Abstract
PURPOSE
Homocysteine is implicated in ganglion cell death associated with glaucoma. To understand mechanisms of homocysteine-induced cell death, we analyzed the sensitivity of the RGC-5 cell line, differentiated using staurosporine, to physiologically-relevant levels of the excitotoxic amino acid homocysteine.
METHODS
RGC-5 cells were differentiated 24 h using 316 nM staurosporine and tested for expression of Thy 1.2 via immunodetection, RT-PCR and immunoblotting. The sensitivity of staurosporine-differentiated RGC-5 cells to physiological levels of homocysteine (50, 100, 250 µM) and to high levels of homocysteine (1 mM), glutamate (1 mM) and oxidative stress (25 µM:10 mU/ml xanthine:xanthine oxidase) was assessed by TUNEL assay and by immunodetection of cleaved caspase-3. The sensitivity of undifferentiated RGC-5 cells to high (1, 5, and 10 mM) homocysteine was also examined.
RESULTS
Undifferentiated RGC-5 cells express Thy 1.2 mRNA and protein. Staurosporine-differentiated RGC-5 cells extend neurite processes and express Thy 1.2 after 24 h differentiation; they express NF-L after 1 and 3 days differentiation. Treatment of staurosporine-differentiated RGC-5 cells with 50, 100 or 250µM homocysteine did not alter neurite processes nor induce cell death (detected by TUNEL and active caspase-3) to a level greater than that observed in non-homocysteine-treated, staurosporine-differentiated cells. The 1 mM dosage of homocysteine in staurosporine-differentiated RGC-5 cells also did not induce cell death above control levels, although 18 h treatment of non-differentiated RGC-5 cells with 5 mM homocysteine decreased survival by 50%.
CONCLUSIONS
RGC-5 cells differentiated for 24 h with 316 nM staurosporine project robust neurite processes and are positive for ganglion cell markers consistent with a more neuronal phenotype than non- staurosporine-differentiated RGC-5 cells. However, concentrations of homocysteine known to induce ganglion cell death in vivo and in primary ganglion cells are not sufficient to induce death of RGC-5 cells, even when they are differentiated with staurosporine.
Keywords: retinal ganglion cells, neuronal differentiation, apoptosis, excitotoxicity, hyperhomocysteinemia, mouse retinal cell line
Introduction
Homocysteine is a non-proteinogenic amino acid that is a homolog of cysteine; it is produced by demethylation of methionine. When elevated, homocysteine is an independent risk factor for certain cardiovascular [1,2] and neurodegenerative diseases [3–5]. Hyperhomocysteinemia is implicated in diseases affecting the visual system including maculopathy, optic atrophy and glaucoma [6–14]. In glaucoma retinal ganglion cells die [15,16], however the mechanisms by which homocysteine induces this death are not known. Our laboratory has been investigating homocysteine-induced ganglion cell death using a variety of experimental tools. Most recently, we examined retinas of a mouse model of hyperhomocysteinemia in which the gene encoding the enzyme cystathionine-β-synthase is deficient (cbs mutant mouse) and found that ganglion cell viability is decreased by ~20% in mice with moderate elevation of homocysteine; even greater neuronal death is observed in mice with extremely high levels of this amino acid [17].
While extremely informative, analysis of the effects of hyperhomocysteinemia on retina in an animal model does not permit testing of certain mechanistic questions. Our understanding of mechanisms of ganglion cell death has been facilitated by the availability of culture systems including mixed cultures of retinal cells, immortalized ganglion cell lines, and purified ganglion cells isolated from retinas and maintained as a primary culture [18]. Mixed cultures allow assessment of the interactions of several retinal cell types, but do not allow assessment of the function/viability of a single type of cell, whereas primary cell culture has enhanced our understanding of function and susceptibility to insult of a specific cell (such as ganglion cells alone). Our laboratory has exploited primary retinal ganglion cell culture in several studies. Among these, primary ganglion cells have been used to understand the role of the transporter protein system xc− in response to oxidative stress [19]; to assess the usefulness of sigma receptor ligands in preventing apoptotic cell death induced by exposure to excitatory amino acids [20] and to determine the expression of various proteins in this neuronal cell type [21–23]. Primary cells are robust and live up to 22 days in culture; they are well-differentiated and develop extensive neuronal processes, demonstrate no proliferative activity, are positive for known neuronal markers and are sensitive to excitotoxins. In particular, freshly isolated ganglion cells from postnatal day 2 mice are sensitive to 50 µM homocysteine resulting in ~50% cell death within 18–24 h exposure [20].
A caveat to the use of primary ganglion cells for research is that they do not proliferate in culture, which though a clear indicator of their neuronal origin and phenotype can limit large scale mechanistic studies or analyses of neuroprotective compounds. The field of ganglion cell research clearly needed a cell line with at least some of the characteristic features of ganglion cells. In 2001, Krishnamoorthy and colleagues reported the development of the RGC-5 cell line [24]. The cell line was reportedly derived by transforming postnatal day 1 rat retinal cells with ψ2 E1A virus [24]. Recent re-characterization of the RGC-5 cell line suggests that it was derived from mouse [25]. When originally described, the RGC-5 cells expressed Thy-1.2, Brn-3C, neuritin, NMDA-R1 and GABA receptors, which are neuronal markers characteristic of intact ganglion cells. RGC-5 cells do not express glial fibrillary acidic protein (GFAP) a marker of glial cells. In early passage, the cells were sensitive to glutamate (5 mM) and that sensitivity could be increased if the cells were treated with succinyl concanavalin A (S Con A) [24]. Our laboratory used the S Con A differentiation protocol and found that the cells were sensitive to 1 mM glutamate resulting in ~50% death after 18 h incubation [26]. As the RGC-5 cells have undergone numerous passages over the last several years, the sensitivity to glutamate has diminished markedly, even when cells were differentiated with S Con A. The survival of the cells in the presence of glutamate has increased from 35% to 95% over a period of about 6 years. [25, 27–29, our unpublished observations]. Van Bergen and co-workers reported that RGC-5 cells no longer express the ganglion cell marker protein Thy 1.2, whether differentiated using S Con A or not [25].
While the S Con A method for inducing differentiation of RGC-5 cells did not sustain the desired neuronal phenotype in these cells, interesting experimental manipulations have been reported by other labs that yield a more ganglion cell-like phenotype. The protein kinase inhibitor staurosporine (produced by the microorganism Streptomyces staurosporeus) has been used to induce a more neuronal phenotype in RGC-5 cells [30,31]. When RGC-5 cells were treated with 316 nM staurosporine, they express neurites; they were reported to become postmitotic and nonapoptotic, and they altered their kinase phosphorylation patterns. In addition, they are positive for a number of neuronal markers including Thy 1.2 [30]. Staurosporine-differentiated RGC-5 cells are sensitive to oxidative stress induced by hydrogen peroxide, ischemia, glucose deprivation and plasminogen activators [32–34].
