. Surg Res. 1970;2:217–20. [Google Scholar]
- 7.Jannetta PJ, Abbasy M, Maroon JC, et al. Etiology and definitive microsurgical treatment of hemifacial spasm. Operative techniques and results in forty-seven patients. J Neurosurg. 1977;47:321–8. doi: 10.3171/jns.1977.47.3.0321. [DOI] [PubMed] [Google Scholar]
- 8.Moller MB, Moller AR, Jannetta PJ, Jho HD. Vascular deompression surgery. Laryngoscope. 1993;103:421–427. doi: 10.1002/lary.5541030410. [DOI] [PubMed] [Google Scholar]
- 9.Jannetta PJ, Moller MB, Moller AR, Sekhar LN. Neurosurgical treatment of vertigo by microvascular decompression of the eighth cranial nerve. Clin Neurosurg. 1986;33:645–65. [PubMed] [Google Scholar]
- 10.Jannetta P. Trigeminal and glossopharyngeal neuralgia. Curr Diagn. 1971;3:849–51. [Google Scholar]
- 11.Laha RK, Jannetta PJ. Glossopharyngeal neuralgia. J Neurosurg. 1977;47:316–20. doi: 10.3171/jns.1977.47.3.0316. [DOI] [PubMed] [Google Scholar]
- 12.Resnick DK, Jannetta PJ, Bissonnette D, et al. Microvascular decompression for glossopharyngeal neuralgia. Neurosurgery. 1995;36:64–8. doi: 10.1227/00006123-199501000-00008. discussion 68–9. [DOI] [PubMed] [Google Scholar]
- 13.Jannetta P, Bissonette DJ. Bell's palsy: a theory as to etilogy. Observations in six patients. Laryngoscope. 1978;88:849–54. doi: 10.1002/lary.1978.88.5.849. [DOI] [PubMed] [Google Scholar]
- 14.Jannetta PJ, Gendell HM. Clinical observations on etiology of essential hypertension. Surg Forum. 1979;30:431–2. [PubMed] [Google Scholar]
- 15.Jannetta PJ, Segal R, Wolfson SK., Jr Neurogenic hypertension: etiology and surgical treatment. I. Observations in 53 patients. Ann Surg. 1985;201:391–8. doi: 10.1097/00000658-198503000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jannetta PJ, Segal R, Wolfson SK, Jr, et al. Neurogenic hypertension: etiology and surgical treatment. II. Observations in an experimental nonhuman primate model. Ann Surg. 1985;202:253–61. doi: 10.1097/00000658-198508000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy EI, Scarrow AM, Jannetta PJ. Microvascular decompression in the treatment of hypertension: review and update. Surg Neurol. 2001;55:2–10. doi: 10.1016/s0090-3019(00)00352-9. discussion 10–11. [DOI] [PubMed] [Google Scholar]
- 18.Jannetta PJ, Hollihan L. Type 2 diabetes mellitus, etiology and possible treatment: preliminary report. Surg Neuro. l 2004;61:422–6. doi: 10.1016/j.surneu.2003.08.028. discussion 426–8. [DOI] [PubMed] [Google Scholar]
- 19.Jannetta PJ, Fletcher LH, Grondziowski PM, et al. Type 2 diabetes mellitus: A central nervous system etiology. Surg Neurol Int. 2010;1:31–31. doi: 10.4103/2152-7806.66460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaver DL, Moses HL, Ganote CE. Electron microscopy of the trigeminal ganglion. III Trigeminal neuralgia. Arch Path. 1965;79:571–82. [PubMed] [Google Scholar]
- 21.Kerr FWL. Pathology of trigeminal neuralgia: light and electron microscopic observations. J Neurosurg. 1967;26:151–6. doi: 10.3171/jns.1967.26.1part2.0151. [DOI] [PubMed] [Google Scholar]
- 22.Jannetta PJ, Hackett ER, Ruby JR. Electromyographic and electron microscopic correlates in hemifacial spasm treated by microsurgical relief of neurovascular compression. Surg Forum. 1970;21:449–51. [PubMed] [Google Scholar]
- 23.Nielsen VK, Jannetta PJ. Pathophysiology of hemifacial spasm. Part III: Effects of facial nerve decompression. Neurology. 1984;34:891–7. doi: 10.1212/wnl.34.7.891. [DOI] [PubMed] [Google Scholar]
- 24.Moeller AR, Jannetta PJ. Physiological abnormalities in hemifacial spasm studied during microvascular decompression operations. Exp Neurol. 1986;93:584–600. doi: 10.1016/0014-4886(86)90178-0. [DOI] [PubMed] [Google Scholar]
- 25.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 26.Mueller J, Skogseid IM, Benecke R, et al. Pallidal deep brain stimulation improves quality of life in segmental and generalized dystonia: results from a prospective, randomized sham-controlled trial. Mov Disord. 2008;23:131–4. doi: 10.1002/mds.21783. [DOI] [PubMed] [Google Scholar]
- 27.Pahwa R LK, Wilkinson SB, Simpson RK, Jr, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104:506–12. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]


