Abstract
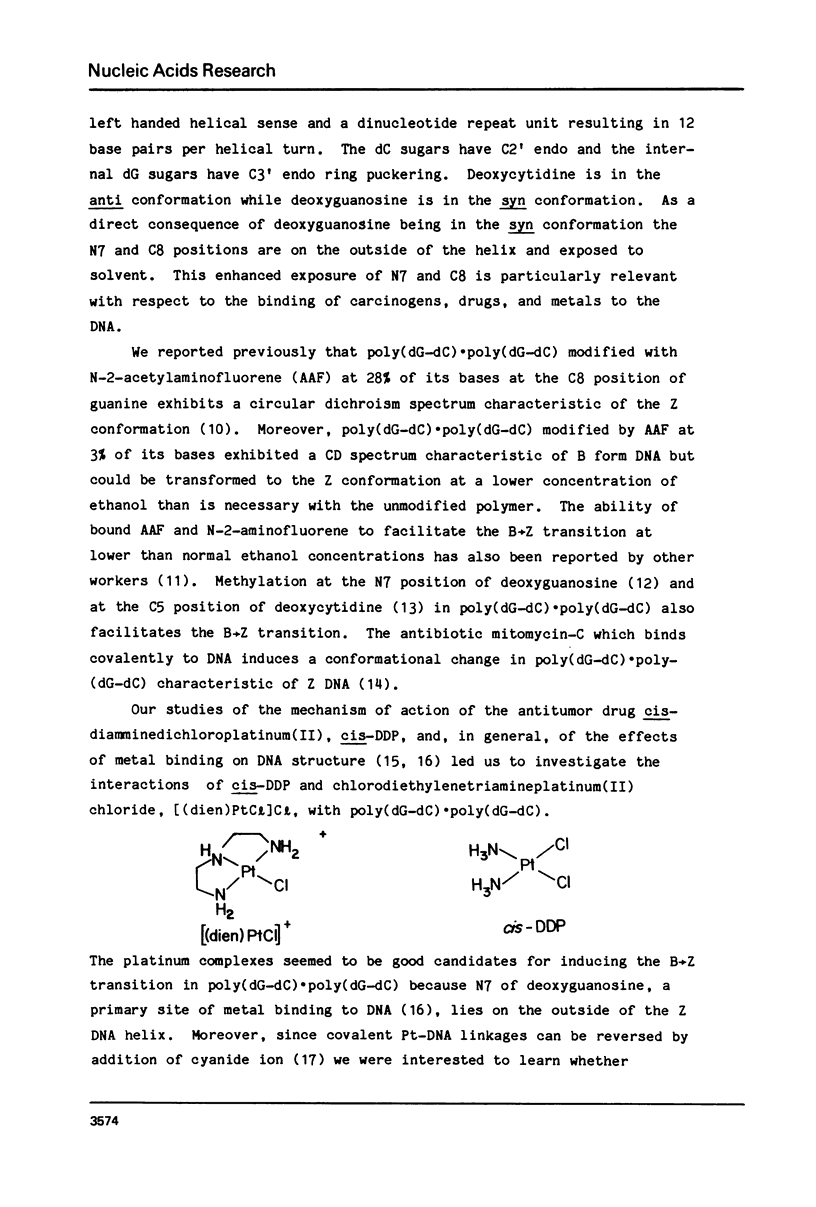
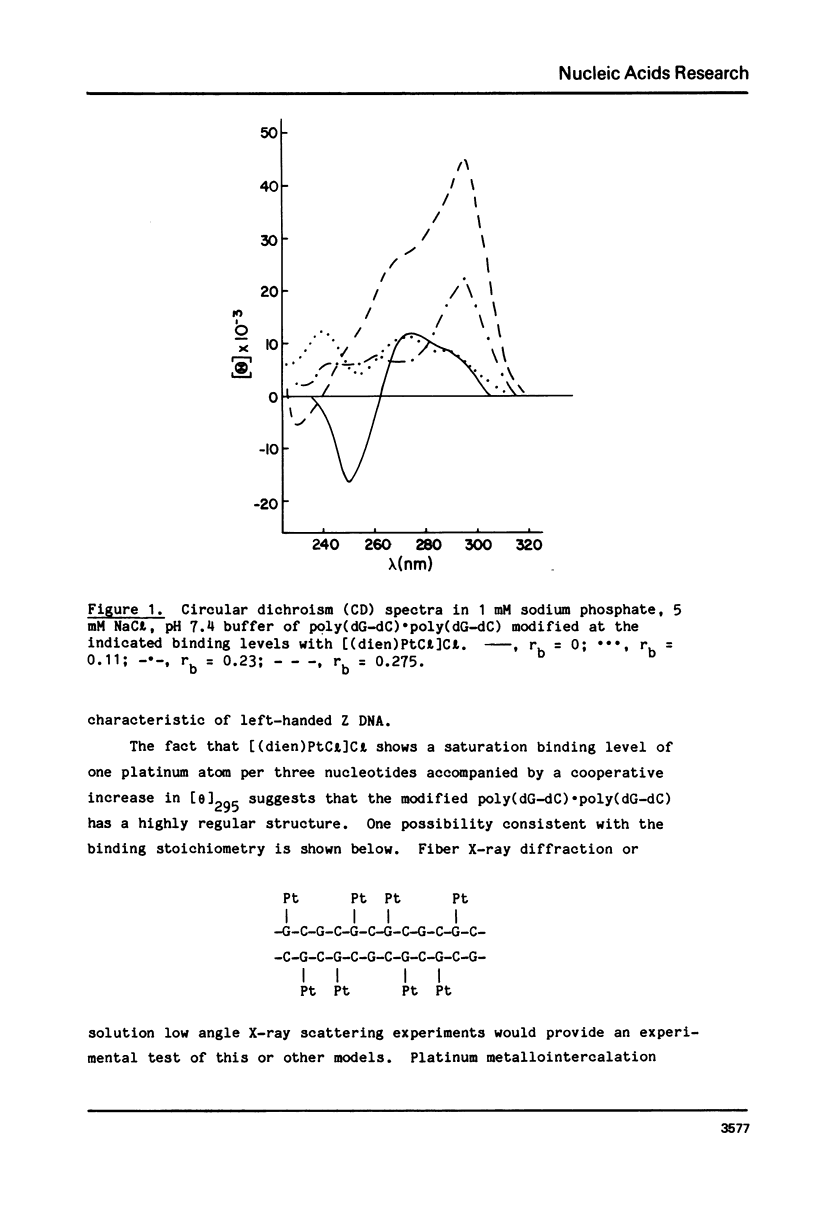
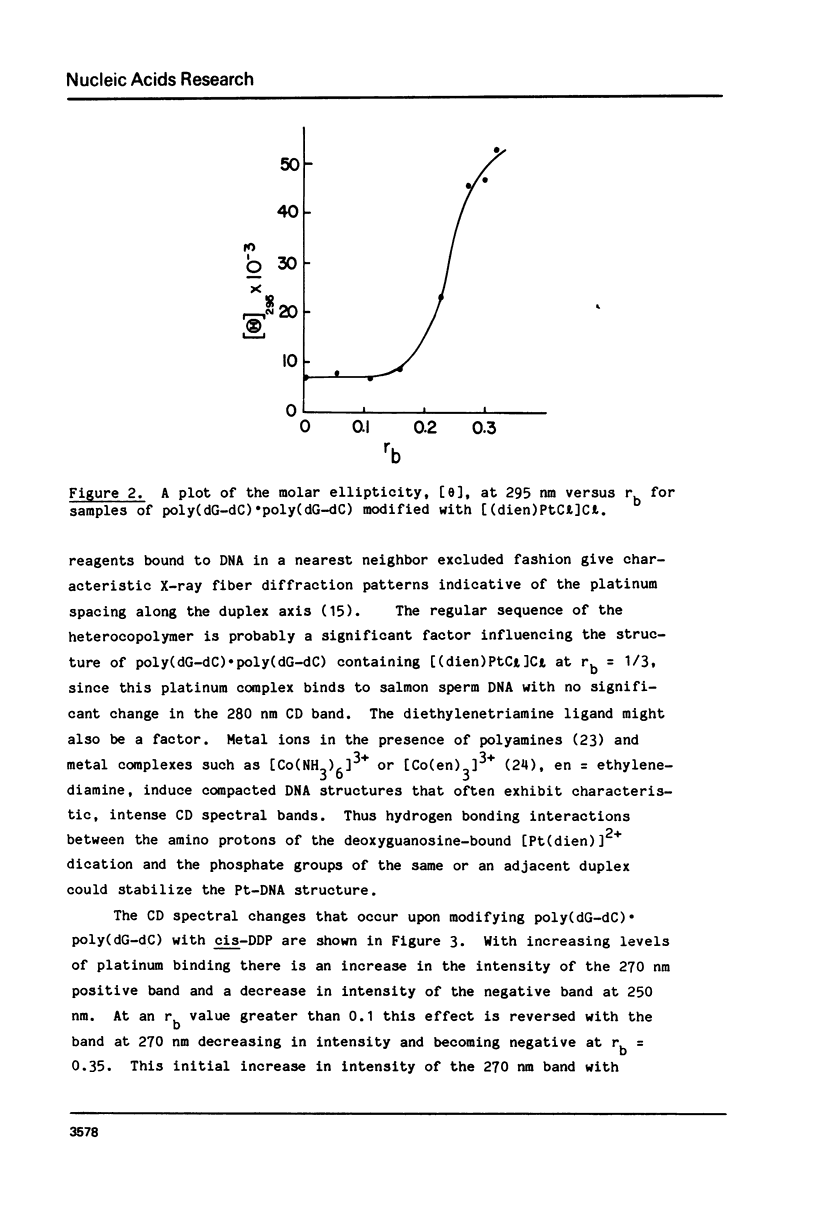
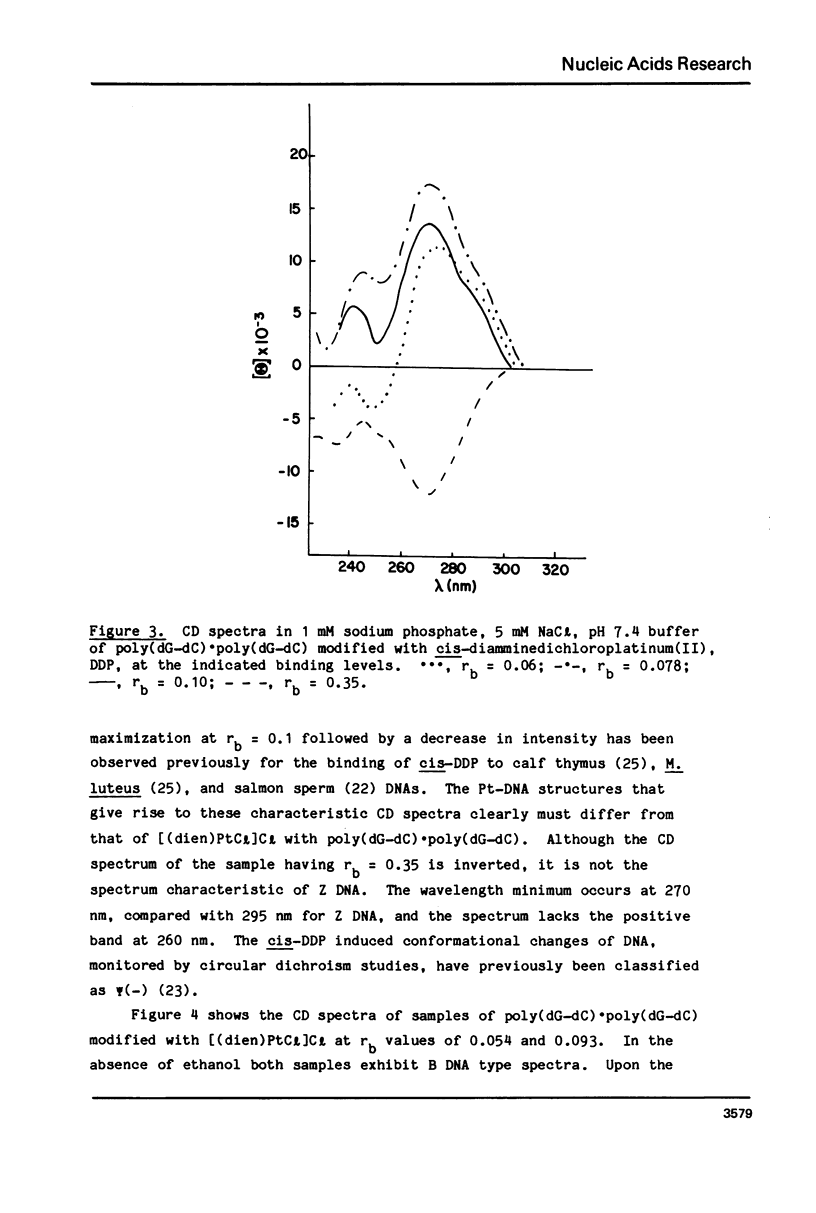
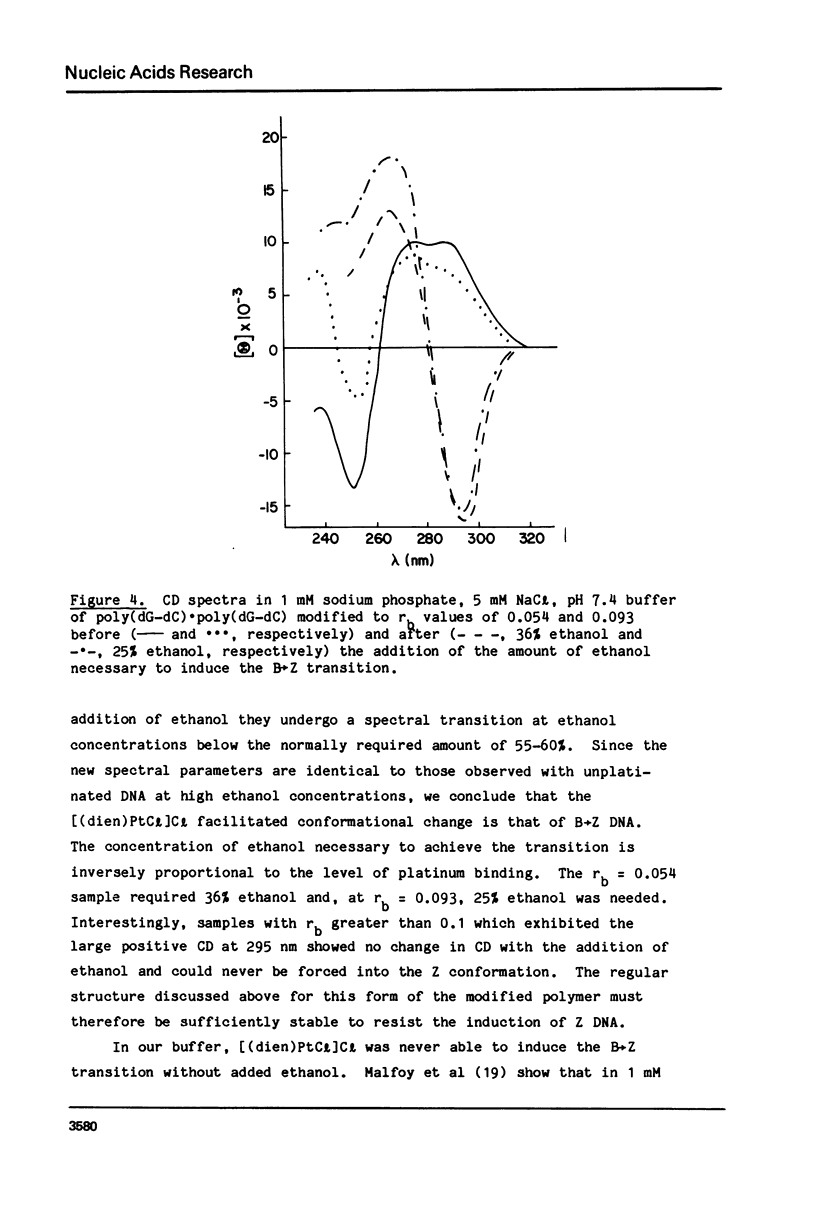
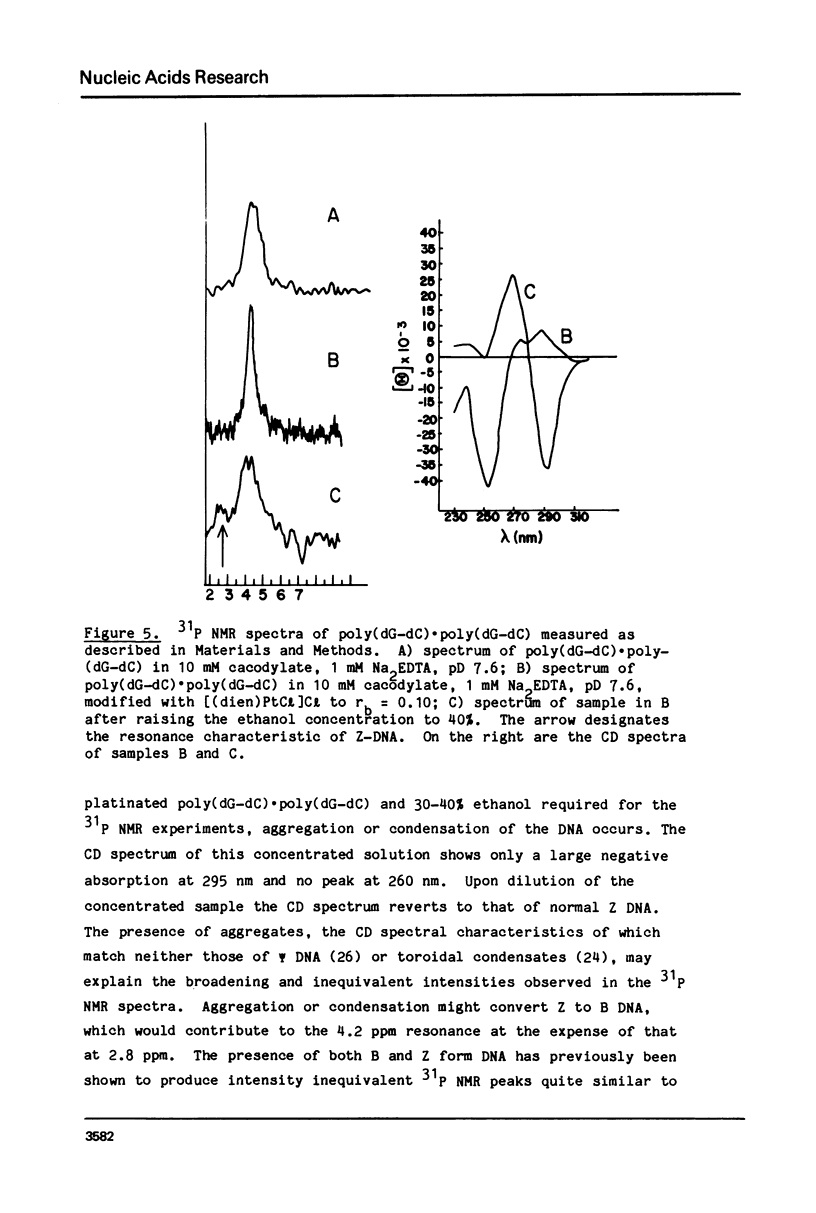
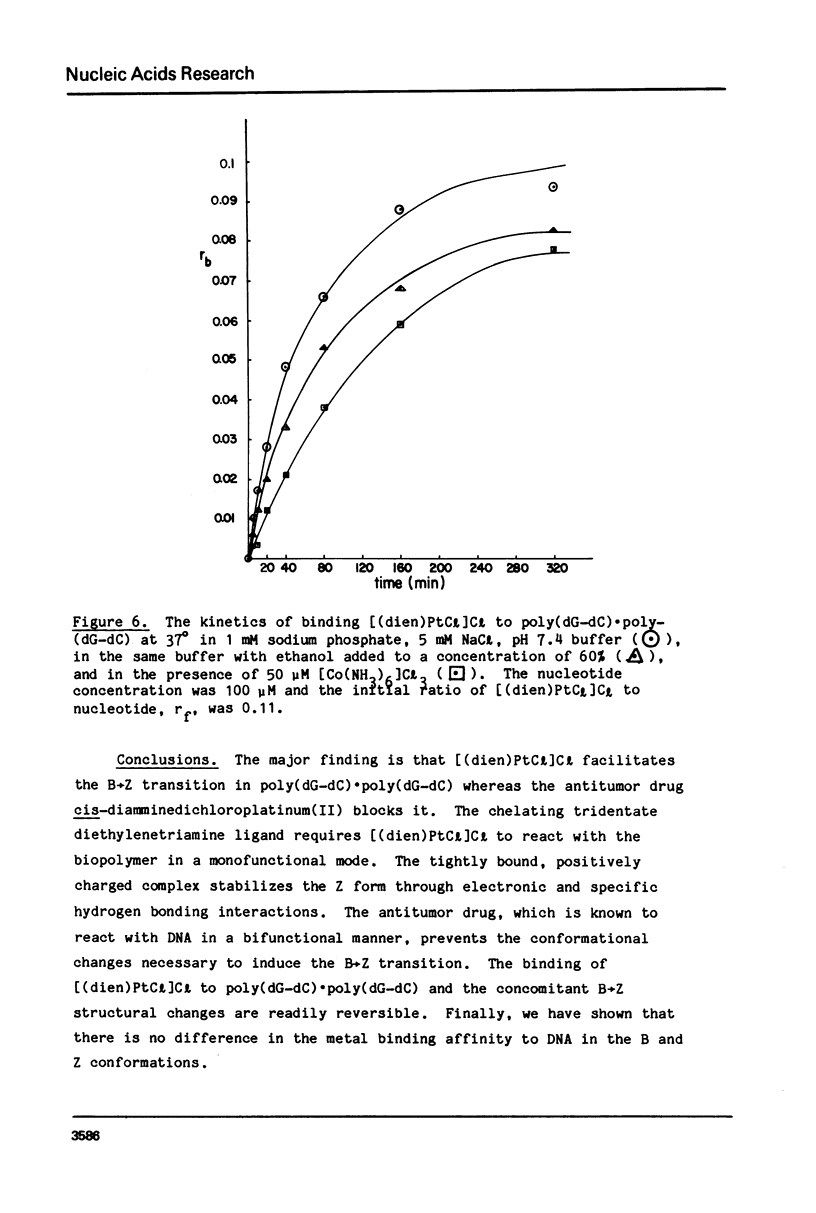
Chlorodiethylenetriamineplatinum(II) chloride, [(dien)PtCl]Cl, bound to less than or equal to 10% of the nucleotide bases of poly(dG-dC) . poly(dG-dC) reduces the amount of ethanol necessary to bring about the B goes to Z conformational transition in proportion to the amount of platinum complex bound as monitored