Our interest in the staurosporine-differentiated RGC-5 cell model, which provided the impetus for the present study, was to determine whether the staurosporine manipulation would render the RGC-5 cells sensitive to physiologically relevant levels of homocysteine. Our earlier studies show that homocysteine can induce death of ganglion cells in vivo at low (micromolar) concentrations [17, 26,35]. Similar sensitivity to homocysteine has been reported in primary cell cultures. Indeed, 18 h exposure of primary mouse ganglion cells to 50 µM homocysteine led to 50% cellular apoptosis [20]. The cells are positive for assays of apoptotic cell death (positive TUNEL reaction, expression of active caspase-3) and they manifest disrupted neurite outgrowths. In the S Con A-differentiated RGC-5 cells, the level of homocysteine required to induce significant cell death in RGC-5 cells is in the millimolar range (1–5 mM) [26]. In the present study, we asked whether the staurosporine-differentiated RGC-5 cells demonstrated sensitivity similar to the in vivo model and primary cell culture and hence would be a useful model for mechanistic analysis of homocysteine-induced death of ganglion cells.
Materials and Methods
Cell culture
The ganglion cell line (RGC-5) [24] was the kind gift of Dr. Neeraj Agarwal (Univ. of North Texas Health Sciences Center, Ft. Worth, Texas). It was transferred to our laboratory in 2001 at which time the cells were grown to allow many early passage preparations. RGC–5 cells were maintained at 37°C in a humidified chamber of 5% CO2. They were cultured in 75 cm2 flasks in DMEM:F12, supplemented with 10% FBS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Culture medium was replaced with fresh medium every other day. To promote differentiation of these cells, they were cultured until 70% confluent and then incubated with the aforementioned-medium containing 316 nM staurosporine (≥ 98% purity; catalog number 380-014, derived from Streptomyces staurosporeus ) from Alexis Biochemicals (San Diego, CA) for 1 or 3 days. RGC-5 cells were grown to approximately 70% confluence on coverslips in 24 well plates for immunohistochemistry and TUNEL assay and on 75 cm2 flasks for protein isolation for immunoblotting analysis.
Immunodetection of neuronal and non-neuronal markers in differentiated RGC-5 cells
To compare expression of retinal cell markers in staurosporine-differentiated versus non-differentiated RGC-5 cells, the cells were processed for immunodetection with antibodies for neuronal markers including Thy 1.2 (BD Biosciences (Catalog Number 553000, San Jose, CA) and NF-L (Santa Cruz, Santa Cruz, CA). In addition to these known markers for neurons, the cells were also processed for immunodetection of vimentin (Chemicon, Temecula, CA), a known marker for Müller glial cells. The cells were cultured on coverslips, air-dried for 5 min, fixed in 4% paraformaldehyde in PBS for 5 min, washed thrice for 5 min with PBS, and incubated for 5 min in 0.1% triton X-100. Cells were blocked with 4% donkey serum plus PowerBlock (BioGenex, San Ramon, CA). Antibody concentrations used were as follows: Thy1.2 (1:100), NF-L (1:50), and vimentin (1:25) followed by appropriate secondary antibodies: Alexa Fluor 488 donkey anti-rat (1:1000), Alexa Fluor 488 donkey anti-goat (1:1000), and Oregon Green 488 goat anti-mouse (1:1000). Cells were analyzed by epifluorescence using a Zeiss Axioplan–2 microscope, equipped with the axiovision program, and an HRM camera.
Immunoblotting of Thy 1.2 and cleaved caspase-3
Western blotting of cleaved caspase-3 (Cell Signaling, Beverly, MA) in treated RGC-5 cells was performed following our published method [19]. Protein samples isolated from cultured RGC-5 cells exposed to various treatments were subjected to SDS-PAGE and transferred to nitrocellulose membranes. Afterwards, these membranes were incubated with a monoclonal rat anti-Thy 1.2 antibody (1:500) at 37°C for 1 h or a polyclonal rabbit anti-cleaved caspase-3 antibody (1:500) at 4°C for 72 h, followed by an HRP-conjugated goat anti-mouse (1:2000) or goat anti-rabbit IgG antibody (1:3000). Proteins were visualized with the ECL Western blot detection system. Membranes were reprobed with mouse monoclonal anti-β-actin antibody (1:5000) as a loading control.
Thy 1.2 gene expression in RGC-5cells
The TRIzol (Invitrogen, Carlsbad, CA) method of RNA extraction was used to collect RNA from RGC-5 cells. Total RNA was converted to cDNA using the GeneAmp RNA PCR Kit (Applied Biosystems, Foster City, CA). PCR was performed using the Takara Taq DNA polymerase kit (Takara Bio USA, Madison, WI). The Thy 1.2 upstream primer 5’-AATGATGGGGAAAGGGGTAG-3’ (sense) and 5’- GAAGAGGCAGGTTGCAAGAC-3’ (antisense) form a 290-base pair product corresponding to nucleotide positions 1252 and 1522 respectively, in mouse (accession number NM 009382). PCR was performed for 30 cycles with a denaturing phase of 1 min at 94°C, an annealing phase of 1 min at 60°C, and an extension phase of 1 min at 72°C. The PCR products were electrophoresed on a 2% agarose gel containing 1:8000 SYBRsafe DNA gel stain, visualized under UV light and photographed.
Sensitivity of RGC-5 cells to insult (TUNEL Assay and DIC imaging)
To determine the sensitivity of staurosporine-differentiated RGC-5 cells to homocysteine toxicity, RGC-5 cells (differentiated 24 h with staurosporine and non-differentiated) were cultured on coverslips in 24-well plates and subsequently exposed to varying concentrations of homocysteine (50, 100, and 250 µM) (D,L-homocysteine thiolactone, Sigma, St. Louis, MO) for 18 h (in the presence of staurosporine). Cells were subjected to detection of DNA strand breaks via terminal dUTP nick end labeling (TUNEL) analysis using the ApopTag kit from Calbiochem (Temecula, CA) following the manufacturer’s instructions. Cell nuclei were counterstained with Hoescht 33258 dye (Molecular Probes, Eugene, OR). The sensitivity of staurosporine-differentiated RGC-5 cells to increased concentrations of excitotoxic amino acids (1 mM glutamate and 1 mM homocysteine) and to an inducer of oxidative stress (xanthine sodium salt/xanthine oxidase at 25 µM and 10 mU/ml, respectively; Sigma, St. Louis, MO) as well as undifferentiated RGC-5 cell sensitivity to homocysteine (1, 5, and 10 mM) was measured using the TUNEL assay. All treatment solutions were prepared fresh just before treatment.
Cells were examined by epifluorescence using standard fluorescein excitation and emission filters and data were captured for image analysis. Each field was examined systematically using an FITC filter for the presence of green fluorescence, indicative of apoptosis, and data were expressed as TUNEL-positive cells per total number of cells in the field. In these experiments, at least three coverslips were prepared. Per coverslip, images were captured from at least 5 fields using the Axiovision software system. Data were analyzed by one-way Analysis of Variance using the NCSS 2007 statistical package. The Tukey-Kramer method was the post-hoc test of significance. A p value of <0.05 was considered significant.
In additional experiments, cells were subjected to differential interference contrast (DIC) microscopy using a Nikon eclipse TE300 inverted microscope. The cell bodies and fibers radiating from them were photographed using a CoolSnap camera.
