Abstract
Acute necrotizing encephalopathy (ANE) is a devastating and rapidly progressive neurologic disorder that occurs in healthy children after common viral infections. Typically, ANE is sporadic and does not recur. However, familial (ANE1) and recurrent cases have been reported and were recently linked to mutations in RANBP2 (RAN-binding protein 2). We report here a multiply affected kindred with recurrent familial ANE. These affected male siblings (a set of twins and their older brother) all presented with prodromal fever and upper respiratory tract infection that progressed within 72 hours to seizures, coma, and ultimately death, a course that is typical of ANE. It should be noted that 1 brother was treated with early aggressive management, including corticosteroids, and he survived for an additional 5 years. This represents the second reported case of familial ANE in the United States and the only case of male siblings with consanguineous parents. We hope that early recognition and growing awareness can lead to more effective treatment and better outcomes in the future. Pediatrics 2010;125: e693–e698
Keywords: acute, recurrent, necrotizing, encephalopathy, ANE, genetic
Acute necrotizing encephalopathy (ANE) is a rapidly progressive encephalopathy that affects healthy children. In this report we explore the cases of 3 male siblings with ANE. One brother was treated with early aggressive management for elevated intracranial pressure, anti-influenza medication, and immunomodulatory therapy. He survived for an additional 5 years. This represents the second reported case of familial ANE in the United States and the only case of male siblings with consanguineous parents. Early recognition of ANE is essential; the results of a retrospective study suggested that diclofenac sodium, a nonsteroidal anti-inflammatory drug, may increase mortality rates and, conversely, that early steroid treatment may improve outcome.1–3 Furthermore, because this disorder is most often associated with influenza infection, vaccinations in susceptible populations may decrease the prevalence of this devastating illness.4
CASE REPORTS
Case 1
An 11-month-old boy was born at 34 weeks’ gestation because of maternal pregnancy-related hypertension. He, and his 2 later-born siblings, had normal growth and development and were up-to-date on immunizations except the influenza vaccine. The parents are first cousins, once removed.
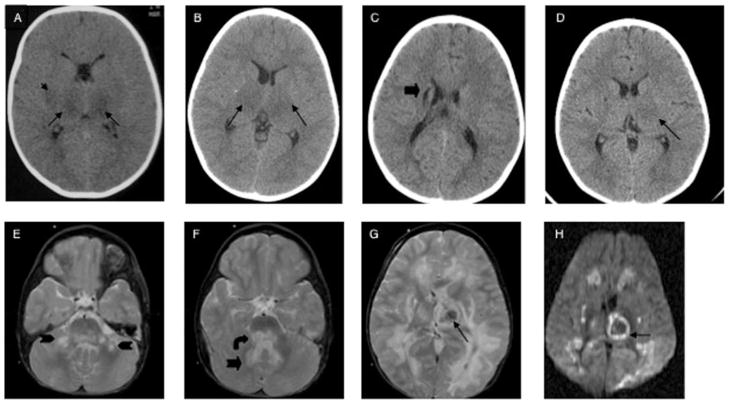
He presented with a low-grade fever, irritability, and emesis. Twenty-four hours after symptom onset, he had a focal seizure with secondary generalization. He was intermittently responsive, with intact cranial nerves, mild left hemiparesis, increased reflexes, and bilateral extensor plantar responses. An intracranial computed tomography (CT) scan demonstrated bilateral symmetrical attenuation of the thalami, putamen, and external capsules (Fig 1). Electroencephalography showed diffuse slow-wave forms without epileptiform activity. A metabolic and infectious workup showed elevation of liver transaminase levels, cerebrospinal fluid (CSF) protein, and glucose (Table 1). Acute care treatment is summarized in Table 2. His neurologic examination showed decorticate posturing and a fixed and dilated left pupil. Intracranial pressure continued to climb despite medical management, and support was withdrawn secondary to brain death on the third day of hospitalization.
FIGURE 1.
A–D, Cranial CT imaging: A, initial CT of patient 1; B, initial CT of patient 2; C, 8-hour CT of patient 3; D, 24-hour CT of patient 3. E–H, Cranial MRI of patient 3, day 7 (E–G, T2-weighted fluid-attenuated inversion-recovery MRI; H, diffusion-weighted MRI). The arrows indicate injury;
 , thalamus;
, thalamus;
 , putamen; ➡ (C), caudate;
, putamen; ➡ (C), caudate;
 , cerebellar nuclei;
, cerebellar nuclei;
 , dorsal brainstem;
, dorsal brainstem;
 (F), white matter.
(F), white matter.
TABLE 1.
