Abstract
A conditioned taste aversion (CTA) is acquired when an animal consumes a novel taste (CS) and then experiences the symptoms of poisoning (US). Following CTA training, animals will avoid the taste that was previously associated with malaise. This defensive reaction to a learned fear can be extinguished by repeated exposure to the CS alone (CS-only; CSO-EXT). However, following a latency period in which the CS is not presented, the CTA will spontaneously recover (SR). Through the use of an explicitly unpaired extinction procedure (EU-EXT) we have shown that we can speed up extinction and attenuate SR of the CTA. Here we compared and contrasted the ability of CSO and EU extinction procedures to affect c-Fos expression in the periaqueductal gray (PAG).
Fluid-deprived Sprague-Dawley rats acquired a strong CTA [via 3 pairings of 0.3% oral saccharin (SAC; the CS) and 81 mg/kg i.p. lithium chloride (LiCl; the US)] followed by extinction trials consisting of multiple exposures to either, (a) the CS every-other day (CSO-EXT), or (b) CS and US on alternate days (EU-EXT). A different group of rats did not receive multiple CS exposures and served as a “no extinction” (NE) control. Both extinction procedures resulted in ≥90% reacceptance of SAC (achieving asymptotic extinction). Some of the animals were sacrificed for c-Fos immunohistochemical analysis following asymptotic extinction. Other rats entered a 30-day latency period where they drank water only. These remaining animals were then tested for SR with a final exposure to SAC before being sacrificed for c-Fos immunohistochemistry.
As reported previously, rats in the CS-only group exhibited a significant SR of the CTA. However, animals in the EU extinction group reached asymptotic extinction more rapidly than did CSO rats and they did not show SR of the CTA. As compared to rats that retained their CTA, both groups of extinguished rats showed suppression in the number of c-Fos-labeled neurons in all 4 longitudinal columns of the PAG. The number of c-Fos-labeled cells in the PAG was generally low but there was a reliable increase in c-Fos expression in dorsolateral PAG (dlPAG) following the SR test in the brains of rats that went through the EU-EXT procedure as compared with those that either went through the more-traditional CSO extinction procedure or experienced no extinction at all. The number of c-Fos-labeled neurons in the dlPAG was significantly correlated with the amount of SAC consumed at the SR test. Surprisingly, the brains of EU-extinguished rats and CSO extinguished rats did not differ in the number of c-Fos-labeled neurons in gustatory neocortex, medial prefrontal cortex, basolateral amygdala, or the central nucleus of the amygdala. Thus, behavioral differences in SR between the EU and CSO extinction animals were not represented by corresponding changes in the neural activity of several brain nuclei classically associated with extinction learning. However a detailed analysis of PAG c-Fos expression provided hints about some of the physiological changes evoked by these 2 extinction paradigms that produce very different behavioral outcomes. The findings are clinically relevant as we seek the development of treatments for deficits in fear extinction (e.g. PTSD, phobias).
Keywords: periaqueductal gray, c-fos, conditioned taste aversion, CTA, extinction, spontaneous recovery, learning, memory, PAG
1. Introduction
Many fears are learned when a previously neutral stimulus (CS) becomes associated with a naturally aversive one (US) through the process of Pavlovian conditioning (Pavlov, 1927). Therapy for anxiety disorders such as phobias and PTSD often utilize techniques based on learning theory that are aimed at breaking or weakening the CS+US bond (Norrholm et al., 2011). These exposure therapies are essentially a type of extinction that typically attempt to reduce fears by presenting the now-aversive CS without the US (CS-only extinction, CSO; Graham et al., 2010). Unfortunately, fears can re-emerge following the passage of time (i.e., spontaneous recovery; SR) or through other forms of relapse (Bouton, 1993; Rescorla and Heth, 1975).
Substantial recent efforts have been made to devise more effective therapies that reduce or eliminate the SR or relapse of fears (Kaplan et al., 2011). Moreover, there has been great interest in identifying the brain areas and neurochemical mechanisms involved in the acquisition, extinction, and SR of conditioned emotional responses (CERs) (for a recent review see Herry et al., 2010) so that more-effective behavioral (Urcelay et al., 2009) and pharmacological therapies (Graham et al., 2010; Debiec et al., 2011) may be developed. This work has been quite fruitful and we now know, for example, that the mPFC, amygdala, hippocampus, and the midbrain periaqueductal gray are involved in various aspects of the extinction of conditioned fears (Sierra-Mercado et al., 2011; Sotres-Bayon and Quirk, 2010; Peters et al., 2009; Li et al., 2009; Quirk and Mueller, 2008; Barad, 2005; McNally et al., 2004).
In order to take advantage of our knowledge about how learning, more generally, could influence development of therapies for fear and anxiety, Groblewski et al. (2009) have called for studies that go beyond the CER. Our recent research has been investigating the conditioned taste aversion (CTA) (Garcia, et al., 1955). Rats will avoid a taste that has been previously associated with malaise (Mickley et al., 2004; 2005). The CTA has been described as a defensive reaction to a learned fear (Parker, 2003) but the extent to which fear mediates the aversion is not settled (Akirav et al., 2009). Still, it is a form of aversive learning that is biologically meaningful and has distinct characteristics (e.g., rapid acquisition and resistance to extinction; Nolan et al., 1997) that may make it a useful model as we seek therapies for anxiety disorders such as phobias and PTSD. Further, CTA extinction employs some of the same neural circuits as does the extinction of CERs. Specifically, we have reported changes in neural activity (as measured through c-Fos immunohistochemistry) in the amygdala and mPFC that correlate with various stages of extinction and SR of a CTA (Mickley et al, 2004; 2005; 2007).
Additional behavioral studies from our laboratory indicate that there are techniques that may be employed to modulate the speed and stability of CTA extinction. For example, we have reported that, by explicitly unpairing the CS and US during extinction training (i.e., through alternate day presentations; EU-EXT), we could speed the rate of extinction and substantially reduce SR (Mickley et al., 2009) when compared to extinction procedures during which the CS-only is presented (CSO-EXT). These studies build on the reports of other labs indicating that the EU-extinction procedure can thwart renewal of conditioned fears (Rauhut et al., 2001; Thomas et al., 2005).
Given the distinctive behavioral outcomes produced by the EU-EXT and CSO-EXT paradigms, the current study was aimed at describing neural correlates that distinguish between these 2 methodologies. In particular, we focused on c-Fos protein expression in the PAG following CTA extinction and SR tests. It is well known that the PAG is involved in defensive reactions to natural and learned fears (Carrive, 1993) and stimulation of the PAG produces an increase in running, jumping, blood pressure, tachycardia, and blood flow redistribution (Morgan et al., 1998). We also selected the PAG for analysis since it has a close functional connection with the mPFC and amygdala - structures that contribute to modulation of emotional responses (Pare et al., 2004; Price, 2005; Peters et al., 2009; Ulrich-Lai and Herman, 2009; Floyd et al., 2000) and extinction of conditioned fears (McNally et al., 2004; 2005). The PAG is not part of the classically identified neural circuit that subserves CTA learning (Yamamoto and Fujimoto, 1991; Yamamoto, 1993; Yamamoto et al., 1994; 1997). However, the mPFC and amygdala that connect with PAG and mediate CER extinction and renewal (Quirk and Mueller, 2008; Bruchey et al., 2007) also play important role in CTA extinction and SR (Mickley et al., 2004; 2005; 2007).
Here we report that CTA extinction, produced by either EU-EXT or CSO-EXT methods, produced suppression in the number c-Fos immunoreactive neurons in the PAG. However, there was a significant increase in c-Fos expression in the dlPAG only in the group of rats that experienced EU-EXT and did not exhibit SR of the CTA.
2. Results
2.1 CTA Acquisition
Following three saccharin (SAC) and Lithium Chloride (LiCl) pairings all rats had acquired a strong CTA. SAC consumption decreased steadily over the three conditioning days and the first day of extinction for all animals and all groups. A t-test revealed that there was a significant drop in the SAC consumed on the first day of naïve SAC exposure as compared to the first day of extinction (i.e., following 3 SAC+LiCl pairings) [t(38) = 9.56; p < 0.001].
To verify that the rats in our 3 treatment groups (CSO-EXT, EU-EXT, and NE; see Table 1) had acquired the same level of aversion to SAC during the CTA acquisition phase of the study, the SAC consumption on the first day of extinction was compared using a one-way ANOVA [treatment groups (CSO-EXT, EU-EXT, and NE)]. The mean volumes of SAC consumed on the first day of extinction training by rats in all 3 groups were not significantly different from one another.
Table 1.