Results
Differentiated RGC-5 cells are positive for neuronal markers
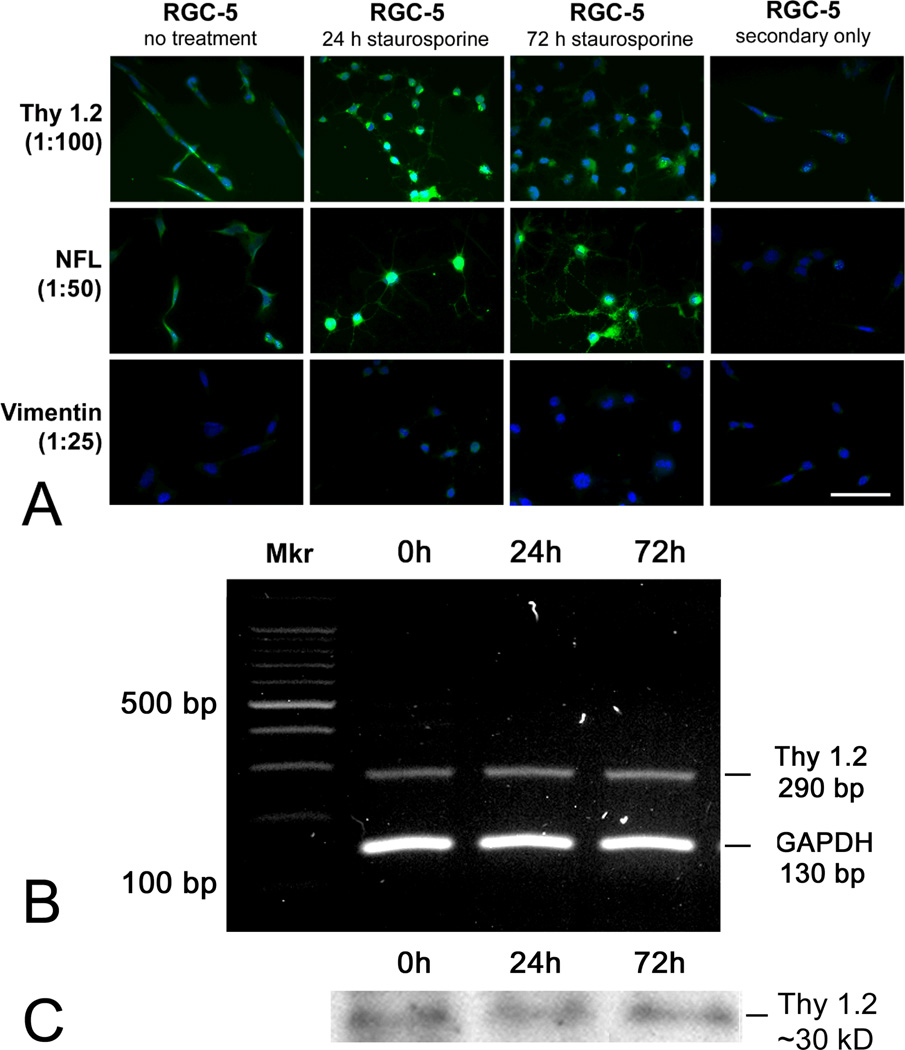
Immunocytochemistry performed in RGC-5 cells to detect Thy 1.2 revealed a low level immunopositive reaction for this ganglion cell marker (Fig. 1A) consistent with immunopositive staining reported by the investigators who developed the cell line [24]. It appears that the early passage RGC-5 cells that were given to our laboratory in 2001 have retained a low level expression of this protein. Treatment of the cells for 24 h with staurosporine led to an apparent increase in Thy 1.2 expression compared with control cells, which were not treated with staurosporine. The peak level of Thy1.2 was at 24 h (at least by immunocytochemical methods); levels decreased by 72 h. We examined RGC-5 cells for the presence of the neuronal marker neurofilament-light (NF-L). The immunostaining was low in undifferentiated RGC-5 cells, but increased after 1 day of treatment with staurosporine (Fig. 1A). The data suggest that RGC-5 cells develop a more neuronal phenotype upon staurosporine treatment that is sustained for at least 3 days. When cells were analyzed for expression of the glial marker vimentin, neither undifferentiated or differentiated RGC-5 cells stained positively. Taken collectively the data are consistent with a neuronal origin for the RGC-5 cells.
Figure 1. Detection of Thy1.2 and other neuronal markers in undifferentiated RGC-5 cells, RGC-5 cells differentiated with staurosporine for 24 and 96 h.
(A) Three retinal cell markers were examined by immunostaining: Thy 1.2, a ganglion cell marker; neurofilament-light (NF-L), a neuronal cell marker; and vimentin, a retinal glial cell marker. Bright whitish fluorescence indicates positive immunodetection of the primary antibody; light gray fluorescence indicates Hoescht 33258 staining of cell nuclei. Scale bar denotes 25 µm. (B) cDNA was isolated from RGC-5 cells cultured in the absence or presence of staurosporine for 24 or 72 h and subjected to RT-PCR using primers specific for mouse Thy 1.2 (290 bp), GAPDH was the internal control (130 bp). “Mkr” = DNA marker. (C) RGC-5 cells were grown to confluence, cultured in the absence or presence of staurosporine for 24 or 72 h, protein isolated, and subjected to immunoblotting using an antibody against Thy 1.2 (30 kD).
To confirm the immunocytochemical detection of Thy 1.2 observed in RGC-5 cells, RT-PCR and western blotting were performed. RGC-5 cells were grown to confluence and RNA extracted; RT-PCR amplified the expected product size of 290 bp confirming the expression of Thy 1.2 in undifferentiated RGC-5 cells as well as cells exposed to staurosporine for 24 and 72 h (Fig. 1B). (We also attempted to grow cells for 96 h in staurosporine-treated media, but the cells did not survive (data not shown)). In companion experiments, RGC-5 cells were grown to confluence in the presence or absence of staurosporine for 24 or 72 h and were subjected to immunoblotting using an antibody against Thy 1.2. Immunoblotting confirmed the immunocytochemistry results showing that Thy 1.2 was expressed in RGC-5 cells (Fig. 1C). Interestingly, while the immunocytochemical studies suggested a transient increase in Thy1.2 in some cells after 24 h of staurosporine treatment, the immunoblotting suggested that protein levels were similar at 24 and 72 h of staurosporine, which may reflect a difference in the sensitivity of the methods and the ability to visualize individual cells in the immunocytochemical experiments.
