Summary
We show constitutive activation of Rho kinase (ROCK) in cells bearing oncogenic forms of KIT, FLT3 and BCR-ABL, which is dependent on PI3K and Rho GTPase. Genetic or pharmacologic inhibition of ROCK in oncogene bearing cells impaired their growth as well as the growth of acute myeloid leukemia patient derived blasts and prolonged the life span of mice bearing myeloproliferative disease. Downstream from ROCK, rapid dephosphorylation or loss of expression of myosin light chain resulted in enhanced apoptosis, reduced growth and loss of actin polymerization in oncogene bearing cells leading to significantly prolonged life span of leukemic mice. In summary, we describe a pathway involving PI3K/Rho/ROCK/MLC which may contribute to myeloproliferative disease and/or acute myeloid leukemia in humans.
Introduction
Although 70–80% acute myeloid leukemia (AML) patients go into remission with standard cytotoxic therapy, most relapse and are unresponsive to subsequent therapies (Druker et al., 2001a). Both AML and myeloproliferative disease (MPD) increase in incidence dramatically in the aging population (Baudard et al., 1994; Brincker, 1985). Unfortunately, elderly patients fare worse than younger patients as a result of co-morbidities (Brincker, 1985). Therefore, it is important to develop therapies with increased efficacy and reduced toxicity for these diseases.
The rational approach to less toxic and more efficacious therapies for many hematologic malignancies is likely to involve targeting molecules that are either mutated or hyperactivated as a result of specific mutations. There are increasing examples of mutations in signaling molecules including KIT mutations in systemic mastocytosis (SM), gastrointestinal stromal tumors (GISTs), and in core binding factor AML (CBF-AML) (Beghini et al., 2000; Hirota et al., 1998; Nagata et al., 1995), FLT3 internal tandem duplications (FLT3-ITD) in AML (Gilliland and Griffin, 2002; Nakao et al., 1996), and BCR-ABL translocations in chronic myelogenous leukemia (CML) (Shtivelman et al., 1985). Oncogenic KIT is constitutively phosphorylated and when expressed in cell lines or primary bone marrow cells demonstrate ligand-independent proliferation (Chian et al., 2001; Kitayama et al., 1995; Piao and Bernstein, 1996). Although KIT mutations within the juxtamembrane region found in GIST are sensitive to inhibition by imatinib mesylate (Gleevec); KIT mutations within the tyrosine kinase domain, such as KITD816V, are imatinib-resistant (Demetri et al., 2002; Frost et al., 2002; Ma et al., 2002).
Similar to the KIT, FLT3 is also a member of the class III subfamily of receptor tyrosine kinase and FLT3 mutations are one of the most frequent somatic alterations in AML occurring in approximately one third of these patients and predict poor prognosis (Frohling et al., 2002; Gilliland and Griffin, 2002; Whitman et al., 2001). FLT3-ITD mutations also result in ligand-independent constitutive activation of the receptor’s tyrosine kinase activity (Kiyoi et al., 1998). Several FLT3 inhibitors have been described, but they vary considerably with respect to selectivity for FLT3 (Pratz et al., 2009). Similarly, nearly all patients with CML express the BCR-ABL fusion protein and stem cells bearing BCR-ABL is sufficient to induce CML (Daley et al., 1990; Koschmieder et al., 2005; Lugo et al., 1990; Shtivelman et al., 1985). Although imatinib has been used successfully to treat CML, emergence of BCR-ABL positive residual stem cells and imatinib-resistant BCR-ABL mutants has resulted in drug resistance and relapse related concerns with this disease (Druker et al., 2001a; Druker et al., 2001b; Graham et al., 2002). Thus, identification of new targets, in particular that might contribute to the initiation and/or progression of multiple hematologic malignancies involving activated tyrosine kinases, is likely to be of therapeutic benefit.
Rho kinases or Rho-associated coiled coil-containing protein kinases (ROCK) are protein serine/threonine kinases. Two isoforms of ROCK have been described which are encoded by two separate genes, ROCK1 and ROCK2 (Nakagawa et al., 1996). ROCK1 and ROCK2 share considerable sequence homology at the protein level; close to 65% overall and nearly 92% in their kinase domains (Nakagawa et al., 1996). Activation of ROCK by GTP bound Rho or by lipid mediators leads to phosphorylation of various downstream target proteins including myosin phosphatase (Kimura et al., 1996), myosin light chain (MLC) (Leung et al., 1996) and LIM kinases 1 and 2 (Ohashi et al., 2000; Sumi et al., 2001). Activation of these substrates results in the recruitment of mediators of actin polymerization and formation of focal adhesions leading to changes in growth, survival and cell motility (Riento and Ridley, 2003).
Emerging data suggests that ROCK is an oncogene. Recently, cancer genome sequencing revealed thxree ROCK1 activating mutations in primary human breast cancer cells and in human non-small-cell lung carcinoma line NCI-H1770 (Greenman et al., 2007). Introducing these mutants to fibroblasts elevated ROCK activity, which lead to changes in actin cytoskeleton, increased motility and decreased adhesion (Lochhead et al., 2010). Taken together, while a role for ROCK in solid tumors is clearly emerging, its role in regulating growth, survival and transformation downstream of tyrosine kinases involved in SM, AML or CML is not known. In the present study, we determined the contribution of PI3K/Rho/ROCK/MLC pathway in regulating the growth, survival and transformation of cells bearing oncogenic forms of KIT, FLT3 and BCR-ABL.
Results
Constitutive activation of ROCK in oncogene bearing cells
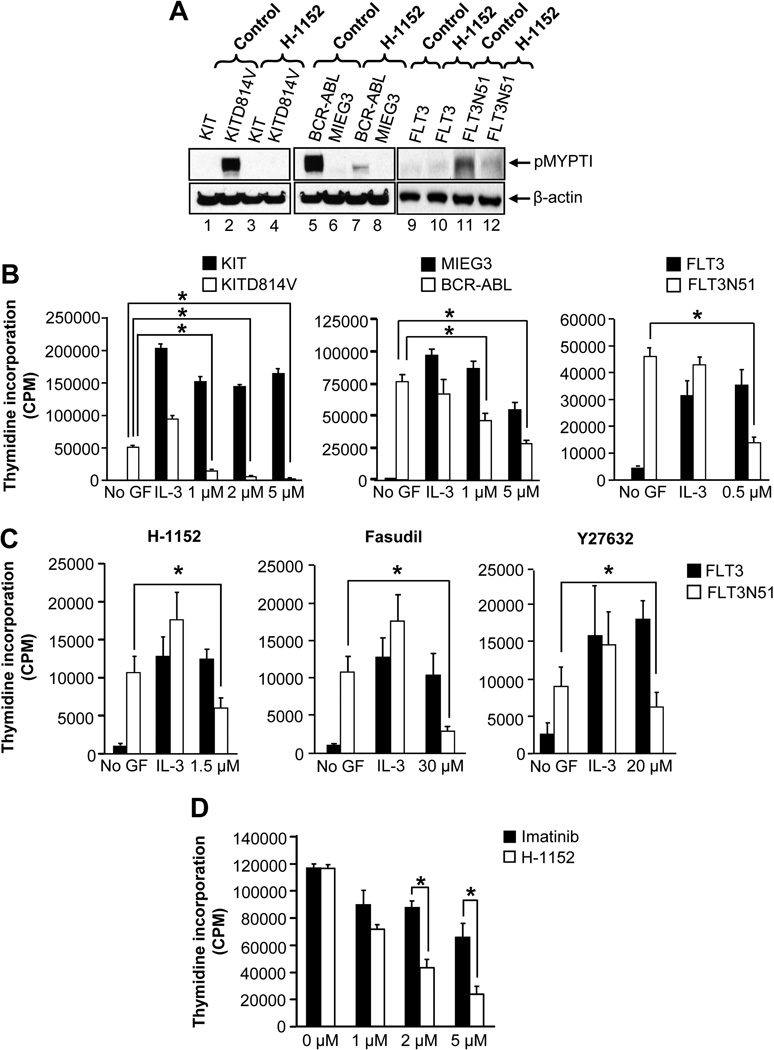
We first investigated the role of ROCK signaling in leukemogenesis mediated by activating mutant tyrosine kinases KITD814V, FLT3N51, and BCR-ABL. 32D cells were starved and ROCK activity was analyzed by assessing the phosphorylation of its substrate myosin phosphatase (MYPT1) in the presence or absence of a highly specific and potent ROCK inhibitor H-1152 (Jacobs et al., 2006). Constitutive activation of ROCK was observed only in oncogene bearing cells, but not in cells bearing the empty vector (MIEG3), KIT or FLT3 (Figure 1A). Importantly, H-1152 treatment rapidly inhibited the ROCK activity in oncogene bearing cells (Figure 1A). Constitutive ROCK activity was also observed in primary bone marrow (BM) cells expressing KITD814V, but not those expressing the wild type KIT and the activity was completely inhibited by H-1152 (Figure S1A). Furthermore, H-1152 treatment has no effect on the activation of AKT, ERK, Stat5, and PKC in oncogene bearing cells (Figure S1). These results suggest that oncogenes such as KITD814V, BCR-ABL, and FLT3N51 induce constitutive ROCK activation, which is inhibited by H-1152.
Figure 1. ROCK activation is essential for constitutive growth of oncogene bearing cells.
