Abstract
Cisplatin is a commonly used chemotherapeutic drug the clinical use of which is limited by the development of dose-dependent nephrotoxicity. Enhanced inflammatory response, oxidative stress and cell death have been implicated in the development of cisplatin-induced nephropathy; however the precise mechanisms are elusive. Overactivation of the nuclear enzyme poly(ADP-ribose)polymerase-1 (PARP-1) by oxidative DNA damage in various pathological conditions promotes cell death and up-regulation of key proinflammatory pathways. In this study, using a well-established model of nephropathy, we have explored the role of PARP-1 in cisplatin-induced kidney injury. Genetic deletion or pharmacological inhibition of PARP-1 markedly attenuated the cisplatin-induced histopathological damage, impaired renal function (elevated serum BUN and creatinine levels), enhanced inflammatory response (leukocyte infiltration, TNF-α, IL-1β, F4/80, adhesion molecules ICAM-1/VCAM-1 expressions) and consequent oxidative/nitrative stress (HNE, 8-OHdG, and nitrotyrosine content, NOX2/NOX4 expressions). PARP inhibition also facilitated the cisplatin-induced cell death in cancer cells. Thus, PARP activation plays an important role in cisplatin-induced kidney injury, and its pharmacological inhibition may represent a promising approach to prevent the cisplatin-induced nephropathy. This is particularly exciting since several PARP inhibitors alone or in combination with DNA-damaging anticancer agents show considerable promise in clinical trials to treat various malignancies (e.g. triple-negative breast cancer).
Keywords: nephropathy, cisplatin, poly(ADP-ribose) polymerase
Introduction
The platinum compound cisplatin is a potent and widely used chemotherapy drug to treat various solid tumors and other malignancies; unfortunately, the major limitation of its clinical use is the development of dose-dependent nephrotoxicity in about one third of patients preventing the use of high doses to take full advantage of the therapeutic efficacy [1, 2]. Cisplatin binds to DNA, leading to the formation of inter- and intrastrand cross-links, resulting in defective DNA templates and arrest of DNA synthesis and replication, particularly in rapidly dividing cancer cells [3]. Enhanced inflammatory response, cell death and oxidative stress appear to be involved in the development of cisplatin-induced nephropathy [4–7]; however the precise mechanisms are elusive and efficient treatment to decrease this devastating complication of the chemotherapy is not available.
Poly(ADP-ribose) polymerase 1 (PARP-1) is the most abundant isoform of the nuclear enzyme PARP family. In various pathological conditions PARP-1 overactivation by oxidative DNA damage depletes its substrate NAD(+), slowing the rate of glycolysis, electron transport, and ATP formation, eventually leading to functional impairment or death of various normal cell types, as well as to up-regulation of various key proinflammatory pathways such as nuclear factor kappa B (NF-κB) [8–10]. Conversely, PARP inhibitors exert multitude of cytoprotective and anti-inflammatory effects in preclinical models of reperfusion injury [9, 10], lung inflammation [11], shock [9, 10, 12, 13], diabetes and diabetic complications [14–20] among many others [9, 10, 21]. In clinical trials inhibition of PARP alone or in combination with DNA-damaging anticancer agents shows considerable promise in facilitating tumor cell death (e.g. in breast cancer) [22–27]. Excitingly, PARP-1 has also been implicated in the chemoresistance of cancer cells to cisplatin [28, 29] and PARP inhibition shows synergistic chemosensitivity of triple-negative breast cancer cell lines to gemcitabine and cisplatin [30].
In this study we investigated the role of PARP-1 in cisplatin-induced kidney injury using a well-established mouse model of cisplatin-induced nephropathy [6, 31–40] and the effects of PARP inhibitors alone or in combination with cisplatin on viability of cancer cells. These results may have important clinical implications for the prevention of the cisplatin-induced nephrotoxicity with PARP inhibitors, which already show potent anticancer activities in clinical trials and synergistic anticancer effect with cisplatin in multiple experimental paradigms.
Material and methods
Animals and drug treatment
All animal experiments conformed to National Institutes of Health (NIH) guidelines and were approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism (NIAAA; Bethesda, MD, USA). Six to 8-week-old male C57Bl/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). PARP-1 knockout mice (PARP-1−/−) and their wild-type littermates (PARP-1+/+) were as described previously backcrossed to a C57Bl/6J background [41]. All animals were kept in a temperature-controlled environment with a 12-h light–dark cycle and were allowed free access to food and water at all times, and were cared for in accordance with National Institutes of Health (NIH) guidelines. Mice were sacrificed 72 hrs following a single injection of cisplatin (cis-Diammineplatinum(II) dichloride 25 mg/kg i.p.; Sigma). Two inhibitors of PARP 5-aminoisoquinoline (5-AIQ) and N-(5,6-Dihydro-6-oxo-2-phenanthridinyl)-2-acetamide hydrochloride (PJ34) [10] were purchased from Axxora LLC (San Diego, CA). The selective PARP inhibitors AIQ and PJ34 were dissolved in saline and administered at 10 mg/kg, i.p. daily, starting 2 hours before the cisplatin administration. In another set of experiments, AIQ and PJ34 treatment started 12 and 24 h after the cisplatin injection and tissues collected for the biochemical and histological measurements at 72 h. For the main study 6 mice were treated with vehicle, 6-6 with PARP inhibitors (AIQ and PJ34), 8 with cisplatin, and 8-8 with cisplatin in combination with PARP inhibitors. For the post-treatment studies additional 4 mice were treated with vehicle, 4 with cisplatin, and 8-8 with cisplatin in combination with PARP inhibitors (4 for each time-point). For the time course experiments 6 mice were treated with vehicle and 28 with cisplatin (following the cisplatin treatment 7-7 mice were sacrificed 6, 24, 48, and 72 hours following the cisplatin exposure). Additional 6-6 PARP-1+/+ and PARP-1−/− mice were treated with vehicle or 8-8 of each with cisplatin.
Renal function monitoring
On the day of the sacrifice, blood was immediately collected and serum levels of creatinine and Blood Urea Nitrogen (BUN) were measured using VetTest 8000 blood chemistry analyzer (Idexx Lab) [5].
Histological examination
Following fixation of the kidneys with 10% formalin, renal tissues were sectioned and stained with periodic acid-Schiff (PAS) reagents (Newcomer Supply, Middleton, WI) for histological examination. Tubular damage in PAS-stained sections was examined under the microscope and scored based on the percentage of cortical tubules showing epithelial necrosis: 0 = normal; 1 = <10%; 2 = 10– 25%; 3 = 26–75%; 4 = >75%. Tubular necrosis was defined as the loss of the proximal tubular brush border, blebbing of apical membranes, tubular epithelial cell detachment from the basement membrane or intraluminal aggregation of cells and proteins as described [5]. For myeloperoxidase (MPO) staining slides were deparaffinized, and hydrated in descending gradations of ethanol, followed by antigen retrieval procedure. Next, sections were incubated in 0.3% H2O2 in PBS to block endogenous peroxidase activity. The sections were then incubated with anti-MPO (Biocare Medical, Concord, CA) antibodies overnight at 4°C in a moist chamber. Biotinylated secondary antibodies and ABC reagent were added as per the kit’s instructions (Vector Laboratories, Burlingame, CA, USA). Color development was induced by incubation with a DAB kit (Vector Laboratories) for 3–5 min, and the sections were counter-stained with nuclear fast red as described [5]. Finally, the sections were dehydrated in ethanol and cleared in xylene and mounted. The specific staining was visualized and images were acquired using microscope IX-81 with 20X, 40X and 100x objectives (Olympus, Center Valley, PA). The morphometric examination was performed in a blinded manner by two independent investigators.
Renal nitrotyrosine (3-NT) content
NT was measured by the NT ELISA kit from Hycult Biotechnology (Cell Sciences, Canton, MA) from tissue homogenates as described [5]. Levels were presented as fold change compared to vehicle-treated control sample.
Renal myeloperoxidase activity assay
Myeloperoxidase [MPO, (EC1.11.1.7)] was measured by InnoZyme™ Myeloperoxidase Activity Kit (EMD Gibbstown, NJ) according to manufacturer’s instruction. Myeloperoxidase activities were expressed as fold change compared to the vehicle-treated control sample [42].
Renal 4-hydroxynonenal (4-HNE) content
4-HNE in the kidney tissues was determined using the kit (Cell Biolabs, San Diego, CA). In brief, BSA or renal tissue extracts (10μg/mL) are adsorbed on to a 96-well plate for 12hrs at 4°C. 4-HNE adducts present in the sample or standard are probed with anti-HNE antibody, followed by an HRP conjugated secondary antibody. The HNE-protein adducts content in an unknown sample is determined by comparing with a standard curve.
Renal caspase3/7 activity
For caspase assay of tissue lysate, caspase-3/7 activity of the lysate was measured using Apo-One Homogenous caspase-3/7 assay kit (Promega, Madison, WI) as described. An aliquot of caspase reagent was added to each well, mixed on a plate shaker for 1 h at room temperature with light protection, and the fluorescence emission was measured in Victor (Perkin Elmer, Waltham, MA).
