Abstract
Background
In developing countries, 8 to 71% of patients initiating highly active antiretroviral therapy (HAART) die within the first year of treatment. Apart from baseline CD4 count, viral load, hemoglobin, BMI and stage of the disease, there may be other variables that contribute to AIDS-related mortality. We investigated the potential role of nutrition, lipids and insulin resistance-related phenotypes in predicting early mortality.
Methods
We recruited 210 HAART-naïve HIV/AIDS patients in Lusaka, Zambia. Dietary intake, anthropometric measurements, fasting serum insulin, glucose, and lipid profiles were assessed at baseline. Mortality was assessed after 90 days of follow-up. We used logistic regression models to identify variables associated with mortality.
Results
The mean±SD for age, BMI and CD4 count at baseline were 34±7.4 y, 20±3 kg/m2 and 138±52 cells/μL, respectively. Sixteen patients (7.6%) died during follow-up. Triglyceride concentrations were associated with increased mortality [odds ratio (OR) for 1 mmol/L increase in triglyceride concentration=2.51; 95% CI: 1.34-4.71]. This association remained significant (OR=3.24; 95% CI: 1.51-6.95) after adjusting for age, gender, smoking, alcohol use, total cholesterol, BMI, CD4 count and n3 fatty acid intake. Apart from higher n3 fat intake which was inversely associated with mortality (survivors: 1.81±0.99% total energy/day vs. non-survivors 1.28±0.66% energy/day, P=0.04), there were no other macronutrients associated with mortality.
Conclusion
Triglyceride concentrations at the time of initiating HAART are independently associated with increased risk for early mortality. If this association is confirmed in larger studies, assessment of triglycerides could become part of routine care of HIV patients initiating HAART in developing countries.
Keywords: Triglycerides, lipids, mortality, HIV, HAART, Africa, Zambia
Background
In Zambia and other developing countries, particularly in Sub-Saharan Africa, about 8 to 71% of patients who start use of Highly Active Anti-retroviral Therapy (HAART) die within the first year of treatment1-6. Prospective studies aimed at identifying patients at high risk of early mortality have focused on WHO clinical staging of the disease at baseline7, 8, gender, hemoglobin level, CD4 cell count, viral load, body mass index9-12 and immune reconstitution inflammatory syndrome (IRIS)13. Lower baseline CD4 count and body mass index (BMI) have emerged as strong predictors of increased mortality among HIV patients on HAART in less-affluent countries14 The known predictors of disease progression do not explain in totality the observed high early mortality in developing compared to developed countries1. Little is known about the role of malnutrition and dyslipidemia on disease progression and possibly mortality among patients newly initiated on HAART in less affluent societies where use of lipid-lowering drugs is not common.
Conditions such as chronic or recurrent diarrhea, opportunistic gastrointestinal infections, malabsorption disorders and depression15, known to play a role in macro- and micronutrient deficiencies in HIV/AIDS infection, may play an important additive role in the decline of immunologic function. These condition may contribute to early mortality in patients initiating HAART16. In addition to nutritional deficiencies or excesses, metabolic derangements like dyslipidemia and insulin resistance, common in chronic conditions like diabetes, hypertension and HIV with or without HAART, could potentially contribute to early mortality17-19 Metabolic complications like lipodystrophy and dyslipidemia in HIV disease have been ascribed in part to factors such as genetics, treatment with protease inhibitors (PI) and nucleoside reverse transcriptase inhibitors (nRTIs)20,22, the refeeding paradox23 , hypogonadism24, male gender and increasing age25.
Furthermore, whole body protein turnover (the rate at which lean body mass is destroyed and replaced) in HIV infection is elevated by about 16-18% and up to 24% in people with Acquired Immunodeficiency Syndrome (AIDS)23. This change in protein metabolism could be further compounded by what has been recently described as the “anabolic block”-wherein new nutritional intake is preferentially shunted to fat synthesis at the detriment of lean body mass, lost during the early phase of infection26. A better understanding of the role of metabolic derangements in early mortality in patients initiated on HAART is important for identifying potential interventions as well as additional clinical measurements that can be used to improve the care of HIV patients, especially those at high risk for early mortality. In this pilot study, we sought to identify nutritional and metabolic markers that are associated with early mortality in HAART-naïve patients who initiate HAART in Lusaka, Zambia.
SUBJECTS AND METHODS
Study participants
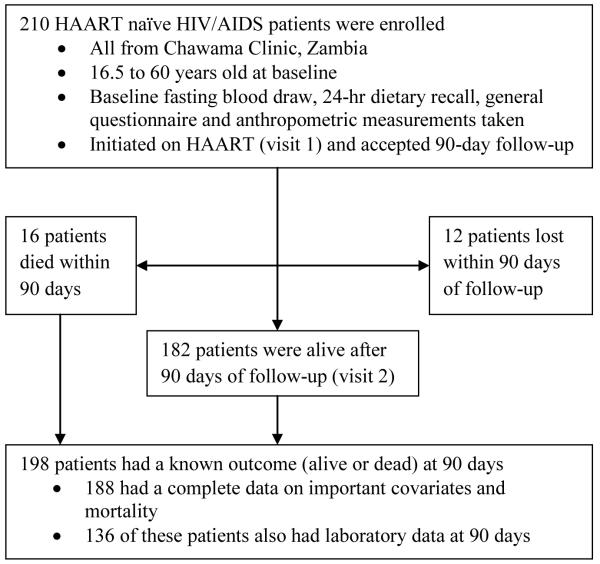
Over a one year period we recruited 210 HAART naïve HIV/AIDS patients from Chawama Clinic in Lusaka, Zambia (Figure 1). Chawama Clinic is a government-run, primary care facility providing HIV care and treatment services, supported by the University of Alabama at Birmingham and the Centre for Infectious Disease Research in Zambia5, 27. All men and women who were HAART-naïve, aged 16.5-60.0 years and who qualified to be initiated on HAART at Chawama Clinic were invited to participate in this study. All eligible patients who started treatment between January and December 2007 and consented to participate were enrolled in the study, which we named DGPLEAD (Diet, Genetic Polymorphisms in Lipid-Metabolizing Enzyme genes, and Antiretroviral Therapy-Related Dyslipidemia). The main goal of DGPLEAD was to generate preliminary data on the role of diet, genetic polymorphisms and metabolic parameters in predicting dyslipidemia and mortality among patients starting HAART in resource-limited settings. Indication for treatment and the regimen employed were determined by a clinical officer in accordance with Zambia HIV National Guidelines28. The recommended treatment regimens consisted of two nucleoside reverse transcriptase inhibitors (NRTI) e.g., zidovudine and lamivudine or stavudine and lamivudine and one non-nucleoside reverse transcriptase inhibitor (nNRTI) e.g., efavirenz or nevirapine. We actively traced patients throughout the study in order to enhance adherence. None of our patients reported use of any lipid-lowering medications. This study was approved by the Human Subjects use in Research Institutional Review Board at the University of Alabama at Birmingham (UAB) and the Research Ethics Committee at the University of Zambia.