Group designations and summary of procedures
| Group Designation | Conditioning | Extinction3 | 30-day SR test4 | Number of rats5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Odd Days | Even Days | |||
| CS-Only Extinction (CSO-EXT) | SAC1+LiCl2 | Water | SAC+LiCl | Water | SAC+LiCl | Water | SAC | Water + SAL | SAC | 12/6/6 |
| Explicitly Unpaired Extinction (EU-EXT) | SAC+LiCl | Water | SAC+LiCl | Water | SAC+LiCl | Water | SAC | Water + LiCl | SAC | 11/5/6 |
| No Extinction (NE) | SAC+LiCl | Water | SAC+LiCl | Water | SAC+LiCl | Water | Water | Water + SAL | SAC | 16/9/7 |
SAC = 30 min presentation of the 0.3%, weight by volume, saccharin salt solution. The SAC was dissolved in deionized water and presented to animals in a single bottle test.
LiCl = Injection of lithium chloride (81 mg/kg; 1 ml/kg; i.p.), dissolved in physiological saline.
Extinction = EU-EXT refers to the method in which SAC and LiCl were administered to animals on alternate days throughout the extinction phase of the study. SAC was administered every-other day to animals designated as CSO-EXT, but physiological saline (SAL, i.p.) injections were administered in lieu of lithium chloride, as a control measure, on water-only days. Note that animals in the NE group were never given extinction training, but were instead given only water throughout this phase of the study to parallel the fluid presentation schedule of the other groups. However, NE rats were offered SAC on the day their yoked counterpart reached asymptotic extinction. SAC offerings were followed by 30-min supplemental water presentations to reduce the chance of dehydration. There was never any indication that EU-EXT rats formed an aversion to water.
SR Test Solution = All animals received water only for 29 days and on the 30th day all received 0.3% SAC solution as the SR test solution. Note that for animals in the NE group this was not a true test of SR, but simply a final CS re-exposure to establish the strength of the CTA prior to sacrifice.
The first number indicates the total number of rats in each group. The second number represents the number of rats that were sacrificed following asymptotic extinction. The third number represents the number of rats sacrificed following the spontaneous recovery test.
2.2 CTA Extinction and Spontaneous Recovery
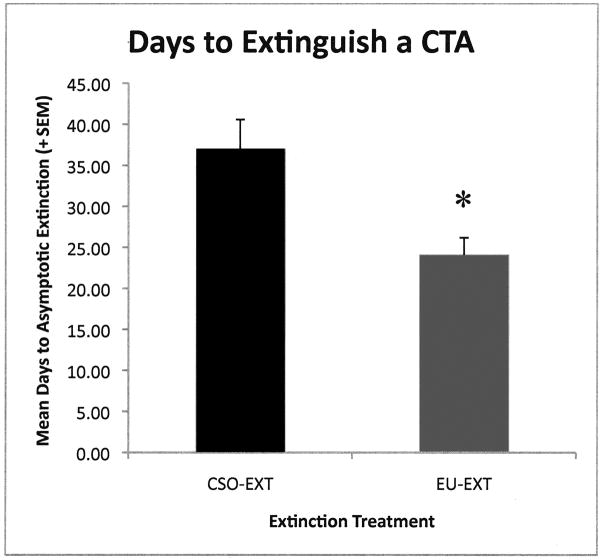
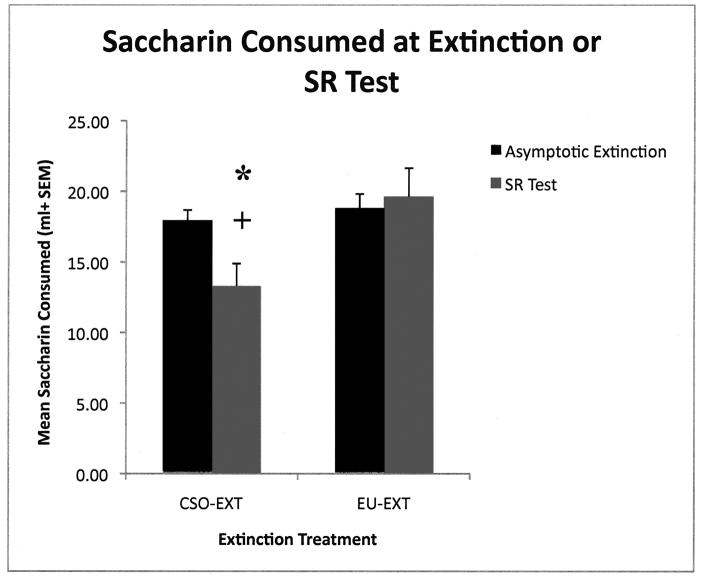
As reported previously (Mickley et al., 2009) rats exposed to the EU-EXT method extinguished the CTA rapidly and did not exhibit spontaneous recovery as compared to rats that experienced the CSO-EXT procedure. A t-test revealed that rats in the EU-EXT group took significantly fewer days to extinguish their CTA than did the CSO-EXT extinction group [t (21) = 3.00; p = 0.007; see Figures 1 and 3]. Further, the CSO-EXT animals exhibited SR of the CTA, but the EU-EXT group did not (see Figure 2). Rats in the explicitly unpaired extinction group drank about the same amount of SAC on the day of extinction as they did on the 30-Day SR test day. However, rats in the CSO-EXT group drank significantly less SAC on the day of the SR test than on the day these animals reached asymptotic extinction [t (5) = 2.72; p = 0.042]. Likewise, SAC drinking at the SR test was significantly less in the CSO-EXT rats than in the EU-EXT rats [t (9) = 2.47; p = 0.036]. There was no indication that rats going through the EU-EXT procedure formed an aversion to the very-familiar water. Rats in the 3 extinction groups (CSO, EU and NE) drank similar amounts of fluid each day.
Figure 1.
Mean days (± SEM) required to extinguish a CTA. Rats in the explicitly unpaired extinction group (EU-EXT) took significantly fewer days to extinguish the learned fear than the CS-only extinction group (CSO-EXT). * = Significantly different from the CSO-EXT Group (p < 0.05).
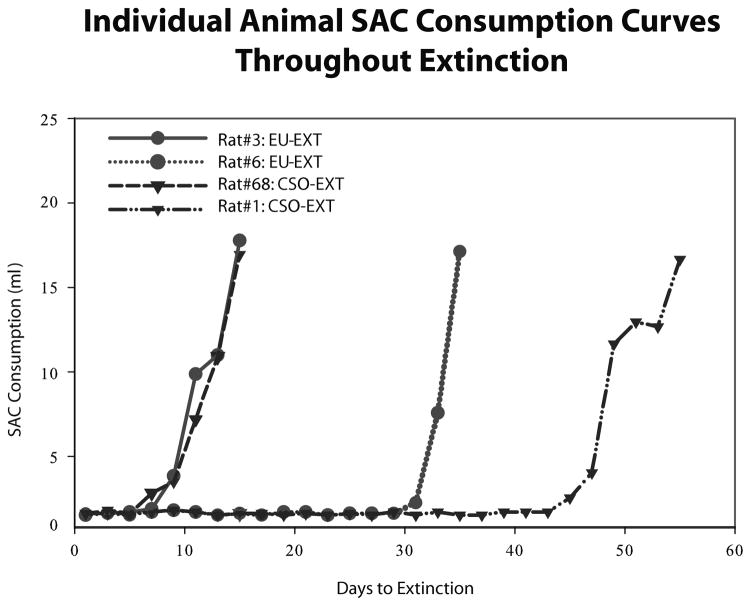
Figure 3.
Selected SAC consumption curves for individual animals throughout extinction. The extinction curves of the slowest animal to extinguish and fastest animal to extinguish are presented from each of the two main experimental groups (EU-EXT = circles; CSO-EXT = inverted triangles). Rats in the EU-EXT group took a smaller range of days to extinguish than did rats in the CSO-EXT group.
Figure 2.
Mean (± SEM) SAC consumption on the day of asymptotic extinction or spontaneous recovery test. Rats in the CSO-EXT extinction group drank significantly less SAC during the SR test than on the day of extinction indicating a spontaneous recovery of the CTA [* = p < 0.05]. Animals in the EU-EXT group did not exhibit a SR. SAC drinking at the SR test was significantly less in the CSO-EXT rats than in the EU-EXT rats [+ = p < 0.05]. These data indicate that the CS-only extinction animals showed a SR of the CTA, but the EU-EXT group did not.
2.3. SAC drinking in rats that did not go through CTA extinction (NE condition)
The non-extinguished (NE) rats did not go through an extinction procedure but were given an opportunity to drink SAC on the same day their yoked pair reached asymptotic extinction or was given a test for SR of the CTA. All of these rats maintained a strong CTA throughout the study. The SAC consumed by rats in the NE group at the test coinciding with the yoked pair’s achievement of asymptotic extinction was 0.456 ml ± 0.295 (Mean ± SEM). At the time of the “SR test” these NE rats drank only 0.086 ml ± 0.026 (Mean ± SEM). These levels of SAC consumption were not significantly different from those observed when the animals had just completed CTA training.