Renal 8-hydroxy-2′-deoxyguanosine (8-OHdG) content
Under conditions of oxidative stress, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is formed when DNA is oxidatively damaged by reactive oxygen species (ROS). DNA from samples were isolated using Qiagen Genomic DNA isolation kit. Extracted DNA was digested by P1 nuclease followed by alkaline phosphatase treatment. Enzymes and other macromolecules were removed through Microcon YM-10 (Millipore, Billerca, MA). The 8-OHdG ELISA assay uses a competitive format wherein a murine monoclonal antibody to 8-OHdG (Primary Anti-8OHdG) and sample or standard are added to a microtiter plate which has been precoated with 8-OHdG (Northwest Life Science Research, Vancouver, WA). Sample or calibrator 8-OHdG competes with plate-bound 8-OHdG for binding with the antibody. Accordingly, higher concentrations of sample or calibrator leads to reduced binding of the antibody to the 8OHdG coated plate. A subsequent wash step removes any free 8-OHdG/antibody adduct leaving stationary plate bound 8-OHdG complexed to antibody for later detection. Anti-murine antibody conjugated to horse radish peroxidase (HRP-Conjugate) is then added to the plate. HRP-conjugate binds to remaining murine anti-8-OHdG and unbound HRP-conjugate is removed in another wash step. Addition of 3,3′,5,5′tetramethylbenzidine (TMB Substrate) results in blue color development proportional to the amount of anti 8-OHdG antibody bound to the plate and inversely proportional to the concentration 8-OHdG in original samples or calibrators applied to the plate. The reaction is terminated by addition of phosphoric acid (Stop Solution) producing yellow color with measurable absorbance at 450 nm.
Real-time PCR analyses
Total RNA was isolated from kidney homogenate using Trizol reagents (Invitrogen, Carlsbad, CA) according to manufacturer’s instruction. The isolated RNA was treated with RNase-free DNase (Ambion, Austin, TX) to remove traces of genomic DNA contamination. One microgram of total RNA of was reverse-transcribed to cDNA using the Super-Script II (Invitrogen, Carlsbad, CA). The target gene expression was quantified with Power Syber Green PCR Master Mix using ABI HT7900 Realtime PCR Instrument. Each amplified sample in all wells was analyzed for homogeneity using dissociation curve analysis. After denaturation at 95 °C for 2 min, 40 cycles were performed at 95 °C for 10 s, 60 °C for 30 s. Relative quantification was calculated using the comparative CT method (2- Ct method: Ct = Ct sample – Ct [5]. Lower CT values and lower CT reflect a relatively higher amount of gene transcript. Statistical analyses were carried out for at least 6 to 8 replicate experimental samples in each set.
Primers used:
TNF-α: 5′AAGCCTGTAGCCCACGTCGTA3′ and 5′AGGTACAACCCATCGGCTGG3′
IL-1β: 5′AAAAAAGCCTCGTGCTGTCG3′ and 5′GTCGTTGCTTGGTTCTCCTTG3′
ICAM: 5′ AACTTTTCAGCTCCGGTCCTG 3′ and 5′ TCAGTGTGAATTGGACCTGCG3′
VCAM: 5′ TTATTGTTGACATCTCCCCCG 3′ and 5′ TCATTCCTTACCACCCCATTG 3′
NOX2(gp91phox): 5′ GACCATTGCAAGTGAACACCC3′ and 5′ AAATGAAGTGGACTCCACGCG3′
NOX4(RENOX): 5′ TCATTTGGCTGTCCCTAAACG3′ and 5′ AAGGATGAGGCTGCAGTTGAG3′
F4/80: 5′ TGTGTCGTGCTGTTCAGAACC3′ and 5′AGGAATCCCGCAATGATGG3′
Actin: 5′TGCACCACCAACTGCTTAG3′ and 5′GGATGCAGGGATGATGTTC3′.
Cell culture of cancer cell line T24
T24, a transitional cell carcinoma from human urinary bladder was purchased from ATCC. The T24 cell line was cultured in McCoy’s medium with 10% foetal calf serum and 1% penicillin–streptomycin. Cells were harvested at 70–80% confluence by washing with prewarmed 37°C phosphate-buffered saline (PBS) and then trypsinisation (0.1% trypsin, 0.4% EDTA in PBS) at 37°C. All cultures were tested for viability following the treatment as described in result section.
Cytotoxicity and cell survival assays
The effect of cisplatin and PARP inhibitors, or their combinations on cell survival were assessed using the XTT assay (Cell Proliferation Kit II, Roche Diagnostics, Indianapolis, IN). Cells were seeded into 96 or 24 well culture plates. Twenty-four hours later, cells were treated with different concentrations of cisplatin, AIQ and PJ34, or their combination as described in the figures. Untreated cells were cultured in parallel as a negative control. After 72h of incubation, cells were treated with 50 μL aliquot of the XTT solution (1 mL XTT labeling/20 μL electron-coupling reagent) to each well at the end of the experiment. After 2h of incubation, the absorbance was measured at both 492 nm and a reference wavelength (690 nm).
Flow cytometry analyses of cell death
After the treatments, total cell death in T24 cancer cell line was determined by using flow cytometry as described [43, 44].
Statistical analysis
Results are expressed as mean±SEM. Statistical significance among groups was determined by one-way ANOVA followed by Newman-Keuls post hoc analysis using GraphPad Prism 5 software (San Diego, CA). Probability values of P<0.05 were considered significant.
Results
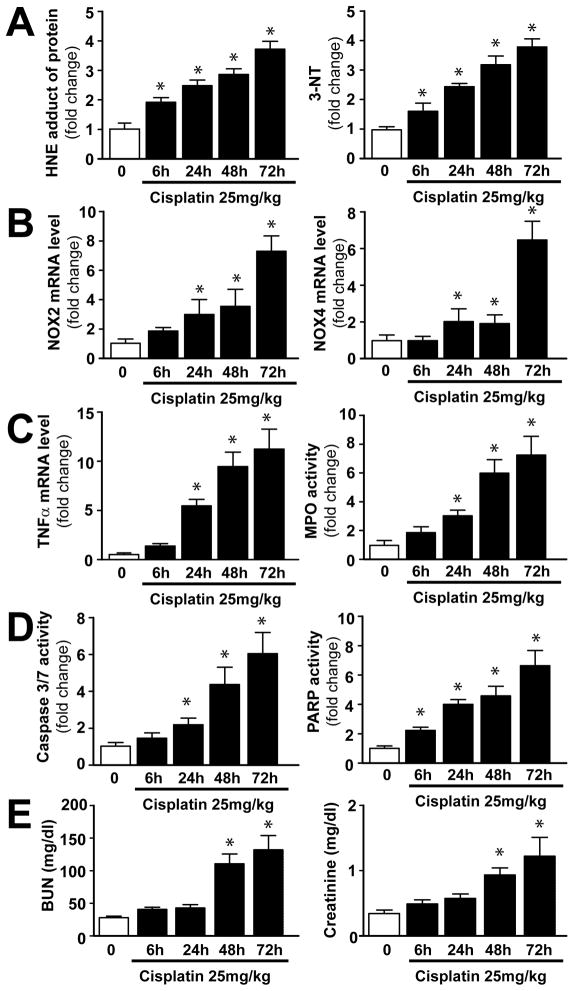
Cisplatin-triggers time-dependent increase in renal oxidative/nitrative stress, PARP activation, and inflammatory response, which correlate with tubular cell death and renal dysfunction
In the kidney cisplatin induced time-dependent increase in HNE and 3-NT from 6 hours of its administration, which paralleled with PARP activation (Figure 1A–D). The expression of mRNAs of reactive oxygen species (ROS) generating NAD(P)H oxidase isoforms NOX2 and NOX4 were increased only from 24–48 hours following cisplatin administration (Figure 1B), which coincided with the enhanced inflammatory response (increased TNFα mRNA levels Figure 1C). The apoptotic cell death occurred at the peak of oxidative/nitrative stress and inflammation (Figure 1D) and also paralleled with the development of renal dysfunction (Figure 1E). These results suggest that the cisplatin induces increased oxidative/nitrative stress and consequent PARP activation in the kidneys preceding inflammatory response.
Figure 1. Time-dependent changes in cisplatin-induced renal oxidative/nitrative stress, inflammation, apoptosis, PARP activation and dysfunction.
Cisplatin time-dependently increased markers of oxidative/nitrative stress, inflammation and cell death (Panels A–D) in the kidneys following its administration to mice, which paralleled with the development of renal dysfunction (Panel E). Results are mean±S.E.M. of 6–7/group. *P<0.05 vs. 0 time point.
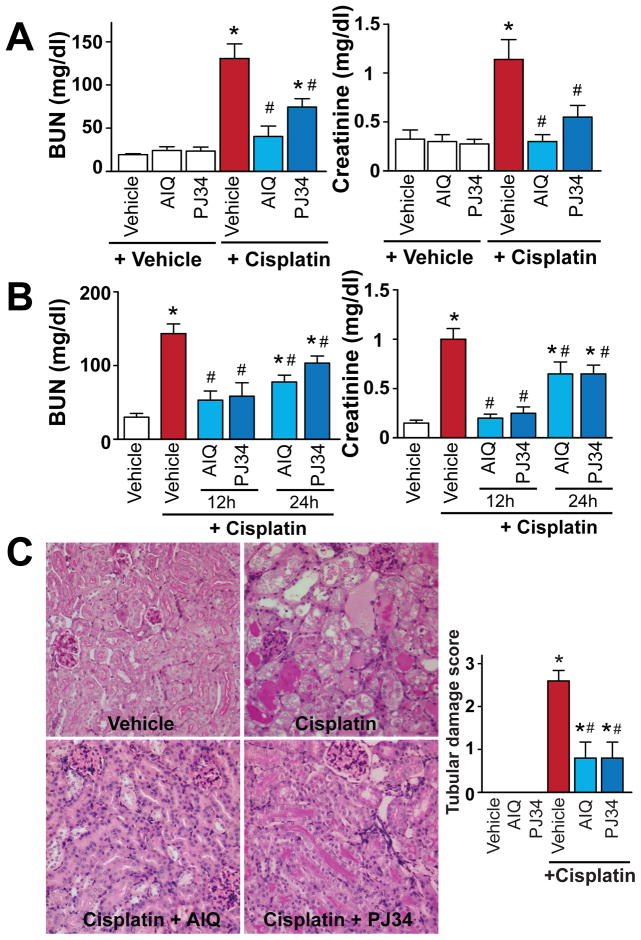
Pharmacologic inhibition of PARP attenuates the cisplatin-induced kidney injury
Pharmacological inhibition of PARP with AIQ or PJ34 administered 2 hours before cisplatin injection (Figure 2A) or 12 or 24 hours after (Figure 2B) reduced cisplatin-induced profound kidney injury as evidenced by the attenuation of the increase in blood urea nitrogen (BUN) and creatinine values (Figure 2A, B) and decrease in tubular necrosis (Figure 2C) determined by PAS staining in the kidney 72 hours after cisplatin administration to mice. PARP inhibitors had no effect in control mice on all variables studied (Figure 2A–C).