Figure 1.
Participant flow in the DGPLEAD cohort of HIV/AIDS patients starting HAART in Lusaka, Zambia (January to December, 2007).
Data collection
At the initial patient visit, standardized questionnaires were used to collect data on smoking, physical activity, alcohol consumption and dietary intake using a 24-hour dietary recall method. Nutrient intakes were then computed using Nutrition Data System for Research (NDSR®) software Zambian foods not in the database were substituted with similar foods in the database using the following recommended guidelines: for 100 g of product, the tolerance guidelines were 85 kcal for calories, 5 g for protein, 2.5 g total fat, 10 g carbohydrate and 100 mg sodium. Zambian food composition data was used for comparison. We also added recipes of frequently consumed foods to the database. This information was gathered from preliminary focus group meetings.
Anthropometric data, including weight, height, waist, hip and mid-upper arm circumferences were measured twice in the clinic by trained staff and an average of the two measurements was recorded. A fasting specimen of blood was drawn from each patient for the measurement of plasma concentrations of total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), triglycerides, insulin and glucose at baseline and then at 3 months after initiating HAART. A buffy coat for DNA extraction was collected at the first visit. Patients were then followed and changes in their anthropometric measures, metabolic profile and vital status, assessed at 90 days, the period within which most of the early mortality (70%) occurs following initiation of antiretroviral therapy in developing countries29.
Laboratory assays
Roche Cobas Integra 400+ (Roche Diagnostics, Indianapolis, IN, USA) auto analyzer was used to measure lipids and glucose. Triglycerides, LDL and total cholesterol concentrations were measured using an enzymatic colorimetric assay, while HDL cholesterol was measured with a homogeneous enzymatic colorimetric assay. Glucose was measured using hexokinase enzyme method. Insulin was measured by chemiluminescence on a Roche Elecsys 2010 autoanalyzer(Roche Diagnostics, Indianapolis, IN, USA).
Statistical analysis
Data were analyzed using SAS software version 9.2 (SAS Institute Inc., Cary NC, USA). Due to inadequate funding, active follow-up was terminated early for patients recruited late in the study. However, patients continued to obtain routine care from the same clinic. Except for 12 patients who were lost to follow-up, the vital status of all patients was recorded on or around their expected 90 day visit regardless of whether a second blood sample was obtained.
For the main analyses on the relation between baseline triglyceride concentrations and early mortality we had 188 patients with complete data on mortality and major potential confounders namely age, sex, BMI, smoking, alcohol use, CD4 counts, total cholesterol concentrations, total energy, n3 fatty acid intake, total fat, saturated fat, monounsaturated fat, polyunsaturated fat, trans fat, protein, sugars and total energy.
Macronutrients, namely carbohydrates, protein, total fat as well as fat subtypes e.g., saturated fat, trans fats, monounsaturated fats, polyunsaturated fats and total n3 fatty acids, were all expressed as a percentage of total energy before use in the analyses. All continuous variables were checked for normality and log- or square-root transformed as appropriate, before hypothesis tests.
To compare survivors and non-survivors at 90 days of follow-up on key variables, we used the unpaired, two-tailed Student’s t-test for continuous variables and the chi-squared test for categorical variables. Variables found to be significantly associated with mortality from univariate analyses were checked for multicollinearity, using linear regression and variance inflation factor (VIF) for continuous and ordinal variables. The Chi-square test was used for categorical variables. Using mortality as an outcome variable, we progressively introduced non-correlated variables into multivariable logistic regression models with the baseline values of the following variables: age, sex, CD4 count and body mass index and obtained adjusted odd ratios for mortality at 90-days. Other variables were added sequentially as described in the results section. Except for models involving HDL and insulin, which had some missing values, all multivariate models had a fixed sample size of 188 patients which included 16 deaths.
RESULTS
Of 210 patients enrolled at baseline (visit 1), vital status at visit 2 was determined for 198 patients, as 12 patients were lost to follow-up (Figure 1). Of the 198 patients with mortality data at 90 days, 136 also had laboratory measurements at visit 2. Laboratory data at visit 2 could not be obtained for the remainder of the patients.
Anthropometric measures, lipid profiles and dietary characteristics of the study participants by outcome status at 90 days are presented in Table 1. At the end of 90 days, 16 patients had died, representing a 90-day cumulative all-cause mortality of 7.6% (95% CI: 6.6 to 8.6%). The mean age of those who survived (34 ± 7.4 years) was not different from that of those who died (36.7 ± 7.5 years; P = 0.20), and the gender distribution of the participants did not differ by survival status (54.1% women among survivors vs. 43.8% women among non-survivors, P = 0.44). Although the baseline mean CD4 cell counts of survivors (140 ± 53 cells/μl) was higher than that of patients who died (125 ± 47cells/μl), this difference was not statistically significant (P = 0.30). The markers of nutritional status, BMI and mid-upper arm circumference (MUAC) were significantly higher among survivors compared to non-survivors (P <0.05). Sixty-five (31%) patients had a BMI ≤18.5 kg/m2 at the time of initiating HAART.