2.4 c-Fos Immunohistochemistry
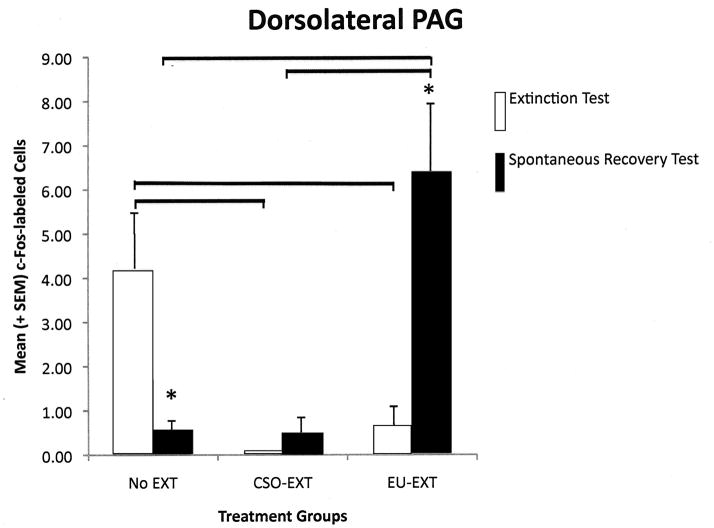
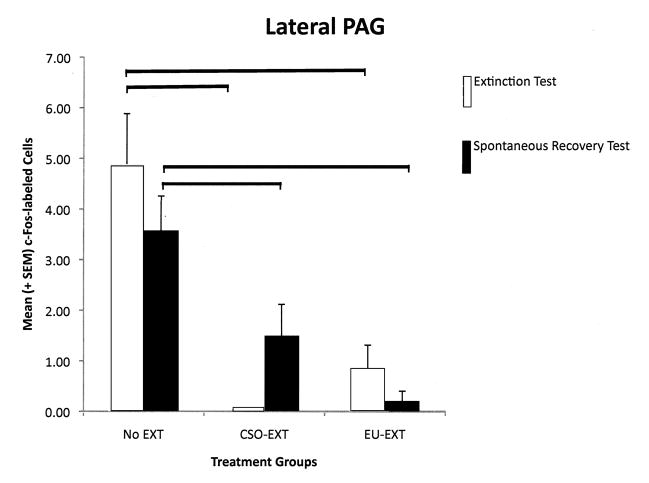
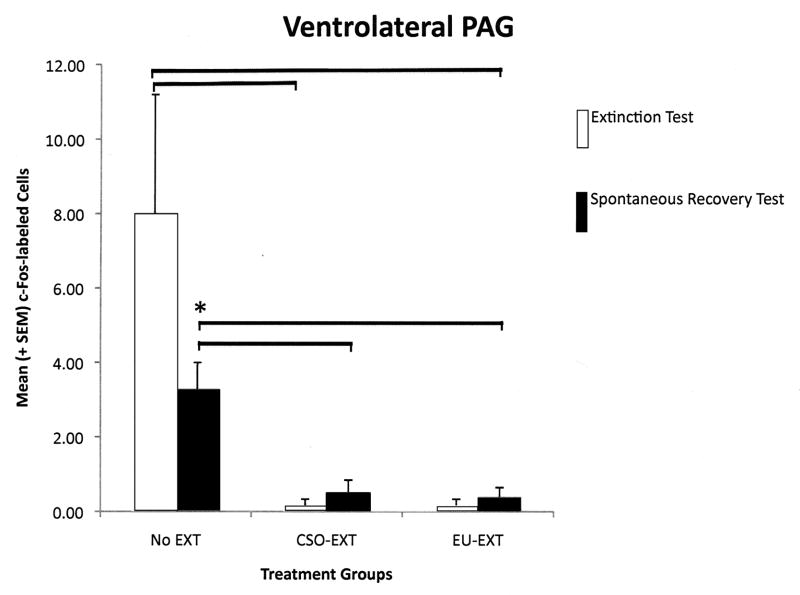
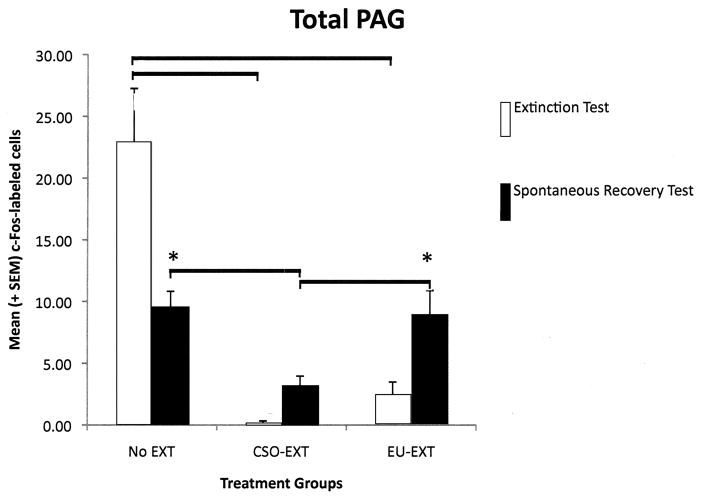
Each longitudinal column of the PAG reflected a different pattern of c-Fos protein expression depending on the extinction method employed and whether the rats were sacrificed following asymptotic extinction or after the SR test. However, several general themes emerged (see Figure 4, A–E). As compared to rats that maintained their CTA (NE group), EU-EXT or CSO-EXT animals, having achieved asymptotic extinction, uniformly suppressed c-Fos expression in all 4 longitudinal columns of the PAG. Rats that underwent the CSO or EU extinction procedures did not differ in the number of cells exhibiting c-Fos expression in the PAG when they reached asymptotic EXT. However following the SR test, the PAG of EU-EXT rats exhibited a small, but reliable, increase in c-Fos expression as compared to the PAG of CSO-EXT animals. Most dramatic were the findings in the dlPAG where c-Fos protein was detected in more neurons after the SR test if the rats experienced the EU-EXT procedure instead of the CSO-EXT procedure. There was also a significant positive correlation between the number of dlPAG cells expressing c-Fos and the amount of SAC consumed at the SR test. Rats that drank more SAC (indicating retention of the extinguished CTA, i.e., a reduced SR) also exhibited more c-Fos-labeled cells in the dlPAG. This behavioral-anatomical relationship was not seen in the cell counts of other portions of the PAG.
Figure 4.
A–E The number of c-Fos-labeled cells counted in the entire PAG or its individual longitudinal columns (A = dmPAG; B = dlPAG; C = lPAG; D = vlPAG; E = Total cell counts for entire PAG). CTA extinction generally caused a suppression of PAG c-Fos expression (in comparison with non-extinguished controls) independent of the extinction methodology employed. The c-Fos expression that accompanied SR of the CTA was more variable and PAG-column-specific with generally no differences between rats that went through CSO-EXT or EU-EXT procedures. However, rats that experienced EU-EXT exhibited a significant increase in dlPAG c-Fos as compared with rats that went through CSO-EXT. * = Statistically significant difference between c-Fos labeling at asymptotic extinction vs. SR test. The horizontal bars represent significant differences between particular treatment groups - evaluated via ANOVAs [Extinction Treatment (CSO or EU) × Test day (Asymptotic EXT or SR)] and Bonferroni post hoc comparisons. α = 0.05.
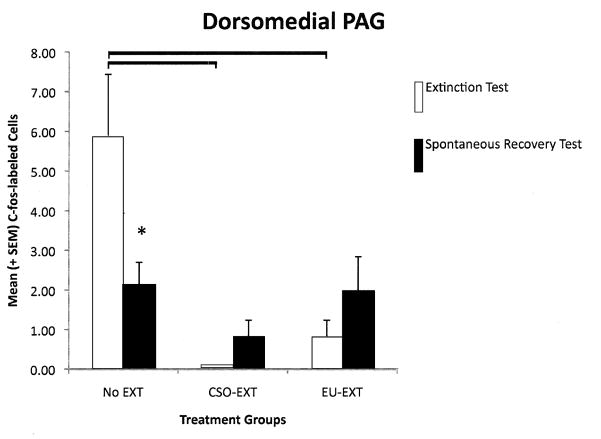
2.4.1 Dorsomedial PAG (dmPAG)
When we analyzed the number of cells expressing c-Fos protein in the dmPAG, a 2-way ANOVA revealed a significant main effect of extinction treatment [F(2,33) = 7.84, p = 0.002] and a significant interaction between extinction treatment and time of testing (at asymptotic extinction or at the SR test) [F(2,33) = 4.39, p = 0.020]. Pair-wise group comparisons indicated that, when rats reached asymptotic extinction through either the CSO or EU methods, these animals expressed fewer c-Fos-labeled cells in the dmPAG than did rats that retained the CTA (NE group). Despite the fact that the NE rats retained a strong CTA throughout the study, there was a significant reduction in the number of neurons expressing c-Fos-like immunoreactivity as rats in the NE group progressed from the “asymptotic extinction” to the “SR” test. See Figure 4A. There was not a significant correlation between the amount of SAC consumed during the SR test and the number of cells in the dmPAG expressing c-Fos protein.
