Abstract
Introduction
The purpose of this study was to determine whether fusion causes adjacent segment degeneration or whether degeneration is due to disease progression.
Materials and methods
Eighty-seven patients that had undergone single level anterior cervical decompression and fusions with at least 5 years of follow-up were enrolled in this retrospective study. Segments adjacent to fusion levels (above or below) were allocated to group A, and all others were allocated to group B. Radiographic evaluations of adjacent level changes included assessments of; disc degenerative changes, anterior ossification formation, and segmental instability. The developments of new clinical symptoms were also evaluated.
Results
In group A, adjacent segment degenerative change developed in 28 segments (16%) and two cases (2%) developed new clinical symptoms. In group B, adjacent segment degenerative change developed in 10 segments (3%), and two cases (0.7%) also developed new clinical symptoms. Additional operations were performed in one patient in each group.
Conclusion
Although, fusion per se can accelerate the severity of adjacent level degeneration, no significant difference was observed between adjacent and non-adjacent segments in terms of the incidence of symptomatic disease. The authors conclude that adjacent segment disease is more a result of the natural history of cervical spondylosis than the presence of fusion.
Keywords: Cervical, Fusion, Adjacent segment disease, Natural history
Introduction
Cervical spondylosis is a common pathological condition that affects the adult spine, and is the most frequent cause of cervical radiculopathy and myelopathy in older patients [13]. Anterior cervical discectomy and fusion is regarded as a gold standard treatment for degenerative cervical spine disease, but it is believed that arthrodesis of spinal segments can lead to excessive stress at unfused adjacent levels. Furthermore, motion-preserving techniques involving the use of artificial disc prostheses are becoming more popular, because these are believed to decrease the incidence of accelerated degenerative disease at levels adjacent to fused regions. The short-term results of disc replacement appear to be satisfactory [2, 7, 16], although this is largely attributed to the effects of decompression and a short period of postoperative immobilization, rather than to disc replacement per se. The rationale for using these disc prostheses and the widening of their indications from radiculopathy to myelopathy [17], must be based on long-term results of the effects of these devices on the incidence of adjacent segment disease. Previous biomechanical and clinical studies on the incidence of adjacent segment degeneration have reported figures ranging from 25 to 89% [1, 8, 14]. However, these changes do not always correlate with clinical findings, and others that have studied degenerative changes during follow-up have postulated that degenerative changes adjacent to fused segments are largely caused by the natural history of the disease process [6, 12]. Furthermore, it is difficult to make conclusions regarding adjacent segment problems because comparative data on segments adjacent to fused and non-fused segment are not available. Hence, we evaluated whether fusion per se can affect adjacent segment degeneration by comparing the radiological and clinical findings of segments adjacent and not adjacent to fused segments in single level anterior cervical fusion cases.
Materials and methods
Materials
We evaluated 457 levels in 87 patients (cases) with at least 5 years of follow-up that had undergone single level anterior discectomy and fusion from February 1999 to March 2004.
Mean patient age was 54.4 years (from 38 to 67), and there were 54 men and 33 women. The mean follow-up period was 84.8 months (from 62 to 121). Fusion was performed in 18 cases at C3–4, in 20 cases at C4–5, in 27 cases at C5–6, and in 22 cases at C6–7.
Operation method
Surgery was performed under general anesthesia in all patients. First, cancellous bone for bone grafting was harvested percutaneously using a trocar (AO Synthesis, diameter 7 mm) via a 1-cm mini-incision placed at least 2 mm from the lateral side of the anterior superior iliac spine. A standard Smith–Robinson method was used to expose the cervical spine. After complete decompression by removing osteophytes and remnant disc materials, endplate cartilage was removed with a high-speed burr and curette until bleeding occurred. Lateral radiographs of the cervical spine were checked to determine cage size, plate lordosis, and screw insertion angles. Finally, a PEEK cage filled with cancellous bone graft was inserted into the intervertebral space and anterior plating was performed. The cages used were Solis cages (Stryker®, EMEA), and the MaximaTM Anterior Cervical Plate System (U&I Corporation®, South Korea) or the CSLP (Cervical Spine Locking Plate®, AO North America) system was used for anterior stabilization. After operations, a Philadelphia cervical orthosis was applied for 4 weeks and a soft collar was recommended for an additional 2 weeks.