TABLE 1.
Baseline characteristics of HIV/AIDS patients initiating HAART by mortality status, Zambia1
| Variable | Total (n) |
Alive (n = 182) |
Dead (n = 16) |
P |
|---|---|---|---|---|
| Age, y | 194 | 34.0 ± 7.4 | 36.6 ± 7.5 | 0.20 |
| Gender, women, n (%) | 197 | 98 (54.1) | 7 (43.8) | 0.44 |
| BMI, kg/m2 | 197 | 20.1 ± 2.7 | 18.7 ± 2.0 | 0.04 |
| Waist circumference, cm | 198 | 72.7 ± 6.5 | 71.0 ± 5.4 | 0.31 |
| Mid-upper arm circumference, cm | 198 | 25.0 ± 2.6 | 23.3 ± 3.1 | 0.02 |
| Current smokers, n (%) | 195 | 10 (5.6) | 1 (6.3) | 1.00 |
| Current alcohol use, n (%) | 195 | 21 (11.7) | 1 (6.3) | 1.00 |
| CD4 count, cells/μl | 197 | 140 ± 53 | 125 ± 47 | 0.30 |
| Fasting plasma insulin, mU/L | 163 | 4.6 ± 5.9 | 4.9 ± 5.1 | 0.87 |
| Fasting blood glucose, mmol/L | 193 | 3.8 ± 1.1 | 3.9 ± 1.0 | 0.86 |
| Total cholesterol, mmol/L | 196 | 3.5 ± 0.9 | 3.2 ± 0.9 | 0.29 |
| Triglyceride, mmol/L | 196 | 1.20 ± 0.6 | 1.7 ± 0.8 | 0.002 |
| LDL-cholesterol, mmol/L | 196 | 2.0 ± 0.8 | 1.5 ± 0.8 | 0.004 |
| HDL-cholesterol, mmol/L | 189 | 0.84 ± 0.5 | 0.48 ± 0.4 | 0.0004 |
| Total cholesterol : HDL-C ratio | 188 | 10.1 ± 39.3 | 25.6 ± 38.7 | 0.0001 |
| Dietary variables | ||||
| Total energy, kcal/d | 197 | 1759 ± 672 | 1413 ± 681 | 0.05 |
| Carbohydrate, % energy/d | 197 | 55.8 ± 13.5 | 61.3 ± 11.2 | 0.12 |
| Protein, % energy/d | 197 | 13.2 ± 4.2 | 11.5 ± 3.7 | 0.11 |
| Total fat, % energy/d | 197 | 31.6 ± 11.3 | 28.2 ± 10.3 | 0.24 |
| Saturated fat, % energy/d | 197 | 6.4 ± 2.9 | 5.8 ± 2.4 | 0.43 |
| Monounsaturated fat, % energy/d | 197 | 9.1 ± 3.8 | 8.6 ± 3.6 | 0.59 |
| Polyunsaturated fat, , % energy/d | 197 | 13.8 ± 6.1 | 11.3 ± 4.9 | 0.12 |
| Trans fat, % energy/d | 197 | 0.53 ± 0.75 | 0.36 ± 0.36 | 0.72 |
| n3 Fatty acids, % energy/d | 197 | 1.81 ± 0.99 | 1.28 ± 0.66 | 0.04 |
| HAART regimen (n, %) | ||||
| Zidovudine + Lamivudine + Nevirapine | 79 | 5 (38.5) | 77 (43.0) | 0.93 |
| Stavudine + Lamivudine + Nevirapine | 60 | 5 (38.5) | 55 (32.0) | |
| Tenofovir + Emtricitabine + Nevirapine | 48 | 3 (23.1) | 43 (25.0) |
Values are means ± SD or percentages; HAART, highly active antiretroviral therapy
Compared to survivors, those who died had significantly lower HDL-cholesterol (P = 0.0004) and LDL-cholesterol (P = 0.04) but significantly higher (P = 0.002) mean triglyceride concentrations (Table 1). The baseline total cholesterol to HDL-cholesterol ratio was significantly higher among those who died compared to survivors (P = 0.0001) and the absolute values for this ratio were quite high, driven mainly by the very low HDL-cholesterol concentrations at baseline. Seventeen percent of the patients had baseline triglyceride concentrations greater than 150 mg/dL, the National Cholesterol Education Program cut-off point for hypertriglyceridemia30. A large number of patients (74%) had HDL-concentrations below 40 mg/dL at baseline and 4% had LDL-cholesterol concentrations above 130 mg/dL (data not shown in tables).
Except for dietary n3 fatty acid intake, which was significantly higher among survivors compared to those non-survivors (P = 0.01), all other macronutrients examined were not significantly associated with all-cause mortality (Table 1).
The distribution of treatment regimens was similar among those who survived and those who died. The most commonly used regimen was the combination: Zidovudine + Lamivudine + Nevirapine, which was used by 42% of patients.
The distribution of potential confounders by tertiles of triglyceride concentrations is shown in Table 2. Apart from HDL-cholesterol which was inversely and significantly associated with triglyceride concentrations (P < 0.0001), and the total cholesterol:HDL-cholesterol ratio which was positively associated with triglyceride concentrations (P < 0.0001), there were no other variables that were significantly associated with triglyceride concentrations.
TABLE 2.