2.4.2 Dorsolateral PAG (dlPAG)
A 2-way ANOVA investigating the number of cells expressing c-Fos protein in the dlPAG, revealed a significant main effect of extinction treatment [F(2,33) = 4.14, p = 0.025] and a significant interaction between extinction treatment and time of testing (at asymptotic extinction or at the SR test) [F(2,33) = 8.80, p = 0.001]. Pair-wise group comparisons indicated that rats achieving asymptotic extinction of their CTA through either the CSO or EU-EXT procedures expressed fewer c-Fos-labeled neurons than did the NE rats that retained the CTA. However, rats in the EU-EXT group significantly increased the number of c-Fos-labeled neurons in the dlPAG following the SR test (as compared to the EU-EXT rats sacrificed following achievement of asymptotic extinction). Further, after the SR test, animals in the EU-EXT group exhibited more c-Fos-labeled neurons than did either the rats in the CSO-EXT or NE groups. See Figure 4B. There was a significant positive correlation between the amount of SAC consumed during the SR test and the number of cells in the dlPAG expressing c-Fos protein [r(16) = 0.56, p = 0.016].
2.4.3 Lateral PAG (lPAG)
When we analyzed the number of cells expressing c-Fos protein in the lPAG, a 2-way ANOVA revealed a significant main effect of extinction treatment [F(2,33) = 7.84, p = 0.002] but not a significant effect of time of testing/sacrifice. Nor was there a significant interaction between extinction treatment and the time of testing (at asymptotic extinction or at the SR test). Pair-wise comparisons among the treatment groups indicated that when rats reached asymptotic extinction through either the CSO or EU methods, these animals expressed fewer c-Fos-labeled cells in the lPAG than did rats that retained the CTA (NE group). This difference persisted and was also observed following the SR test. However, there were no differences in lPAG c-Fos expression between the rats in the EU-EXT groups vs. those in the CSO-EXT groups. See Figure 4C. There was a significant negative correlation between the amount of SAC consumed during the SR test and the number of cells in the lPAG expressing c-Fos protein [r(16) = 0.69, p = 0.001].
2.4.4 Ventrolateral PAG (vlPAG)
The pattern of c-Fos labeled cells in the vlPAG closely paralleled those in the lPAG. A 2-way ANOVA investigating the number of cells expressing c-Fos protein in the vlPAG, revealed a significant main effect of extinction treatment [F(2,33) = 5.75, p = 0.007]but not a significant effect of time of testing/sacrifice. Nor was there a significant interaction between extinction treatment and the time of testing (at asymptotic extinction or at the SR test). Pair-wise comparisons among the treatment groups indicated that when rats reached asymptotic extinction through either the CSO or EU methods, these animals expressed fewer c-Fos-labeled cells in the vlPAG than did rats that retained the CTA (NE group). This difference persisted and was also observed following the SR test. However, there were no differences in vlPAG c-Fos expression between the rats in the EU-EXT groups vs. those in the CSO-EXT groups (see Figure 4D). There was a significant negative correlation between the amount of SAC consumed during the SR test and the number of cells in the vlPAG expressing c-Fos protein [r(16) = 0.72, p = 0.001].
2.4.5 Total PAG (tPAG)
In a final analysis we combined the cell counts for all the longitudinal columns of the PAG. A 2-way ANOVA revealed a significant main effect of extinction treatment [F(2,33) = 17.90, p < 0.001] and a significant interaction between extinction treatment and time of testing (at asymptotic extinction or at the SR test) [F(2,33) = 8.60, p = 0.001]. Pair-wise group comparisons indicated that, when rats reached asymptotic extinction through either the CSO or EU methods, these animals expressed fewer c-Fos-labeled cells in the tPAG than did rats that retained the CTA (NE group). Despite the fact that the NE rats retained a strong CTA throughout the study, there was a significant reduction in the number of neurons expressing c-Fos-like immunoreactivity as rats in the NE group progressed from the “asymptotic extinction” to the “SR” test. Following SAC consumption at asymptotic extinction, there was no difference between the number of c-Fos-labeled neurons in the EU-EXT or CSO-EXT groups. However, rats in the EU-EXT group expressed more c-Fos after the SR test than they did when they reached asymptotic extinction. Further, the animals that went through the EU-EXT procedure exhibited more c-Fos-labeled neurons at the SR test than did the CSO-EXT rats. See Figure 4E. There was not a significant correlation between the amount of SAC consumed during the SR test and the number of cells in the tPAG expressing c-Fos protein.
2.4.6 Analysis of other brain areas
We previously published data representing patterns of c-Fos expression following CTA extinction (Mickley et al., 2004; 2005) and SR (Mickley et al., 2007) using the CSO method. The data from the current study closely replicated those findings and are not reproduced here. Compared to rats that retained a CTA, extinction and SR of the CTA modulated the number of c-Fos-labeled neurons in several of the brain areas known to be engaged in CTA extinction learning: medial prefrontal cortex (mPFC)(prelimbic or infralimbic), gustatory neocortex (GNC), and amygdala (basolateral or central nuclei) (Mickley et al., 2004; 2005) and SR (Mickley et al., 2007). But the current study identified no significant differences between EU-EXT and CSO-EXT brains in the number of neurons exhibiting c-Fos expression in these brain areas (data not presented here). This was true for both animals sacrificed at asymptotic extinction and following the SR test. Therefore, as long as asymptotic extinction is achieved, the method of CTA extinction employed does not modify the pattern of c-Fos immunoreactivity in several brain areas that have been closely associated with both CER and CTA extinction learning (Herry et al., 2010; Li et al., 2009; Quirk and Mueller, 2008; Mickley et al., 2004; 2005).
3. Discussion
3.1 General summary
As we reported previously (Mickley et al., 2009), extinction learning that employed the EU-EXT procedure of disassociating the CS and US produced more rapid extinction of a CTA and also inhibited spontaneous recovery of this defensive reaction to a learned fear. Our immunohistochemical analysis revealed that c-Fos expression throughout the PAG was significantly lower in rats that underwent either CTA extinction procedure (CSO or EU) as compared to animals that maintained a CTA (NE group). Further, our data indicated that the number of neurons expressing c-Fos protein in the dlPAG was higher in rats failing to show a significant SR (EU-EXT group) as compared to those exhibiting a SR of the CTA (CSO group). The number of c-Fos-labeled neurons in the dlPAG was significantly correlated with the amount of SAC consumed at the SR test. These data are consistent with other studies indicating that the midbrain PAG, and the dlPAG in particular, are important in modulating defensive responses to conditioned fears (Bittencourt et al., 2004; Moreira et al., 2009). However, it is the first study to identify a possible role for the PAG in CTA extinction and SR.
3.2 The PAG and defensive behaviors
The midbrain PAG is a functionally heterogeneous structure (Carrive, 1993). Classic studies of this brain area indicate that it plays important roles in CNS mechanisms of pain (Willis, 1988), cardiovascular functioning (Carrive et al., 1988), and production of defensive/protective behaviors (Carrive, 1993). Considerable recent attention has focused on active and passive emotional coping (Bandler et al., 2000) and conditioned and non-conditioned fear reactions that may be mediated by neurons in the PAG (Vianna and Brandao, 2003). The PAG is well situated to receive projections from various limbic forebrain areas (e.g., central amygdala, mPFC) and send afferents to the spinal cord in the expression of fear behaviors (LeDoux et al., 1988). Thus, evidence builds supporting the idea that the PAG is an important site for the registration of fear, fear memories, defensive reactions to these memories, or even changes in the representations of these memories.
This work has been supplemented by interest in the PAG’s longitudinal columns and the distinct functional roles they may play in the production, or elimination, of fear (Bandler et al., 2000; Vianna & Brandao, 2003). The different longitudinal columns of the PAG likely play different roles in producing different defensive behaviors. For example, Borelli et al. (2006) have reported that c-Fos expression in the dmPAG is prominent during freezing responses whereas the dlPAG exhibits more activity during escape. Other investigators have suggested a broader role for dlPAG indicating that it mediates both passive and active emotional coping (Keay and Bandler, 2001) and fight or flight reactions (e.g., running, jumping, blood pressure increase, tachycardia, and blood flow redistribution; Morgan et al., 1998). The vlPAG has been confirmed as an important structure for the expression of fear responses (De Oca et al., 1998; LeDoux et al., 1988) and for extinction learning (McNally et al., 2004; 2005).