(A) Oncogene bearing 32D cells were starved for 6 hours in serum- and cytokine-free medium and incubated in the presence or absence of H-1152 (2 µM) for 1 hour. Equal amount of protein was subjected to western blot analysis using an anti-phospho-MYPT1 antibody. Similar results were observed in three independent experiments. (B) Cells in (A) were treated with indicated amounts of H-1152. After 48 hours, proliferation was evaluated by [3H] thymidine incorporation. Assay was performed in the presence of IL-3 (10 ng/mL) for vector and WT receptor bearing cells, and in the absence of IL-3 for oncogenic receptor bearing cells. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in quadruplicate.*p<0.01. (C) HSC/Ps bearing FLT3 or FLT3N51 were treated with indicated amount of ROCK inhibitors. After 48 hours, proliferation was evaluated. Assays were performed in the presence of IL-3 (10 ng/mL) for FLT3 bearing cells, and in the absence of IL-3 for FLT3N51 bearing cells. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in quadruplicate.*p<0.05. (D) Starved 32D cells bearing BCR-ABLT315I were cultured in the presence of indicated amounts of H-1152 or imatinib. After 48 hours, proliferation was evaluated by [3H] thymidine incorporation. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in quadruplicate.*p<0.05. See also Figure S1.
ROCK inhibitors suppress the constitutive growth of oncogene bearing cells
Next, we assessed whether ROCK inhibitors suppress the growth of KITD814V, FLT3N51, and BCR-ABL expressing cells. 32D cells bearing MIEG3 or KIT, or BaF3 cells bearing FLT3 showed minimal thymidine incorporation in the absence of growth factors. IL-3 enhances the growth of these cells. In contrast, cells expressing KITD814V, BCR-ABL or FLT3N51 showed constitutive growth in the absence of growth factors, which was repressed by H-1152 in a dose dependent manner. Importantly, treatment of cells bearing MIEG3, KIT or FLT3 with H-1152 in the presence of IL-3 showed minimal suppression in proliferation (Figure 1B). Other ROCK inhibitors fasudil and Y27632 similarly repress the growth of cells bearing KITD814V or FLT3N51 (data not shown).
To validate whether the suppression in growth of oncogene bearing cells by ROCK inhibitors seen in 32D cells also occurs in primary hematopoietic stem and progenitor cells (HSC/Ps); we transduced primary HSC/Ps from C57BL/6 mice with FLT3 or FLT3N51 and analyzed proliferation in the presence or absence of ROCK inhibitors. While primary HSC/Ps bearing the FLT3 grown in the absence of growth factors demonstrated minimal thymidine incorporation, cells bearing the FLT3N51 demonstrated a significant increase in thymidine incorporation in the absence of cytokine (Figure 1C). When stimulated with IL-3, FLT3 bearing cells demonstrated a significant increase in growth. When these cells were treated with H-1152, a significant reduction in proliferation was observed in cells bearing FLT3N51 but not those expressing FLT3. Consistent with these results, treatment of these cells with less potent ROCK inhibitors fasudil and Y27632 also resulted in significant reduction in the growth of cells expressing FLT3N51 but not cells expressing FLT3 (Figure 1C). Similar repression in the constitutive growth of primary HSC/Ps was observed in cells bearing KITD814V and BCR-ABL in the presence of ROCK inhibitors (data not shown).
To determine whether the ROCK pathway is also involved in the constitutive growth of cells bearing the imatinib-resistant BCR-ABLT315I mutant, we treated 32D cells expressing this mutant with H-1152. As expected, imatinib treatment showed minimal effect on the growth of cells bearing BCR-ABLT315I. In contrast, treatment of these same cells with H-1152 demonstrated dose dependent suppression in growth (Figure 1D). These results suggest that ROCK may play a prominent role in supporting the growth of oncogene bearing cells, but only a modest role in supporting the growth of non oncogene bearing hematopoietic cells.
ROCK inhibitor suppresses the growth of primary bone marrow derived blasts from AML patients
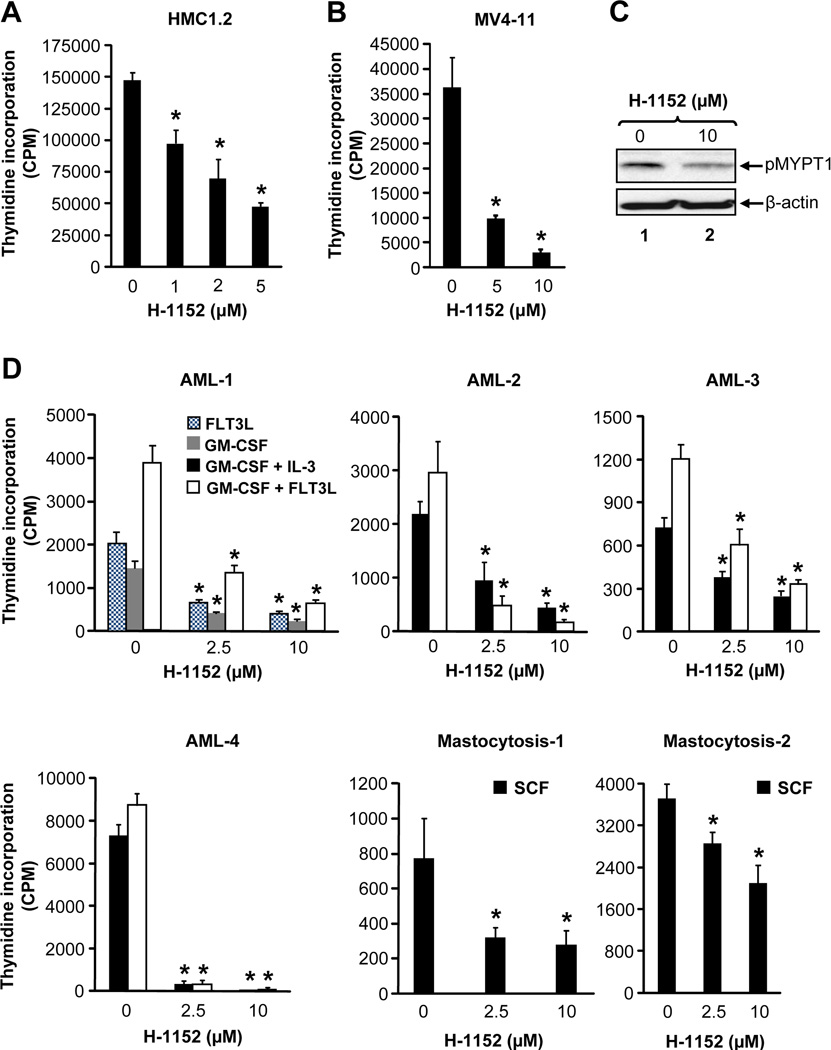
We next performed studies in HMC1.2 cells bearing the activating KIT mutation (KITV560G and KITD816V) and in MV4-11 cells bearing the activating FLT3 mutation (FLT3-ITD) (Butterfield et al., 1988; Lange et al., 1987). In both instance, H-1152 showed a dose dependent reduction in growth of HMC1.2 and MV4-11 cells (Figure 2A and 2B). Likewise, cells derived from AML and mastocytosis patients also demonstrated repression in ROCK activity and growth in the presence of H-1152 (Figure 2C, 2D, S2 and Table S1). Because we have not been able to verify the status of KIT mutations in all AML samples, it is conceivable that the growth of all AML cells to some extent is repressed by ROCK inhibitors.
Figure 2. ROCK inhibitor suppresses the growth of primary BM derived AML blasts.
(A) HMC 1.2 or (B) MV4-11 cells were starved and treated with indicated amounts of H-1152. After 48 hours, proliferation was evaluated. Bars denote the mean thymidine incorporation ± SD from a representative experiment performed in quadruplicate. n=3,*p<0.001. (C) AML patient sample was incubated in the absence or presence of H-1152 (10 µM) for 1 hour and equal amount of protein lysate was subjected to western blot analysis using an anti-phospho-MYPT1 antibody. (D) Primary AML patient samples positive for FLT3-ITD and mastocytosis patient samples positive for KIT mutation were grown in the presence of indicated cytokines including FLT3L (10 ng/mL), GM-CSF (1 ng/mL), GM-CSF + IL-3 (1 + 10 ng/mL), GM-CSF + FLT3L (1 + 50 ng/mL) or SCF (100 ng/mL) and treated with indicated amounts of H-1152. After 48 hours, proliferation was evaluated. Bars denote the mean thymidine incorporation ± SD performed in triplicate or quadruplicate.*p<0.01. See also Figure S2 and Table S1.