Characteristics of the study participants by tertiles of fasting triglyceride concentrations1
| Tertiles of plasma triglyceride levels |
||||
|---|---|---|---|---|
| 1 (Low) | 2 | 3 (High) | P | |
| n | 68 | 70 | 70 | |
| Age, y | 33.2 ± 7.5 | 34.6 ± 7.8 | 34.3 ± 7.0 | 0.46 |
| Gender, % women | 47 | 60 | 56 | 0.31 |
| BMI, kg/m2 | 20.00 ± 2.69 | 20.39 ± 2.74 | 19.74 ± 2.53 | 0.33 |
| Waist circumference, cm | 71.92 ± 6.02 | 73.05 ± 7.10 | 72.66 ± 5.98 | 0.70 |
| Mid-upper arm circumference, cm | 24.89 ± 2.72 | 25.40 ± 2.70 | 24.25 ± 2.54 | 0.06 |
| Current smokers, n (%) | 2(3.1) | 5 (7.1) | 4 (6.1) | 0.63 |
| Current alcohol use, n (%) | 8 (12.3) | 11 (15.7) | 3 (4.6) | 0.09 |
| Fasting plasma insulin, mU/L | 3.81 ± 3.37 | 4.62 ± 6.42 | 5.84 ± 6.94 | 0.30 |
| Fasting blood glucose, mmol/L | 3.67 ± 0.70 | 3.73 ± 0.61 | 3.98 ± 1.61 | 0.25 |
| CD4 count, cells/μl | 131 ±48 | 152 ± 59 | 130 ± 47 | 0.08 |
| Triglycerides, mmol/L | 0.74 ±0.15 | 1.08 ±0.11 | 1.88±0.62 | N/A |
| Total cholesterol, mmol/L | 3.24 ±0.90 | 3.48 ± 0.90 | 3.63 ± 0.95 | 0.06 |
| LDL-cholesterol, mmol/L | 1.98 ±0.58 | 2.10 ± 0.78 | 1.90 ± 0.89 | 0.18 |
| HDL-cholesterol, mmol/L | 0.95 ±0.47 | 0.89 ± 0.52 | 0.58 ± 0.35 | <0.0001 |
| Total cholesterol : HDL ratio | 4.82 ± 4.89 | 5.79 ± 5.75 | 22.74 ± 63.37 | <0.0001 |
| Dietary variables | ||||
| Total energy, kJ/d | 7713 ± 2642 | 7266 ± 3194 | 6947 ± 2870 | 0.18 |
| Carbohydrate, % energy/d | 56.59 ± 13.24 | 53.84 ±13.56 | 57.87 ± 13.02 | 0.32 |
| Protein, % energy/d | 13.28 ± 3.94 | 13.60 ± 4.34 | 12.53 ± 4.13 | 0.19 |
| Total fat, % energy/d | 30.51 ± 11.12 | 33.14 ±11.78 | 30.78 ± 11.01 | 0.66 |
| Saturated fat, % energy/d | 6.41 ± 2.53 | 6.54 ±3.12 | 5.99 ± 2.42 | 0.51 |
| Monounsaturated fat, % energy/d | 9.03 ± 3.93 | 9.47 ±3.57 | 8.74 ± 3.64 | 0.66 |
| Polyunsaturated fat, , % energy/d | 12.77 ± 5.78 | 14.63 ± 6.65 | 13.81 ± 5.77 | 0.35 |
| Trans fat, % energy/d | 0.56 ± 0.71 | 0.46 ± 0.68 | 0.48 ± 0.77 | 0.25 |
| n3 Fatty acids, % energy/d | 1.74 ± 0.98 | 1.86 ± 1.03 | 1.75 ± 0.94 | 0.80 |
| Common HAART regimen, n (%) | ||||
| Zidovudine,+Lamivudine+ Nevirapine | 30 (48.4) | 26 (39.4) | 23 (37.1) | 0.17 |
| Stavudine+Lamivudine+ Nevirapine | 14 (22.6) | 22 (33.3) | 27 (43.6) | |
| Tenofovir+Emtricitabine+Nevirapine | 18 (29.0) | 18 (27.3) | 12 (19.4) | |
Values are means ± SD or percentages.
Table 3 shows results from univariate and multivariate adjusted analyses of the association between baseline fasting triglycerides and all-cause mortality. Higher triglyceride concentrations were strongly associated with increased mortality [odds ratio (OR) for 1 mmol/L increase in triglyceride concentration = 2.51; 95% CI: 1.34-4.71]. This association became stronger (OR = 3.24; 95% CI: 1.51-6.95) after adjusting for age, sex, smoking, alcohol use, total cholesterol, BMI, CD4 count and n3 fatty acid intake. The relation between triglyceride and all-cause mortality remained significant after adjusting for insulin, glucose or other dietary macronutrients (data not shown). Apart from higher n3 fatty acid intake, which was inversely associated with all-cause mortality, there were no other significant associations detected in multivariable models. When total cholesterol was replaced by HDL and LDL in the models, neither HDL nor LDL was found to be significantly associated with mortality. In these analyses, the association between triglycerides and mortality remained significant (OR for 1 mmol/L increase in triglycerides = 2.67; 95% CI: 1.03-6.91). All models were adequate as shown by the Hosmer-Lemeshow test for goodness-of-fit P>0.05.
TABLE 3.
Adjusted odds ratios (95% CI) for the association between baseline fasting triglyceride concentrations and all-cause mortality in patients on HAART in Lusaka, Zambia1,2
| Model | Adjusted OR | 95% CI | |
|---|---|---|---|
| M1 | Triglyceride | 2.51 | 1.34 - 4.71 |
| M2 | M1 + Age + Sex + CD4 count + BMI | 2.63 | 1.33-5.20 |
| M3 | M2 + Total cholesterol | 2.90 | 1.43-5.88 |
| M4 | M3 + Smoking + Alcohol use | 3.03 | 1.44-6.36 |
| M53 | M4 + % energy from n3 fatty acids | 3.24 | 1.51-6.95 |
The total sample size for all models was 188 which includes 16 patients who died.