In particular, McNally and colleagues have built a strong case that extinction of conditioned fear is mediated through activation of neurons in the vlPAG (McNally, 2005) and that endogenous opioids and μ-opioid receptors are critical to fear extinction (McNally et al., 2004; McNally et al., 2005). Our data are, in some ways, consistent with the proposition that the vlPAG plays a role in extinction because we saw a significant difference between the numbers of c-Fos-positive cells in the vlPAG of extinguished vs. non-extinguished rats. However, here we also report that this difference was not specific since it was seen in all 4 longitudinal columns of the PAG. There are numerous intrinsic connections within the PAG (Beitz, 1982) that may have helped distribute activity in this structure and mediate common functions. For example, Vianna et al. (2001) reported that common functions (e.g., the species-specific defense response of freezing) may be mediated by both dorsal PAG and vlPAG. On the other hand, reciprocal interactions between dlPAG and ventral PAG have been proposed (De Oca et al., 1998) that would suggest that we should expect quite different activity in these portions of the structure at least during acquisition and expression of defensive responses. Further, McNally et al., (2004) have reported that microinjections of naloxone into the dorsal PAG do not impair the extinction of fear conditioning whereas the same injections in the vlPAG block the extinction process. For a more-complete discussion of the functional differentiation between the vlPAG and dlPAG subdivisons see Vianna and Brandao (2003) who have proposed that defensive reactions mediated by the PAG subdivisions may depend on the perceived proximity of the danger. Perhaps a CTA produces a fear or anxiety that could be perceived as more-remote, in time or probability, than that produced by a predator or through experimental manipulation of foot shocks delivered in a CER paradigm. Further exploration of this theory is warranted.
3.3 PAG and CTA acquisition, extinction, and SR
We observed relatively high numbers of c-Fos expressing neurons in the rats that retained a CTA (NE group) and a consistent decrease in PAG c-Fos-labeled neurons that accompanied CTA extinction. These data are consistent with other reports suggesting that enhanced c-Fos-like immunoreactivity occurs when rats are anxious or fearful, e.g., during exposure to a natural predator (Canteras and Goto, 1999) or when they have developed a conditioned place aversion (Zanoveli et al., 2007). Reversal of these effects may be expected if a fear was extinguished. We observed such a reduction in c-Fos expression in the brains of our rats that had undergone CTA extinction.
We present evidence that c-Fos expression in the PAG does not merely represent the amount of SAC consumed or the sensory aspects of this sweet taste. For example, the NE rats retained their CTA (and drank almost no SAC) but the number of c-Fos-labeled neurons in most of the longitudinal columns (except lPAG) changed in the time between the end of “extinction” and the “SR” measures for these animals. Likewise, the EU-EXT rats did not exhibit a SR and drank similar amounts of SAC during asymptotic extinction and at the SR test while simultaneously exhibiting different levels of c-Fos expression in the dlPAG. Thus the volume of SAC consumed was not always a good predictor of the number of c-Fos-labeled PAG neurons we observed.
If the change in the number of c-Fos-labeled PAG cells does not simply represent SAC ingestion or sensation then what does this alteration in c-Fos expression from extinction to SR actually mean? There is some evidence that the pattern of PAG c-Fos labeling we observed is a reflection of associative/disassociative processes. For example, our data suggest that extinction of a CTA is accompanied by a general reduction of PAG neuronal activity and that enhancement of the dlPAG c-Fos expression, in particular, may be correlated with protection from SR. However, we should recognize that other explanations are possible as well. During the 30-day latency period following asymptotic extinction rats drank only water. However, on the SR test day they were again confronted with SAC. The shock of being re-exposed to the once-aversive (or still aversive) taste might be reflected in our c-Fos data. In fact, McNally et al. (2004) proposed that the vlPAG contributes information about the mismatch between the actual and expected outcome of a CS presentation and sends it to the amygdala via pathways employing NMDA-glutamate receptors (Cole and McNally, 2010; McNally and Westbrook, 2010). Re-experiencing a SAC CS after a 30-day hiatus may produce such a mismatch.
C-Fos protein expression in the PAG, and the dlPAG in particular, is enhanced in rats that underwent EU-EXT and show no SR of the CTA. However, it is important to note that our data are correlative and do not indicate that more c-fos in the dlPAG causes suppression of SR. Future studies in our laboratory are aimed at determining if manipulation of dlPAG can reverse or potentiate these effects thereby revealing the extent to which dlPAG controls SR of this defensive reaction to a learned fear.
In a related study, Bruchey and Gonzalez-Lima (2006) used flurodeoxyglucose mapping and structural equation modeling to assess brain activity that is associated with context-dependent fear extinction and renewal. They noted that activity of vlPAG neurons was significantly correlated with freezing behavior associated with the re-emergence (renewal) of a once-extinguished conditioned fear response. Infralimbic mPFC effects on the vlPGA were found to be negative following extinction and near zero at renewal. Influences coming from the amygdala showed a reversal of sign indicating that an increase in activity in the lateral and central-medial amygdala may lead to a proportional decrease in vlPAG activity after extinction while an increase in amygdala activity at renewal may lead to an increase in vlPAG activity (Bruchey et al., 2007). These effects were not seen in dorsal PAG and dlPAG, specifically, was not sampled. Still, the data suggest that renewal, a learning phenomenon that has several common features with SR, engages portions of the PAG through connections with forebrain structures.
As described in the introduction, CTA extinction is mediated by several brain areas that also subserve classical fear conditioning (e.g., produced by tone+shock pairings) (Pare et al., 2004; Price, 2005; Peters et al., 2009; Ulrich-Lai and Herman, 2009; Floyd et al., 2000) and the extinction of conditioned fears (Quirk and Mueller, 2008). For example, c-Fos protein immunohistochemical analyses have demonstrated that the mPFC, basolateral amygdala (BLA), and GNC play a significant role in CTA extinction (Mickley et al., 2004; 2005). Furthermore, it has been shown that neural activity in both the mPFC and GNC is significantly altered during SR of a CTA (Mickley et al, 2007). The anatomical connections between these structures and the PAG (for review, see Saper, 1995; Jasmin et al., 2004; Mota-Ortiz et al., 2009) lead us to investigate a possible role for the PAG in CTA extinction and SR.
The PAG is not part of the classic neuroanatomical pathway known to mediate CTA acquisition (Yamamoto and Fujimoto, 1991; Yamamoto, 1993; Yamamoto et al., 1994;1997) although it communicates with brain areas that are part of that pathway (e.g., amygdala, parabrachial nucleus; see Beitz, 1982; Krout et al., 1998; Saper, 1995). In fact, lesion studies indicate that neither vlPAG nor dlPAG lesions impair the acquisition of a CTA (De Oca et al., 1998). On the contrary, Blair and Amit (1981) reported that PAG lesions reversed a CTA – but only if it was produced using morphine as the US. Therefore, the extent to which CTAs may be mediated by PAG neurons remains ambiguous.
Although CTA has been described as a defensive reaction to a learned fear (Parker, 2003), the extent to which fear mediates the aversion is also not settled (Akirav et al., 2009). The CTA is a form of aversive learning that is certainly biologically meaningful and it has distinct characteristics (Domjan, 1993) that may make it a useful model as we seek therapies for anxiety disorders such as phobias and PTSD. For example, robust CTAs are readily acquired with few CS+US pairings even when CS and US are not presented in close temporal proximity. Moreover, like cases of PTSD, CTAs can be quite tenacious and extinction may take weeks or months (Domjan, 1993; Nolan et al. 1997). It has yet to be determined if these distinct characteristics of the CTA phenomenon help explain the differences in anatomical substrate reported between extinction/SR of CTAs and conditioned fears produced through tone+footshock pairings.
It should be noted that the differences in c-Fos labeling in the dlPAG observed between rats that experienced an SR of a CTA and those that did not exhibit an SR were small, but reliable. The generally low number of cells in the PAG expressing c-Fos protein immunoreactivity may be attributed somewhat to our strict cell counting criteria (see Procedures) but it could also reflect quite subtle functional alterations. There is a natural tendency to associate functional alterations with greatly enhanced c-Fos expression. But how many cells must show “activity” before a function change can be expected? Recently, it has been noted that very small populations (e.g., <1%) of neurons in other brain areas can produce dramatic behavioral changes (see Witten et al., 2010).
3. 4 Summary and Conclusions
We present data indicating that, as compared to rats that retained their CTA, rats that extinguished their CTA showed suppression in the number of c-Fos-labeled neurons in all 4 longitudinal columns of the PAG. We also report column-specific changes in c-Fos protein expression at the time of the SR test. There was a reliable increase in c-Fos expression in dorsolateral PAG (dlPAG) in the brains of rats that went through the EU-EXT procedure (and did not show SR of the CTA) as compared with those that went through the more-traditional CSO extinction procedure (and exhibited a significant SR of the CTA). The number of c-Fos-labeled neurons in the dlPAG was significantly correlated with the amount of SAC consumed at the SR test. Surprisingly, the brains of EU-extinguished rats and CS-only extinguished rats did not differ in the number of c-Fos-labeled neurons in GNC, mPFC, BLA, or the central nucleus of the amygdala. To the best of our knowledge, this is the first report of changes in neural activity indicating a possible role for PAG neurons in CTA extinction and SR.
4. Experimental Procedure
4.1 Subjects
The subjects were male Sprague-Dawley rats (weight = 436.71+ 12.93; mean + SEM) supplied by Charles-River Laboratories; Wilmington, MA. Throughout the experiment the animals were housed in plastic ‘shoe box’ cages (20 cm × 22 cm × 20 cm deep) equipped with wire tops and filled with corncob bedding (Bed o’cobbs, Andersons Industrial Products, Maumee, OH). Home cage temperature was maintained at 23–26°C with 30% humidity, ±5%. The animals were kept on a 12:12 hour light:dark schedule (lights on at 0600h). Rats had free access to food (Purina Rodent Chow, No. 5001, PMI Nutrition International, Brentwood, MO), and underwent 23-hour fluid deprivation as described below. All animals were handled briefly during daily weighings and injections.