Figure 2. Pharmacologic inhibition of PARP attenuates cisplatin-induced kidney damage.
Pretreatment with PARP inhibitors (Panels A and C) ameliorates cisplatin-induced profound kidney damage as evidenced by the attenuation of the increase in blood urea nitrogen (BUN) and creatinine values (Panel A) and histological tubular damage (Panel C, PAS Staining) in the kidney 72h after cisplatin administration to mice. PARP inhibitors were also effective in decreasing cisplatin-induced renal dysfunction (B) when administered 12 or 24 hours following the drug injection. Results are mean±S.E.M. of 6–8 experiments/group for panel A, 4–8 for panel B. Panel C (400x magnification) depicts representative sections from 4 kidneys of 4 animals/group, and quantifications from 8 representative fields with 400x magnification/group. *P<0.05 v.s. Vehicle, #P<0.05 Vehicle+Cisplatin vs. Cisplatin+AIQ/PJ34.
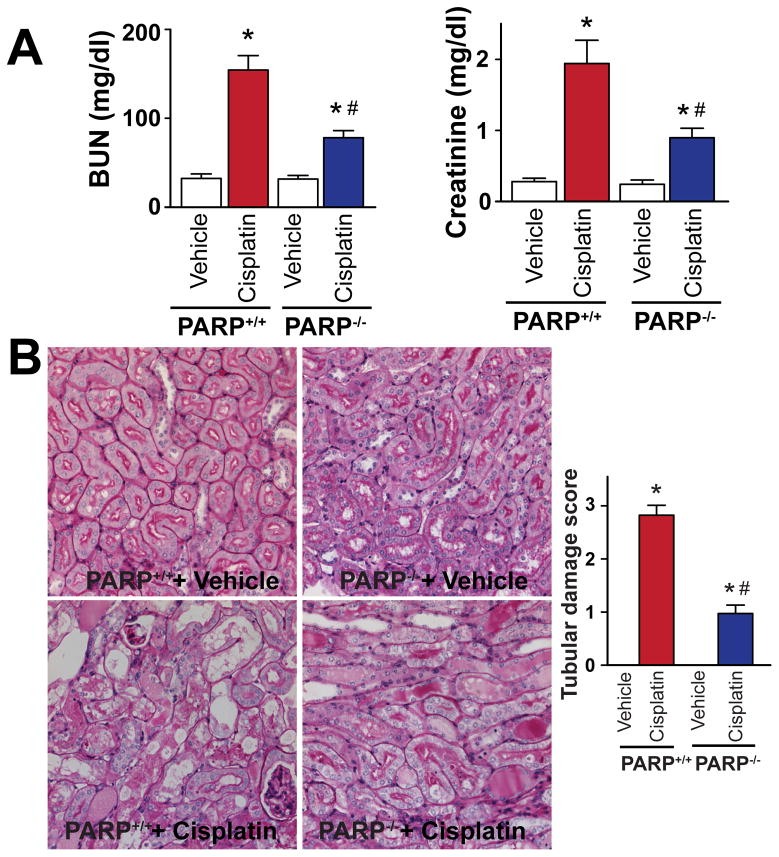
Genetic deletion of PARP-1 attenuates the cisplatin-induced kidney injury
Genetic deletion of PARP-1 reduced cisplatin-induced profound kidney damage as evidenced by the attenuation of the increase in blood urea nitrogen (BUN) and creatinine values (Figure 3A) and decrease in tubular necrosis (Figure 3B) determined by PAS staining in the kidney 72 hours after cisplatin administration to mice. PARP-1−/− and PARP-1+/+ mice treated with vehicle only had normal kidney function and histology (Figure 3A, B).
Figure 3. Genetic deletion of PARP-1 attenuates cisplatin-induced kidney damage.
Genetic deletion of PARP-1 ameliorates the cisplatin-induced profound kidney damage as evidenced by the attenuation of the increase in blood urea nitrogen (BUN) and creatinine values (Panel A) and histological tubular damage (Panel B, PAS Staining) in the kidney 72h after cisplatin administration to mice. Results are mean±S.E.M. of 6–8 experiments/group. *P<0.05 vs. Vehicle, #P<0.05 Cisplatin in PARP+/+ vs. Cisplatin in PARP−/−mice.
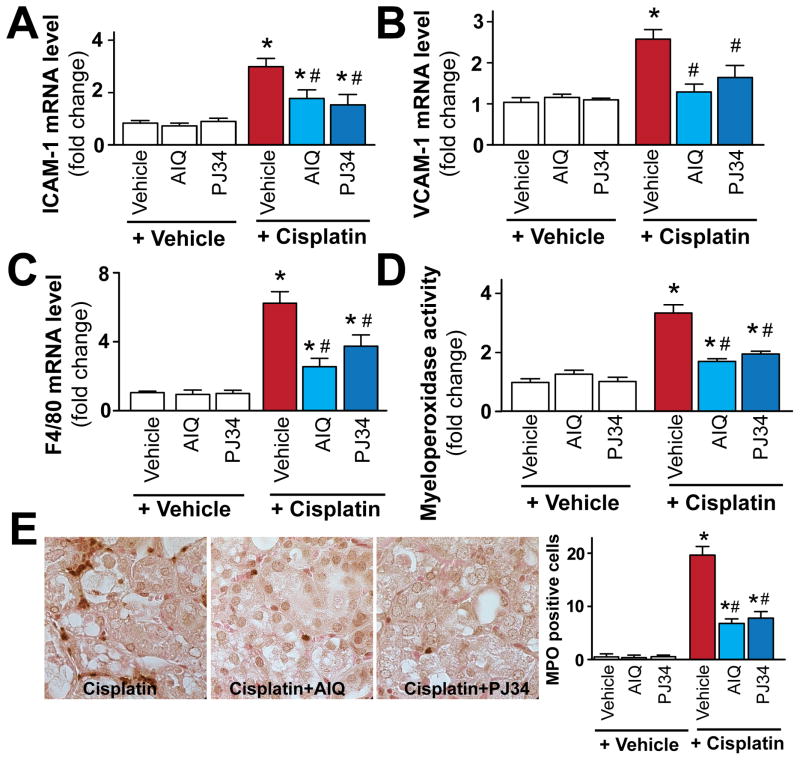
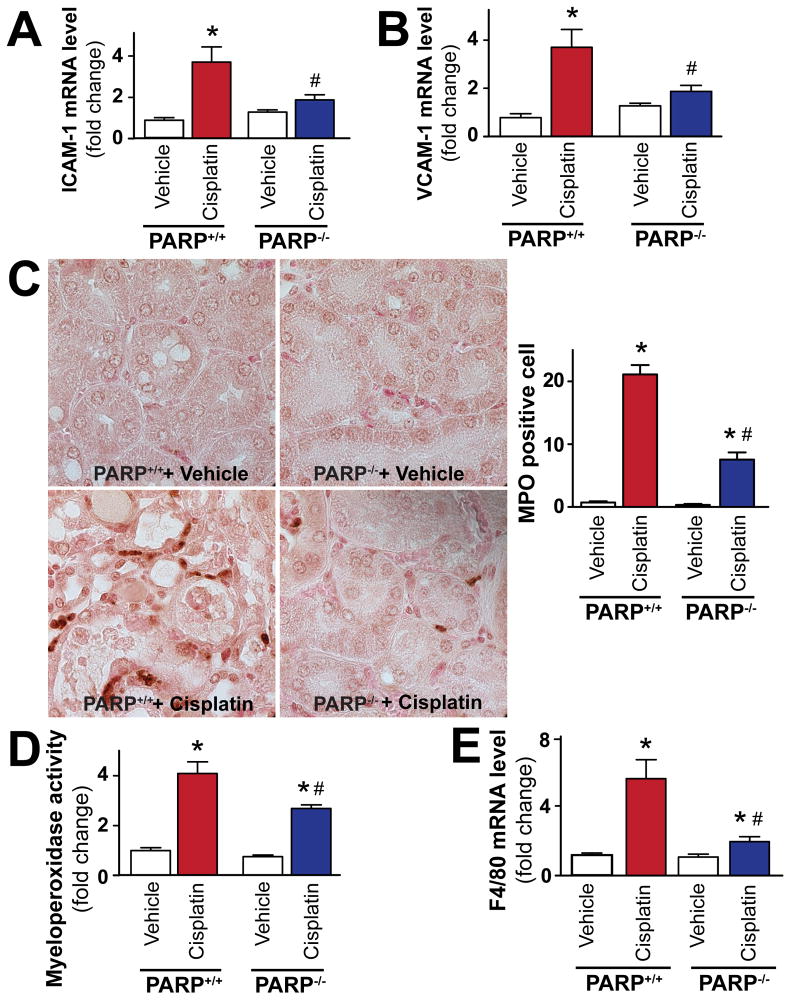
Pharmacologic inhibition of PARP attenuates cisplatin-induced adhesion molecules expression, leukocyte and macrophage infiltration in the kidney
Since pharmacological inhibition or genetic deletion of PARP-1 is known to attenuate adhesion molecules expression during ischemic-reperfusion injury [10, 45], and increased adhesion molecules expressions are important in mediating inflammatory response (leukocyte and other inflammatory cell attachment to activated endothelium and/or parenchyma cells) in cisplatin-induced nephropathy, we also investigated the effects of pharmacological inhibition or genetic deletion of PARP-1 on cisplatin-induced adhesion molecules ICAM-1 and VCAM-1 expressions. Cisplatin significantly increased mRNA expression of adhesion molecules ICAM-1 and VCAM-1 (Figure 4A, B), F4/80 (marker of macrophage infiltration; Figure 4C), and myeloperoxidase activity (an indicator of leukocyte infiltration; Figure 4D, E). These changes were attenuated by treatment with AIQ or PJ34 (Figure 4A–E). PARP inhibitors had no effects in control mice on all variables studied (Figure 4A–E).