Differentiated RGC-5 cells are not susceptible to physiologically relevant doses of homocysteine
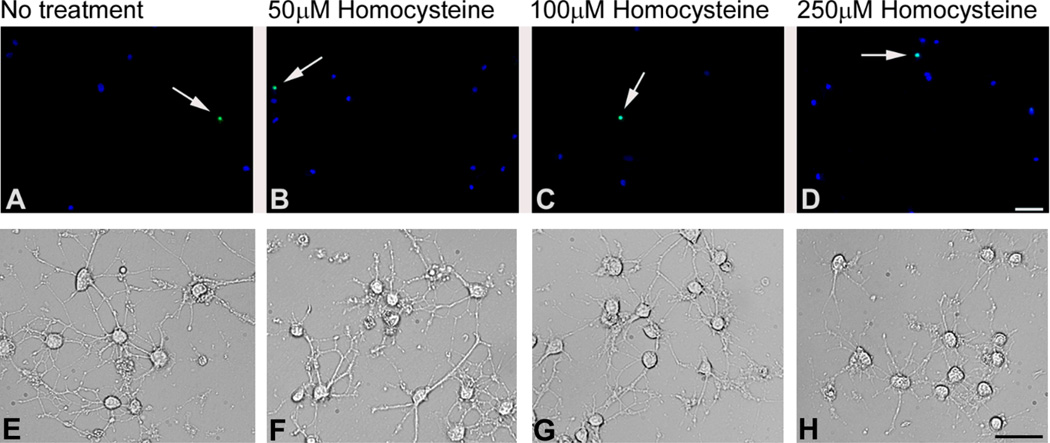
To determine whether staurosporine-differentiation of RGC-5 cells would provide a useful model for studies of homocysteine-induced toxicity to retinal ganglion cells, RGC-5 cells were differentiated with 316 nM staurosporine for 24 h and were then exposed to 50, 100, and 250 µM homocysteine for 18 h. These dosages were selected based on previous work showing that ~50% of primary cells die upon 18 h exposure to 50 µM D,L-homocysteine thiolactone [20] and that intravitreal injection of 75–250 µM D,L-homocysteine thiolactone in mice induced profound death of cells in the ganglion cell layer within 3 days of the injection [35]. (Levels of hyperhomocysteinemia determined in plasma are classified as moderate (16–30 µmol/l), intermediate (31–100 µmol/l), and severe (>100 µmol/l) [36]). When staurosporine-differentiated RGC-5 cells were treated with physiologically relevant doses of homocysteine there were very few TUNEL-positive cells detected at either 50, 100 or 250 µM dosages (Fig. 2B–D). Indeed the paucity of TUNEL-positive cells in homocysteine-treated cells was similar to the few TUNEL-positive cells observed in the untreated staurosporine-differentiated cells (Fig. 2A). These data suggest that staurosporine-differentiation of RGC-5 cells does not render them more susceptible to physiological doses of homocysteine.
Figure 2. TUNEL and differential interference contrast (DIC) microscopic analyses of staurosporine-differentiated RGC-5 cells treated with physiological doses of homocysteine.
Cells were differentiated for 24 h with staurosporine and subjected to no treatment (A,E) or treated with 50 µM homocysteine (B,F), 100 µM homocysteine (C,G), or 250 µM homocysteine (D,H) for 18 h. TUNEL positive cells in panels A–D (arrows) are indicated by bright white (green when in color) fluorescence; Hoescht 33258 staining (blue fluorescence) allows visualization of all cell nuclei. In DIC imaging (E–H), the staurosporine-treated cells demonstrate neurite-like processes, which were not altered by the 50, 100 and 250 µm dosages of homocysteine. Scale bar A–D = 50 µm, E–H = 25 µm).
One of the key features of the staurosporine treatment is that cells develop a more neuronal phenotype characterized by extension of neurite-like processes [30]. Following treatment with physiologically relevant dosages of homocysteine in staurosporine-differentiated RGC-5 cells, we examined cells by differential interference contrast (DIC) microscopy to determine whether homocysteine affected the neuronal processes. There appeared to be no difference in the processes at these homocysteine concentrations (Fig. 2E–H). The cell bodies were also similar in size to the non-homocysteine-treated staurosporine-differentiated cells.
Staurosporine-differentiation of RGC-5 cells induces cell death independent of excitotoxic or oxidative insult
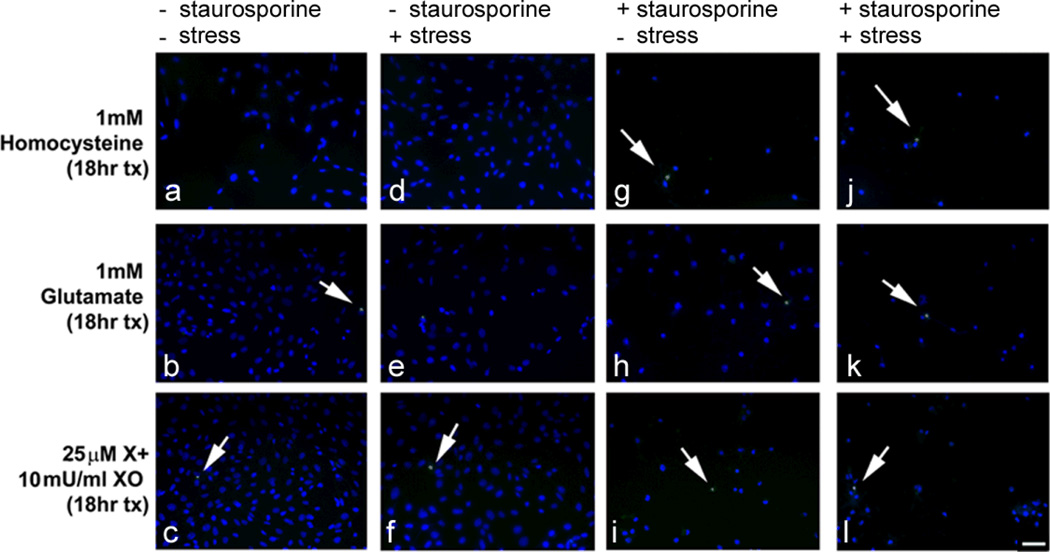
To determine whether staurosporine-differentiated RGC-5 cells were sensitive to higher doses of homocysteine (1 mM) and other toxic insults, RGC-5 cells were differentiated for 24 h in staurosporine and then were exposed for 18 h to excitotoxins (1 mM homocysteine, 1 mM glutamate) or to an inducer of oxidative stress xanthine:xanthine oxidase (25 µM:10 mU/ml). They were subjected to the TUNEL assay to detect fragmented DNA characteristic of dying cells. There were many more cells in the non-staurosporine-differentiated group (Fig. 3a–f) compared to cells that were differentiated with staurosporine (Fig. 3g–l). The number of TUNEL-positive cells was similar regardless whether the cells were differentiated or not or whether they were insulted with homocysteine, glutamate or X:XO (or not). This finding suggested that the staurosporine-differentiated cells do not demonstrate increased sensitivity to elevated levels of homocysteine that would mimic observations in primary cells [26] or in in vivo studies [17].
Figure 3. TUNEL analysis of undifferentiated RGC-5 cells and staurosporine-differentiated RGC-5 cells treated with high doses of homocysteine, and excitotoxic and oxidative stress.
Cells remained undifferentiated or were differentiated with staurosporine for 24 h and subsequently treated with 1 mM homocysteine, 1 mM glutamate, or 25 µM xanthine:10 mU/ml xanthine oxidase for 18 h. TUNEL positive cells (arrows) are indicated by green fluorescence; Hoescht 33258 staining (blue fluorescence) allows visualization of all cell nuclei. Scale bar denotes 50 µm. The labels at the top of the figure: “+ or − staurosporine” indicates whether the cells were (or were not) differentiated with staurosporine; and the “+ or − stress” refers to whether or not the cells were exposed to the excitotoxic insult (homocysteine, glutamate) or oxidative insult (X:XO).