Careful physical examinations and radiological examinations, including plain radiography and MRI, were performed preoperatively in all patients. Radiographic data were evaluated at 6 weeks and at 3, 6, 9, 12, and 18 months postoperatively and then annually using AP, lateral, and flexion/extension lateral plain radiographs. In cases that developed additional radiculopathy or myelopathic symptoms during follow-up, MRI was performed for evaluation purposes.
Methods
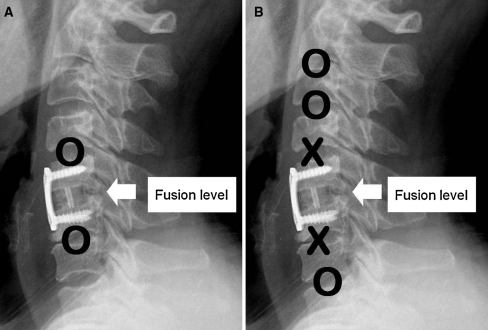
Group A (the adjacent segment group) included segments above and below the fused segments (Fig. 1). In group A, the case distribution of segments above the fusion levels were; C2–3 (18 segments), C3–4 (20 segments), C4–5 (27 segments), and C5–6 (22 segments), and the case distribution of segments below the fusion levels were C4–5 (18 segments), C5–6 (20 segments), C6–7 (27 segments), and C7–T1 (22 segments). In group B (the non-adjacent segment group), the distribution of segments was C2–3 (69 segments), C3–4 (49 segments), C4–5 (22 segments), C5–6 (18 segments), C6–7 (38 segments), and C7–T1 (87 segments).
Fig. 1.
Diagram showing the segments which were included for the evaluation according to each group (O included, X excluded). a Group A, b group B
Radiological degenerative changes was evaluated by comparing preoperative and last follow-up (mean 64.8 months postop) lateral, flexion/extension lateral plain radiographs. Radiological evaluations included: (1) disc degeneration (Criteria I), (2) anterior ossification formation (Criteria II), and (3) segmental instability (Criteria III). Criteria I, disc degeneration, was evaluated using a modification of Hilibrand’s radiographic grading of degenerative change, according to which degeneration was assessed as grade 0 (normal), grade 1 (mild; narrowing of disc space of <50% and no posterior osteophytes), grade 2 (moderate; narrowing of disc space from 50 to 75% and posterior osteophytes) or grade 3 (severe; narrowing of disc space by >75%) [12]. Criteria II, anterior ossification formation was evaluated using Park’s classification and was graded as; grade 0 (none), grade 1 (mild; ossification extended across <50% of the disc space), grade 2 (moderate; ossification extended across ≥50% of the disc space) or grade 3 (severe; complete bridging of the adjacent disc space) [15]. When the grade of Criteria I or II increased as comparing with the preoperative condition, it was regarded to indicate progression of radiological degeneration. For Criterion III, segmental instability, cases showing >3 mm of antero-posterior displacement or 20° of sagittal plane rotation on follow-up dynamic (flexion/extension) lateral radiographs were regarded as having developed degenerative change [4]. No instability was observed during preoperative evaluations.
Two spinal surgeons with no knowledge of clinical outcomes assessed radiological findings twice independently. To evaluate the reliability of findings, we used standardized confidence analysis to determine ICC values (Cronbach’s α), and these were categorized as; poor (α < 0.4), fair to good (0.4–0.7), excellent (α < 0.7) [10]. Interbody fusion can lead to increases in mechanical stress at adjacent disc levels, and thereby accelerate degenerative changes and produce clinical symptoms with time, that is, it can induce “adjacent segment disease” [8]. To evaluate the development of adjacent segment disease, newly generated radicular or myelopathic symptoms were evaluated clinically.
Developments of radiological and clinical degenerative cases were analyzed with respect to an age of less or greater than 50 years.
The Chi-square test in SPSS 12.0 version (Chicago, Illinois) was used to compare the incidences of new radiological and clinical adjacent degenerative change cases. Statistical significance was accepted for p values of <0.05.
Results
ICC (Cronbach’s α) showed that intraobserver (0.76) and interobserver (0.71) correlations were excellent.