Diagnostic Evaluation
| Test | Normal Value | Case 1 | Case 2 | Case 3 |
|---|---|---|---|---|
| Infectious | ||||
| WBCs: blood, 109 per L/CSF, 106 per L | 7/2 | 12/0 | 12/8 | |
| CSF glucose, mg/dL | 40–70 | 161 | 98 | − |
| Influenza A DFA | 3 | − | ||
| Blood, urine, CSF, and stool cultures | − | − | − | |
| CSF HSV and VZV PCR | − | − | ||
| Rabies antibody | − | − | ||
| HHV-6 | 1:20 | |||
| Enterovirus IgM | Increased | |||
| Toxic | ||||
| Lead, iron, and salicylates | − | |||
| Plasma and urine toxin screen | − | |||
| Methemoglobin and carboxyhemoglobin | − | − | − | |
| Carboxyhemoglobin | − | − | ||
| Metabolic | ||||
| Ammonia, μmol/L | <49 | − | 55 | − |
| Plasma ALT, U/L | 12–59 | 205 | − | 67 |
| Plasma AST, U/L | 16–41 | 141 | − | 105 |
| CSF and plasma AAs and urine OAs | − | − | − | |
| VLCFAs | − | − | ||
| Carnitine, total, μmol/L | 31–79 | − | 150 | |
| Acylcarnitine | consistent with ketosis | Mild increased C4 | ||
| Acylglycine | − | − | ||
| Mitochondrial antibody | − | |||
| CK, U/L | 55–380 | − | − | |
| CSF lactate, mmol/L | 1.1–2.8 | − | ||
| Muscle biopsy | ragged red fibers, increased No./size mitochondria | normal | ||
| Mitochondrial point mutations | − | |||
| MCAD | − | |||
| Neurotransmitters: 5-HIAA | 129–520 | 115 | ||
| Homovanillic acid (HVA) | 249–1115 | 209 | ||
| Hematology, rheumatology, and immunology | ||||
| CSF protein, mg/dL | 15–50 | 112 | 80 | − |
| Coagulopathy panel | − | − | − | |
| Flow cytometry | − | |||
| IgE and IgA levels | − | |||
| Genetic | ||||
| Chromosomes | 46XY | |||
− indicates normal results; +, abnormal results; WBCs, white blood cells; DFA, direct immunofluorescence; HSV, herpes simplex virus; VZV, varicella zoster virus; Ig, immunoglobulin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PCR, polymerase chain reaction; HHV-6, human herpesvirus 6; AAs, amino acids; OAs, organic acids; VLCFAs, very long-chain fatty acids; CK, creatinine kinase; MCAD, medium-chain acyl dehydrogenase; 5-HIAA, 5-hydroxyindoleacetic acid test.
TABLE 2.
Acute Care Treatment
| Medication/Treatment | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Antipyretic | Acetaminophen Ibuprofen |
Acetaminophen Ibuprofen |
Acetaminophen Ibuprofen |
| Antibiotic/antiviral | Cephalexin Ceftriaxone Acyclovir Erythromycin |
Ceftriaxone acyclovir | Oseltamivir Azithromycin |
| Anticonvulsant | Lorazepam Fosphenytoin |
Lorazepam Fosphenytoin |
Fosphenytoin |
| Metabolic | − | − | Coenzyme Q10 carnitine Biotin Vitamin B12 Zinc Vitamin C, riboflavin Thiamine |
| Intracranial pressure management | Hyperventilation Mannitol Phenobarbital |
Hyperventilation Mannitol Phenobarbital |
Hyperventilation Mannitol Phenobarbital Saline solution |
| Hemodynamic support | Dopamine | Dopamine Phenylephrine |
Dopamine epinephrine |
| Immunomodulatory | − | − | Dexamethasone Plasmapheresis |
Case 2
The younger sibling of patient 1 was a 23-month-old boy born at 32 weeks’ gestation. The pregnancy was complicated by gestation-induced hypertension and cholestasis. The neonatal course was remarkable for Escherichia coli urinary tract infection. He had grade 3 vesiculoureteric reflux and a pelvic left kidney with duplication of the right collecting system. Cardiac evaluation revealed an atrial septal defect.
After 2 days of rhinorrhea and fever, he presented with a focal seizure. A CT scan showed symmetrical hypodensities of the thalamus (Fig 1). A nasal aspirate was positive for influenza A virus, and he also had an elevation of CSF protein and glucose levels. Over 16 hours, he became unresponsive, lost cranial nerve function, and exhibited decorticate posturing. Care was withdrawn secondary to brain death. His treatments are summarized in Table 2.