Hosmer-Lemeshow statistic for goodness-of-fit P>0.05 for all models.
When total cholesterol was replaced by HDL and LDL-cholesterol in model 5, the sample size reduced to 178 due to missing values in HDL. However, the association between triglycerides and mortality remained significant (OR for 1 mmol/L increase in triglycerides = 2.67; 95% CI: 1.03-6.91). Neither HDL- nor LDL-cholesterol was significantly associated with mortality (P>0.05).
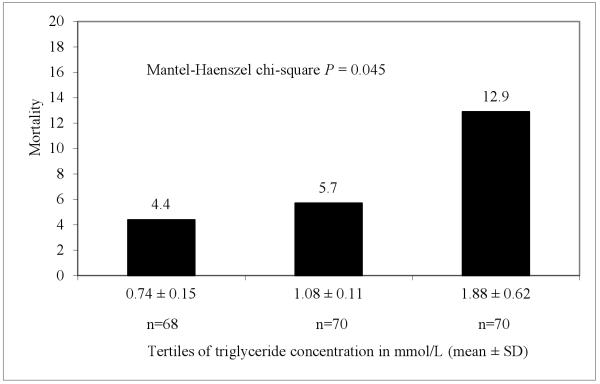
In additional analyses, we investigated the distribution of mortality by tertiles of triglyceride concentrations so as to explore existence of a dose-response relationship (Fig. 2). The number of deaths (mortality rate) in the first, second and third tertile of triglyceride concentrations was 3 (4.4%), 4 (5.7%) and 9 (12.9%), respectively. Compared to the lower tertile, patients with triglycerides concentrations in the upper tertile had a 3-fold incresase in mortality.
Figure 2.
Absolute mortality at 90 days of follow-up by tertiles of triglyceride concentrations. To convert mmol/L to mg/dL divide by 0.0113.
DISCUSSION
From this study, we have demonstrated that higher baseline fasting triglyceride concentrations are significantly associated with increased risk for early mortality among treatment-naïve adult HIV/AIDS patients starting HAART in Zambia. The 90-day all-cause mortality rate of 7.6% found is this study is lower than rates reported from most other developing nations3,6,12; however it still remains 3 times higher than rates reported for developed countries1. Prior to the HAART era, similar findings were reported in a few observational studies31-33.
While, only 17% of the patients had baseline triglyceride concentrations greater than the National Cholesterol Education Program cut-point for hypertriglyceridemia (150 mg/dL), a large number of patients (74%) had HDL-concentrations below 40 mg/dL at baseline. Very few patients (4%) had LDL-cholesterol concentrations above 130 mg/dL. The observed distribution of lipids is consistent with the lipid pattern described among HIV-infected individuals before the widespread use of HAART (1996 pre-HAART pattern). The hallmark of this pre-HAART pattern is hypertriglyceridemia, low HDL- and low LDL-cholesterol concentrations (mostly small dense particles)34, while the hallmark of the post-HAART pattern is increased total cholesterol and LDL-cholesterol levels and small changes in HDL-cholesterol35-37 Previous studies have explicated the underpinning mechanisms as increased hepatic de novo synthesis of fatty acids leading to increased release of very low density lipoprotein (VLDL)-cholesterol, with decreased clearance of triglyceride-rich lipid fractions or infection-induced lipolysis and increased release of fatty acids for hepatic re-esterification 33, 38. These mechanisms are supported by earlier studies that have implicated cytokines (e.g. interferon-α (IFN-α) and tumor necrosis factor (TNF)-alpha/beta, secreted in response to HIV infection) as inducers of hypertriglyceridemia33, 39 This is not surprising given the reported suppression of lipoprotein lipase activity by inflammatory markers such as IL1, IL2, IL6 and TNF40. The lipid profiles in our study patients are similar to pre-HAART lipid profiles previously reported in developed countries34.
The main finding of this pilot study is the independent positive association between fasting triglyceride concentrations and early mortality in patients initiating HAART in Zambia. Given that hypertriglyceridemia is a component of the metabolic syndrome and a known risk factor for coronary heart disease (CHD) and the improved survival of patients due to wider use of HAART, dyslipidemia due to HIV-infection by itself or HAART could become a common problem in developing countries like Zambia. This could result in an increase in cardiovascular disease burden in affected countries.
The finding of a positive relationship between total cholesterol:HDL-choleterol ratio and early all-cause mortality (P < 0.0001) is of interest given that this ratio is strongly predictive of cardiovascular disease outcomes41, 42. The total cholesterol:HDL-cholesterol ratio is the mathematical equivalent of non-HDL-cholesterol level, currently considered the sum total of cholesterol carried by the lipoproteins that are atherogenic. This is because a high serum triglyceride level (>2.3 mmol/L) occurring in 4.8% (10/210) of our patients could be accounted for by non-HDL-cholesterol sources of triglycerides like VLDL, chylomicrons and remnant lipoproteins. Furthermore, low mean levels of HDL-cholesterol, in and of itself an independent cardiovascular risk factor, half as low in survivors (P = 0.01), makes for high total cholesterol:HDL-C ratios (TC:HDL); three times greater in non-survivors. This ratio has been shown from prior studies to have greater coronary heart disease predictive value than increased LDL-cholesterol alone41, 42. As shown in Table 1, the higher TC:HDL ratio observed among non-survivors is mainly due to lower HDL in this group and more advanced disease and poorer nutritional status compared to survivors. Similarly, the significantly low mean LDL-cholesterol among non-survivors compared to survivors is of interest and may be related to the poorer nutritional status of non-survivors as manifest by a lower mean upper arm circumference in this group of participants (P = 0.02).