Rho GTPase regulates ROCK in oncogene bearing cells
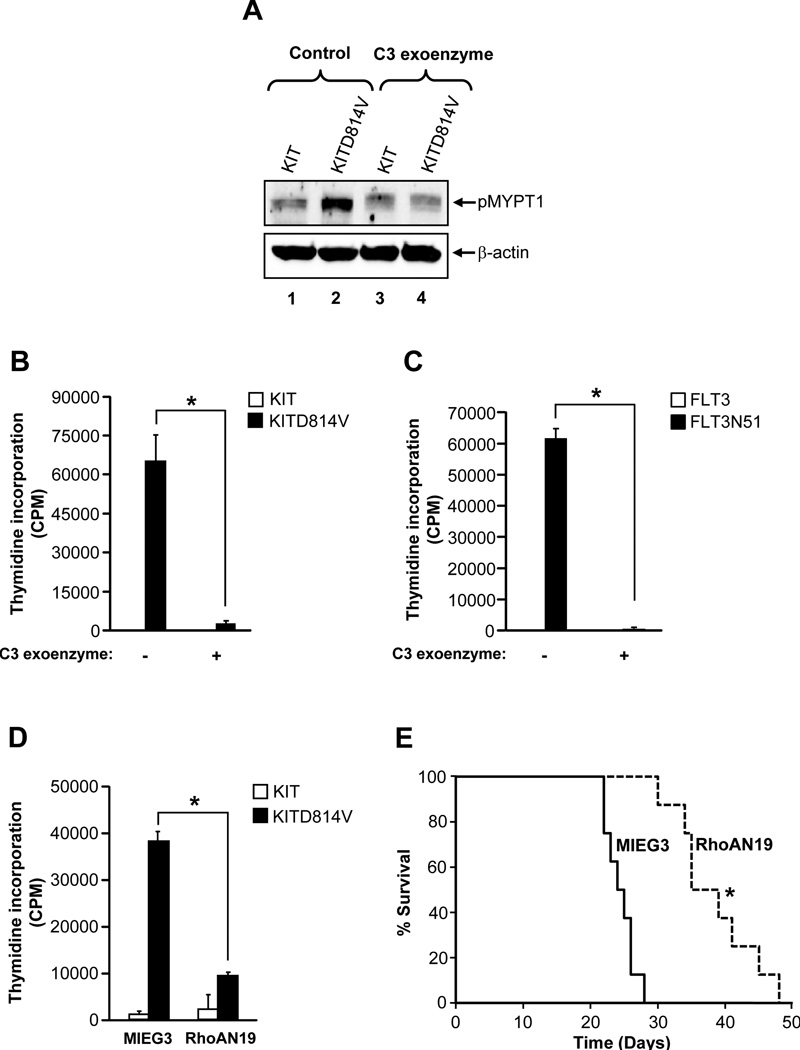
We next determined the mechanism of activation of ROCK in oncogene bearing cells. We analyzed whether the small Rho GTPase, which is upstream of ROCK, is involved in KITD814V induced growth and ROCK activation. C3 exoenzyme (a Rho inhibitor) inhibited the activation of ROCK and the growth of cells bearing KITD814V or FLT3N51 (Figure 3A–C). In addition, cells co-infected with KITD814V and a dominant negative mutant of RhoA (RhoAN19) showed significantly reduced growth compared to cells expressing KITD814V and MIEG3 (Figure 3D). Consistent with in vitro findings, mice transplanted with cells bearing KITD814V and RhoAN19 showed significantly prolonged survival compared to cells bearing KITD814V and MIEG3 (Figure 3E). These results suggest that RhoA is involved in KITD814V induced constitutive growth in vitro and MPD in vivo in part by regulating the activation of ROCK.
Figure 3. Rho GTPase is required for constitutive growth and activation of ROCK in oncogene bearing cells.
(A) & (B) KIT or KITD814V expressing 32D cells were treated with Rho inhibitor C3 exoenzyme (5 µg/mL) for 2 hours and assessed for MYPT1 phosphorylation (A) or for 48 hours and proliferation was evaluated (B). Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in quadruplicate.*p < 0.01. (C) FLT3 or FLT3N51 expressing 32D cells were treated with C3 exoenzyme (5 µg/mL). After 48 hours, proliferation was evaluated. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in triplicate.*p < 0.01. (D) Cells as in (B) were infected with a dominant negative mutant of RhoA (RhoAN19) and subjected to proliferation assay. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments in quadruplicate.*p<0.001. (E) Kaplan-Meier survival curves of mice transplanted with cells co-infected with KITD814V and MIEG3 or RhoAN19. 1 × 106 cells were injected into mice and monitored for MPD and survival (n=8 in each group).*p < 0.01.
PI3K signaling is essential for activation of ROCK in cells bearing KITD814V
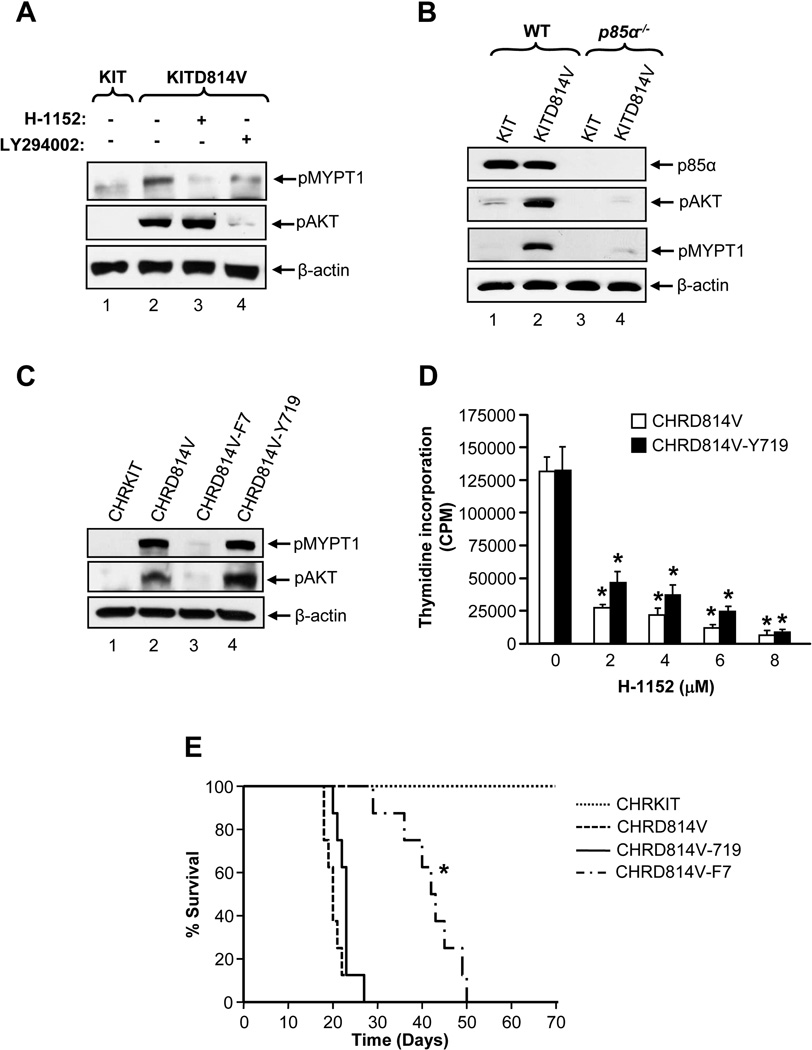
PI3K mediated generation of PIP3 activates Rho GTPases by regulating guanine exchange factors (Han et al., 1998; Schuebel et al., 1998). PI3K plays an important role in KITD814V induced constitutive growth in vitro and MPD in vivo (Munugalavadla et al., 2007; Munugalavadla et al., 2008). We determined if PI3K induced MPD of KITD814V bearing cells involves ROCK. After starved of serum and cytokine for 6 hours, PI3K and ROCK activities were observed only in KITD814V bearing cells, but not in KIT expressing cells (Figure 4A). Treatment of these cells with H-1152 for 1 hour completely inhibited the activation of ROCK, but had no effect on the activation of AKT. In contrast, one hour treatment with PI3K inhibitor LY294002 completely inhibited the activity of AKT and significantly reduced the activity of ROCK in KITD814V bearing cells. These results suggest that PI3K is important for the activation of ROCK downstream from KITD814V.
Figure 4. PI3K signaling is essential for constitutive activation of ROCK in cells bearing KITD814V.
(A) 32D cells bearing KIT or KITD814V starved for 6 hours in serum- and cytokine-free medium were treated as indicated (h-1152, 2 µM; LY294002, 2 µM) for 1 hour and subjected to western blot analysis. Similar results were observed in two independent experiments. (B) Starved primary WT or p85α−/− HSC/Ps expressing KIT or KITD814V were subjected to western blot analysis. Similar results were observed in two independent experiments. (C) Starved 32D cells bearing CHRKIT, CHRD814V, CHRD814V-F7 or CHRD814V-Y719 were subjected to western blot analysis. Similar results were observed in 4 independent experiments. (D) 32D cells bearing CHRD814V and CHRD814V-Y719 were treated with indicated amounts of H-1152 for 48 hours. After 48 hours, proliferation was evaluated. Bars denote the mean thymidine incorporation ± SD from 1 of 4 independent experiments in quadruplicate.*p<0.01. (E) Kaplan-Meier survival curves of mice transplanted with 32D cells bearing CHRKIT, CHRD814V, CHRD814V-F7 or CHRD814V-Y719. CHRD814V-F7 vs. CHRD814V or CHRD814V-Y719.*p<0.01. n=8 mice in each group.
To further analyze the role of PI3K in ROCK activation, primary BM cells from WT and p85α−/− mice transduced with KIT or KITD814V were starved for 6 hours in serum- and cytokine-free medium and the activity of AKT and of ROCK were measured. Constitutive activation of PI3K and ROCK was observed in WT cells transduced with KITD814V but not KIT (Figure 4B). Importantly, deletion of p85α resulted in significant inhibition of both AKT and ROCK activity in KITD814V bearing cells. These results further support the notion that PI3K is required for the activation of ROCK in KITD814V bearing cells.