Case 3
The 23-month-old monoamniotic, dichorionic male twin sibling of patient 2 had an episode of fever with abdominal pain at 18 months associated with transient ataxia and myoclonic jerks of his lower extremities. This lasted <12 hours, and the patient was then completely well. A screening CT scan, performed when his twin became symptomatic, revealed a remote punctate lesion of the right internal capsule with no acute changes (Fig 1). Twenty hours after presentation, after a clinical seizure, his CT scan showed thalamic hypodensities with more notable injury to the left thalami. Despite aggressive care (Table 2), his clinical and radiographic condition worsened. On day 4, he had no spontaneous eye-opening with withdrawal only to deep painful stimuli. His pupils were minimally responsive, and he had an intact oculovestibular reflex with continuous sucking. He developed decorticate posturing and diffuse increase in tone and reflexes. Laboratory evaluation showed a slight elevation of liver transaminase levels but normal CSF protein and glucose levels.
Over the next 2 weeks, he became more arousable, with spontaneous eye-opening and sleep-wake cycles. He was left with severe residual cortical hearing loss, vision deficits, and a spastic quadriparesis. He was unable to follow commands or construct verbal communication other than crying. Over the next 5 years, he became able to visually fix and follow and to smile in response to voice and touch. He was able to take steps with support. He had multiple gastrointestinal bleeds that generally followed viral illnesses. He continued to have daily to weekly brief seizure activity, and at 8 years of age he presented with 3 episodes of prolonged status epilepticus. Brain imaging after the third episode revealed new areas of injury that involved both gray and white matter regions with marked cerebral edema. He died when aggressive care was discontinued. Genetic testing of patient 3 revealed no mutations in the RAN-binding protein 2 (RANBP2) coding regions.
DISCUSSION
ANE affects healthy children between the ages of 5 months and 11 years. Mortality rates reach 30%, with severe neurologic handicap in 15% of survivors.5 The incidence of ANE is unknown; however, more than 240 cases have been reported from Asia, 5 from North America, and 10 from Europe.6 These cases highlight the genetic contribution to ANE, the possibility of recurrence, and the potential for ameliorating the outcome with early aggressive intervention.
The clinical presentation is nonspecific; however, the combination of acute encephalopathy and seizures with the radiographic findings of symmetric, edematous, necrotic lesions of the bilateral thalami makes the diagnosis of ANE likely.7–9 For a review of ANE pathogenesis and differential diagnosis, see ref 10. A radiograph of Case 3 taken 8 hours after the onset of respiratory symptoms and before the onset of encephalopathy or seizures showed no evidence of new brain injury. There is evidence of an old injury, which highlights the recurrent nature of this family’s disease. In general and for this family, the putamina, cerebrum, cerebellum, and brainstem tegmentum can also be involved. Diffuse brain edema leads to medically refractory elevation of intracranial pressure, and the thalamic lesions undergo hemorrhagic conversion. However, the affected white matter does not appear to have this vascular leakage, and there is a conspicuous absence of inflammatory cells in and around the white matter tracts.
On histologic examination, the central nervous system lesions were in a laminar pattern, with central neurons and glial cells appearing necrotic, and perivascular petechial hemorrhage, peripheral vascular congestion with swelling of oligodendrocytes, an edge with plasma extravasation and myelin pallor, and outside congestion of arteries with evidence of diffuse cerebral edema were seen.11 The brain biopsy from our index case shared these reported features. For patient 2, we report evidence of ragged-red fibers and increased mitochondrial number and size. However, muscle biopsy from patient 3 did not show evidence of mitochondrial stress or proliferation, and the results of a genetic test for known mitochondrial mutations were negative. A previous report suggested that oxidative phosphorylation may become loosely coupled or uncoupled in response to certain inflammatory states.12 The role of the mitochondria in this disorder remains to be further elucidated as we discover the underlying genetic pathways.
ANE may result from a cytokine storm in response to a viral precipitant in susceptible individuals. Cytokines (interleukin 6 and tumor necrosis factor μ) can be markedly elevated in the CSF and serum.13–16 Upper respiratory tract infection or preceding febrile illness has been documented in more than 90% of affected children.5 Influenza A (H3N2) and B are the most commonly identified viral etiologies; however, there have been other etiologies reported, such as human herpes virus 6.13,17,18 Although brainstem lesions appear more commonly in influenza-related cases, documentation of influenza does not seem to predict outcome.19 In our series, patient 2 was documented to have an acute infection with influenza A, but we were unable to determine a pathogen for the upper respiratory infection symptoms in his brothers. This is not surprising, because in the seminal case series, only 24% of cases had documentation of influenza infection.7 All 3 brothers demonstrated clinical signs of respiratory infection.