Figure 4. Pharmacologic inhibition of PARP attenuates cisplatin-induced adhesion molecules expression and leukocyte and macrophage infiltration.
Cisplatin significantly increased mRNA expression of adhesion molecules ICAM-1 and VCAM-1 mRNA (panels A and B), F4/80 (a marker of macrophages, panel C) and renal myeloperoxidase (MPO) activity/staining (panel D, E; an indicator of leukocyte infiltration), indicating enhanced inflammatory response. These were attenuated by treatment with AIQ or PJ34 (panels A–E; n=6–8/group). Panel E shows representative MPO staining (brown) of 4-4 kidneys/group (1000x magnification) from mice treated with cisplatin or cisplatin in combination with PARP inhibitors, and quantifications from 8 representative fields with 400x magnification/group. Results are mean±S.E.M. *P<0.05 vs. Vehicle, #P<0.05 Vehicle+Cisplatin vs. Cisplatin+AIQ/PJ34.
Genetic deletion of PARP attenuates cisplatin-induced adhesion molecules expression and leukocyte infiltration in the kidney
Cisplatin significantly increased mRNA expression of adhesion molecules ICAM-1 and VCAM-1 in PARP-1+/+ mice (Figure 5A, B), F4/80 (Figure 5E), and renal myeloperoxidase activity and staining (Figure 5C, D); these changes were attenuated in PARP-1−/− mice compared to PARP-1+/+ littermates (Figure 5A–E). PARP-1−/− and PARP-1+/+ mice treated with vehicle only had similar low levels of adhesion molecules and inflammatory cell markers (Figure 5A–E).
Figure 5. Genetic deletion of PARP-1 attenuates cisplatin-induced adhesion molecules expression, leukocyte and macrophage infiltration.
Cisplatin significantly increased mRNA expression of adhesion molecules ICAM-1 and VCAM-1 (panels A and B), F4/80 (marker of macrophages, panel E), and renal myeloperoxidase staining (panel C) or activity (panel D) (an indicator of leukocyte infiltration), in PARP-1+/+ mice (n=8). Panel D: Quantitative MPO assay indicating enhanced inflammatory cell infiltration in the kidneys of cisplatin-treated PARP-1+/+ mice (n=8). These changes were attenuated in PARP-1−/− mice (n=8). Panel C shows representative MPO staining (brown) of 4-4 kidneys/group (1000x magnification) from PARP-1+/+ and PARP-1 mice treated with vehicle (n=6) or cisplatin (n=8), and quantifications from 8 representative fields with 400x magnification/group. Results are mean±S.E.M. *P<0.05 vs. Vehicle in PARP+/+, #P<0.05 +Cisplatin in PARP+/+ vs. Cisplatin+PARP−/−
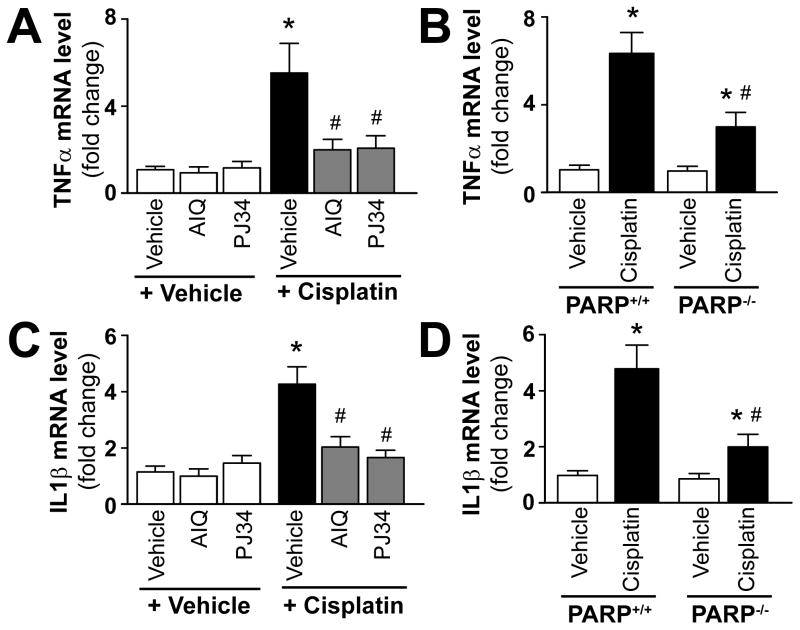
Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced kidney inflammatory response
Consistent with the increased inflammatory response cisplatin significantly increased mRNA expression of inflammatory cytokines TNF-α and IL1β (Figure 6A–D) in the kidneys. These changes were attenuated by treatment with AIQ or PJ34 (Figure 6A, C) and also in PARP-1−/− mice compared to PARP-1+/+ littermates (Figure 6B, D). PARP inhibitors had no effects in control mice on all variables studied, likewise there were no significant differences in kidney TNF-α and IL1β mRNA levels in PARP-1−/− and PARP-1+/+ mice treated with vehicle only (Figure 6A–D).
Figure 6. Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced increased expression of mRNA of TNF-α and IL-1β.
Cisplatin significantly increased renal mRNA expression of TNF-α and IL1β mRNA (panels AD), indicating enhanced inflammatory response 72 h following its administration to mice. These were attenuated by treatment with AIQ or PJ34 (left side: panels A and C), and also in PARP-1−/− mice compared to PARP-1+/+ mice (right side: panels B and D). Results are mean±S.E.M. of 6–8/group *P<0.05 vs. Vehicle in C57Bl/6J/PARP+/+, #P<0.05 Cisplatin in C57Bl/6J/PARP+/+ vs. Cisplatin+AIQ/PJ34/PARP−/−
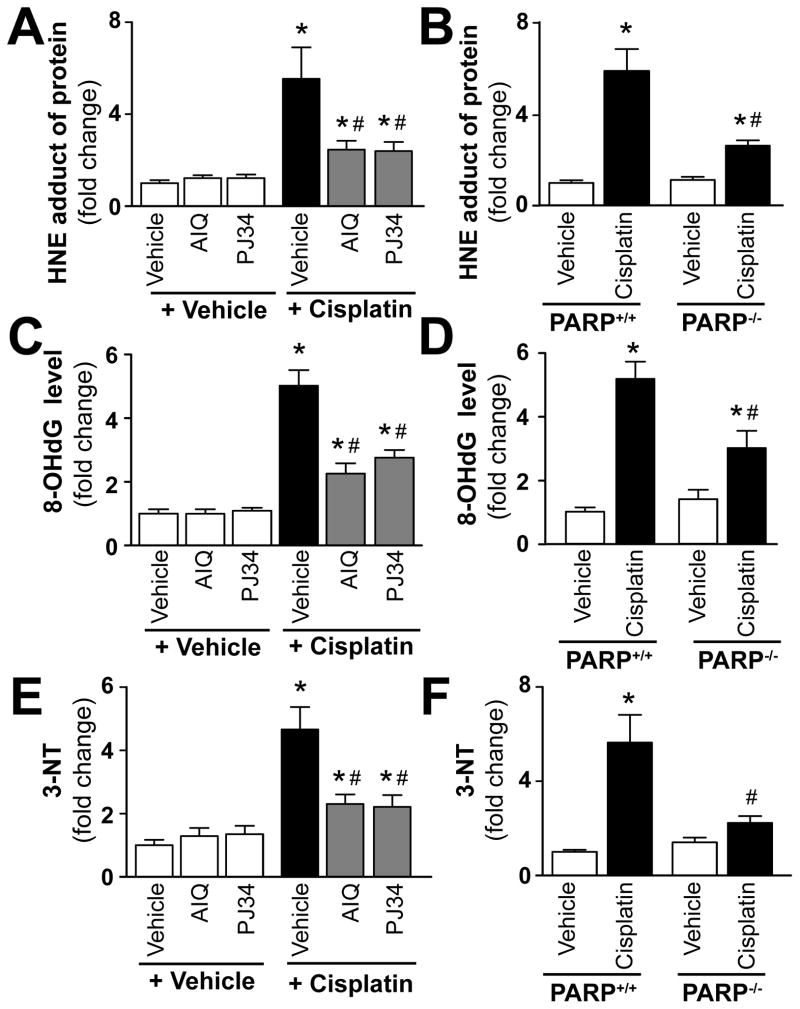
Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced increased oxidative and nitrative stress
Lipid peroxides are unstable indicators of oxidative stress in cells that decompose to form more complex and reactive compounds such as 4-hydroxynonenal (HNE), which has been shown to be capable of binding to proteins and forming stable adducts. Recent evidence also implicates HNE in various key signaling processes [46, 47]. 8-hydroxy-2′-deoxyguanosine (8-OHdG) or 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) is a marker of free radical-induced oxidative damage in nuclear and mitochondrial DNA, which has been widely used as a biomarker for oxidative stress and carcinogenesis [48–53]. Nitrotyrosine (3-NT) formation was initially considered a specific marker of in vivo peroxynitrite generation, but now it is rather used as a collective index of protein nitration, because other pathways have also been proposed to be involved in its formation (e.g., myeloperoxidase under certain inflammatory conditions [21, 54–58]. Cisplatin significantly increased renal HNE, 8-OHdG and nitrotyrosine levels (Figure 7A–F) 72 h following its administration to mice. These increases were attenuated by treatment with PARP inhibitors AIQ or PJ34 (Figure 7A, C, E), and also in PARP-1−/− mice compared to PARP-1+/+ littermates (Figure 7B, D, F). PARP inhibitors had no effects in control mice on all variables studied, likewise there were no significant differences in kidney levels of HNE, 8-OHdG, and 3-NT in PARP-1−/− and PARP-1+/+ mice treated with vehicle only (Figure 7A–F).