Another significant finding is the inverse association between intake of n3 fatty acids and mortality. Previous studies have shown that intake of n3 fatty acids lowers triglyceride concentrations43, 44 and thus beneficial action is expected among individuals with hypertriglyceridemia. Our analyses show that triglyceride concentrations remained significantly associated with mortality even after adjusting for intake of n3 fatty acids. In the same multivariable model (M5, Table 3), intake of n3 fatty acids showed an independent and significant inverse association with all-cause mortality, suggesting additional benefits on all-cause mortality that are not via lowering of triglycerides. However, these data are to be interpreted with caution given that dietary intakes were estimated from a 24-hr dietary recall which may not be a good estimator of habitual diet. Our future studies will use stored serum to examine fatty acid profiles and their relation with all-cause mortality.
We examined immunologic status as measured by CD4 count, as a risk factor for mortality in this study since this variable has been shown to be inversely associated with mortality in several studies10, 11, 45. Although the mean CD4 counts of survivors (140 ± 53 cells/μL) and non-survivors (125 ± 47 cells/μL) were not significantly different (P>0.05), the counts are below the cut-off of 200 cells/μL, indicative of advanced immunosuppression. This finding may indirectly indicate late presentation to seek care by HIV-infected patients or barriers to accessing HAART that could in turn be contributory to early mortality. Unfortunately, we did not have additional measures of immunosuppression such as HIV viral load so as to explore the relation between immunosuppression and all-cause mortality.
Although we did not assess the HIV viral subtype distribution in this study population, HIV subtype C is the most prevalent in Zambia46, 47 and, has been shown by Fourie CM et al to be responsible for dyslipidemia of a pattern similar to pre-HAART, among treatment naive patients48. However, unlike subtype D, HIV subtype C infection has not been shown to be independently associated with a significant increase in risk of disease progression and mortality49, 50.
It is important to interpret the findings from this pilot study with caution, given the small sample size and limited number of deaths (16 deaths). Though fasting triglycerides levels were determined for the study, it is worth noting that triglyceride concentrations can be affected by many factors including duration of fasting, alcohol consumption, vitamin supplementation, use of antibiotics and a patient’s hydration status. However all patients in the study reported fasting and non-use of lipid-lowering medications. We further adjusted for variables such as smoking and alcohol use -- approaches that we expect to have improved our assessment of the association between triglyceride concentrations and mortality. The small sample size precluded additional analyses in which we would use multivariable models with multiple categories of triglyceride concentrations to adequately assess the potential dose-response relationship between triglyceride concentrations and all-cause mortality. Nonetheless, we performed a univariable analysis with triglyceride concentrations distributed into tertiles and determined whether there is a linear relation between triglycerides and mortality (Figure 2). This analysis disclosed a significant monotonic association between increasing triglyceride concentrations and higher all-cause mortality (P = 0.045).
Furthermore, we had only two visits 90 days apart that were scheduled a priori. Thus, if a patient missed the second visit, assigning follow-up time as the middle of the interval between the two visits could grossly bias the estimate of time-to-event and therefore the estimate of hazard ratios. This limitation, precluded use of Cox-regression approaches to estimate incidence densities and hazard ratios. The odds ratios estimated from multivariable logistic regression models and reported in this paper are robust for assessing the association between baseline triglyceride concentrations and mortality after 90 days of follow-up.
The role of specific opportunistic infections in determining our study results cannot be determined effectively because screening for opportunistic infections was not routinely done in this setting with limited resources, except on grounds of clinical suspicion or radiographic signs of diseases like tuberculosis. Furthermore, data on other clinical parameters such as HIV viral load or specific causes of death were not available for this pilot study so as to assess the relation between triglycerides and cause-specific mortality. Since HIV viral load is inversely correlated with CD4 counts we believe that by adjusting our models for CD4 counts we captured some of the variance explained by the viral load. Another limitation is lack of data on the prevalence of immune reconstitution syndrome which may increase the risk for early mortality, especially given the low mean CD4 count in our study patients at initiation of HAART. Finally, dietary intake was assessed by a single 24-hour dietary recall. Thus, associations between diet and mortality in this study must be interpreted with caution until they are replicated in other studies with more comprehensive dietary assessment such as use of biomarkers. None-the-less, the results from the current pilot study are of importance in paving way for more studies on dyslipidemia and early mortality in patients initiating HAART in developing countries.
Conclusion
Elevated fasting plasma triglyceride concentrations at baseline are associated with increased early mortality in HIV/AIDS patients who start HAART. This is a new finding that will need to be replicated in a sufficiently powered prospective study. If confirmed, lipid measurements at baseline which are currently not part of routine care in a number of developing countries could become an important parameter to predict survival at the time of initiating HAART. A large scale, primary prospective investigation is warranted to better understand the role of triglyceride concentrations in response to HAART and to elucidate the mechanism underlying this association.
ACKNOWLEDGMENTS
We are grateful to Mr. Dixon Mutatula, Ms. Bernadette Nyemba, Mr. Hilary Lumano and the staff of Kalingalinga laboratory for their help with data collection and sample processing. We thank Dr. Sandeep Misra for quality control checks in the data. The help of Mr. Andrew Westfall in verifying follow-up data is highly appreciated. We are grateful to the patients and staff at Chawama Clinic for their cooperation. This study was supported by the University of Alabama at Birmingham, Department of Epidemiology and by the Nutrition Obesity Research Center grant # DK056336.
ABBREVIATIONS
- HAART
Highly active antiretroviral therapy
- HDL
High density lipoprotein cholesterol
- IFN-α
Interferon-α
- IL1
Interleukin-1
- IL2
Interleukin-2
- IL6
Interleukin-6
- LDL
Low density lipoprotein cholesterol
- TNF
Tumor necrosis factor
- VLDL
Very low density lipoprotein
Footnotes
COMPETING INTERESTS
There are no competing interests to disclose.
Authors’ contributions
JNN performed analyses and drafted the manuscript; EKK, DCH and DKA conceived the idea; CNN processed 24-hr dietary recalls; CKN, DP, IZ, SB and BHC contributed to patient recruitment and overall execution of the study in Zambia; JY managed the study database and performed some analyses; All authors contributed to the scientific content, edited and approved the various versions of manuscript. EKK oversaw the overall execution of the DGPLEAD study and the writing process.