A predominance of cases are isolated and sporadic, but recurrent and/or familial cases such as ours have been recognized, some with mutations in RANBP2 and others in patients who do not harbor this mutation.12,20–23 This family, which is of eastern European ethnicity, does not have a mutation in the coding region of RANBP2. Although this analysis does not preclude the possibility of an unidentified intronic mutation, the familial consanguinity and lack of other affected family members raises the possibility of an unidentified autosomal recessive ANE locus. Finally, there have been 2 reports of ANE linked to hemophagocytic syndromes, which can be familial and are characterized by elevated cytokine levels.24,25
Treatments for ANE have shown limited efficacy. Vaccinations for influenza may be helpful, because routine vaccination has coincided with an increase in ANE.26 Carnitine, coenzyme Q10, and pyridoxine have been tried to buttress mitochondrial functions. Infectious agents have been targeted with antiviral agents. Methylprednisolone pulses, ulinastatin, high-dose γ-globulin, and plasmapheresis have been used to modulate immune-mediated neurovascular and cell injury.1,27,28 Hypothermia has also been initiated. Use of these interventions in Japan since 2001 has coincided with a decrease in the mortality rate from 30% to 15%.29 A recent report showed that treatment with steroids in the first 24 hours in children without brainstem involvement may improve outcome, whereas the use of diclofenac sodium has been correlated with poor prognosis.3
CONCLUSIONS
ANE presents as a severe neurologic complication after a mild viral illness during which the young child rapidly progresses to having seizures and profound coma. Bilaterally symmetric lesions in the thalamus can point to the diagnosis early in the course of this illness, which allows pediatricians and neurologists to begin intensive supportive treatment and attempt immunomodulatory interventions, antiviral medication, and metabolic supplementation as indicated. Although there has been a suggestion that early intervention may ameliorate clinical outcome, it remains unclear whether early and aggressive therapy can eliminate neurologic sequelae of this disease. It is hoped that genetic discoveries will lead to more targeted and successful treatment options in the future.
Acknowledgments
Dr Neilson’s genetic research is funded by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant K08NS50331.
ABBREVIATIONS
- ANE
acute necrotizing encephalopathy
- CT
computed tomography
- CSF
cerebrospinal fluid
- RANBP2
RAN-binding protein 2
Footnotes
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
References
- 1.Kawashima H, Togashi T, Yamanaka G, et al. Efficacy of plasma exchange and methylprednisolone pulse therapy on influenza-associated encephalopathy. J Infect. 2005;51(2):E53–E56. doi: 10.1016/j.jinf.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Nagao T, Morishima T, Kimura H, et al. Prognostic factors in influenza-associated encephalopathy. Pediatr Infect Dis J. 2008;27(5):384–389. doi: 10.1097/INF.0b013e318162a13b. [DOI] [PubMed] [Google Scholar]
- 3.Okumura A, Mizuguchi M, Kidokoro H, et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009;31(3):221–227. doi: 10.1016/j.braindev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994–2002. Virus Res. 2004;103(1–2):75–78. doi: 10.1016/j.virusres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19(2):81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 6.Mastroyianni SD, Gionnis D, Voudris K, Skardoutsou A, Mizuguchi M. Acute necrotizing encephalopathy of childhood in non-Asian patients: report of three cases and literature review. J Child Neurol. 2006;21(10):872–879. doi: 10.1177/08830738060210101401. [DOI] [PubMed] [Google Scholar]
- 7.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58(5):555–561. doi: 10.1136/jnnp.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuguchi M, Hayashi M, Nakano I, et al. Concentric structure of thalamic lesions in acute necrotizing encephalopathy. Neuroradiology. 2002;44(6):489–493. doi: 10.1007/s00234-002-0773-3. [DOI] [PubMed] [Google Scholar]
- 9.Goo HW, Choi CG, Yoon CH, Ko TS. Acute necrotizing encephalopathy: diffusion MR imaging and localized proton MR spectroscopic findings in two infants. Korean J Radiol. 2003;4(1):61–65. doi: 10.3348/kjr.2003.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl. 2007;186:45–56. [PubMed] [Google Scholar]