Procedures were approved by the Baldwin-Wallace College Institutional Animal Care and Use Committee. Animals were procured and cared for according to the recommendations in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and in compliance with the Animal Welfare Act.
4.2 Drugs and Solutions
All chemicals were purchased from the Sigma-Aldrich Chemical Company (St. Louis, MO). Lithium chloride (LiCl) was dissolved in physiological saline to produce a final concentration of 81mg/ml and was administered at a dose of 81mg/kg (i.p.). Saccharin salt was dissolved in deionized water to create a final concentration of 0.3%, by mass, saccharin solution (SAC). Vehicle control injections were 0.9% saline given in the same volume as was the LiCl. All consummatory tests (SAC or water) involved a single bottle. A 1-bottle test is more sensitive for detecting between-group differences in the strength of CTA learning than is a 2-bottle test (Batsell and Best, 1993; Liang et al., 2011).
4.3 CTA Conditioning
Rats were conditioned and tested in their home cages. All contextual cues were kept constant throughout each phase of the study. Two days prior to the start of the experiment, rats began a 23-hour water deprivation cycle. Rats were given a 1-hr presentation of liquid at the same time each day (1200–1300 hrs). This brief acclimation to the fluid deprivation schedule ensured that animals were motivated to drink SAC (the CS) when it was first presented. Fluid consumption was recorded daily to the nearest tenth of a gram. CTA conditioning lasted 6 days. Animals were presented with 0.3% saccharin solution (SAC) for 30 minutes on days 1, 3, and 5. During a 15-minute latency animals were injected with lithium chloride (LiCl; 81 mg/kg, i.p.), which was followed by a presentation of tap water for 30 minutes to prevent dehydration. Days 2, 4, and 6 served as rest days to further facilitate hydration of the rats. On these days the animals received two 30-minute presentations of water separated by 15 minutes, to mimic the conditioning days.
4.4 CTA Extinction
After day 6 of the conditioning phase, the extinction phase of our study began. The animals were maintained on the 23-hr fluid deprivation schedule and were randomly assigned to one of 2 CTA extinction conditions: (1) CS-Only Extinction (CSO-EXT) or (2) Explicitly Unpaired Extinction (EU-EXT). Other rats were randomly assigned to the No Extinction (NE) condition. Both of the extinction procedures effectively reduce the strength of the CR (saccharin water avoidance; see Mickley et al., 2009) but may do so via very different means that are still being explored. Unlike the more-traditional CS-only extinction procedure, the EU procedure, for example, may inhibit the conditioned response by causing habituation of the US or by the CS losing its excitatory value due to the emergence of reinstatement effects during US-only trials (Rauhut et al., 2001; Thomas et al., 2005).
During this phase of the study, rats were presented with SAC (odd days) or water (even days) for 30 min every-other day, followed by a 15-minute latency (in which no fluid was offered), and then by a 30-minute presentation of water. On water-only (odd) days, animals were also given an injection of LiCl (81 mg/kg, i.p.) if they were assigned to the EU group or SAL if they were assigned to the CSO group. Animals in the NE group were not re-exposed to SAC during this phase but instead received water only on a schedule that paralleled the fluid availability for the other groups of animals. The NE rats were randomly yoked to animals going through the CSO or EU-EXT procedures and given a single SAC exposure on the same day their pair reached asymptotic extinction or were tested for SR of the CTA (see below). The NE rats were also sacrificed on the same day as their yoked counterpart. Refer to table 1 for group assignments and corresponding treatments throughout extinction. The animals were maintained in this regimen until they reached the asymptotic extinction criterion of >90% reacceptance of SAC (i.e., 90% of baseline SAC consumption).
As a first step in evaluating the degree to which the rats in this study had extinguished their CTA, we needed to estimate levels of baseline familiar SAC drinking. However, recording several days of baseline SAC pre-exposure in our animals would have impeded future CTA training, due to latent inhibition effects (Bakner et al., 1991). Moreover, we also wanted to record SAC consumption over several days to avoid the bias associated with the rat’s initial hesitation to consume novel substances (i.e., neophobia; Gillan & Domjan, 1977). Therefore, baseline SAC consumption was determined by averaging SAC consumption on the third day of exposure from a separate group (N = 10) of similarly-sized rats (mean consumption ± SEM = 17.57± 1.29 ml) (Mickley et al., 2004). In order to confirm that this method of determining baseline SAC consumption was appropriate, we also measured the SAC consumption of a group of rats that were exposed to SAC and LiCl but did not have the US and CS paired. The SAC consumption of this group represented normal enhanced acceptance of the sweet tasting liquid in the absence of conditioned avoidance. The animals that experienced these explicitly unpaired CS-US exposures over 3 days drank amounts of SAC equivalent to those animals that only drank SAC over the same time period. These data validated our method of estimating baseline SAC consumption as a comparison point to determine 90% reacceptance of SAC as asymptotic extinction.
4.5 Spontaneous Recovery (SR)
After reaching asymptotic extinction, animals (and their yoked NE counterparts) entered a latency period of 29 days, during which they received two 30-minute presentations of only tap water daily (separated by 15 minutes), without injections of any sort. This procedure corresponded to the temporal characteristics of the previous CTA and EXT training regimens. On day 30 following asymptotic extinction, animals were presented with SAC for 30 minutes, in order to test the degree of spontaneous recovery of the CTA.
4.6 c-Fos protein immunohistochemical analysis
The immediate early gene c-fos has been proposed to be a “third messenger” molecule that bridges the gap between short-term intracellular signals and long-term physical changes in pre- and post-synaptic membranes (Sagar et al., 1988). As such, c-Fos protein labeling has frequently been used as a metabolic marker of synaptic stimulation (Herrera and Robertson, 1996). Further, c-Fos protein immunohistochemistry has been used to identify brain areas involved in not only the sensory experiences of a CS and US but also the associative processes that mediate a CTA (Swank & Bernstein, 1994; Swank, Schafe & Bernstein, 1995). Moreover, the use of c-fos antisense has been helpful in determining that c-Fos protein is a specific physiological substrate of CTA formation and EXT (Lamprecht and Dudai, 1996; Swank, Ellis, & Cochran, 1996).
Rats were sacrificed 90 minutes following the end of the last SAC exposure (following asymptotic extinction, or following the SR test – see Table 1) in preparation for c-Fos protein immunohistochemical analysis. This timing was employed because c-Fos expression is highest between 90 and 120 minutes after post-synaptic neuronal activity (Herrera and Robertson, 1996). All rats were given access to SAC water before perfusion to control for c-Fos expression that may be directly caused by the sensation of a sweet taste. All rats in this study drank at least some SAC on the last day of the experiment.
Before perfusion, rats were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.). Each rat was then transcardially perfused with 300–500 ml of heparinized saline followed by 300–500 ml of 4% paraformaldehyde. The brains were immediately removed, placed in 4% paraformaldehyde, and stored at ~ 4 °C. Eight hours later, the brains were transferred to a cryoprotectant solution (30% sucrose mixed in phosphate buffered saline with 0.01% thimerosal) and kept at ~ 4 °C until they were sliced. Brains were sliced in the coronal plane at 40 μm using a freezing microtome and stored in phosphate buffered saline containing 0.2% sodium azide until they were assayed for c-Fos protein immunoreactivity as previously described (Hsu et al., 1981; Mickley et al., 2004). Afterward, brain sections were mounted on gelatin and chrom-alum coated slides, dehydrated, counterstained with neutral red, and cover slipped with Permount™.
To ensure that negative c-Fos expression was not a result of faulty staining procedures, we employed a positive control procedure as described by Rinaman et al. (1997). Adult male rats (not engaged in the CTA study) received an injection of 8% saline solution (2.0 ml/100g, i.p.) to produce an osmotic thirst, which leads to certain c-Fos expression in the periventricular nucleus (PVN) of the hypothalamus. Ninety minutes later, the rats were given a supra-lethal dose of sodium pentobarbital (100 mg/kg, i.p.), perfused, and prepared for c-Fos immunohistochemical procedures. Brain slices including the PVN from these animals were included in all the immunohistochemical assays of the experimental and control groups to ensure that successful staining had occurred. If positive staining was not observed in the positive control sections, the entire assay was discarded. Likewise, in order to ensure that artifacts associated with counter-staining were not counted, negative control brain sections were included along with each assay and treated as described above but were not incubated in the primary antibody.