References
- 1.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004 Apr 9;18(6):887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Veloso VG, Friedman RK, et al. Early mortality and cause of deaths in patients using HAART in Brazil and the United States. AIDS. 2009 Oct 23;23(16):2107–2114. doi: 10.1097/QAD.0b013e32832ec494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006 Sep 15;43(6):770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JSA, Zulu I, Levy J, et al. Rapid Scale-up of Antiretroviral Therapy at Primary Care Sites in Zambia: Feasibility and Early Outcomes. JAMA. 2006 Aug 16;296(7):782–793. doi: 10.1001/jama.296.7.782. 2006. [DOI] [PubMed] [Google Scholar]
- 6.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008 Apr;24(4):555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 7.Etard JF, Ndiaye I, Thierry-Mieg M, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006 May 12;20(8):1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 8.Fetzer BC, Hosseinipour MC, Kamthuzi P, et al. Predictors for mortality and loss to follow-up among children receiving anti-retroviral therapy in Lilongwe, Malawi. Tropical Medicine & International Health. 2009;14(8):862–869. doi: 10.1111/j.1365-3156.2009.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mzelini MO, L MB, Chephe TJ. Mortality and causes of death in HIV-positive patients receiving antiretroviral therapy at Tshepang Clinic in Doctor George Mukhari Hospital. Pol Arch Med Wemn. 2008;118(10):9. 2008. [PubMed] [Google Scholar]
- 10.Sieleunou I, Souleymanou M, Schonenberger AM, Menten J, Boelaert M. Determinants of survival in AIDS patients on antiretroviral therapy in a rural centre in the Far-North Province, Cameroon. Trop Med Int Health. 2009 Jan;14(1):36–43. doi: 10.1111/j.1365-3156.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 11.French ALMD, Gawel SHP, Hershow RMD, et al. Trends in Mortality and Causes of Death Among Women With HIV in the United States: A 10-Year Study. :1525–4135. doi: 10.1097/QAI.0b013e3181acb4e5. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johannessen A, Naman E, Ngowi B, et al. Predictors of mortality in HIV-infected patients starting antiretroviral therapy in a rural hospital in Tanzania. BMC Infectious Diseases. 2008;8(1):52. doi: 10.1186/1471-2334-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. doi: 10.1186/1742-6405-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egger M, Boulle A, Schechter M, Miotti P. Antiretroviral therapy in resource-poor settings: scaling up inequalities? nt. J. Epidemiol. 2005 Jun 1;34(3):509–512. doi: 10.1093/ije/dyi110. 2005. [DOI] [PubMed] [Google Scholar]
- 15.Isaac R, Jacobson D, Wanke C, Hendricks K, Knox TA, Wilson IB. Declines in dietary macronutrient intake in persons with HIV infection who develop depression. Public Health Nutr. 2008 Feb;11(2):124–131. doi: 10.1017/S1368980007000067. [DOI] [PubMed] [Google Scholar]
- 16.Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005 Jan;21(1):96–99. doi: 10.1016/j.nut.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Maahs DM, Rewers M. Editorial: Mortality and renal disease in type 1 diabetes mellitus--progress made, more to be done. J Clin Endocrinol Metab. 2006 Oct;91(10):3757–3759. doi: 10.1210/jc.2006-1730. [DOI] [PubMed] [Google Scholar]
- 18.Sowers JR. Update on the cardiometabolic syndrome. Clin Cornerstone. 2001;4(2):17–23. doi: 10.1016/s1098-3597(01)90026-2. [DOI] [PubMed] [Google Scholar]
- 19.Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004 May;286(5):H1597–1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 20.Barbaro G. Highly active antiretroviral therapy-associated metabolic syndrome: pathogenesis and cardiovascular risk. Am J Ther. 2006 May-Jun;13(3):248–260. doi: 10.1097/01.mjt.0000162013.66614.16. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua M, Dominguez LJ, Barbagallo M. Insulin Resistance and the cardiometabolic syndrome in HIV infection. J Cardiometab Syndr. 2009;4(1):40–43. doi: 10.1111/j.1559-4572.2008.00027.x. Winter. [DOI] [PubMed] [Google Scholar]
- 22.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998 Jun 20;351(9119):1881–1883. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 23.Harrington M. [Accessed October 30, 2009];Anabolic Block, Metabolic Disturbances in HIV Infection Find Friend Not Foe in Protease Inhibitor Regimens, Refeeding paradox. 1997 :1–5. Available at: http://www.treatmentactiongroup.org/assets/0/16/42/122/242/fb6d92c6-db9a-481c-b2aa-171d33e975e8.pdf.