- 11.Yagishita A, Nakano I, Ushioda T, Otsuki N, Hasegawa A. Acute encephalopathy with bilateral thalamotegmental involvement in infants and children: imaging and pathology findings. AJNR Am J Neuroradiol. 1995;16(3):439–447. [PMC free article] [PubMed] [Google Scholar]
- 12.Neilson DE, Eiben RM, Waniewski S, et al. Autosomal dominant acute necrotizing encephalopathy. Neurology. 2003;61(2):226–230. doi: 10.1212/01.wnl.0000073544.28775.1a. [DOI] [PubMed] [Google Scholar]
- 13.Ito Y, Ichiyama T, Kimura H, et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58(4):420–425. doi: 10.1002/(sici)1096-9071(199908)58:4<420::aid-jmv16>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Ichiyama T, Isumi H, Ozawa H, Matsubara T, Morishima T, Furukawa S. Cerebrospinal fluid and serum levels of cytokines and soluble tumor necrosis factor receptor in influenza virus-associated encephalopathy. Scand J Infect Dis. 2003;35(1):59–61. doi: 10.1080/0036554021000026986. [DOI] [PubMed] [Google Scholar]
- 15.Kawada J, Kimura H, Ito Y, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infect Dis. 2003;188(5):690–698. doi: 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 16.Ichiyama T, Morishima T, Isumi H, Matsufuji H, Matsubara T, Furukawa S. Analysis of cytokine levels and NF-κB activation in peripheral blood mononuclear cells in influenza virus-associated encephalopathy. Cytokine. 2004;27(1):31–37. doi: 10.1016/j.cyto.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Sugaya N. Influenza-associated encephalopathy in Japan. Semin Pediatr Infect Dis. 2002;13(2):79–84. doi: 10.1053/spid.2002.122993. [DOI] [PubMed] [Google Scholar]
- 18.Skelton BW, Hollingshead MC, Sledd AT, Phillips CD, Castillo M. Acute necrotizing encephalopathy of childhood: typical findings in an atypical disease. Pediatr Radiol. 2008;38(7):810–813. doi: 10.1007/s00247-008-0823-z. [DOI] [PubMed] [Google Scholar]
- 19.Okumura A, Abe S, Kidokoro H, Mizuguchi M. Acute necrotizing encephalopathy: a comparison between influenza and non-influenza cases. Microbiol Immunol. 2009;53(5):277–280. doi: 10.1111/j.1348-0421.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 20.Neilson DE, Feiler HS, Wilhelmsen KC, et al. Autosomal dominant acute necrotizing encephalopathy maps to 2q12.1–2q13. Ann Neurol. 2004;55(2):291–294. doi: 10.1002/ana.10849. [DOI] [PubMed] [Google Scholar]
- 21.Neilson DE, Adams MD, Orr CM, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84(1):44–51. doi: 10.1016/j.ajhg.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gika AD, Rich P, Gupta S, Neilson DE, Clarke A. Recurrent acute necrotizing encephalopathy following influenza A in a genetically predisposed family. Dev Med Child Neurol. 2009 doi: 10.1111/j.1469-8749.2009.03405.x. In press. [DOI] [PubMed] [Google Scholar]
- 23.López-Laso E, Mateos-González ME, Pérez-Navero JL, Camino-León R, Briones P, Neilson DE. Infection-triggered familial or recurrent acute necrotizing encephalopathy [in Spanish] An Pediatr (Barc) 2009;71(3):235–239. doi: 10.1016/j.anpedi.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Akiyoshi K, Hamada Y, Yamada H, Kojo M, Izumi T. Acute necrotizing encephalopathy associated with hemophagocytic syndrome. Pediatr Neurol. 2006;34(4):315–318. doi: 10.1016/j.pediatrneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Astigarraga I, Prats JM, Navajas A, Fernández-Teijeiro A, Urberuaga A. Near fatal cerebellar swelling in familial hemophagocytic lymphohistiocytosis. Pediatr Neurol. 2004;30(5):361–364. doi: 10.1016/j.pediatrneurol.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Grose C. The puzzling picture of acute necrotizing encephalopathy after influenza A and B virus infection in young children. Pediatr Infect Dis J. 2004;23(3):253–254. doi: 10.1097/01.inf.0000114901.70040.33. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai T, Kimura A, Tanaka Y, Hozumi I, Ogura S, Inuzuka T. Case of adult influenza type A virus-associated encephalopathy successfully treated with primary multidisciplinary treatments[in Japanese] Rinsho Shinkeigaku. 2007;47(10):639–643. [PubMed] [Google Scholar]
- 28.Munakata M, Kato R, Yokoyama H, et al. Combined therapy with hypothermia and anticytokine agents in influenza A encephalopathy. Brain Dev. 2000;22(6):373–377. doi: 10.1016/s0387-7604(00)00169-8. [DOI] [PubMed] [Google Scholar]
- 29.Morishima T. Treatment of influenza-associated encephalopathy [in Japanese] Nippon Rinsho. 2003;61(11):2006–2012. [PubMed] [Google Scholar]