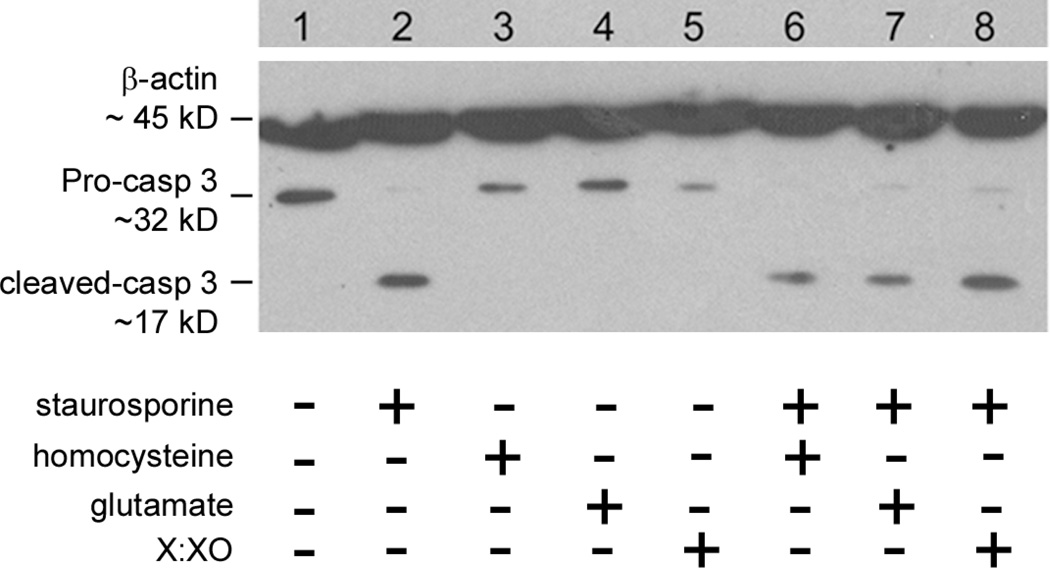
Since there were significantly fewer RGC-5 cells in the staurosporine-treated group, we applied a second measure of cell death, i.e. detection of the pro- versus cleaved forms of caspase-3. Cells undergoing apoptosis typically have a higher expression of the cleaved form (Mr ~ 17 kDa) while cells that are not undergoing apoptosis express predominantly the pro-form of caspase 3 (Mr ~ 32 kDa). Undifferentiated and staurosporine-differentiated RGC-5 cells were treated with homocysteine (1 mM), glutamate (1 mM) or xanthine:xanthine oxidase (25 µM:10 mU/ml) and protein was isolated for detection of the pro- and cleaved forms of caspase-3. Undifferentiated RGC-5 cells (Fig. 4, lane 1), receiving no toxic insult, expressed pro-caspase-3 but not the cleaved form of this protein. Treatment of these non-differentiated cells with homocysteine, glutamate or xanthine:xanthine oxidase did not increase detection of cleaved caspase-3 (Fig. 4, lanes 3–5). We anticipated that differentiating the RGC-5 cells with staurosporine in the presence of excitotoxic insult would increase detection of cleaved caspase-3. We found that the level of cleaved caspase-3 detected in staurosporine-differentiated RGC-5 cells, which received no treatment (Fig. 4, lane 2), was virtually identical to levels detected in treated cells (Fig. 4, lanes 6–8). Thus, the staurosporine-differentiation was associated with induction of cell death independent of toxic insult. Owing to the finding that cleaved caspase-3 is as high in untreated staurosporine-differentiated RGC-5 cells as in homocysteine-treated staurosporine-differentiated cells, the staurosporine-differentiated RGC-5 model does not appear a suitable model for studies of homocysteine-induced ganglion cell death.
Figure 4. Immunodetection of cleaved caspase-3 in undifferentiated and staurosporine-differentiated RGC-5 cells treated with homocysteine, glutamate or X:XO for 18 h.
Protein was isolated from cells and subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an antibody against cleaved caspase-3. β-actin served as the loading control. β-actin: Mr ~ 45 kDa, pro-form caspase-3: Mr ~ 32 kDa, cleaved form caspase-3: Mr ~ 17 kDa. Lanes: (1) RGC-5 cells, no staurosporine differentiation; (2) RGC-5 cells + staurosporine, (3) RGC-5 cells + 1 mM homocysteine, (4) RGC-5 cells + 1 mM glutamate, (5) RGC-5 cells + 25 µM xanthine:10 mU/ml xanthine oxidase (6) RGC-5 cells + staurosporine + 1 mM homocysteine, (7) RGC-5 cells + staurosporine + 1 mM glutamate, (8) RGC-5 cells + staurosporine + 25 µM xanthine:10 mU/ml xanthine oxidase.
Taken together the data from TUNEL analysis and immunodetection of cleaved caspase-3 suggest that 316 nM staurosporine is sufficient to induce apoptosis and activation of caspase-3 in RGC-5 cells, but does not render the cells sensitive to homocysteine treatment.
Undifferentiated RGC-5 cells are susceptible to high levels of homocysteine
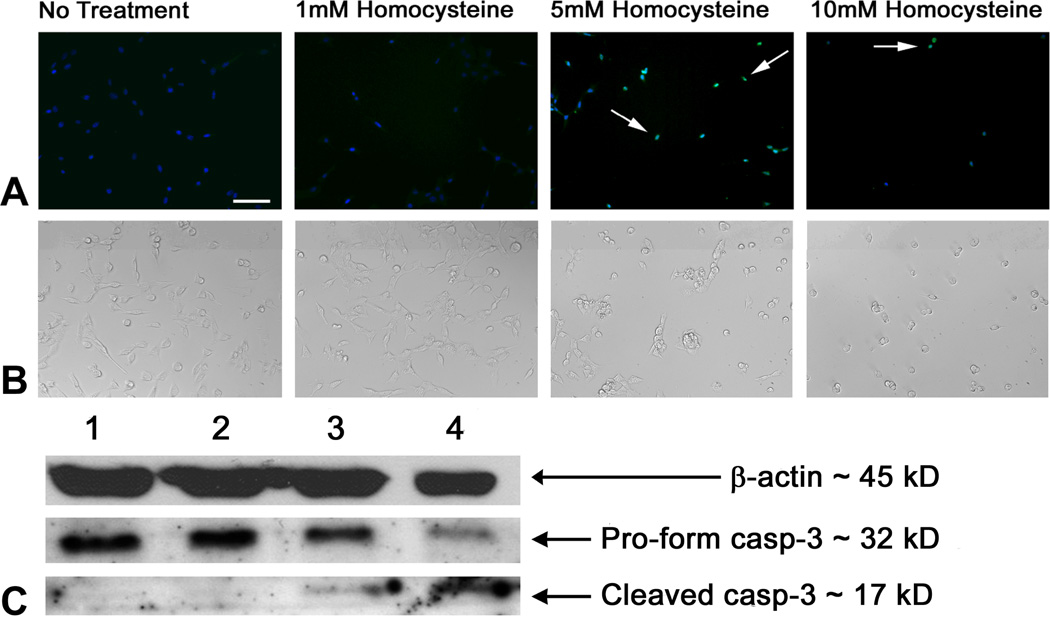
Since the staurosporine-differentiation of RGC-5 cells did not seem promising as a model for testing mechanisms of homocysteine-induced ganglion cell death, we examined the susceptibility of the undifferentiated RGC-5 cells to very high concentrations of homocysteine. Roybal and colleagues examined the role of homocysteine in increasing vascular endothelial growth factor (VEGF) in immortalized (undifferentiated) ARPE-19 cells and showed that homocysteine-induced VEGF increase was mediated by ER-stress [37]. They used concentrations of 10 mM homocysteine for their mechanistic experiments. To determine whether undifferentiated RGC-5 cells were susceptible to such high concentrations of homocysteine, cells were treated with 5 and 10 mM homocysteine for 18 h and subjected to TUNEL analysis and detection of pro- and cleaved-caspase-3. There were significantly more TUNEL-positive cells in RGC-5 cells treated with 5 mM homocysteine (49.54% ± 6.37) compared to untreated (control) and 1 mM homocysteine-treated cells (1.89% ± 0.88 and 1.84% ± 0.96, respectively) and an even higher percentage of TUNEL positive cells when treated with 10 mM homocysteine (75.09% ± 4.10, p<0.0001). After 18 h exposure of RGC-5 cells to 5 mM homocysteine there were ~50% fewer cells (20.5 ± 2.24 cells/field) compared to non-treated cells and to cells treated with only 1 mM homocysteine (42.87 ± 3.41 and 34.27 ± 3.70 cells/field, respectively). In the 10 mM homocysteine-treated cells the number of cells that survived 18 h treatment was only ~25% that of control cells (9.67 ± 0.73 cells/field). The differences in cell numbers in 5 and 10 mM homocysteine-treated cells was highly significant (p<0.0001). When cells were viewed by differential interference contrast microscopy (Fig. 5B), there was little difference in cells treated with 1 mM homocysteine compared to control cells. Cells treated with 5 mM homocysteine showed rounded nuclei consistent with increased cell death. The 10 mM treatment was extremely toxic to cells and very few remained. In additional experiments, cells were treated with 1, 5 or 10 mM homocysteine and levels of pro- and cleaved caspase-3 determined by immunoblotting (Fig. 5C). Treatment with 1 mM homocysteine for 18 h caused no change in the pro- or cleaved forms of caspase-3 when compared to control RGC-5 cells (Fig. 5C, lane 2). Treatment with 5 mM homocysteine resulted in a detectable increase in levels of cleaved-caspase-3 compared to untreated control cells (Fig. 5C, lane 3). Treatment with 10 mM homocysteine, which was highly toxic to cells, resulted in a marked decrease in the pro-form of caspase-3 and pronounced increase in the cleaved form of caspase-3 (Fig 5C, lane 4). The data suggest that undifferentiated RGC-5 cells were sensitive to 5 mM homocysteine as indicated by two measures of apoptosis.