- 24.Wunder DM, Bersinger NA, Fux CA, et al. Hypogonadism in HIV-1-infected men is common and does not resolve during antiretroviral therapy. Antivir Ther. 2007;12(2):261–265. [PubMed] [Google Scholar]
- 25.El-Sadr WM, Mullin CM, Carr A, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Med. 2005 Mar;6(2):114–121. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein MK. Pathophysiology of body composition and metabolic abnormalities in HIV-infection: therapeutic implications. Int J Sport Nutr Exerc Metab. 2001 Dec;11(Suppl):S105–110. doi: 10.1123/ijsnem.11.s1.s105. [DOI] [PubMed] [Google Scholar]
- 27.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical Outcomes and CD4 Cell Response in Children Receiving Antiretroviral Therapy at Primary Health Care Facilities in Zambia. JAMA. 2007 Oct 24;298(16):1888–1899. doi: 10.1001/jama.298.16.1888. 2007. [DOI] [PubMed] [Google Scholar]
- 28.JHPIEGO . In: Zambia HIV national guidelines. Gallant J, Pham P, Mwaba P, et al., editors. Zambia; Lusaka: [Accessed November, 2009]. 2009. http://www.zambiahivguide.org/ [Google Scholar]
- 29.Zachariah R, Fitzgerald M, Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006 Nov 28;20(18):2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 30.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002 Dec 17;106(25):3143. 2002. [PubMed] [Google Scholar]
- 31.Constans J, Marchand JM, Conri C, et al. Asymptomatic atherosclerosis in HIV-positive patients: A case-control ultrasound study. Ann Med. 1995 Dec;27(6):683–685. doi: 10.3109/07853899509019256. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Miranda C, Pulido F, Carrillo JL, et al. Lipoprotein alterations in patients with HIV infection: relation with cellular and humoral immune markers. Clin Chim Acta. 1998 Jun 8;274(1):63–70. doi: 10.1016/s0009-8981(98)00050-3. [DOI] [PubMed] [Google Scholar]
- 33.Grunfeld C, Pang M, Doerrler W, Shigenaga J, Jensen P, Feingold K. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992 May 1;74(5):1045–1052. doi: 10.1210/jcem.74.5.1373735. 1992. [DOI] [PubMed] [Google Scholar]
- 34.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005 Jan 6;352(1):48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 35.Constans J, Pellegrin JL, Peuchant E, et al. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. European Journal of Clinical Investigation. 1994;24(6):416–420. doi: 10.1111/j.1365-2362.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 36.Lenhard JM, Croom DK, Weiel JE, Winegar DA. HIV Protease Inhibitors Stimulate Hepatic Triglyceride Synthesis. Arterioscler Thromb Vasc Biol. 2000 Dec 1;20(12):2625–2629. doi: 10.1161/01.atv.20.12.2625. 2000. [DOI] [PubMed] [Google Scholar]
- 37.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003 Jun 11;289(22):2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 38.Grunfeld C, Feingold KR. The Role of the Cytokines, Interferon Alpha and Tumor Necrosis Factor in the Hypertriglyceridemia and Wasting of AIDS. J. Nutr. 1992 Mar 1;122(3_Suppl):749–753. doi: 10.1093/jn/122.suppl_3.749. 1992. [DOI] [PubMed] [Google Scholar]
- 39.Grunfeld C, Kotler DP, Shigenaga JK, et al. Circulating interferon-alpha levels and hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1991 Feb;90(2):154–162. [PubMed] [Google Scholar]
- 40.Khovidhunkit W, Kim M-S, Memon RA, et al. Thematic review series: The Pathogenesis of Atherosclerosis. Effects of infection and inflammation on lipid and lipoprotein metabolism mechanisms and consequences to the host. J. Lipid Res. 2004 Jul 1;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. 2004. [DOI] [PubMed] [Google Scholar]
- 41.Lemieux I, Lamarche B, Couillard C, et al. Total Cholesterol/HDL Cholesterol Ratio vs LDL Cholesterol/HDL Cholesterol Ratio as Indices of Ischemic Heart Disease Risk in Men: The Quebec Cardiovascular Study. Arch Intern Med. 2001 Dec 10;161(22):2685–2692. doi: 10.1001/archinte.161.22.2685. 2001. [DOI] [PubMed] [Google Scholar]
- 42.Scranton R, Sesso HD, Stampfer MJ, Levenson JW, Buring JE, Gaziano JM. Predictors of 14-year changes in the total cholesterol to high-density lipoprotein cholesterol ratio in men. Am Heart J. 2004 Jun;147(6):1033–1038. doi: 10.1016/j.ahj.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 43.De Luis DA, Conde R, Aller R, et al. Effect of omega-3 fatty acids on cardiovascular risk factors in patients with type 2 diabetes mellitus and hypertriglyceridemia: an open study. Eur Rev Med Pharmacol Sci. 2009 Jan-Feb;13(1):51–55. [PubMed] [Google Scholar]
- 44.Holub DJ, Holub BJ. Omega-3 fatty acids from fish oils and cardiovascular disease. Mol Cell Biochem. 2004 Aug;263(1-2):217–225. doi: 10.1023/B:MCBI.0000041863.11248.8d. [DOI] [PubMed] [Google Scholar]
- 45.Kitchen M, Quigley MA, Mwinga AM, et al. HIV Progression and Predictors of Mortality in a Community-Based Cohort of Zambian Adults. J Int Assoc Physicians AIDS Care (Chic Ill) 2008 Feb 1;7(1):17–26. doi: 10.1177/1545109707303989. 2008. [DOI] [PubMed] [Google Scholar]
- 46.Morison L, Buve A, Zekeng L, et al. HIV-1 subtypes and the HIV epidemics in four cities in sub-Saharan Africa. AIDS. 2001 Aug;15(Suppl 4):S109–116. doi: 10.1097/00002030-200108004-00012. [DOI] [PubMed] [Google Scholar]
- 47.Handema R, Terunuma H, Kasolo F, et al. Emergence of new HIV-1 subtypes other than Subtype C among antenatal women in Lusaka, Zambia. AIDS Res Hum Retroviruses. 2001 May 20;17(8):759–763. doi: 10.1089/088922201750237031. [DOI] [PubMed] [Google Scholar]
- 48.Fourie CM, Van Rooyen JM, Kruger A, Schutte AE. Lipid Abnormalities in a Never-Treated HIV-1 Subtype C-Infected African Population. Lipids. 2009 Nov 15; doi: 10.1007/s11745-009-3369-4. [DOI] [PubMed] [Google Scholar]
- 49.Vasan A, Renjifo B, Hertzmark E, et al. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin Infect Dis. 2006 Mar 15;42(6):843–852. doi: 10.1086/499952. [DOI] [PubMed] [Google Scholar]
- 50.Kiwanuka N, Laeyendecker O, Robb M, et al. Effect of human immunodeficiency virus Type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008 Mar 1;197(5):707–713. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]