Figure 7. Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced increased oxidative and nitrative stress.
Cisplatin significantly increased renal HNE protein adduct, 8-OHdG, and 3-NT levels in the kidneys (panels A–F), indicating enhanced oxidative/nitrative stress 72 h following its administration to mice. These were attenuated by treatment with AIQ or PJ34 (left side of panels A, C and E), and also in PARP-1−/− mice compared to PARP-1+/+ mice (right side of panels B, D and F). Results are mean±S.E.M. of 6–8/group *P<0.05 vs. Vehicle vs. Cisplatin, #P<0.05 C57Bl/6J/PARP+/++Cisplatin vs. Cisplatin+AIQ/PJ34/PARP−/−
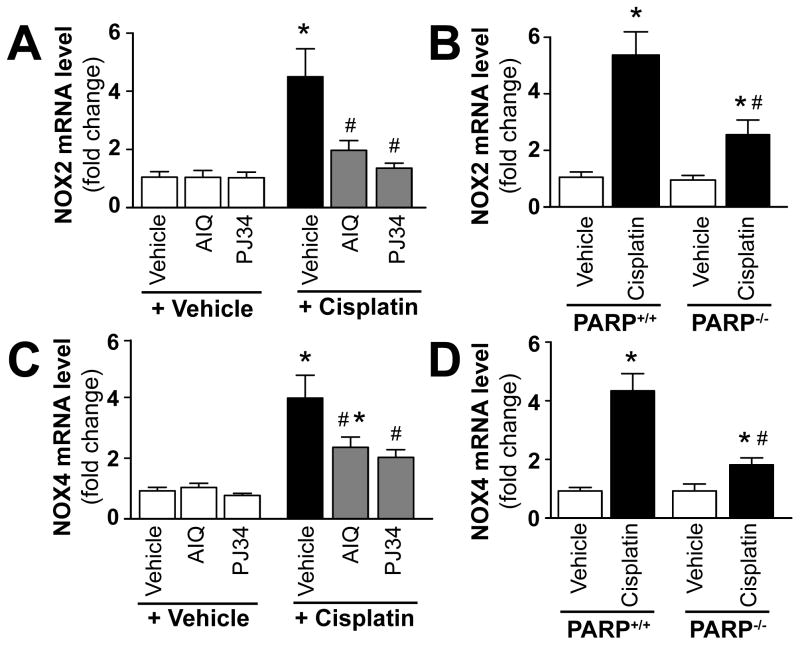
Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced increased expression of NOX2 and NOX4
Cisplatin significantly increased renal mRNA expression of ROS generating enzymes NOX4 (renox) and NOX2 (gp91phox) (Figure 8A–D) 72 h following its administration to mice. These effects were significantly attenuated by treatment with AIQ or PJ34 (Figure 8A and C), and also in PARP-1−/− mice compared to PARP-1+/+ ones (Figure 8B and D). PARP inhibitors had no effects in control mice on all variables studied, likewise there were no significant differences in kidney levels of NOX2/4 mRNA in PARP-1−/− and PARP-1+/+ mice treated with vehicle only (Figure 8A–D).
Figure 8. Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced increased expression of ROS generating NADPH oxidase isoforms (NOX4 (RENOX), NOX2 (gp91phox)).
Cisplatin significantly increased renal mRNA expression of NOX4 and NOX2 (Panels A–D) 72 h following its administration to mice. These were attenuated by treatment with AIQ or PJ34 (left side of panels A and C), and also in PARP-1−/− mice compared to PARP-1+/+ littermates (right side of panels B and D). Results are mean±S.E.M. of 6–8/group *P<0.05 Vehicle/PARP+/+ vs. Cisplatin, #P<0.05 C57Bl/6J/PARP+/++Cisplatin vs. Cisplatin+AIQ/PJ34/PARP−/−
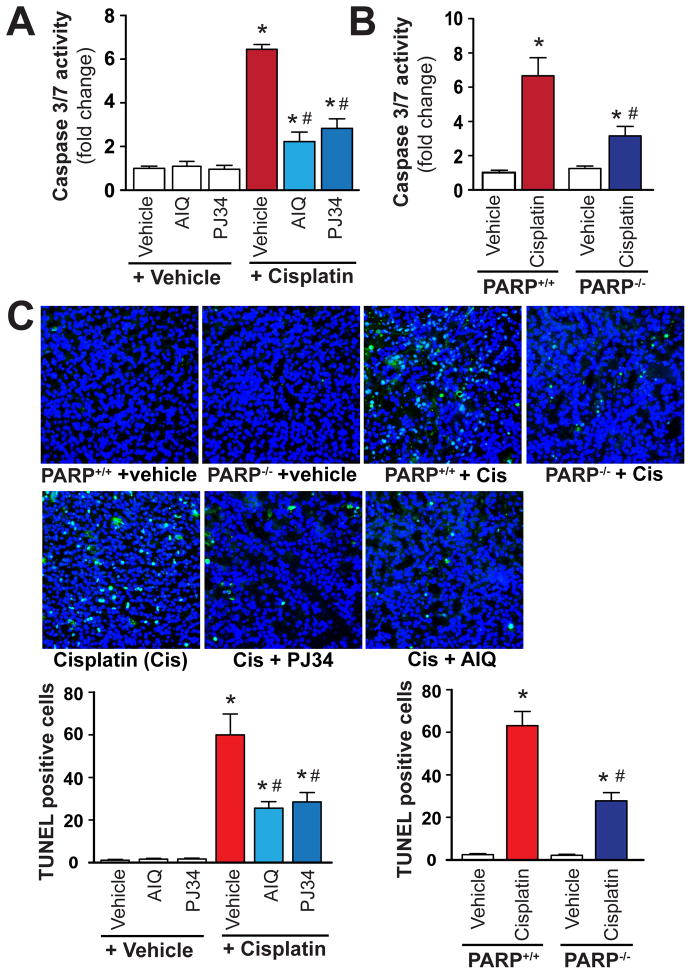
PARP inhibition attenuates cisplatin-induced renal apoptosis
We have also evaluated the effect of pharmacological inhibition or genetic deletion of PARP on cisplatin-induced apoptotic cell death. As revealed by our time-course experiments apoptotic cell death (caspase 3/7 activity) was increased by 48 and 72 hours following cisplatin administration (Figure 1D) and paralleled with enhanced inflammatory response and oxidative/nitrative stress (Figure 1A–C). Cisplatin significantly increased renal apoptosis (caspase 3/7activity and TUNEL staining) (Figure 9A–C) 72 h following its administration to mice. These increases were attenuated by treatment with PARP inhibitors AIQ or PJ34 (Figure 9A, C), and also in PARP-1−/− mice compared to PARP-1+/+ littermates (Figure 9B, C). PARP inhibitors had no effects in control mice on all variables studied, likewise there were no significant differences in kidney caspase 3/7 activity and TUNEL staining in PARP-1−/− and PARP-1+/+ mice treated with vehicle only (Figure 9A–C). Panel C shows representative TUNEL staining of kidney sections. The light blue/green TUNEL positive cells (overlaid nuclear staining (blue) and TUNEL (green)) were markedly increased by cisplatin, and attenuated by pharmacological inhibition or genetic deletion of PARP. The reduction of apoptotic cell death was consistent with the attenuation of inflammation and oxidative/nitrative stress.
Figure 9. Pharmacologic inhibition or genetic deletion of PARP-1 attenuates cisplatin-induced apoptosis.
Cisplatin significantly increased markers of renal apoptosis (caspase 3/7 activity and TUNEL staining; Panels A–C) 72 h following its administration to mice. These were attenuated by treatment with AIQ or PJ34 (Panels A and C), and also in PARP-1−/− mice compared to PARP-1+/+ littermates (panels B and C) (n= 6–8/group for Panels A and B).
Panel C shows representative TUNEL staining of 4-4 kidneys/group (400x magnification) from mice treated with cisplatin or cisplatin in combination with PARP inhibitors, and quantifications from 12 representative fields/group with 400x magnification. The images are overlays of nuclear staining (blue) and TUNEL (green) and the TUNEL positive cells are shown in light blue/green. Results are mean±S.E.M. *P<0.05 Vehicle vs. Cisplatin, #P<0.05 C57Bl/6J/PARP+/++Cisplatin vs. Cisplatin+AIQ/PJ34/PARP−/−.