Incidence of newly developed degenerative changes
Thirty-eight (8%) of the 457 segments developed a radiological degenerative change. In group A (the adjacent segment group), 28 (16%) of the 174 segments showed degenerative change, and in group B, 10 (3%) of the 283 segments showed degenerative change (p < 0.01). In group A, 15 cases degenerative change developed above the fused segments and in 13 cases below the fused segments (Table 1).
Table 1.
Number of cases showing degenerative changes at last follow-up visits
| Group A | Group B | Total | p Value | |
|---|---|---|---|---|
| C2–3 | 1/18 (5%) | 0/69 (0%) | 1 | 0.21 |
| C3–4 | 2/20 (10%) | 3/49 (6%) | 5 | 0.62 |
| C4–5 | 11/45 (24%) | 0/22 (0%) | 11 | 0.01 |
| C5–6 | 11/42 (26%) | 2/18 (11%) | 13 | 0.38 |
| C6–7 | 3/27 (11%) | 5/38 (13%) | 8 | 0.8 |
| C7–T1 | 0/22 (0%) | 0/87 (0%) | 0 | |
| 28/174 (16%) | 10/283 (3%) | 38/457 (8%) | <0.01 |
Incidences of newly developed degenerative disease
Newly developed radiating and myelopathic symptoms were evaluated by follow-up MRI or post-myelographic CT. Four patients developed new symptoms (4%); 2 cases in group A (1%) and 2 cases in group B (0.7%) (p = 0.52). In two patients (one in each group) symptoms were relieved by conservative management, and in the other two symptoms responded to surgery.
Analysis according to the radiological criteria of degeneration
Progressions of anterior ossification formation (Criteria II) occurred in 20 segments and segmental instability (Criteria III) in 4 cases, and degeneration occurred at fusion levels above in all 4 (Table 2). There were more cases of anterior ossification (Criteria II) than disc degeneration (Criteria I) in group A than in group B, but this was not significant (Table 2).
Table 2.
Number of cases showing degenerative changes for each of the radiological criteria
| Disc degenerative change | Anterior osteophyte formation | Instability | ||||
|---|---|---|---|---|---|---|
| Group A | Group B | Group A | Group B | Group A | Group B | |
| C2–3 | 0 | 0 | 1 | 0 | 0 | 0 |
| C3–4 | 1 | 2 | 1 | 1 | 0 | 0 |
| C4–5 | 2 | 0 | 6 | 0 | 3 | 0 |
| C5–6 | 5 | 0 | 5 | 2 | 1 | 0 |
| C6–7 | 1 | 3 | 2 | 2 | 0 | 0 |
| C7–T1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 5 | 15 | 5 | 4 | 0 | |
In terms of disc degeneration (Criteria I), there are 14 cases of progression. Thirteen of these 14 cases had a preoperative degenerative condition of grade I or II. On the other hand, 7 of 20 cases showed progression according to Criteria II, and these had no preoperative anterior ossification (grade 0); 6 of these 7 cases were in group A.
Analysis according to the age
The patients were divided in two groups according to age. There were 38 patients younger than 50 years (mean age 47.7 years, range from 38 to 49), and 49 patients older than 50 years (mean age 61.1 years, range from 52 to 67). The number of cases that showed new degenerative development are shown in Table 3. No significant difference was observed between these two groups in terms of the development of new radiological degenerative changes (p = 0.83). The percentage of patients that developed adjacent segment disease was greater in older group (6%) than in the younger group (2%), but this was not statistically significant (p = 0.62).
Table 3.
Number of cases of degeneration analyzed by age
| Younger than 50 years | Older than 50 years | |||
|---|---|---|---|---|
| Group A | Group B | Group A | Group B | |
| Disc degenerative change | 3 | 1 | 6 | 4 |
| Anterior osteophyte formation | 7 | 2 | 8 | 3 |
| Instability | 3 | 0 | 1 | 0 |
| Adjacent segment disease | 1 | 0 | 1 | 2 |
Discussion
Since anterior cervical discectomy and fusion were introduced by Smith and Robinson, it has been used as the standard for the care for cervical degenerative disease [18]. However, the widespread use of cervical fusion has raised concerns about donor site morbidity, pseudoarthrosis, and adjacent segment degeneration. Furthermore, the usages of bone graft substitutes, such as, allografts, cages, demineralized bone matrix (DBM), and bone morphogenic protein (BMP), have reduced donor site morbidities, and additional plating or the use of osteoinductive materials could solve pseudoarthrosis problems [3]. However, fusion per se may increase mechanical stress at adjacent disc levels, accelerate degenerative changes, and eventually lead to ‘adjacent segment disease’.