Figure 5. TUNEL analysis and immunodetection of cleaved caspase-3 in undifferentiated RGC-5 cells treated with various doses of homocysteine for 18 h.
(A) RGC-5 cells were seeded onto coverslips and processed for TUNEL analysis with either no treatment, or treatment with 1 mM homocysteine, 5 mM homocysteine, and 10 mM homocysteine. TUNEL positive cells (arrows) are indicated by bright white (green when in color) fluorescence; Hoescht 33258 staining (light gray (blue when in color) fluorescence) allows visualization of all cell nuclei. Scale bar denotes 25 µm. (B) DIC images of cells treated as described in panel A. (C) Protein isolated from cells were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and probed with an antibody against cleaved caspase-3. β-actin served as the loading control. Lanes: (1) RGC-5 cells, no treatment; (2) RGC-5 cells + 1 mM homocysteine; (3) RGC-5 cells + 5 mM homocysteine; (4) RGC-5 cells + 10 mM homocysteine. β-actin: Mr ~ 45 kDa, pro-form caspase-3: Mr ~ 32 kDa, cleaved form caspase-3: Mr ~ 17 kDa.
Discussion
There is considerable interest in understanding mechanisms of ganglion cell death associated with sight-threatening diseases and in developing strategies to intervene in this cell death. To that end, the availability of the RGC-5 ganglion cell line [24] was a breakthrough in the field of retinal ganglion cell research. A drawback of the cell line was its highly proliferative activity and morphological features that were non-neuronal. These observations led to experimentation of the cell line that could trigger a more neuronal phenotype. Recent observations that RGC-5 cell susceptibility to glutamate-induced death has diminished over the past several years [25, 27–29] raised questions about the usefulness of these cells in studies of toxicity and neuroprotection. The encouraging reports that staurosporine-differentiation induced development of neuronal processes and decreased cell proliferation in RGC-5 cells [30,31] prompted our study to determine whether staurosporine-differentiated RGC-5 cells would be useful for the analysis of mechanisms of homocysteine-induced ganglion cell toxicity. It is critical that cellular models demonstrate features of the cell type of interest and mimic toxic susceptibility similar to that known to occur in vivo. Hence, we were particularly interested in determining whether the staurosporine-differentiated RGC-5 cells were susceptible to physiologically relevant dosages of homocysteine. We found that staurosporine-differentiation of RGC-5 cells led to transient increased expression of neuronal and ganglion cell markers and to robust formation of processes. However, staurosporine-differentiated RGC-5 cells did not exhibit increased sensitivity to physiologically relevant levels of homocysteine nor to homocysteine in the 1 mM range. Indeed, staurosporine itself induced apoptosis as indicated by marked increase of cleaved caspase-3, but homocysteine did not increase the cell death.
Regarding the finding that staurosporine-differentiated RGC-5 cells express the ganglion cell marker Thy 1.2, Frassetto et al [30] were the first to report that the RGC-5 cell line could be differentiated by treatment with the nonspecific protein kinase inhibitor staurosporine at a dose of 316 nM for 24 h to induce a more ganglion cell-like phenotype. They reported increased NMDA receptor and Thy 1.2 expression after treatment with staurosporine and an induction of neurite extensions [29]. Our studies confirmed this report and we were able to demonstrate Thy 1.2 expression by immunocytochemistry as well as by gene and protein expression analysis in staurosporine-differentiated cells. Regarding undifferentiated RGC-5 cells, Van Bergen et al found that their RGC-5 cell line did not express Thy 1.2, even after treatment with the differentiating agent S ConA [25]. Interestingly, we were able to detect Thy 1.2, albeit at low levels, in our undifferentiated RGC-5 cells consistent with the original report by Krishnamoorthy et al [24]. The findings are intriguing and suggest that progressive sub-culturing of cell lines alters gene expression, a finding reported by others [38–39]. For example, continued passage of the LNCaP prostatic adenocarcinoma cell line has been shown to mimic the transformation of prostatic cancer from an androgen-dependent to an androgen-independent phenotype [40]. Thus, it is possible that the varying reports of Thy 1.2 expression in the RGC-5 cells reflect alterations due to continued sub-culturing of the cell line.