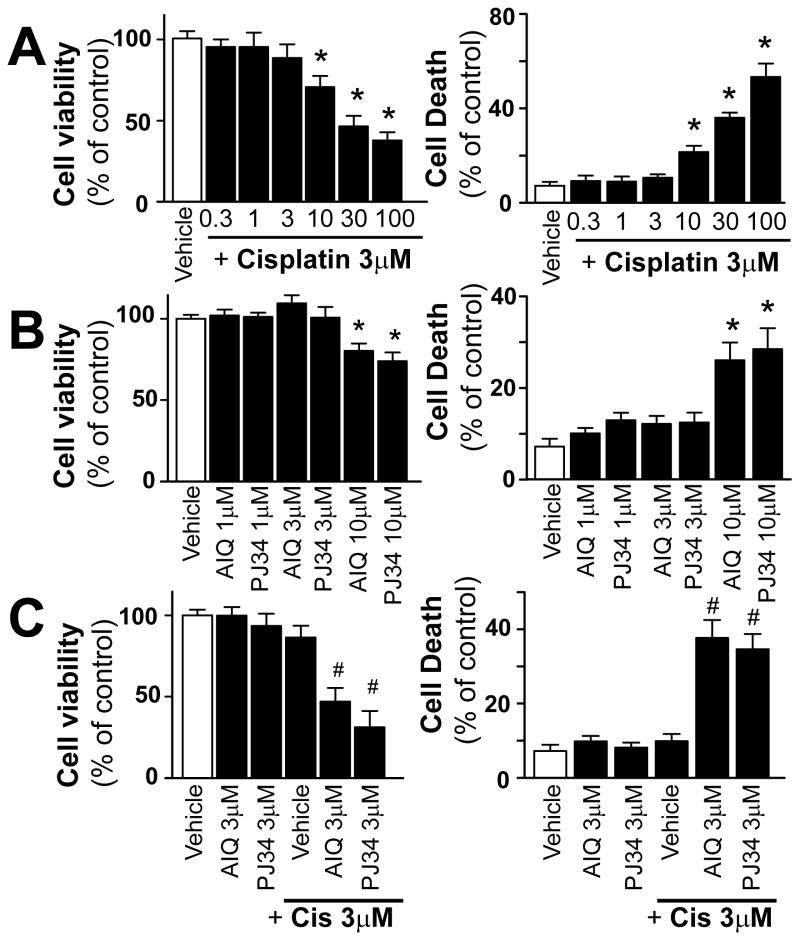
PARP inhibitors enhance the cisplatin-induced cell death in tumor cells
Since cisplatin is widely used in various urinary tract cancers (because of its primary excretion by kidneys and therefore high concentration reached in the urinary tract), we also wanted to determine if PARP inhibition interferes with its anticancer effects using human bladder carcinoma cell line (T24). Cisplatin induced concentration-dependent cell death of T24 cancer cells following 72h (Figure 10A), as well as PARP inhibitors AIQ and PJ34 (Figure 10B). Simultaneous administration of PARP inhibitors and cisplatin at doses which did not induce cell death by themselves promoted cell death in T24 cancer cells (Figure 10C) determined by XTT assay (Figure 10 left panels) and flow cytometry (Figure 10 right panels). Thus, these results support the view and ongoing clinical trials that PARP inhibitors together with DNA damaging anticancer drugs, such as cisplatin, can accelerate tumor cell killing.
Figure 10. Pharmacologic inhibition of PARP promotes cisplatin-induced cell demise in cancer cells.
Cisplatin (panel A) and PARP inhibitors AIQ and PJ34 (panel B) induced concentration-dependent cell death in T24 cancer cells measured by XTT assay (left panels) and flow cytometry (right panels). Cisplatin combined with PARP inhibitor even at concentrations which did not induce cell death by themselves, decreased cell viability of cancer cells. Results are mean±S.E.M. of 4–8/group *P<0.05 vs. corresponding Vehicle, #P<0.05 vs. Vehicle+Cisplatin..,.,
Discussion
In this study, we have investigated the role of PARP-1 in a clinically relevant model of cisplatin-induced nephropathy by using two pharmacological antagonists, as well as PARP-1 knockout mice. We demonstrate that genetic deletion of PARP-1 or its pharmacological inhibition with PJ34 or AIQ attenuates cisplatin-induced increased histopathological injury, inflammation and oxidative/nitrative stress in the kidney, leading to marked improvement in cisplatin-induced compromised renal function. These findings suggest that PARP activation plays a key role in promoting cisplatin-induced renal inflammation and injury. We also demonstrate that PARP inhibition does not interfere with the antitumor activity of cisplatin, in fact it rather promotes it, in a human cancer cell line, which is consistent with results of numerous recent in vitro and in vivo experimental and clinical reports [30, 59], overviewed in [8, 60–67].
Consistent with previous reports we found that cisplatin induced marked tubular necrosis, increased inflammatory cell infiltration, oxidative/nitrative stress and impaired renal function [5, 6, 31–40, 68, 69]. NAD(P)H oxidases may represent very significant source of reactive oxygen species (e.g. superoxide) generation in various pathological conditions. In the kidneys of cisplatin-treated animals we found marked overexpression of phagocyte NAD(P)H oxidase isoform gp91phox/NOX2, as well as NOX4/renox (the NAD(P)H oxidase isoform considered to be a major source of ROS generation in the kidneys under pathological conditions). The increased NOX2 expression was consistent with enhanced leukocyte infiltration in the kidneys of cisplatin-treated mice around damaged tubular structures. The cisplatin-induced ROS generation may also facilitate the expression of adhesion molecules and iNOS through the activation of NF-κB [70]. Indeed, in the kidneys of cisplatin-treated mice we found increased expression of adhesion molecules ICAM-1 and VCAM-1, which may facilitate adhesion of inflammatory cells to the activated endothelium or damaged parenchyma cells. These activated immune cells then may release various chemokines, cytokines ROS/RNS further amplifying the inflammatory cascade and injury [58, 71]. Consistently with an important role of inflammation in cisplatin-induced nephropathy [6, 31, 34, 72], we also found marked increases in mRNA expressions of proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), and markers of macrophage (F4/80) and neutrophil (MPO activity) infiltration. The simultaneous increases in superoxide and NO production may also lead to the formation of the potent oxidant peroxynitrite via a diffusion-limited reaction, which in turn impairs various proteins, lipids leading to cellular dysfunction and/or demise [21, 58, 73]. Previous studies have demonstated increased nitrotyrosine (3-NT) formation (marker of peroxynitrite generation and more broadly nitrative stress) in cisplatin-treated mouse kidneys localized in the damaged tubular cells, endothelium, as well as infiltrating immune cells [5]. Consistently with the above discussed observations we also found several fold increases in renal 3-NT and HNE levels in cisplatin-treated mice.
Oxidative and nitrative stress and/or peroxynitrite-induced DNA damage is a potent trigger of PARP-1 activation, which is in turn also involved in the activation of various pro-inflammatory transcription factors such as NF-κB (in addition to its well-known role in cell death processes), and thus in the regulation of consequent inflammatory response (e.g. expression of adhesion molecules, leukocyte infiltration, etc.) [8, 10]. Indeed, pharmacological inhibition or genetic deletion of PARP-1 not only decreases the acute necrotic cell death, but also attenuates adhesion molecules expression and inflammatory cell infiltration and the consequent secondary oxidative/nitrative injury in models of ischemic-reperfusion injury [10, 45]. In agreement with the above, pharmacological inhibition or genetic deletion of PARP-1 markedly attenuated the cisplatin-induced impaired renal function (elevated serum BUN and creatinine levels), histopathological injury, and reduced the inflammatory response (adhesion molecules ICAM-1/VCAM-1 expression, leukocyte infiltration, TNF-α, IL-1β) and the consequent oxidative/nitrative stress (HNE and nitrotyrosine content, NOX2/NOX4 expressions). Apoptotic cell death and autophagy have also been importantly implicated in cisplatin-induced tubular cell death [7, 35]. We also found enhanced apoptotic cell death in kidneys of cisplatin-treated mice, which was attenuated by PARP inhibitors or in PARP-1 knockout mice. PARP-1 can be a switch between apoptotic and necrotic cell death [10] and depending on the degree of oxidant-induced DNA injury and various other factors it may promote both of these forms of cell demise. In addition, PARP activation has also been implicated in nuclear-mitochondrial crosstalk involving apoptosis inducing factor mediated cell death [74–77]. However, the beneficial effect of PARP inhibition or genetic deletion of PARP-1 on apoptotic cell death in our model (which occur only relatively late following the administration of cisplatin) is most likely related to the attenuation of the cisplatin-induced inflammatory response and second wave of oxidative stress generated by inflammatory cells. In addition to inducing damage of tubular cells, cisplatin also promotes endothelial injury with dysregulation of renal blood flow and secondary functional hypoxia [7]. Consistent with this, and the importance of the oxidative injury in cisplatin-induced nephrotoxicity, increased nitrotyrosine formation was also reported not only in damaged tubular, but also in endothelial cells of cisplatin treated mice [5]. Since the reactive oxygen and nitrogen species-PARP pathway importantly contributes to the developments of endothelial and cardiac dysfunction in reperfusion injury [78–83], diabetes [14, 20, 84–87], hypertension [88], shock [89–92], aging [93], and various forms of cardiomyopathies and heart failure [42, 94–98], it is very likely that inhibition of PARP or genetic deletion of PARP-1 also afforded protection by preventing cisplatin-induced vascular injury. The latter is also supported by our results demonstrating attenuation of cisplatin-induced vascular pro-inflammatory response (VCAM expression) in kidneys of mice deficient of PARP-1 or treated with PARP inhibitors.
Increased oxidative/nitrative [95] [96] stress and PARP activation [97, 99–101] has also been implicated in the cardiotoxicity of another important antineoplastic drug doxorubicin. Furthermore the inhibition of PARP enhances doxorubicin’s activity against liver cancer cells [102], and the efficacy and safety of combination of PARP inhibitor AZD2281 with doxorubicin (Doxil) is being recently evaluated in advanced ovarian cancer (see ClinicalTrials.gov).