Sufficient evidence is available to prove the occurrence of adjacent segment change. Goffin et al. [6] reported a 92% incidence of radiological adjacent segment change after cervical fusion with a mean follow-up of 8 years, and Teramoto et al. [20] reported radiological adjacent segment disease in 51.1% of their patients at a mean 10 years, though only 6.7% required further surgery at an adjacent level. Furthermore, biomechanical studies have produced evidence of increased stress and hypermobility at adjacent segments [5]. However, controversy exists as to whether adjacent segment degenerative change is a result of fusion or of the natural history of cervical spondylosis. Despite a number of reports about adjacent degeneration, it is widely accepted that radiological degenerative adjacent change does not lead to symptomatic change, and that it is not related to adjacent segment disease. Hilibrand et al. [12] concluded that adjacent segment disease is more likely to be a product of the natural history of the disease based on the finding that adjacent segment disease occurred at a relatively constant rate, was more likely at C5/6 and C6/7, occurred in older patients, and was significantly less likely after multilevel fusion. Our previous study showed that multi-level fusion using a cage and plate augmentation procedure accelerated the severity of adjacent level degeneration as compared with single level fusion, but we found no correlation between the incidence of symptomatic adjacent level disease and numbers of fusion levels after anterior cervical arthrodesis in degenerative cervical diseases [19].
Scare reports have been issued on the natural history of adjacent segment disease without fusion in cases of degenerative cervical disease. Herkowitz et al. [11] reported that 39% of patients developed radiological adjacent segment degeneration, although this was not necessarily associated with clinical symptoms, and in the same study, 50% of patients that underwent posterior foraminotomy also developed radiological disc degeneration at operated and adjacent levels. Gore [9] studied the natural history of cervical spondylotic disease in 159 asymptomatic patients, and found that 12% developed symptomatic spondylotic disease over a 10-year period, suggesting a high incidence of naturally occurring degenerative disease. According to the results of the present study, fusion per se can accelerate the severity of adjacent level degeneration. However, no significant difference was found between segments adjacent to and not adjacent to fused segments with respect to the incidence of symptomatic adjacent segment disease.
Another issue that should be considered is whether anterior ossification formation adjacent to fused segments should be viewed as an adjacent degenerative change induced by fusion. Nowadays, the majority of patients are treated by plate augmentation, and plating is known to be associated with the development of adjacent-level ossification [15]. In the present study, we found much more anterior ossification formation at segments adjacent to fused segments than in non-adjacent segments. It was notable that 7 of the 20 cases with no ossification before operation subsequently developed anterior ossification, and that in 6 of these 7 cases ossification occurred at adjacent segments. On the other hand, 13 of 14 cases that showed disc degenerative changes according to the modified Hilibrand’s criteria (Criteria I) had a previous degenerative condition before surgery. Of the four cases that developed adjacent segment disease in our study, three had disc degeneration and one had instability. Thus, the radiological degenerative condition related to symptom development was disc degenerative change (Criteria I) and not anterior ossification (Criteria II). Furthermore, fusion was only found to be marginally related to disc degeneration. Thus, we postulate that in previous studies, including some recent studies, anterior ossification was viewed as an adjacent degenerative change, which could have lead to an overestimation of the rate of radiological adjacent segment degenerative change.
The limitations of this study are its relatively short-term follow-up and the unevenness of the case distribution. In particular, the number of fusion cases with C5–6 and C6–7 involvement outnumbered those with C3–4 or C4–5 involvements, and case distributions in groups I and II were uneven. Furthermore, it has been established that involvements of C2–3 and C5–6 differentially affect the development of new degenerative changes. In fact, it is our opinion that disc degeneration occurs at greater frequency in central levels in the cervical spine than at its ends. In the present study, the number of cases showing radiological degenerative change was significant only in C4–5. Furthermore, our results do not support a relation between adjacent segment radiological findings and symptomatic adjacent disease. No previous study has compared segments adjacent and not adjacent to fused levels in same cases, and thus, we believe our results contribute to our understanding of adjacent segmental disease. Another limitation is that we could not perform follow-up MRI scan on all patients due to the economic burden. We believe that MRI may be more helpful than plain radiography for detecting ‘degenerative changes’, and thus, this limitation may have biased results.