After confirming the neuronal and ganglion cell-like phenotype of staurosporine-differentiated RGC-5 cells, their susceptibility to homocysteine treatment was determined. We anticipated that upon staurosporine-differentiation of RGC-5 cells, neuronal processes would extend and that there would be fewer cells due to decreased proliferation. We further expected that when cells were treated with homocysteine, neuronal processes would be altered and more cells would be TUNEL-positive. We observed considerably fewer cells when RGC-5 cells were differentiated with staurosporine, but the numbers of cells present under conditions of homocysteine were comparable to non-homocysteine treated cells. There did not appear to be an alteration or disruption of neuronal processes due to homocysteine as we had observed with primary ganglion cells treated with homocysteine [20]. Thus, we concluded that concentrations of homocysteine known to induce ganglion cell death in vivo and in primary cells did not induce death of the staurosporine-differentiated cells. Using higher (1 mM) dosages of homocysteine yielded similar results, i.e. no significant increase in cell death compared to non-treated staurosporine-differentiated cells. Importantly, the staurosporine treatment induced expression of cleaved caspase-3, which has been reported in other cellular systems [41,42]. When characterizing the ability of staurosporine to differentiate RGC-5 cells, Frassetto et al indicated that a low dose of staurosporine (1 µM) failed to induce apoptosis after 24 h as determined by assessment of nuclear condensation using Hoescht 33258 staining [29]. Our data demonstrate that 316 nM staurosporine induces cell death after 36 h exposure (24 h differentiation plus 18 h treatment).
Very recently, investigators have manipulated the experimental conditions for culturing differentiated RGC-5 cells by shortening the treatment with staurosporine to only an hour and have shown sensitivity to serum deprivation and exogenous glutamate [43]. Others have imposed experimental conditions rendering the RGC-5 cells useful for studies testing neuroprotective agents. Chen et al reported that taurine prevented hypoxia-induced apoptosis in RGC-5 cells by preventing mitochondrial dysfunction [44]. Harper et al differentiated RGC-5 cells using 1 µM staurosporine for one hour to promote neurite outgrowth, returned the cells to non-staurosporine media and found that glutamate as well as hydrogen peroxide could induce cell death, which was attenuated by brain-derived neurotrophic factor [43]. Kanamori and coworkers found that tafluprost protected RGC-5 cell death induced by serum deprivation and exogenous glutamate [45]. Thus, it is possible that the experimental paradigm for staurosporine-differentiation of RGC-5 cells reported in the present study and that of Frassetto [30], may not be optimal for testing homocysteine-induced neuronal cell death as we had hoped, however alternative experimental manipulations may prove very useful for this purpose. Taken collectively, the data underscore a critical point in using model cellular systems to simulate in vivo conditions: manipulations, which induce phenotypic changes and even gene changes, do not necessarily reflect functional changes within cells. Future studies will investigate this in detail particularly analyzing susceptibility various ganglion cell model systems to physiologically relevant concentrations of homocysteine.
Acknowledgments
Supported by: NIH R01 EY014560 and EY012830
References
- 1.Wierzbicki AS. Homocysteine and cardiovascular disease: a review of the evidence. Diab Vasc Dis Res. 2007;4:143–150. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. 2009;35:120–129. doi: 10.1002/biof.17. [DOI] [PubMed] [Google Scholar]
- 3.Obeid R, McCaddon A, Herrmann W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin Chem Lab Med. 2007;45:1590–1606. doi: 10.1515/CCLM.2007.356. [DOI] [PubMed] [Google Scholar]
- 4.Martignoni E, Tassorelli C, Nappi G, Zangaglia R, Pacchetti C, Blandini F. Homocysteine and Parkinson's disease: a dangerous liaison? J Neurol Sci. 2007;257:31–37. doi: 10.1016/j.jns.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Staehelin HB. Micronutrients and Alzheimer's disease. Proc Nutr Soc. 2005;64:565–570. doi: 10.1079/pns2005459. [DOI] [PubMed] [Google Scholar]
- 6.Heuberger RA, Fisher AI, Jacques PF, et al. Relation of blood homocysteine and its nutritional determinants to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2002;76:897–902. doi: 10.1093/ajcn/76.4.897. [DOI] [PubMed] [Google Scholar]
- 7.Axer-Siegel R, Bourla D, Ehrlich R, et al. Association of neovascular age-related macular degeneration and hyperhomocysteinemia. Am J Ophthalmol. 2004;137:84–89. doi: 10.1016/s0002-9394(03)00864-x. [DOI] [PubMed] [Google Scholar]
- 8.Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and retinal degeneration in cobalamin C methylmalonic aciduria and homocystinuria. Arch Ophthalmol. 2005;123:1143–1146. doi: 10.1001/archopht.123.8.1143. [DOI] [PubMed] [Google Scholar]
- 9.Seddon JM, Gensler G, Klein ML, Milton RC. Evaluation of plasma homocysteine and risk of age-related macular degeneration. Am J Ophthalmol. 2006;141:201–203. doi: 10.1016/j.ajo.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Bleich S, Jünemann A, von Ahsen N, et al. Homocysteine and risk of open-angle glaucoma. J Neural Transm. 2002;109:1499–1504. doi: 10.1007/s007020200097. [DOI] [PubMed] [Google Scholar]
- 11.Roedl JB, Bleich S, Reulbach U, et al. Homocysteine levels in aqueous humor and plasma of patients with primary open-angle glaucoma. J Neural Transm. 2007;114:445–450. doi: 10.1007/s00702-006-0556-9. [DOI] [PubMed] [Google Scholar]
- 12.Roedl JB, Bleich S, Reulbach U, et al. Vitamin deficiency and hyperhomocysteinemia in pseudoexfoliation glaucoma. J Neural Transm. 2007;114:571–575. doi: 10.1007/s00702-006-0598-z. [DOI] [PubMed] [Google Scholar]
- 13.Roedl JB, Bleich S, Reulbach U, et al. Homocysteine in tear fluid of patients with pseudoexfoliation glaucoma. J Glaucoma. 2007;16:234–239. doi: 10.1097/IJG.0b013e31802d6942. [DOI] [PubMed] [Google Scholar]
- 14.Clement CI, Goldberg I, Healey PR, Graham SL. Plasma homocysteine, MTHFR gene mutation, and open-angle glaucoma. J Glaucoma. 2009;18:73–78. doi: 10.1097/IJG.0b013e31816f7631. [DOI] [PubMed] [Google Scholar]
- 15.Vrabec JP, Levin LA. The neurobiology of cell death in glaucoma. Eye. 2007 Suppl 1:S11–S14. doi: 10.1038/sj.eye.6702880. [DOI] [PubMed] [Google Scholar]
- 16.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res. 2008;173:495–510. doi: 10.1016/S0079-6123(08)01134-5. [DOI] [PubMed] [Google Scholar]