Slides were viewed with 20X magnification using an Olympus™ BX-60 microscope equipped with an AxioCam MRc5 camera connected to a computer running Carl Zeiss AxioVision™ v. 4.5 software (Carl Zeiss Microimaging, Germany). The PAG was located using standard demarcations (Paxinos and Watson, 1998) between −7.2 and −8.2 mm posterior to bregma. For detailed analysis, we further divided the PAG into the four recognized major longitudinal columns: Dorsomedial PAG (dmPAG); Dorsolateral PAG (dlPAG); Lateral PAG (lPAG) and Ventrolateral PAG (vlPAG). Cells were counted as expressing positive c-Fos protein immunoreactivity based on the visualization of a black, punctate, round and uniformly stained neuronal nucleus using standards as described in Mickley et al. (2007). The observer was blind to the experimental condition of the rats.
Although a single coronal section was analyzed per brain area for each animal, we have determined that this method produces cell counts not significantly different from those in close-by sections. Parametric studies indicate a high correlation [r(15) = 0.98; p < 0.01] between the cell counts in one of our brain sections and counts from the same brain area found in sections 80–160 μm anterior or posterior. Moreover, the cell counts in these close-by sections are not significantly different from one another.
4.7 Statistical Analysis
Pair-wise group comparisons of the behavioral data were accomplished using t-tests. We used two-way ANOVAs to assess the effects of the extinction methods on c-Fos cell counts in various brain areas at the end of extinction or after the SR test [Extinction treatment (CSO-EXT, EU-EXT or NE) × Test (Asymptotic extinction or SR test)]. Post-hoc pairwise comparisons were performed via Bonferroni-corrected t-tests. We predicted changes in PAG c-Fos expression as rats moved from asymptotic extinction to the SR test. Therefore, in some cases a priori planned comparisons between the c-Fos cell counts in rats sacrificed following asymptotic extinction vs. those sacrificed following the SR tests also employed Bonferroni-corrected t-tests. Finally, in order to evaluate the relationship between the behavioral indicators of CTA SR and the number of c-Fos-labeled neurons we performed Pearson r multivariate correlations between data representing the SAC consumption at the SR test and the c-Fos cell counts in each longitudinal column of the PAG. All analyses were facilitated through the use of the PASW, Statistics 17.0 (IBM Corp, Somers, NY). An α = 0.05 was adopted throughout.
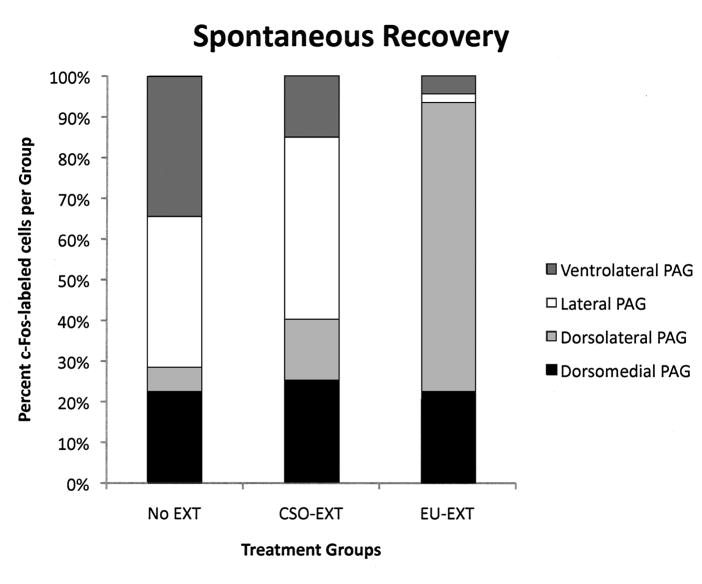
Figure 5.
Distribution of c-Fos immunoreactivity within the PAG following the SR test. Each of the bars represents 100% of the c-Fos-labeled neurons and the shaded areas represent the distribution among the longitudinal columns of the PAG. Following the SR test, brains of rats that went through the EU extinction procedure contained proportionally more c-Fos-labeled cells in the dlPAG than in other longitudinal columns of this midbrain structure.
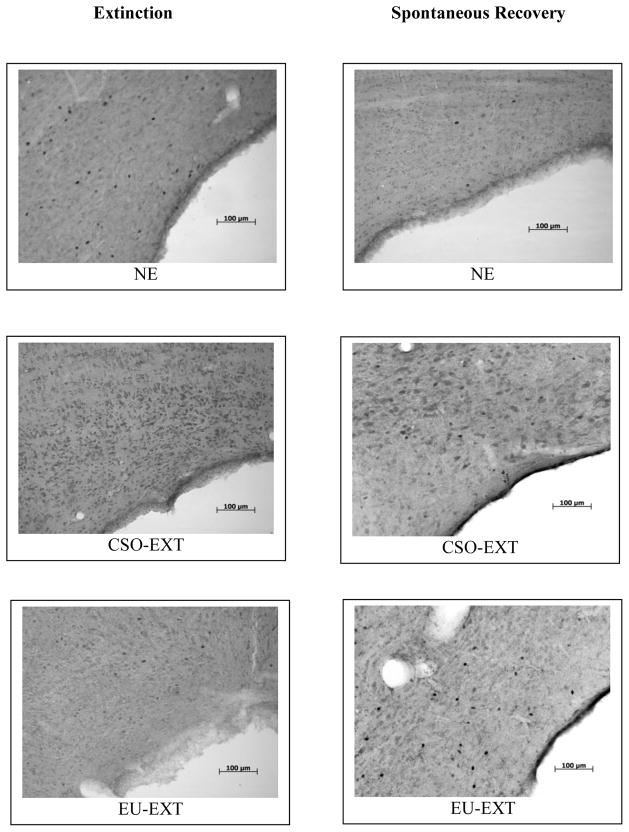
Figure 6.
C-Fos expression in the dlPAG following either asymptotic extinction op a CTA or a spontaneous recovery test. Either method of CTA extinction employed (CSO or EU) reduced c-Fos expression in the dlPAG as compared to non-extinguished (NE) rats. This reduction was also observed in several other longitudinal columns of the PAG (see Figure 4). After the SR test, rats that underwent the EU-EXT procedure failed to show a spontaneous recovery of their CTA and also expressed more c-Fos-labeled neurons in the dlPAG than did CSO-EXT or NE control animals. The space on the right of each image is the cerebral aqueduct.
Highlights.
We studied different methods of conditioned taste aversion (CTA) extinction (EXT).
Explicitly unpairing a CS & US during EXT produces faster and more profound EXT.
We report reductions in periaqueductal gray (PAG) c-Fos expression that accompany CTA extinction.
Region-specific PAG c-Fos changes accompany SR of a CTA following different EXT methods.
Possible role for PAG neurons in CTA extinction and SR.
Acknowledgments
The authors wish to acknowledge the following students and technicians for their excellent contributions to this work: Sarah Clark, Jennifer Dunger, Sarah Frischmann, Jennifer Graebert, Nick Grisak, Natalie Hogan, Sarah Hummel, Ye-Hyun Kim, Bruce Kinley, Kim Parkinson, Doug Placko, Morgan Rogers, Dave Revta, Ashley Sandmann, and James Romanchik.
Supported by NIMH grant: 2-R15-MH063720-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
G. Andrew Mickley, Email: amickley@bw.edu.
Gina N. Wilson, Email: ginanwilson@gmail.com.
Jennifer L. Remus, Email: JenniferLRemus@Gmail.com.
Linnet Ramos, Email: lramos1984@gmail.com.
Kyle D. Ketchesin, Email: kketches@umich.edu.
Orion R. Biesan, Email: orionbiesan@gmail.com.
Joseph R. Luchsinger, Email: joeluchsinger@gmail.com.
Suzanna Prodan, Email: sprodan@mail.bw.edu.