Conclusion
Radiological adjacent segment change was found to be greater than at non-adjacent segments in single level anterior fusion cases. However, no significant difference was observed between adjacent and non-adjacent segments in terms of the incidence of symptomatic disease. Accordingly, we conclude that fusion per se has only a small effect on the development of adjacent segment disease.
Conflict of interest
None.
References
- 1.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnoli R, Yue JJ, Pfeiffer F, et al. Early results after ProDisc-C cervical disc replacement. J Neurosurg Spine. 2005;2:403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 3.Caspar W, Geisler FH, Pitzen T, Johnson TA. Anterior cervical plate stabilization in one- and two-level degenerative disease: overtreatment or benefit? J Spinal Disord. 1998;11:1–11. doi: 10.1097/00002517-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Dvorak J, Froehlich D, Penning L, Baumgartner H, Panjabi MM. Functional radiographic diagnosis of the cervical spine: flexion/extension. Spine. 1988;13:748–755. doi: 10.1097/00007632-198807000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 6.Goffin J, Geusens E, Vantomme N. Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech. 2004;17:79–85. doi: 10.1097/00024720-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Goffin J, Calenbergh F, Loon J, et al. Intermediate follow-up after treatment of degenerative disc disease with the Bryan cervical disc prosthesis: single-level and bi-level. Spine. 2003;28:2673–2678. doi: 10.1097/01.BRS.0000099392.90849.AA. [DOI] [PubMed] [Google Scholar]
- 8.Goffin J, Loon J, Calenbergh F, Plets C. Long-term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:500–508. doi: 10.1097/00002517-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Gore DR. Roentgenographic findings in the cervical spine in asymptomatic persons. A ten year follow-up. Spine. 2001;26:2463–2466. doi: 10.1097/00007632-200111150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Henriksen M, Lund H, Moe-Nilssen R, et al. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture. 2004;19:288–297. doi: 10.1016/S0966-6362(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 11.Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation: a comparison between the anterior and posterior approach. Spine. 1990;15:1026–1030. doi: 10.1097/00007632-199015100-00009. [DOI] [PubMed] [Google Scholar]
- 12.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kalfas IH. Role of corpectomy in cervical spondylosis. Neurosurg Focus. 2002;12:E11. doi: 10.3171/foc.2002.12.1.12. [DOI] [PubMed] [Google Scholar]
- 14.McGrory BJ, Klassen RA. Arthrodesis of the cervical spine for fractures and dislocations in children and adolescents. A long-term follow-up study. J Bone Joint Surg Am. 1994;76:1606–1616. doi: 10.2106/00004623-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Park JB, Cho YS, Riew KD. Development of adjacent-level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005;87:558–563. doi: 10.2106/JBJS.C.01555. [DOI] [PubMed] [Google Scholar]
- 16.Pimenta L, McAfee PC, Cappuccino A, Bellera FP, Link HD. Clinical experience with the new artificial cervical PCM (Cervitech) disc. Spine J. 2004;4:315S–321S. doi: 10.1016/j.spinee.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Sekhon LH. Cervical arthroplasty in the management of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17:E8. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 18.Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607–624. [PubMed] [Google Scholar]
- 19.Song KJ, Lee SK, Song JH, Choi BY. The comparison of multi-level fusion versus one-level fusion to the development of adjacent level degeneration in anterior cervical arthrodesis with PEEK cage and plate augmentation for the degenerative cervical spinal disorders. J Korean Orthop Assoc. 2009;44(6):613–618. doi: 10.4055/jkoa.2009.44.6.613. [DOI] [Google Scholar]
- 20.Teramoto T, Ohmori K, Takatsu T, et al. Long-term results of the anterior cervical spondylosis. Neurosurgery. 1994;35:64–68. doi: 10.1227/00006123-199407000-00010. [DOI] [PubMed] [Google Scholar]