by CD spectroscopy. The transition may be effected by 25% ethanol with 9.3% of the bases modified polymer an ethanol with 5.4% of the bases modified. With an unmodified polymer an ethanol concentration of 55-60% is necessary to bring about the transition. The assignment of the Z conformation was supported by 31P NMR spectroscopy. This covalent modification of the DNA is reversed by treatment with cyanide ion after which the normal amount of ethanol is necessary to achieve the transition. The platinum complex shows no enhanced binding to DNA in the Z versus the B conformation. Between 20 and 33% (saturation binding) modification, [(dien)PtCl]Cl binds cooperatively to the heterocopolymer as judged by CD spectroscopy. At this high level of modification it is no longer possible to induce the Z DNA structure with ethanol. When [(dien)PtCl]Cl is bound to preformed (with ethanol) Z DNA at saturating levels the CD spectrum is altered but reverts to the spectrum of highly modified DNA upon removal of ethanol. The antitumor drug cis-diaminedichloroplatinum(II), cis-DDP, binds to poly(dG-dC) . poly(dG-dC) and alters the CD spectrum. It does not facilitate the B goes to Z conformational change, however, and actually prevents it from happening even at very high ethanol concentrations.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Gonias S. L., Kam S. K., Wu K. C., Lippard S. J. Binding of the antitumor drug platinum uracil blue to closed and nicked circular duplex DNAs. Biochemistry. 1978 Mar 21;17(6):1060–1068. doi: 10.1021/bi00599a019. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Wooten J. B., Chatterjee C. L. Characterization of alternating deoxyribonucleic acid conformations in solution by phosphorus-31 nuclear magnetic resonance spectroscopy. Biochemistry. 1981 May 26;20(11):3049–3055. doi: 10.1021/bi00514a010. [DOI] [PubMed] [Google Scholar]