References
- Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not on conditioned taste aversion. Learn Mem. 2009;16:682–686. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- Bakner L, Strohen K, Marvin N, Riccio DC. Postconditioning recovery from the latent inhibition effect in conditioned taste aversions. Physiol Behav. 1991;50:1269–1272. doi: 10.1016/0031-9384(91)90595-f. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Batsell WR, Best MR. One bottle too many? Method of testing determines the detection of overshadowing and retention of taste aversions. Anim Learn Behav. 1993;2:154–158. [Google Scholar]
- Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC. Organization of single components of defensive behaviors within distinct columns of Periaqueductal gray matter of the rat: Role of N-methyl-D-Aspartic acid glutamate receptors. Neuroscience. 2004;125:71–89. doi: 10.1016/j.neuroscience.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Blair R, Amit Z. Morphine conditioned taste aversion reversed by periaqueductal gray lesions. Pharmacol Biochem Behav. 1981;15:651–653. doi: 10.1016/0091-3057(81)90224-0. [DOI] [PubMed] [Google Scholar]
- Borelli KG, Ferrieira-Netto C, Brandao ML. Distribution of Fos immunoreactivity in the rat brain after freezing or escape elicited by inhibition of glutamic acid decarboxylase or antagonism of GABA-A receptors in the inferior colliculus. Behav Brain Res. 2006;170:84–93. doi: 10.1016/j.bbr.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Gonzalez-Lima F. Brain activity associated with fear renewal. Eur J Neurosci. 2006;24:3567–3577. doi: 10.1111/j.1460-9568.2006.05229.x. [DOI] [PubMed] [Google Scholar]
- Bruchey AK, Schumake J, Gonzalez-Lima F. Network model of fear extinction and renewal functional pathways. Neuroscience. 2007;145:423–437. doi: 10.1016/j.neuroscience.2006.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. NeuroReport. 1999;10:413–418. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Anatomical evidence that hypertension associated with the defense reaction in the cat is mediated by a direct projection from a restricted portion of the midbrain periaqueductal grey to the subretrofacial nucleus of the medulla. Brain Res. 1988;460:339–345. doi: 10.1016/0006-8993(88)90378-2. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Cole S, McNally GP. Complementary roles for amygdala and periaqueductal gray in temporal-difference fear learning. Learn Mem. 2010;16:1–7. doi: 10.1101/lm.1120509. [DOI] [PubMed] [Google Scholar]
- Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fears in rats—a possible mechanism for the persistence of traumatic memories in PTSD. Depress Anxiety. 2011;28:186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oca BM, DeCola JP, Maren S, Fanselow MS. Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci. 1998;18:3426–3432. doi: 10.1523/JNEUROSCI.18-09-03426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M. The principles of learning and behavior. 3. Brooks/Cole Publishing Company; Pacific Grove, CA: 1993. [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the Periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ, Knelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–158. [PubMed] [Google Scholar]
- Gillan DJ, Domjan M. Taste-aversion conditioning with expected versus unexpected drug treatment. J Exp Psychol Anim B. 1977;3:297–309. doi: 10.1037//0097-7403.3.4.297. [DOI] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R. Pharmacological enhancement of fear reduction: preclinical models. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2010.01175.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groblewski PA, Lattal MK, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fangler H. Use of avidin-biotin peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: A tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: neural mechanisms and their treatment implications. Pharmacol Biochem Behav. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Krout KE, Jansen ASP, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht R, Dudai Y. Transient expression of c-fos in rat amygdala during training is required for encoding conditioned taste aversion memory, Learn. Mem. 1996;3:31–41. doi: 10.1101/lm.3.1.31. [DOI] [PubMed] [Google Scholar]
- Liang NC, Bello NT, Moran TH. Experience with activity based anorexia enhances conditioned taste aversion learning in rats. Physiol Behav. 2011;102:51–57. doi: 10.1016/j.physbeh.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. Facilitation of fear extinction by midbrain periaqueductal gray infusions of RB101 (S), an inhibitor of enkephalin-degrading enzymes. Behav Neurosci. 2005;119:1672–1677. doi: 10.1037/0735-7044.119.6.1672. [DOI] [PubMed] [Google Scholar]
- McNally GP, Lee BW, Chiem JY, Choi EA. The midbrain periaqueductal gray and fear extinction: opioid receptor subtype and roles of cyclic AMP, protein kinase A, and mitogen-activated protein kinase. Behav Neurosci. 2005;119:1023–1033. doi: 10.1037/0735-7044.119.4.1023. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Opioid receptors in the midbrain periaqueductal gray regulate extinction of Pavlovian fear conditioning. J Neurosci. 2004;24:6912–6919. doi: 10.1523/JNEUROSCI.1828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Predicting danger: The nature, consequences, and neural mechanisms of predictive fear learning. Learn Mem. 2010;13:245–253. doi: 10.1101/lm.196606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, McMullen CA, Yocom AM, Valentine EL, Dengler-Crish CM, Weber B, Wellman JA, Remmers-Roeber DR. Dynamic Processing of Taste Aversion Extinction in the Brain. Brain Res. 2004;1061:79–89. doi: 10.1016/j.brainres.2004.04.071. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Kenmuir CL, Yocom AM, Wellman JA, Biada JM. A Role for prefrontal cortex in extinction of a conditioned taste aversion. Brain Res. 2005;1051:176–182. doi: 10.1016/j.brainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, Biada JM, DiSorbo A. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–157. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Disorbo A, Wilson GN, Huffman J, Bacik S, Hoxha Z, Biada JM, Kim YH. Explicit disassociation of a conditioned stimulus and unconditioned stimulus during extinction training reduces both time to asymptotic extinction and spontaneous recovery of a conditioned taste aversion. Learn Motiv. 2009;40:209–220. doi: 10.1016/j.lmot.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AI, Resstel LB, Guimaraes FS. Antiaversive effects of cannabinoids: Is the periaqueductal gray involved? Neural Plast. 2009 doi: 10.1155/2009/625469. Retrieved from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2593468/pdf/NP2009-625469.pdf. [DOI] [PMC free article] [PubMed]
- Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat Periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- Mota-Ortiz SR, Sukikara MH, Felicio LF, Canteras NS. Afferent Connections to the Rostrolateral Part of the Periaqueductal Gray: A Critical Region Influencing the Motivation Drive to Hunt and Forage. Neural Plasticity. 2009;2009:Article ID 612698. doi: 10.1155/2009/612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington D.C: 1996. [Google Scholar]
- Nolan LJ, McCaughey SA, Giza BK, Rhinehart-Doty JA, Smith JC, Thomas RS. Extinction of a conditioned taste aversion in rats: I. Behavioral effects. Physiol Behav. 1997;61:319–323. doi: 10.1016/s0031-9384(96)00411-8. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parker LA. Taste avoidance and taste aversion: evidence for two different processes. Learn Behav. 2003;31:165–172. doi: 10.3758/bf03195979. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned reflexes. Oxford University Press; Oxford: 1927. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Free will versus survival: brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493:132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Thomas BL, Ayres JJ. Treatments that weaken Pavlovian conditioned fear and thwart its renewal in rats: implications for treating human phobias. J Exp Psychol Anim Behav Process. 2001;27:99–114. [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exptl Psychol. 1975;1:88–96. [PubMed] [Google Scholar]
- Rinaman L, Stricker EM, Hoffman GE, Verbalis JG. Central c-Fos expression in neonatal and adult rats after subcutaneous injection of hypertonic saline. Neuroscience. 1997;79:1165–1175. doi: 10.1016/s0306-4522(97)00022-5. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: Metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Saper CB. Central autonomic system. In: Paxinos G, editor. The Rat Nervous System. Academic Press; 1995. pp. 107–135. [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GL. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GL. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank MW, Bernstein IL. c-Fos induction in response to a conditioned stimulus after single trial taste aversion learning. Brain Res. 1994;636:202–208. doi: 10.1016/0006-8993(94)91018-9. [DOI] [PubMed] [Google Scholar]
- Swank MW, Schafe GE, Bernstein IL. c-Fos induction in response to taste stimuli previously paired with amphetamine or LiCl during taste aversion learning. Brain Res. 1995;673:251–261. doi: 10.1016/0006-8993(94)01421-d. [DOI] [PubMed] [Google Scholar]
- Swank MW, Ellis AE, Cochran BN. c-Fos antisense blocks acquisition and extinction of conditioned taste aversion in mice. NeuroReport. 1996;7:1866–1870. doi: 10.1097/00001756-199607290-00036. [DOI] [PubMed] [Google Scholar]
- Thomas BL, Longo CL, Ayres JJB. Thwarting the renewal (relapse) of conditioned fear with the explicitly unpaired procedure: Possible interpretations and implications for treating human fears and phobias. Learn Motiv. 2005;36:374–407. [Google Scholar]
- Urcelay GP, Wheeler DS, Miller RR. Spacing extinction trials alleviates renewal and spontaneous recovery. Learn Behav. 2009;37:60–73. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DM, Graeff FG, Brandao MI, Landeira-Fernandez J. Defensive freezing evoked by electrical stimulation of the periaqueductal gray: comparison between dorsolateral and ventrolateral regions. NeuroReport. 2001;12:4109–4112. doi: 10.1097/00001756-200112210-00049. [DOI] [PubMed] [Google Scholar]
- Vianna DML, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- Willis WD. Anatomy and physiology of descending control of nociceptive responses of dorsal horn neurons: comprehensive review. Prog Brain Res. 1988;77:1–29. doi: 10.1016/s0079-6123(08)62776-4. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Fujimoto Y. Brain mechanisms of taste aversion learning in the rat. Brain Res Bull. 1991;27:403–406. doi: 10.1016/0361-9230(91)90133-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Neural mechanisms of taste aversion learning. Neurosci Res. 1993;16:181–185. doi: 10.1016/0168-0102(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sako N, Yasoshima Y, Sakai N. Neural substrates for conditioned taste aversion in the rat. Behav Brain Res. 1994;65:123–137. doi: 10.1016/0166-4328(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Let. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- Zanoveli JM, Ferreira-Netto C, Brandao ML. Conditioned place aversion organized in the dorsal periaqueductal gray recruits the laterodorsal nucleus of the thalamus and the basolateral amygdala. Exp Neurol. 2007;208:127–136. doi: 10.1016/j.expneurol.2007.08.007. [DOI] [PubMed] [Google Scholar]