To further study the contribution of p85α in ROCK induced MPD, we generated a chimeric KIT receptor CHRKIT and three derivatives CHRD814V, CHRD814V-F7, and CHRD814V-Y719. CHRD814V is corresponding to KITD814V. CHRD814V-F7 has all seven tyrosine residues corresponding to those in KITD814V known to bind SH2 containing proteins (tyrosines 567, 569, 702, 719, 728, 745, and 934) mutated to phenylalanine. CHRD814V-Y719 is similar to CHRD814V-F7 except that tyrosine residue 719 (the binding site for p85α) is preserved. Loss of all tyrosine residues in KITD814V resulted in complete loss of its ability to activate PI3K and ROCK (Figure 4C). Restoration of the p85α binding site alone in KITD814V was sufficient to completely restore the activation of both AKT and ROCK. Furthermore, cells bearing CHRD814V or CHRD814V-Y719 showed similar level of ligand independent growth, compared to cells expressing CHRKIT or CHRD814V-F7 (Figure 4D and data not shown). Treatment of cells bearing CHRD814V or CHRD814V-Y719 with H-1152 demonstrated a dose dependent inhibition in constitutive growth (Figure 4D). In contrast, lack of all tyrosine residues in KITD814V, which cannot activate PI3K or ROCK, results in complete suppression of ligand independent growth. Consistent with cells bearing CHRKIT, H-1152 treatment showed only a moderate suppression in growth of cells bearing CHRD814V-F7 in the presence of IL-3 (data not shown). Consistent with in vitro findings, mice transplanted with cells bearing CHRD814V or CHRD814-Y719 succumbed to MPD and died relatively early (within three and a half weeks) after transplantation (Figure 4E). In contrast, mice transplanted with cells bearing CHRD814V-F7 survived for a significantly longer time and most mice died after 6 to 7 weeks of transplantation. These results demonstrate that p85α mediated activation of PI3K is vital for constitutive activation of ROCK and growth of KITD814V bearing cells in vitro and transformation in vivo.
ROCK inhibitor prolongs the survival of leukemic mice and modulates MPD in vivo
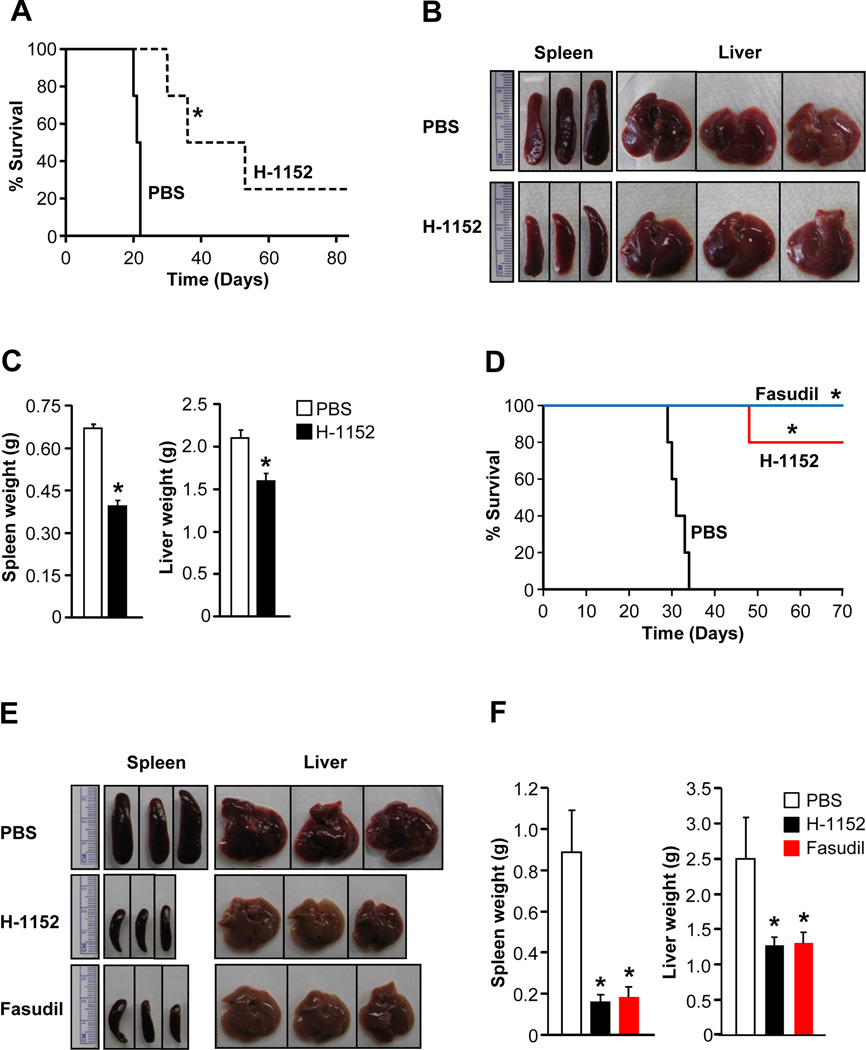
We next assessed the in vivo impact of ROCK inhibitor treatment on KITD814V induced MPD. Mice transplanted with cells bearing KITD814V were treated with PBS or H-1152 at 24 hour intervals via oral gavage for 21 days and monitored for MPD and survival. While mice treated with PBS died within 21 days of transplantation, mice treated with H-1152 showed significantly prolonged survival (Figure 5A). Mice treated with H-1152 showed significantly reduced spleen and liver weight compared to PBS treated mice (Figure 5B and 5C). Similar results were observed in an independent experiment (Figure S3).
Figure 5. In vivo ROCK inhibitor treatment of oncogene bearing mice enhances their survival and modulates MPD.
(A) Kaplan-Meier curves of mice transplanted with 1 × 106 32D cells bearing KITD814V through tail vein and treated with PBS (n=4) or H-1152 (n=4) at 24 hour intervals via oral gavage for 21 days.*p<0.01. (B) & (C) Reduced splenomegaly and hepatomegaly in mice treated with H-1152. Mean ± SEM. n=3, *p<0.01. (D) Kaplan-Meier curves of mice transplanted with 1 × 106 32D cells bearing FLT3N51 through tail vein and treated with PBS (n=5), H-1152 (n=5, oral gavage) or fasudil (n=5, intraperitoneal) at 24 hour intervals for 21 days. .*p<0.01. (E) & (F) Reduced splenomegaly and hepatomegaly in mice treated with H-1152 or Fasudil. Spleen and liver were harvested from transplanted mice treated with PBS (at moribund) or H-1152 or fasudil (after five weeks of prolonged survival) and weights were measured. Mean ± SD. n=4–5, *p<0.01. See also Figure S3.
To further determine the efficacy of ROCK inhibitors in treating MPD due to activating mutations in receptor tyrosine kinases, we performed similar pharmacological studies using a different oncogene and a distinct ROCK inhibitor. We transplanted 32D cells bearing FLT3N51 into syngenic C3H/HeJ mice through tail vein. After two weeks of transplantation, mice were treated with PBS (vehicle), H-1152, or fasudil for 21 days and monitored for MPD and survival. While mice treated with PBS died within 34 days of transplantation, treatment with H-1152 significantly prolonged the survival of mice (Figure 5D). Treatment with fasudil also showed similar efficacy in enhancing the survival of mice bearing FLT3N51 (Figure 5D). After 5 weeks of prolonged survival compared to PBS treated mice, H-1152 or fasudil treated mice were sacrificed and further analysis were performed. Mice treated with H-1152 or fasudil showed significantly reduced spleen and liver weights compared to PBS treated mice (Figure 5E and 5F).
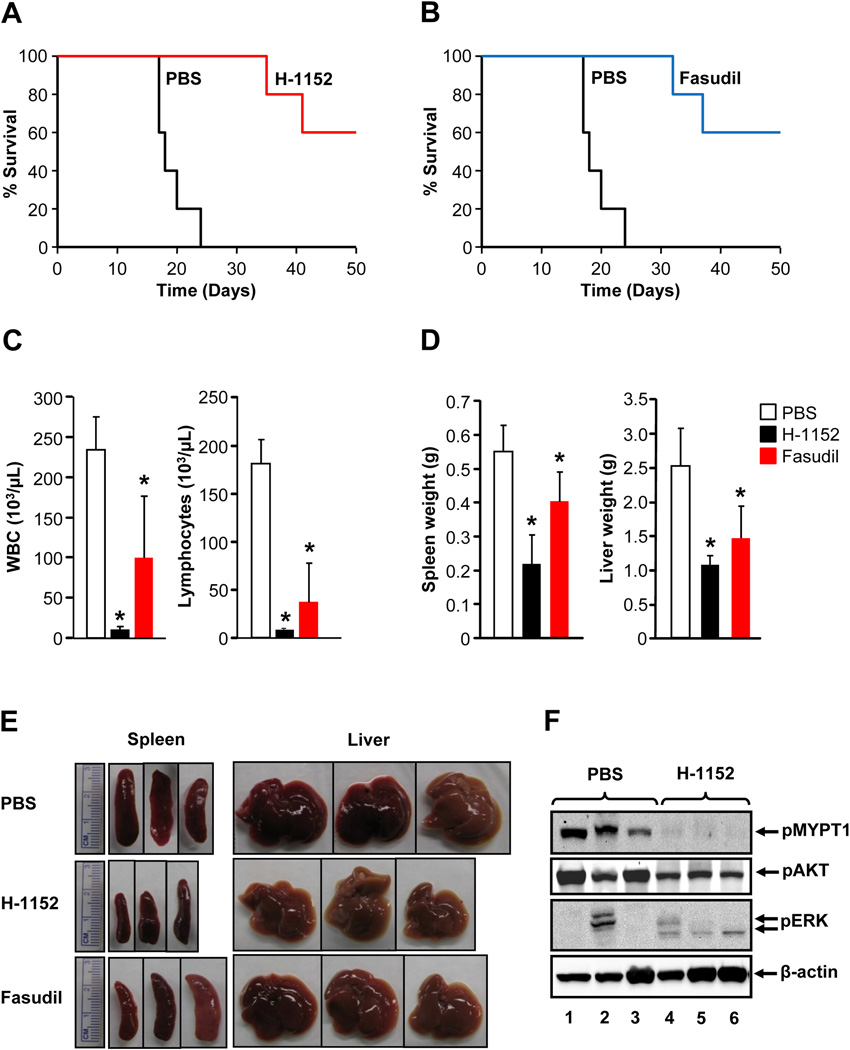
To further evaluate the anti-leukemic activity of ROCK inhibitors, additional studies were performed using primary HSC/Ps bearing KITD814V. After 10 days of transplantation, mice were treated with PBS, H-1152, or fasudil for 21 days and monitored for MPD and survival. While PBS treated mice died within 24 days of transplantation, mice treated with H-1152 or fasudil survived significantly longer (Figure 6A–B). Only 2 out of 5 mice treated with either H-1152 or fasudil died within 37 days of transplantation and the remaining 3 surviving mice were harvested at day 49 post transplant for further analysis. Consistent with the studies with 32D cells, treatment with H-1152 or fasudil significantly modulates the pathological features associated with MPD such as increased white blood and lymphocyte counts as wells as splenomegaly and hepatomegaly (Figure 6C–F). These results suggest that ROCK inhibitors significantly modulate MPD development in mice transplanted with KITD814V or FLT3N51 bearing cells and prolong the survival of these mice.