- Kłysik J., Stirdivant S. M., Larson J. E., Hart P. A., Wells R. D. Left-handed DNA in restriction fragments and a recombinant plasmid. Nature. 1981 Apr 23;290(5808):672–677. doi: 10.1038/290672a0. [DOI] [PubMed] [Google Scholar]
- Lerman L. S. Chromosomal analogues: long-range order in psi-condensed DNA. Cold Spring Harb Symp Quant Biol. 1974;38:59–73. doi: 10.1101/sqb.1974.038.01.009. [DOI] [PubMed] [Google Scholar]
- Lippard S. J., Hoeschele J. D. Binding of cis- and trans-dichlorodiammineplatinum(II) to the nucleosome core. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6091–6095. doi: 10.1073/pnas.76.12.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Möller A., Nordheim A., Nichols S. R., Rich A. 7-Methylguanine in poly(dG-dC).poly(dG-dC) facilitates z-DNA formation. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4777–4781. doi: 10.1073/pnas.78.8.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T., Shindo H. Conformation of 145 base pair length poly (dG-dC) . poly (dG-dC) in solution and in association with histones. Nucleic Acids Res. 1980 May 10;8(9):2093–2103. doi: 10.1093/nar/8.9.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushay H. M., Tullius T. D., Lippard S. J. Inhibition of the BamHI cleavage and unwinding of pBR322 deoxyribonucleic acid by the antitumor drug cis-dichlorodiammineplatinum(II). Biochemistry. 1981 Jun 23;20(13):3744–3748. doi: 10.1021/bi00516a012. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang A. J., Quigley G. J., Kolpak F. J., van der Marel G., van Boom J. H., Rich A. Left-handed double helical DNA: variations in the backbone conformation. Science. 1981 Jan 9;211(4478):171–176. doi: 10.1126/science.7444458. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]