Importantly, we found that PARP inhibitors exerted synergistic effect with cisplatin in killing T24 cancer cells, which is consistent with numerous reports demonstrating synergistic chemosensitivity of various breast and other cancer cell lines to PARP inhibition and cisplatin or other antineoplastic drugs [8, 30, 60–67]. Thus, it is unlikely that PARP inhibition would decrease the chemotherapeutic efficacy of cisplatin. Indeed, in several clinical trials inhibition of PARP alone or in combination with DNA-damaging anticancer agents shows considerable promise in facilitating tumor cell death [22–27]. Furthermore, several PARP inhibitors (alone or in combination with other antineoplastic agents) from Pfizer (PF-01367338/AG014699), AstraZeneca (olaparib/AZD2281/KU-0059436), Sanofi-Aventis (iniparib/BSI-201/SAR240550), Abbott Laboratories (veliparib/ABT-888), Merck (MK4827), and Cephalon (CEP-9722) are in clinical development for various forms of primary breast, ovarian, brain, pancreatic, peritoneal, intestinal, colorectal cancer, leukemia, lymphoma, and neoplasm metastasis (see: ClinicalTrials.gov). Several of these recent clinical trials are aiming to evaluate the efficacy of several PARP inhibitors (AVT-888, PF-01367338, BSI-201, AZD2281) in combination with the platinum compound carboplatin to treat ovarian, Fallopian tube, primary peritoneal, triple negative primary or metastatic breast cancer (see: ClinicalTrials.gov). Excitingly, PARP-1 has recently been implicated in the chemoresistance of cancer cells to cisplatin, which is a significant clinical problem, giving and additional reasons for optimism regarding the clinical trials evaluating the efficacy of the combination of PARP inhibitors in combination with cisplatin for various malignancies [28, 29]. The mechanisms of the beneficial effects of PARP inhibitors in cancer are multiple and may involve attenuation of cancer cell proliferation and migration, promotion of cancer cell demise, decrease of angiogenesis, and modulation of the tumor environment (e.g. attenuation of inflammation) [8, 60–67, 103–106].
The selective promotion of cell death in cancer, but not in normal cells by PARP inhibitors is based on the novel approach of “synthetic lethality” in cancer therapy. The rational for this is that in cancers with selective defects in homologous recombination repair (cancer cells frequently harbor defects in DNA repair pathways leading to genomic instability), inactivation of PARPs directly causes cell death (overviewed in [8, 60–67]).
In summary, PARP activation plays a key role in promoting the cisplatin-induced cell death and tissue injury by amplifying inflammation and oxidative/nitrative stress. Thus, pharmacological inhibition of PARP may exert beneficial effects against cisplatin-induced nephrotoxicity. This is particularly exciting in light of the recent studies suggesting that PARP inhibitors not only chemosensitize cancer cells to cisplatin (and to other chemotherapeutic agents) but may also be useful in combating development of chemoresistance of cancer cells to cisplatin. Furthermore, numerous high-potency PARP inhibitors are currently evaluated as cancer therapeutics in clinical trials by major pharmaceutical companies.
Acknowledgments
This study was supported by the Intramural Research Program of NIH/NIAAA (to P.P.) and HL072889 (to A.H.B). Dr. Horvath is a recipient of Hungarian Scientific Research Fund Fellowship (OTKA-NKTH-EU MB08 80238). Dr. Pacher dedicates this study to his beloved mother Iren Bolfert, who died from complications of chemotherapy.
Footnotes
Disclosures: No conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ries F, Klastersky J. Nephrotoxicity induced by cancer chemotherapy with special emphasis on cisplatin toxicity. Am J Kidney Dis. 1986;8:368–379. doi: 10.1016/s0272-6386(86)80112-3. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW. Cancer therapy and renal injury. J Clin Invest. 2002;110:743–745. doi: 10.1172/JCI16568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 4.Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates Cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- 5.Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med. 2010;48:457–467. doi: 10.1016/j.freeradbiomed.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 8.Peralta-Leal A, Rodriguez-Vargas JM, Aguilar-Quesada R, Rodriguez MI, Linares JL, de Almodovar MR, Oliver FJ. PARP inhibitors: new partners in the therapy of cancer and inflammatory diseases. Free Radic Biol Med. 2009;47:13–26. doi: 10.1016/j.freeradbiomed.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 10.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 11.Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165:372–377. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 12.Soriano FG, Liaudet L, Szabo E, Virag L, Mabley JG, Pacher P, Szabo C. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–292. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Evgenov OV, Liaudet L. Role of nitrosative stress and activation of poly(ADP-ribose) polymerase-1 in cardiovascular failure associated with septic and hemorrhagic shock. Curr Vasc Pharmacol. 2005;3:293–299. doi: 10.2174/1570161054368580. [DOI] [PubMed] [Google Scholar]
- 14.Soriano FG, Pacher P, Mabley J, Liaudet L, Szabo C. Rapid reversal of the diabetic endothelial dysfunction by pharmacological inhibition of poly(ADP-ribose) polymerase. Circ Res. 2001;89:684–691. doi: 10.1161/hh2001.097797. [DOI] [PubMed] [Google Scholar]
- 15.Lupachyk S, Shevalye H, Maksimchyk Y, Drel VR, Obrosova IG. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: Correlation with peripheral nerve function. Free Radic Biol Med. 2011;50:1400–1409. doi: 10.1016/j.freeradbiomed.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shevalye H, Maksimchyk Y, Watcho P, Obrosova IG. Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency alleviates diabetic kidney disease. Biochim Biophys Acta. 2010;1802:1020–1027. doi: 10.1016/j.bbadis.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevalye H, Stavniichuk R, Xu W, Zhang J, Lupachyk S, Maksimchyk Y, Drel VR, Floyd EZ, Slusher B, Obrosova IG. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model. Biochem Pharmacol. 2010;79:1007–1014. doi: 10.1016/j.bcp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drel VR, Xu W, Zhang J, Kador PF, Ali TK, Shin J, Julius U, Slusher B, El-Remessy AB, Obrosova IG. Poly(ADP-ribose)polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:1778–1790. doi: 10.1167/iovs.08-2191. [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, Pavlov IA, Zhang J, Slusher B, Drel VR. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med. 2008;44:972–981. doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 21.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. The New England Journal of Medicine. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 23.O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. The New England Journal of Medicine. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 24.Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, Mukhopadhyay A, Los G, Hostomsky Z, Plummer ER, Edmondson RJ, Curtin NJ. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 25.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 26.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler RK, Wardley A, Mitchell G, Earl H, Wickens M, Carmichael J. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 27.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A’Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–2519. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 28.Ganesan S. MYC, PARP1, and Chemoresistance: BIN There, Done That? Sci Signal. 2011;4:pe15. doi: 10.1126/scisignal.2001946. [DOI] [PubMed] [Google Scholar]
- 29.Pyndiah S, Tanida S, Ahmed KM, Cassimere EK, Choe C, Sakamuro D. c-MYC Suppresses BIN1 to Release Poly(ADP-Ribose) Polymerase 1: A Mechanism by Which Cancer Cells Acquire Cisplatin Resistance. Sci Signal. 2011;4:ra19. doi: 10.1126/scisignal.2001556. [DOI] [PubMed] [Google Scholar]
- 30.Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70:7970–7980. doi: 10.1158/0008-5472.CAN-09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tadagavadi RK, Reeves WB. Renal dendritic cells ameliorate nephrotoxic acute kidney injury. J Am Soc Nephrol. 2010;21:53–63. doi: 10.1681/ASN.2009040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Moon SO, Kim W, Sung MJ, Kim DH, Kang KP, Jang YB, Lee JE, Jang KY, Lee SY, Park SK. Protective role of L-2-oxothiazolidine-4-carboxylic acid in cisplatin-induced renal injury. Nephrol Dial Transplant. 2006;21:2085–2095. doi: 10.1093/ndt/gfl209. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009;76:277–285. doi: 10.1038/ki.2009.157. [DOI] [PubMed] [Google Scholar]
- 34.Tadagavadi RK, Reeves WB. Endogenous IL-10 attenuates cisplatin nephrotoxicity: role of dendritic cells. J Immunol. 2010;185:4904–4911. doi: 10.4049/jimmunol.1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, Agarwal A. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21:1702–1712. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basnakian AG, Apostolov EO, Yin X, Napirei M, Mannherz HG, Shah SV. Cisplatin nephrotoxicity is mediated by deoxyribonuclease I. J Am Soc Nephrol. 2005;16:697–702. doi: 10.1681/ASN.2004060494. [DOI] [PubMed] [Google Scholar]
- 38.Han X, Yue J, Chesney RW. Functional TauT protects against acute kidney injury. J Am Soc Nephrol. 2009;20:1323–1332. doi: 10.1681/ASN.2008050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol. 2008;19:923–932. doi: 10.1681/ASN.2007090982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol. 2006;17:765–774. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 41.Oumouna-Benachour K, Hans CP, Suzuki Y, Naura A, Datta R, Belmadani S, Fallon K, Woods C, Boulares AH. Poly(ADP-ribose) polymerase inhibition reduces atherosclerotic plaque size and promotes factors of plaque stability in apolipoprotein E-deficient mice: effects on macrophage recruitment, nuclear factor-kappaB nuclear translocation, and foam cell death. Circulation. 2007;115:2442–2450. doi: 10.1161/CIRCULATIONAHA.106.668756. [DOI] [PubMed] [Google Scholar]