Figure 6. ROCK inhibitor enhances survival and modulates MPD of mice transplanted with KITD814V bearing cells.
(A) & (B) Kaplan-Meier curves of mice transplanted with primary HSC/Ps bearing KITD814V (1 × 106) through tail vein and treated with PBS or H-1152 or fasudil. After 10 days of transplantation, mice were treated with PBS (n=5) or H-1152 (n=5, oral gavage) or fasudil (n=5, intraperitoneal) at 24 hour interval for 21 days.*p<0.05. (C) Reduced white blood cell and lymphocyte counts in mice treated with H-1152 or fasudil. Mean ± SD. *p<0.05. (D) & (E) Reduced splenomegaly and hepatomegaly in mice treated with H-1152 or fasudil. Mean ± SD. *p<0.01. (F) Splenocytes from three mice transplanted with KITD814V bearing cells treated with PBS or H-1152 were lysed and equal amount of protein lysates were subjected to western blot analysis using indicated antibodies.
Deficiency of ROCK1 suppresses the growth of KITD814V bearing cells
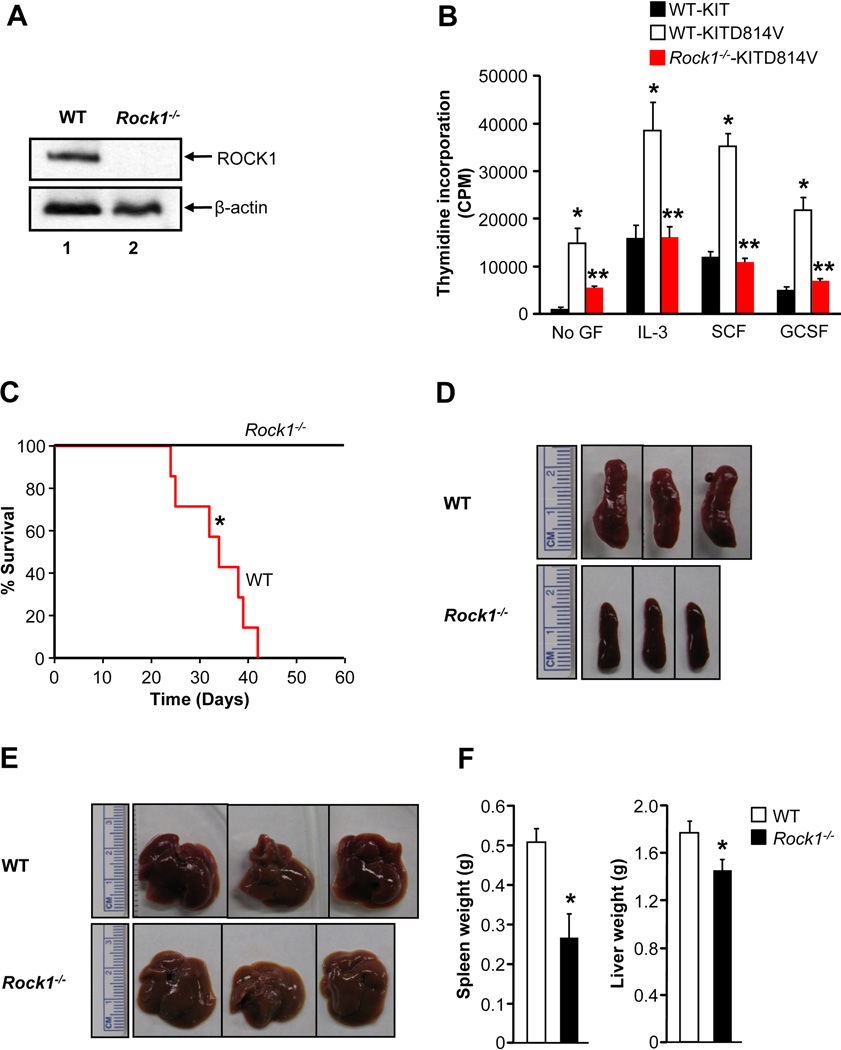
To validate the pharmacologic findings using H-1152 genetically, we transduced primary HSC/Ps from WT and Rock1−/− mice with KIT or KITD814V and analyzed proliferation. Figure 7A shows deletion of ROCK1 in Rock1−/− HSC/Ps. As expected, WT cells transduced with KITD814V, but not KIT, showed constitutive growth in the absence of growth factors (Figure 7B). We also observed hyperproliferation of WT cells transduced with KITD814V compared to KIT in the presence of IL-3, SCF and G-CSF. In contrast, deficiency of ROCK1 resulted in correction in the growth of cells bearing KITD814V compared to WT cells in the presence or absence of IL-3, SCF, and G-CSF. In addition, treatment of Rock1−/− cells bearing KITD814V with H-1152 showed no significant suppression in growth (Figure S4A). These results suggest that ROCK1 is likely to function as the predominant isoform of ROCK in regulating KITD814V induced growth and MPD.
Figure 7. Deficiency of ROCK1 prolongs the survival of KITD814V bearing mice.
(A) HSC/Ps from WT and Rock1−/− mice were subjected to western blot analysis to confirm the deletion of ROCK1. (B) KITD814V bearing HSC/Ps from WT or Rock1−/− mice were starved for 6 hours, cultured in the presence and absence of IL-3 (10 ng/mL), SCF (50 ng/mL) or GCSF (20 ng/mL) for 48 hours, then proliferation was evaluated. Bars denote the mean thymidine incorporation ± SD from 1 of 5 independent experiments in quadruplicate.*p<0.05, KIT vs. KITD814V; **p<0.05, KITD814V vs. Rock1−/−KITD814V. (C) Kaplan-Meier survival curves of WT mice transplanted with WT or Rock1−/− cells bearing KITD814V. n=7, *p<0.01. (D), (E) & (F) Reduced splenomegaly and hepatomegaly in mice transplanted with Rock1−/− cells bearing KITD814V. Mean ± SD. n=6–7, *p<0.01. See also Figure S4.
To further determine the contribution of ROCK1 in KITD814V induced MPD in vivo, we transduced primary HSC/Ps from 5-FU-treated WT or Rock1−/− mice with KITD814V and transplanted into recipient mice and monitored for MPD and survival. While all recipient mice transplanted with WT cells bearing KITD814V died within 42 days of transplantation, all recipient mice transplanted with Rock1−/− cells bearing KITD814V survived for the entire duration of the experiment (Figure 7C). 63 days post transplantation mice expressing KITD814V in Rock1−/− BM were harvested for further analysis. Mice transplanted with Rock1−/− cells bearing KITD814V showed reduced spleen and liver weights as well as white blood counts compared to mice transplanted with WT cells bearing KITD814V (Figure 7D–7F, data not shown). Furthermore, mice bearing an activating version of ROCK1 also resulted in MPD and hypersensitivity to cytokines (Figure S4).
ROCK inhibitors induce cell death in oncogene bearing cells
To understand how ROCK inhibitors might inhibit the growth of oncogene bearing cells, we evaluated the survival of oncogene bearing cells in the presence or absence of ROCK inhibitors. While H-1152 treatment induced only 5–10% cell death in KIT or MIEG3 bearing 32D cells, treatment of 32D cells bearing the KITD814V or BCR-ABL with H-1152 resulted in significantly greater and a dose dependent increase in cell death (Figure S5A–B). Similar results were observed using BaF3 cells bearing FLT3N51 and Y27632 (Figure S5C). These results suggest that the reduced growth observed in cells bearing the oncogenes treated with ROCK inhibitors is in part due to enhanced cell death. Furthermore, ROCK inhibitors are more selective inducers of cell death in oncogene bearing cells relative to WT receptor bearing cells.