- 17.Ganapathy PS, Moister B, Roon P, Mysona B, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB. Endogenous Elevation of Homocysteine Induces Retinal Ganglion Cell Death in the Cystathionine-β-synthase Mutant Mouse. Invest Ophthal Vis Sci. 2009;50:4460–4470. doi: 10.1167/iovs.09-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodyear E, Levin LA. Model systems for experimental studies: retinal ganglion cells in culture. Prog Brain Res. 2008;173 doi: 10.1016/S0079-6123(08)01120-5. 279-184. [DOI] [PubMed] [Google Scholar]
- 19.Dun Y, Mysona B, Van Ells TK, Amarnath L, Ola MS, Ganapathy V, Smith SB. Expression of the glutamate-cysteine (xc−) exchanger in cultured retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tiss Res. 206;324:189–202. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dun Y, Thangaraju M, Prasad P, Ganapathy V, Smith SB. Prevention of excitotoxicity in primary retinal ganglion cells by (+)-Pentazocine, a sigma receptor-1-specific ligand. Invest Ophthamol Vis Sci. 2007;48:4785–4794. doi: 10.1167/iovs.07-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, Ganapathy V, Prasad P. Cloning and functional characterization of the proton-coupled electrogenic folate transporter from mouse retina and analysis of its expression in retinal cell types. Invest Ophthamol Vis Sci. 2007;48:5299–5305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dun Y, Duplantier JN, Roon P, Martin P, Ganapathy V, Smith SB. Serine Racemase Expression and D-serine content are Developmentally Regulated in Retinal Ganglion Cells. J. Neurochemistry. 2008;104:970–978. doi: 10.1111/j.1471-4159.2007.05015.x. [DOI] [PubMed] [Google Scholar]
- 23.Kutty RK, Samuel W, Chen S, Vijayasarathy C, Dun Y, Mysona B, Smith SB. Immunofluorescence analysis of the expression of Norpeg (Rai14) in retinal Müller and ganglion cells. Neuroscience Letters. 2006;404:294–298. doi: 10.1016/j.neulet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 25.Van Bergen NJ, Wood JP, Chidlow G, Trounce IA, Casson RJ, Ju WK, Weinreb RN, Crowston J. Re-characterisation of the RGC-5 retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2009;50:4267–4272. doi: 10.1167/iovs.09-3484. [DOI] [PubMed] [Google Scholar]
- 26.Martin PM, Ola MS, Agarwal N, Ganapathy V, Smith SB. The Sigma Receptor (σR) Ligand (+)-pentazocine prevents retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Research Mol Brain Res. 2004;123:66–75. doi: 10.1016/j.molbrainres.2003.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aoun P, Simpkins JW, Agarwal N. Role of PPAR-gamma ligands in neuroprotection against glutamate-induced cytotoxicity in retinal ganglion cells. Invest Ophthalmol Vis Sci. 2003;44:2999–3004. doi: 10.1167/iovs.02-1060. [DOI] [PubMed] [Google Scholar]
- 28.Fan W, Agarwal N, Kumar MD, Cooper NG. Retinal ganglion cell death and neuroprotection: Involvement of the CaMKIIalpha gene. Brain Res Mol Brain Res. 2005;139:306–316. doi: 10.1016/j.molbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Maher P, Hanneken A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Invest Ophthalmol Vis Sci. 2005;46:749–757. doi: 10.1167/iovs.04-0883. [DOI] [PubMed] [Google Scholar]
- 30.Frassetto LJ, Schlieve CR, Lieven CJ, Utter AA, Jones MV, Agarwal N, Levin LA. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 31.Lieven CJ, Millet LE, Hoegger MJ, Levin LA. Induction of axon and dendrite formation during early RGC-5 cell differentiation. Exp Eye Res. 2007;85:678–683. doi: 10.1016/j.exer.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang SS, Zhang JH, Liu HX, Zhou D, Qi R. Pro-protein convertase-2/carboxypeptidase-E mediated neuropeptide processing of RGC-5 cell after in vitro ischemia. Neurosci Bull. 2009;25:7–14. doi: 10.1007/s12264-009-1027-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Iizuka Y, Hong S, Kim CY, Kim SK, Seong GJ. Agmatine pretreatment protects retinal ganglion cells (RGC-5 cell line) from oxidative stress in vitro. Biocell. 2008;32:245–250. [PubMed] [Google Scholar]
- 34.Harvey R, Chintala SK. Inhibition of plasminogen activators attenuates the death of differentiated retinal ganglion cells and stabilizes their neurite network in vitro. Invest Ophthalmol Vis Sci. 2007;48:1884–1891. doi: 10.1167/iovs.06-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore P, El-Sherbeny A, Roon P, Schoenlein PV, Ganapathy V, Smith SB. Apoptotic retinal ganglion cell death is induced in vivo by the excitatory amino acid homocysteine. Exp Eye Res. 2001;73:45–57. doi: 10.1006/exer.2001.1009. [DOI] [PubMed] [Google Scholar]
- 36.Graeme JH, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999;354:407–413. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- 37.Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, Abcouwer SF. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279:14844–14852. doi: 10.1074/jbc.M312948200. [DOI] [PubMed] [Google Scholar]
- 38.Fourrier MC, Arnold MF, Collet B, Munro ES. The Effect of sub-culturing on the basal level of type I interferon (IFN) gene expression in the Salmon Head Kidney (SHK-1) cell line. Fish Shellfish Immunol. 2009;27:535–538. doi: 10.1016/j.fsi.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Pavan B, Dalpiaz A, Biondi C, Nieddu M, De Luca A, Prasad PD, Paganetto G, Favaloro B. An RPE cell line as a useful in vitro model for studying retinoic acid receptor beta: expression and affinity. Biosci Rep. 2008;28:327–334. doi: 10.1042/BSR20080103. [DOI] [PubMed] [Google Scholar]
- 40.Youm YH, Kim S, Bahk YY, Yoo TK. Proteomic analysis of androgen-independent growth in low and high passage human LNCaP prostatic adenocarcinoma cells. BMB Rep. 2008;41:722–727. doi: 10.5483/bmbrep.2008.41.10.722. [DOI] [PubMed] [Google Scholar]
- 41.Lan L, Wong NS. Phosphatidylinositol 3-kinase and protein kinase C are required for the inhibition of caspase activity by epidermal growth factor. FEBS Lett. 1999;444:90–96. doi: 10.1016/s0014-5793(99)00032-0. [DOI] [PubMed] [Google Scholar]
- 42.Chakravarthy BR, Walker T, Rasquinha I, Hill IE, MacManus JP. Activation of DNA-dependent protein kinase may play a role in apoptosis of human nueroblastoma cells. J Neurochem. 1999;72:933–942. doi: 10.1046/j.1471-4159.1999.0720933.x. [DOI] [PubMed] [Google Scholar]
- 43.Harper MM, Adamson L, Blits B, Bunge MB, Grozdanic SD, Sakaguchi DS. Brain-derived neurotrophic factor released from engineered mesenchymal stem cells attenuates glutamate- and hydrogen peroxide-mediated death of staurosporine-differentiated RGC-5 cells. Exp Eye Res. 2009;89:538–548. doi: 10.1016/j.exer.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen K, Zhang Q, Wang J, Liu F, Mi M, Xu H, Chen F, Zeng K. Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res. 2009;1279:131–138. doi: 10.1016/j.brainres.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 45.Kanamori A, Naka M, Fukuda M, Nakamura M, Negi A. Tafluprost protects rat retinal ganglion cells from apoptosis in vitro and in vivo. Graefes Arch Clin Exp Ophthalmol. 2009;247:1353–1360. doi: 10.1007/s00417-009-1122-6. [DOI] [PubMed] [Google Scholar]