- 42.Mukhopadhyay P, Horvath B, Rajesh M, Matsumoto S, Saito K, Batkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Hasko G, Pacher P. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011;50:179–195. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc. 2007;2:2295–2301. doi: 10.1038/nprot.2007.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajesh M, Mukhopadhyay P, Batkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horvath B, Mukhopadhyay B, Becker L, Hasko G, Liaudet L, Wink DA, Veves A, Mechoulam R, Pacher P. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. 2010;56:2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- 46.Forman HJ, Dickinson DA. Introduction to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule. Free Radic Biol Med. 2004;37:594–596. doi: 10.1016/j.freeradbiomed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 49.Floyd RA, Watson JJ, Wong PK, Altmiller DH, Rickard RC. Hydroxyl free radical adduct of deoxyguanosine: sensitive detection and mechanisms of formation. Free Radical Research Communications. 1986;1:163–172. doi: 10.3109/10715768609083148. [DOI] [PubMed] [Google Scholar]
- 50.Floyd RA, Watson JJ, Harris J, West M, Wong PK. Formation of 8-hydroxydeoxyguanosine, hydroxyl free radical adduct of DNA in granulocytes exposed to the tumor promoter, tetradecanoylphorbolacetate. Biochem Biophys Res Commun. 1986;137:841–846. doi: 10.1016/0006-291x(86)91156-3. [DOI] [PubMed] [Google Scholar]
- 51.Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–1450. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 52.Park JW, Floyd RA. Lipid peroxidation products mediate the formation of 8-hydroxydeoxyguanosine in DNA. Free Radic Biol Med. 1992;12:245–250. doi: 10.1016/0891-5849(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 53.Birnboim HC, Maidt L, Raynor T, Floyd RA. 8-Hydroxydeoxyguanosine in DNA from TPA-stimulated human granulocytes. Free Radic Res. 1994;20:113–117. doi: 10.3109/10715769409147508. [DOI] [PubMed] [Google Scholar]
- 54.Eiserich JP, Baldus S, Brennan ML, Ma W, Zhang C, Tousson A, Castro L, Lusis AJ, Nauseef WM, White CR, Freeman BA. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 55.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, van der Vliet A. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 56.van der Vliet A, Eiserich JP, Kaur H, Cross CE, Halliwell B. Nitrotyrosine as biomarker for reactive nitrogen species. Methods in enzymology. 1996;269:175–184. doi: 10.1016/s0076-6879(96)69019-3. [DOI] [PubMed] [Google Scholar]
- 57.Schopfer FJ, Baker PR, Freeman BA. NO-dependent protein nitration: a cell signaling event or an oxidative inflammatory response? Trends Biochem Sci. 2003;28:646–654. doi: 10.1016/j.tibs.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 59.Zheng YD, Xu XQ, Peng F, Yu JZ, Wu H. The poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide suppresses cell growth and migration, enhancing suppressive effects of cisplatin in osteosarcoma cells. Oncol Rep. 2011;25:1399–1405. doi: 10.3892/or.2011.1212. [DOI] [PubMed] [Google Scholar]
- 60.Kruse V, Rottey S, De Backer O, Van Belle S, Cocquyt V, Denys H. PARP inhibitors in oncology: a new synthetic lethal approach to cancer therapy. Acta Clinica Belgica. 2011;66:2–9. doi: 10.2143/ACB.66.1.2062507. [DOI] [PubMed] [Google Scholar]
- 61.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nature Reviews Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burstein HJ. Novel agents and future directions for refractory breast cancer. Semin Oncol. 2011;38(Suppl 2):S17–24. doi: 10.1053/j.seminoncol.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Underhill C, Toulmonde M, Bonnefoi H. A review of PARP inhibitors: from bench to bedside. Ann Oncol. 2011;22:268–279. doi: 10.1093/annonc/mdq322. [DOI] [PubMed] [Google Scholar]
- 64.Calvert H, Azzariti A. The clinical development of inhibitors of poly(ADP-ribose) polymerase. Ann Oncol. 2011;22(Suppl 1):i53–59. doi: 10.1093/annonc/mdq667. [DOI] [PubMed] [Google Scholar]
- 65.Graziani G, Szabo C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–118. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 66.Lewis C, Low JA. Clinical poly(ADP-ribose) polymerase inhibitors for the treatment of cancer. Curr Opin Investig Drugs. 2007;8:1051–1056. [PubMed] [Google Scholar]
- 67.Mangerich A, Burkle A. How to kill tumor cells with inhibitors of poly(ADP-ribosyl)ation. Int J Cancer. 2011;128:251–265. doi: 10.1002/ijc.25683. [DOI] [PubMed] [Google Scholar]
- 68.Mukhopadhyay P, Pan H, Rajesh M, Batkai S, Patel V, Harvey-White J, Mukhopadhyay B, Hasko G, Gao B, Mackie K, Pacher P. CB1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br J Pharmacol. 2010;160:657–668. doi: 10.1111/j.1476-5381.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, Hasko G, Pacher P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–714. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerrero-Beltran CE, Mukhopadhyay P, Horvath B, Rajesh M, Tapia E, Garcia-Torres I, Pedraza-Chaverri J, Pacher P. Sulforaphane, a natural constituent of broccoli, prevents cell death and inflammation in nephropathy. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.02.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 72.Ramesh G, Reeves WB. Inflammatory cytokines in acute renal failure. Kidney Int Suppl. 2004:S56–61. doi: 10.1111/j.1523-1755.2004.09109.x. [DOI] [PubMed] [Google Scholar]
- 73.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos) Sci Signal. 2011;4:ra20. doi: 10.1126/scisignal.2000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.David KK, Andrabi SA, Dawson TM, Dawson VL. Parthanatos, a messenger of death. Front Biosci. 2009;14:1116–1128. doi: 10.2741/3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 77.Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL. Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szabo G, Bahrle S, Stumpf N, Sonnenberg K, Szabo EE, Pacher P, Csont T, Schulz R, Dengler TJ, Liaudet L, Jagtap PG, Southan GJ, Vahl CF, Hagl S, Szabo C. Poly(ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–106. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 79.Szabo G, Buhmann V, Andrasi T, Stumpf N, Bahrle S, Kekesi V, Hagl S, Szabo C, Juhasz-Nagy A. Poly-ADP-ribose polymerase inhibition protects against myocardial and endothelial reperfusion injury after hypothermic cardiac arrest. J Thorac Cardiovasc Surg. 2003;126:651–658. doi: 10.1016/s0022-5223(02)73235-2. [DOI] [PubMed] [Google Scholar]
- 80.Szabo G, Liaudet L, Hagl S, Szabo C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004;61:471–480. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 81.Szabo G, Seres L, Soos P, Flechtenmacher C, Zsengeller Z, Sack FU, Szabo C, Hagl S. Poly-ADP-ribose polymerase inhibition reduces mesenteric injury after cardiopulmonary bypass. Thorac Cardiovasc Surg. 2004;52:338–343. doi: 10.1055/s-2004-821274. [DOI] [PubMed] [Google Scholar]
- 82.Szabo G, Soos P, Heger U, Flechtenmacher C, Bahrle S, Zsengeller Z, Szabo C, Hagl S. Poly(ADP-ribose) polymerase inhibition attenuates biventricular reperfusion injury after orthotopic heart transplantation. Eur J Cardiothorac Surg. 2005;27:226–234. doi: 10.1016/j.ejcts.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 83.Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- 84.Szabo C. Roles of poly(ADP-ribose) polymerase activation in the pathogenesis of diabetes mellitus and its complications. Pharmacol Res. 2005;52:60–71. doi: 10.1016/j.phrs.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 86.Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 87.Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 88.Szabo C, Pacher P, Zsengeller Z, Vaslin A, Komjati K, Benko R, Chen M, Mabley JG, Kollai M. Angiotensin II-mediated endothelial dysfunction: role of poly(ADP-ribose) polymerase activation. Mol Med. 2004;10:28–35. doi: 10.2119/2004-00001.szabo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szabo C, Cuzzocrea S, Zingarelli B, O’Connor M, Salzman AL. Endothelial dysfunction in a rat model of endotoxic shock. Importance of the activation of poly (ADP-ribose) synthetase by peroxynitrite. J Clin Invest. 1997;100:723–735. doi: 10.1172/JCI119585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szabo C. Potential role of the peroxynitrate-poly(ADP-ribose) synthetase pathway in a rat model of severe hemorrhagic shock. Shock. 1998;9:341–344. doi: 10.1097/00024382-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 91.Szabo A, Hake P, Salzman AL, Szabo C. 3-Aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase, improves hemodynamics and prolongs survival in a porcine model of hemorrhagic shock. Shock. 1998;10:347–353. doi: 10.1097/00024382-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 92.Szabo C, Zingarelli B, Salzman AL. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 93.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, Szabo C. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure, and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–898. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 95.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 96.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–1483. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 98.Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 99.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006;17:369–375. [PMC free article] [PubMed] [Google Scholar]
- 100.Szenczi O, Kemecsei P, Holthuijsen MF, van Riel NA, van der Vusse GJ, Pacher P, Szabo C, Kollai M, Ligeti L, Ivanics T. Poly(ADP-ribose) polymerase regulates myocardial calcium handling in doxorubicin-induced heart failure. Biochem Pharmacol. 2005;69:725–732. doi: 10.1016/j.bcp.2004.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartha E, Solti I, Szabo A, Olah G, Magyar K, Szabados E, Kalai T, Hideg K, Toth K, Gero D, Szabo C, Sumegi B, Halmosi R. Regulation of kinase cascade activation and heat shock protein expression by poly(ADP-ribose) polymerase inhibition in doxorubicin-induced heart failure. J Cardiovasc Pharmacol. 2011 doi: 10.1097/FJC.0b013e318225c21e. in press. [DOI] [PubMed] [Google Scholar]
- 102.Munoz-Gamez JA, Quiles-Perez R, Ruiz-Extremera A, Martin-Alvarez AB, Sanjuan-Nunez L, Carazo A, Leon J, Oliver FJ, Salmeron J. Inhibition of poly (ADP-ribose) polymerase-1 enhances doxorubicin activity against liver cancer cells. Cancer Lett. 2011;301:47–56. doi: 10.1016/j.canlet.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 103.Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L, Pacher P. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun. 2006;350:352–357. doi: 10.1016/j.bbrc.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajesh M, Mukhopadhyay P, Godlewski G, Batkai S, Hasko G, Liaudet L, Pacher P. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tentori L, Lacal PM, Muzi A, Dorio AS, Leonetti C, Scarsella M, Ruffini F, Xu W, Min W, Stoppacciaro A, Colarossi C, Wang ZQ, Zhang J, Graziani G. Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer. 2007;43:2124–2133. doi: 10.1016/j.ejca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 106.Pyriochou A, Olah G, Deitch EA, Szabo C. Papapetropoulos, A. Inhibition of angiogenesis by the poly(ADP-ribose) polymerase inhibitor PJ-34. Int J Mol Med. 2008;22:113–118. [PubMed] [Google Scholar]