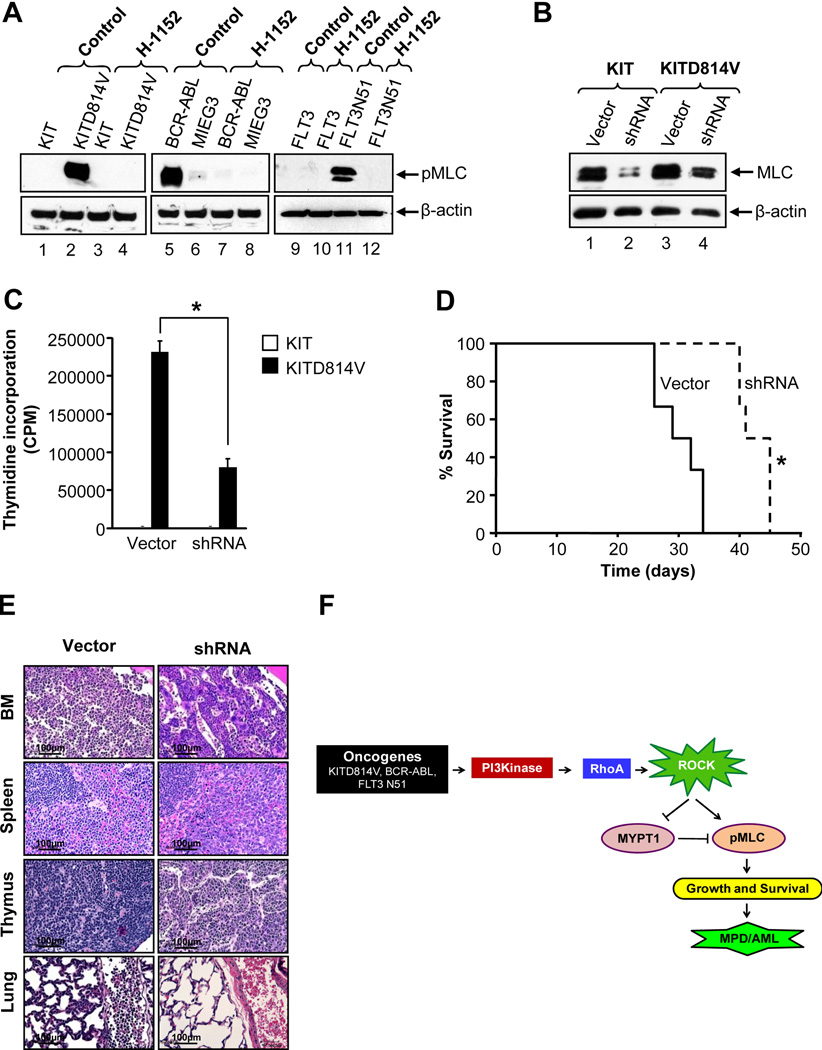
To determine the mechanism(s) by which suppression of ROCK activity induces cell death in oncogene bearing cells, we investigated the activation of ERK, AKT, Stat5, PKA and PKC in H-1152 treated cells. Activation of all of these molecules in oncogene bearing cells was relatively unperturbed in the presence of H-1152 (Figure 4A, Figure S1 and data not shown,). While cells bearing KIT, MIEG3 or FLT3 did not show constitutive phosphorylation of MLC, in contrast, cells bearing the KITD814V, BCR-ABL or FLT3N51 demonstrated constitutive phosphorylation of MLC (Figure 8A). H-1152 completely inhibited the constitutive phosphorylation of MLC within an hour of treatment. These results suggest that constitutive activation of ROCK and phosphorylation of MLC, which is inhibited by H-1152, might contribute to the growth and survival of oncogene bearing cells.
Figure 8. MLC contributes to the growth of oncogene bearing cells.
(A) 32D cells bearing KIT, KITD814V, vector (MIEG3) or BCR-ABL and BaF3 cells bearing FLT3 or FLT3N51 were starved for 6 hours, then incubated in the presence or absence of H-1152 (2 µM) for 1 hour and equal amount of protein lysates were subjected to western blot analysis. Similar results were observed in three independent experiments. (B) 32D cells bearing KIT or KITD814V were co-infected with vector or shRNA against MLC and analyzed for MLC level by western blotting. Similar results were obtained in two independent experiments. (C) Cells as in (B) were exposed to thymidine incorporation assay. Bars denote the mean thymidine incorporation ± SD from 1 of 3 independent experiments performed in quadruplicate.*p<0.01. (D) Kaplan-Meier survival curves of mice transplanted with 1 × 106 cells described in (B) and (C). n=6 in each group.*p<0.01. (E) Histopathological analysis of bone marrow, spleen, thymus, and lungs of mice in (D) by hematoxylin and eosin staining. Shown are representative tissue sections from various groups of transplanted mice. (F) Model of ROCK activation in oncogene bearing cells. Oncogenes such as KITD814V, BCR-ABL or FLT3N51 induce constitutive activation of PI3K, which further activates Rho GTPase leading to constitutive activation of ROCK. Activation of ROCK causes phosphorylation of MLC and inactivation of myosin phosphatase MYPT1 through phosphorylation of myosin binding subunit (MBS). Phosphorylation of MLC promotes cytoskeletal contractility leading to cell growth and survival. Inhibition of ROCK activity by inhibitors suppresses phosphorylation of MLC and causes destabilization of actin filaments there by leading to cell death. See also Figure S5.
To investigate the role of MLC in MPD, we knocked down MLC expression using shRNA in cells bearing KIT or KITD814V. Figure 8B shows significantly reduced expression of MLC in shRNA bearing cells compared to scrambled vector bearing cells. In addition, cells bearing KITD814V and shRNA showed a significant reduction in constitutive growth compared to cells bearing KITD814V and scrambled vector (Figure 8C). Furthermore, mice transplanted with cells co-infected with KITD814V and shRNA survived significantly longer compared to mice bearing cells co-infected with KITD814V and scrambled vector (40–45 vs. 26–34 days, *p<0.05, Figure 8D). Histopathological analysis of bone marrow, spleen, thymus and lungs from transplanted mice showed significantly increased infiltration of tumor cells in mice transplanted with cells co-infected with KITD814V and scrambled vector compared to KITD814V and shRNA (Figure 8E). In contrast, mice transplanted with cells co-infected with KIT and scrambled vector or shRNA did not die and showed no signs of MPD (data not shown). These results demonstrate in vitro and in vivo involvement of MLC in KITD814V induced MPD downstream from ROCK.
To further understand the mechanism behind cell death in ROCK inhibitor treated oncogene bearing cells, we measured F-actin content in 32D cells bearing KIT or KITD814V treated with or without H-1152. Cells bearing KIT showed minimal F-actin in the absence of growth factors (Figure S5D). In contrast, cells bearing KITD814V showed constitutive F-actin in the absence of growth factors which was repressed by H-1152 treatment. Treatment of cells bearing KIT with H-1152 showed no effect on F-actin content in the presence of IL-3. These results suggest that inhibition of ROCK in KITD814V bearing cells but not in normal cells results in dephosphorylation of MLC, actin filament destabilization and disruption of cytoskeleton leading to cell death. Similar findings were observed when these same cells were treated with actin polymerization inhibitor cytochalasin D (data not shown).
Discussion
RhoA and its downstream target ROCK are frequently deregulated in many human cancers. ROCK1 mutations have been recently identified in human tumors that result in elevated kinase activity and contribute to cancer progression (Lochhead et al., 2010; Sahai and Marshall, 2002). While data with respect to the role of ROCK in solid tumors is slowly emerging, the involvement of ROCK in AML and MPD remains unclear. We provide in vitro and in vivo genetic, biochemical as well as pharmacologic evidence to suggest that ROCK plays an essential role in regulating transformation via oncogenic forms of KIT, FLT3 as well as BCR-ABL. Collectively, our results identify PI3K/RhoA/ROCK/MLC pathway in regulating hematologic malignancies via the activating mutations of KIT, FLT3 and BCR-ABL.
With the success of imatinib for the treatment of CML, which targets the Abelson kinase component of the BCR-ABL translocation, a rationale approach to less toxic and more efficacious therapies for many hematologic malignancies including AML and SM would likely involve targeting fundamental signaling molecules bearing mutations in these diseases such as FLT3-ITDs in AML and KIT mutations in SM and AML. Although KIT mutations within the juxtamembrane region found in GISTs are sensitive to inhibition by imatinib, KIT mutations within the tyrosine kinase domain in AML and SM patients, such as KITD814V, stabilize the KIT activation loop conformation in its active form, which precludes sufficient imatinib binding for tyrosine kinase inhibition (Demetri et al., 2002; Frost et al., 2002; Ma et al., 2002). Our results suggest that inhibition of constitutively active ROCK in KITD814V bearing cells is a highly efficacious alternative approach for treating hematologic malignancies involving KITD814V mutation. In addition, inhibition of ROCK in BCR-ABL expressing cells result in growth inhibition and apoptosis, which is associated with dephosphorylation of MLC. Furthermore, we show that inhibition of ROCK also results in growth suppression of an imatinib-resistant BCR-ABLT315I mutant.
While several cell type specific mechanisms of ROCK activation have been described; how ROCK is activated in hematopoietic cells bearing oncogenic mutations such as KITD814V is not known. In addition to protein oligomerization (Doran et al., 2004), other direct activators of ROCK have been described including intracellular second messengers such as arachidonic acid and sphingosylphosphorylcholine which can activate ROCK independently of Rho (Fu et al., 1998; Shirao et al., 2002). Furthermore, ROCK1 activity can also be induced during apoptosis. Cleavage of the carboxy terminal auto-inhibitory region of ROCK1 has been reported by caspase 3. This type of cleavage results in constitutively activated ROCK1 (Sebbagh et al., 2001). Our results in KITD814V bearing cells point to an essential role for RhoA in regulating ROCK activation. We show that treatment of KITD814V expressing cells with C3 exoenzyme (a Rho inhibitor) not only inhibits ROCK activation but also profoundly inhibits the growth of oncogene bearing cells. This observation is further confirmed using cells bearing KITD814V and a dominant negative version of Rho (RhoAN19). Thus, RhoA is likely to regulate ROCK activity in KITD814V bearing cells by directly binding to the RBD domain of ROCK. Binding of activated RhoA to the Rho binding domain (RBD) is thought to disrupt the negative regulatory interaction between the catalytic domain and the C-terminal induced auto-inhibition thereby activating ROCK. Upstream of RhoA, we provide strong evidence to suggest that PI3K contributes to ROCK activation. Utilizing a PI3K inhibitor, BM cells deficient in the expression of p85α and a mutant form of KITD814V receptor that contains only the binding site for p85α, our results show that p85α induced PI3K activity is essential for ROCK activation. It is likely that PIP3 generated via the interaction between KITD814V and p85α recruits and activates the GTPase exchange factor, which in turn contributes to the activation of RhoA and subsequently ROCK. Since PI3K pathway regulates multiple downstream substrates as well as functions, our results suggest that perhaps using ROCK inhibitors may be an alternative strategy for treating hematologic malignancies in which ROCK is constitutively activated.
Our pre-clinical studies, using a mouse model of KITD814V or FLT3N51 driven MPD and treatment with H-1152 or fasudil suggests that inhibiting ROCK in vivo in oncogene bearing cells is of potential therapeutic significance. We show that H-1152 or fasudil treated mice show no signs of toxicity, which is consistent with previous studies demonstrating lack of toxicity upon ROCK inhibition in vivo using fasudil in human trials for cardiovascular indications (Shibuya et al., 2005). Based on these observations, along with our genetic studies demonstrating that constitutive growth of KITD814V bearing cells in the setting of ROCK1 deficient BM cells is normalized, targeting ROCK for treatment of hematologic malignancies due to activating mutations of KIT, FLT3 and BCR-ABL is likely to be a viable therapeutic option.
Activation of ROCK by Rho leads to phosphorylation of various target proteins, including the LIM kinases as well as ERM. Several other cytoskeletal associated proteins have also been described as ROCK substrates. In our studies, we found MLC to be constitutively hyperphosphorylated on Ser19 in all three oncogene bearing cells. Importantly, constitutive Ser19 phosphorylation of MLC was rapidly inhibited in the presence of H-1152. Previous studies have shown that the organization of cellular cytoskeleton is determined to a large extent by interactions between actin and myosin (Elson, 1988) and that these interactions are particularly crucial in cells undergoing rapid growth and proliferation such as leukemic cells. The actin-myosin interaction is largely regulated by the phosphorylation of MLC on Ser19 (Adelstein, 1983; de Lanerolle and Paul, 1991; Somlyo and Somlyo, 1994). While in smooth muscles cells, MLC phosphorylation and dephosphoryaltion is required for muscle contraction and relaxation, our studies suggest that dephosphorylation of MLC by H-1152 in leukemic cells is associated with rapid F-actin depolymerization followed by membrane blebbing and rapid cell death. Further, we show that this process is somewhat unique to oncogene bearing cells. These data suggest that unlike normal hematopoietic cells, proliferation of oncogene bearing cells such as KITD814V is likely to be highly dependent on cytoskeleton deformation and reformation, which is regulated by phosphorylation of MLC on Ser19 by ROCK. While our results suggest that MLC is clearly involved in regulating aspects of MPD; ROCK has many additional targets which are also likely to contribute to MPD.
In conclusion, our results suggest that KITD814V, FLT3N51 and BCR-ABL induce constitutive activation of PI3K/Rho/ROCK/MLC pathway which increases actin-myosin responses promoting cell growth and survival leading to MPD (Figure 8C). Inhibition of ROCK using pharmacological inhibitors or suppressing the expression of MLC is sufficient to inhibit constitutive growth of oncogene bearing cells. ROCK1 appears to be sufficient for inducing oncogene (at least for KITD814V) induced transformation.
Experimental procedures
Antibodies and reagents
Rabbit anti-phospho-MYPT1 antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Rabbit anti-phospho-AKT, anti-AKT, anti-phospho-ERK, anti-ERK, anti-phospho-Stat5, anti-Stat5, anti-phospho-PKC, mouse anti-phospho-MLC and anti-MLC antibodies were purchased from Cell Signaling Technology (Beverly, MA). Rabbit anti-ROCK1 antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Phycoerythrin (PE)-conjugated annexin V antibody and 7-amino actinomycin D (7-AAD) were purchased from BD Biosciences Pharmingen (San Jose, CA). ROCK inhibitors (fasudil and Y-27632) were purchased from Calbiochem (San Diego, CA). Imatinib was purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada). C3 exoenzyme was purchased from Cytoskeleton Inc. (Denver, CO). Recombinant murine and human IL-3, FLT3, GM-CSF, SCF, IL-6, and Tpo were purchased from Peprotech (Rocky Hill, NJ). Retronectin was obtained from Takara (Madison, WI). Iscove`s modified Dulbecco`s medium (IMDM) was purchased from Invitrogen (Carlsbad, CA). Monothioglycerol was purchased from Sigma (St. Louis, MO). [3H] Thymidine was purchased from PerkinElmer (Boston, MA). ROCK inhibitor H-1152 was synthesized as described (US patent 6153600, Nov 28, 2000). Retroviral expression plasmids of dominant negative mutant of RhoA (RhoAN19) and Imatinib-resistant BCR-ABLT315I mutant were a gift from Dr. Yi Zhang and Dr. Jose Cancelas, respectively, from Cincinnati Children’s Hospital Medical Center, Cincinnati.
Mice
C57BL/6 mice and C3H/HeJ mice were purchased from Jackson Laboratory (Bar Harbor, ME). p85α+/– and Rock1−/− mice have been previously described (Terauchi et al., 1999; Vemula et al., 2010b; Zhang et al., 2006). These mice were maintained under specific-pathogen-free conditions at the Indiana University Laboratory Animal Research Center, Indianapolis, IN. All animal procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees (IACUCs) at Indiana University School of Medicine.
Patient samples
Blast cells from the bone marrow of patients with AML were obtained at the time of diagnostic testing after informed consent. Approval was obtained from the institutional review boards of Indiana University School of Medicine. The buoyant fraction was isolated over Ficoll-Hypaque, and then washed with phosphate-buffered saline (PBS) before processing as described previously (Hartman et al., 2006).
Cells
The murine IL-3 dependent myeloid cell line 32D cells bearing MIEG3 vector, KIT, KITD814V, BCR-ABL, BCR-ABLT315I mutant or RhoAN19 were cultured in medium containing IMDM supplemented with 10% fetal bovine serum (FBS) and murine IL-3 (10 ng/mL). The murine IL-3 dependent and G418 resistant pro-B cell line BaF3 bearing FLT3 and FLT3N51 were obtained from Dr. Seiji Fukuda (Shimane University, Izumo, Japan) and cultured in medium containing IMDM supplemented with 10% FBS, G418 (2 mg/mL), and murine IL-3 (5 ng/mL). The Human mast cell leukemia line, bearing the KITV560G as well as KITD816V mutations, HMC1.2 (Butterfield et al., 1988) and acute myeloid leukemia (AML) cell line, bearing the FLT3-ITD mutation, MV4-11(Lange et al., 1987) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in medium containing IMDM supplemented with 15% FBS and 1.2 mM monothioglycerol.
Expression of WT and oncogenic receptors
Transduction of 32D and primary HSC/Ps was performed as described previously (Munugalavadla et al., 2007). After infection, 32D and primary HSC/Ps bearing the WT or oncogenic receptors were sorted and used to perform all experiments.
shRNA silencing of myosin light chain (MLC)
The myosin light chain (MLC)-specific shRNA (CGCGCAACCTCCAATGTGTTCGCCATGTT) expression plasmid in retroviral vector pGFP-V-RS was purchased from OriGene Technologies (Rockville, MD). Purified and sequence verified plasmid containing a non-effective 29-mer sh eGFP cassette (Scrambled vector) was used as a negative control. Cells were transduced with scrambled vector or shRNA plasmid as described above. After infection, cells were grown in the presence of puromycin (10 ng/mL) to select the transduced cells.
Proliferation assay
Proliferation was assessed by conducting a thymidine incorporation assay as previously described (Munugalavadla et al., 2007).
Western blotting
Western blotting was performed as previously described (Munugalavadla et al., 2007).
Analysis of cell death
Cell death was assessed as previously described (Vemula et al., 2010a).
F-actin measurement
Cells were starved in serum- and cytokine-free media for 6 hours and cultured in the presence or absence of IL-3 and H-1152 for 12 or 24 hours. After treatment, cells were fixed with 4% paraformaldehyde in PBS for 15 min and washed with PBS. After fixing, cells were quenched with 0.1 M glycine in PBS for 15 min and washed with PBS. Then, cells were permeabilized with 0.2% Triton X-100 (w/v) in PBS for 10 min and washed with PBS followed by blocking non-specific binding sites with 5% rat serum containing 0.2% BSA in PBS. Cells were stained with FITC-conjugated phalloidin for 30 min, washed with 0.2% BSA in PBS, and analyzed by flow cytometry.
Mouse leukemia induction and In vivo drug treatment
1×106 32D cells bearing KIT or KITD814V in 200 µL PBS were injected into C3H/HeJ mice via tail vein. After 48 hours of transplantation, mice were treated with vehicle (PBS) or H-1152 (66 mg/kg body weight) by oral gavage at 12 hours interval for 14 days. In the second study, mice were treated with vehicle, H-1152 (50 mg/kg body weight, oral gavage) or fasudil (25 mg/kg body weight, intraperitoneal) at 24 hour intervals for 21 days. In a separate study, primary HSC/P’s bearing KITD814V were transplanted into syngenic C57BL/6 mice as previously described (Munugalavadla et al., 2008). After 10 days of transplantation, mice were treated with vehicle, H-1152 (50 mg/kg body weight, oral gavage) or fasudil (25 mg/kg body weight, intraperitoneal) at 24 hour intervals for 21 days. In all studies, mice were closely monitored for MPD and harvested at moribund. Bone marrow, spleen, liver and lungs were fixed in 10% buffered formalin and sections were stained with hematoxylin and eosin for histopathologic analysis.
Statistics
All graphical data was evaluated by paired Student t- test and results were considered significantly different with p-value <0.05. All data are represented as mean values ± standard deviations (SD). Survival probability of transplanted mice groups was compared using a Kaplan-Meier Survival Analysis in which statistical significance was determined as p-values <0.05 by log rank test.
Highlights.
-
▪
Constitutive activation of ROCK in oncogene bearing cells.
-
▪
Pharmacologic inhibition of ROCK impairs the growth of oncogene bearing cells.
-
▪
Genetic or pharmacologic inhibition of ROCK prolongs the survival of leukemic mice.
-
▪
Knockdown of MLC modulates MPD and prolongs the survival of leukemic mice.
Significance.
There are increasing examples of mutations in tyrosine kinases that contribute to myeloproliferative disease (MPD), acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) including KIT mutations in AML as well as in over 90% cases of systemic mastocytosis (SM), FLT3 internal tandem duplications (ITDs) in AML, and BCR-ABL in CML. Although hyperactivation of several signaling molecules downstream from these tyrosine kinases has been reported; little is known about the relative importance of downstream signaling molecules among these mutations. We show here that activating mutants of KIT, FLT3 and BCR-ABL contribute to hematopoietic cell transformation to a large extent via the hyperactivation of ROCK. ROCK is a potential therapeutic target for treating hematologic malignancies involving these mutations.
Supplementary Material
Acknowledgements
We would like to thank Marilyn Wales for her administrative support. This work was supported in part by grants from National Institutes of health (NIH): R01 HL077177 (R.K), R01 HL08111 (R.K), R01 HL075816 (R.K.), R01 CA134777 (R.J.C. and R.K.) and HL085098 (L.W.), and Riley Children’s Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental Information includes five figures and one table.
References
- Adelstein RS. Regulation of contractile proteins by phosphorylation. J Clin Invest. 1983;72:1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudard M, Marie JP, Cadiou M, Viguie F, Zittoun R. Acute myelogenous leukaemia in the elderly: retrospective study of 235 consecutive patients. Br J Haematol. 1994;86:82–91. doi: 10.1111/j.1365-2141.1994.tb03256.x. [DOI] [PubMed] [Google Scholar]
- Beghini A, Peterlongo P, Ripamonti CB, Larizza L, Cairoli R, Morra E, Mecucci C. C-kit mutations in core binding factor leukemias. Blood. 2000;95:726–727. [PubMed] [Google Scholar]
- Brincker H. Estimate of overall treatment results in acute nonlymphocytic leukemia based on age-specific rates of incidence and of complete remission. Cancer Treat Rep. 1985;69:5–11. [PubMed] [Google Scholar]
- Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12:345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- Chian R, Young S, Danilkovitch-Miagkova A, Ronnstrand L, Leonard E, Ferrao P, Ashman L, Linnekin D. Phosphatidylinositol 3 kinase contributes to the transformation of hematopoietic cells by the D816V c-Kit mutant. Blood. 2001;98:1365–1373. doi: 10.1182/blood.v98.5.1365. [DOI] [PubMed] [Google Scholar]
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P, Paul RJ. Myosin phosphorylation/dephosphorylation and regulation of airway smooth muscle contractility. Am J Physiol. 1991;261:L1–L14. doi: 10.1152/ajplung.1991.261.2.L1. [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- Doran JD, Liu X, Taslimi P, Saadat A, Fox T. New insights into the structure-function relationships of Rho-associated kinase: a thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem J. 2004;384:255–262. doi: 10.1042/BJ20040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001a;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001b;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Elson EL. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, Dohner H, Dohner K. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- Frost MJ, Ferrao PT, Hughes TP, Ashman LK. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1:1115–1124. [PubMed] [Google Scholar]
- Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Greenman C, Stephens P, Smith R, Dalgliesh GL, Hunter C, Bignell G, Davies H, Teague J, Butler A, Stevens C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- Hartman AD, Wilson-Weekes A, Suvannasankha A, Burgess GS, Phillips CA, Hincher KJ, Cripe LD, Boswell HS. Constitutive c-jun N-terminal kinase activity in acute myeloid leukemia derives from Flt3 and affects survival and proliferation. Exp Hematol. 2006;34:1360–1376. doi: 10.1016/j.exphem.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- Jacobs M, Hayakawa K, Swenson L, Bellon S, Fleming M, Taslimi P, Doran J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J Biol Chem. 2006;281:260–268. doi: 10.1074/jbc.M508847200. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kitayama H, Kanakura Y, Furitsu T, Tsujimura T, Oritani K, Ikeda H, Sugahara H, Mitsui H, Kanayama Y, Kitamura Y, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85:790–798. [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia. 1998;12:1333–1337. doi: 10.1038/sj.leu.2401130. [DOI] [PubMed] [Google Scholar]
- Koschmieder S, Gottgens B, Zhang P, Iwasaki-Arai J, Akashi K, Kutok JL, Dayaram T, Geary K, Green AR, Tenen DG, Huettner CS. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105:324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I, Griffin C, Emanuel B, Finan J, Nowell P, et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood. 1987;70:192–199. [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L, et al. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Molecular and cellular biology. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead PA, Wickman G, Mezna M, Olson MF. Activating ROCK1 somatic mutations in human cancer. Oncogene. 2010 doi: 10.1038/onc.2010.3. [DOI] [PubMed] [Google Scholar]
- Lugo TG, Pendergast AM, Muller AJ, Witte ON. Science. Vol. 247. New York, NY: 1990. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products; pp. 1079–1082. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zeng S, Metcalfe DD, Akin C, Dimitrijevic S, Butterfield JH, McMahon G, Longley BJ. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99:1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- Munugalavadla V, Sims EC, Borneo J, Chan RJ, Kapur R. Genetic and pharmacologic evidence implicating the p85 alpha, but not p85 beta, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood. 2007;110:1612–1620. doi: 10.1182/blood-2006-10-053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munugalavadla V, Sims EC, Chan RJ, Lenz SD, Kapur R. Requirement for p85alpha regulatory subunit of class IA PI3K in myeloproliferative disease driven by an activation loop mutant of KIT. Exp Hematol. 2008;36:301–308. doi: 10.1016/j.exphem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Nagata H, Worobec AS, Oh CK, Chowdhury BA, Tannenbaum S, Suzuki Y, Metcalfe DD. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92:10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS letters. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. The Journal of biological chemistry. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Piao X, Bernstein A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood. 1996;87:3117–3123. [PubMed] [Google Scholar]
- Pratz KW, Sato T, Murphy KM, Stine A, Rajkhowa T, Levis M. FLT3 mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood. 2009 doi: 10.1182/blood-2009-09-242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2:133–142. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- Schuebel KE, Movilla N, Rosa JL, Bustelo XR. Phosphorylation-dependent and constitutive activation of Rho proteins by wild-type and oncogenic Vav-2. EMBO J. 1998;17:6608–6621. doi: 10.1093/emboj/17.22.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E. Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci. 2005;238:31–39. doi: 10.1016/j.jns.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T, Todoroki-Ikeda N, Mogami K, Mizukami Y, Kuriyama S, et al. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Tsuji Y, Satoh S, Minoura H, Murakami K, Okuno A, Inukai K, Asano T, Kaburagi Y, Ueki K, et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85 alpha subunit of phosphoinositide 3-kinase. Nat Genet. 1999;21:230–235. doi: 10.1038/6023. [DOI] [PubMed] [Google Scholar]
- Vemula S, Ramdas B, Hanneman P, Martin J, Beggs HE, Kapur R. Essential role for focal adhesion kinase in regulating stress hematopoiesis. Blood. 2010a;116:4103–4115. doi: 10.1182/blood-2010-01-262790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010b;115:1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, Carroll AJ, Mrozek K, Vardiman JW, George SL, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.