Abstract
Purpose
There are few prospective studies on surgical outcomes and survival in patients with metastatic disease to the spine. The magnitude and duration of effect of surgery on pain relief and quality of life remains uncertain. Therefore, the aim of this clinical study was to prospectively evaluate clinical, functional, quality of life and survival outcomes after palliative surgery for vertebral metastases.
Methods
118 consecutive patients who underwent spinal surgery for symptomatic vertebral metastases were prospectively followed up for 12 months or until death. Clinical data and data from the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire were obtained pre- and post-operatively and at regular follow-up intervals.
Results
Surgery was effective in achieving rapid improvement in axial and radicular pain, neurological deficit, sphincteric dysfunction and ambulatory status, with a complication rate of 26% and a 12 month mortality rate of 48%. Almost 50% of patients had complete resolution of back pain, radiculopathy and neurological deficit. Of the patients who were non-ambulant and incontinent, over 50% regained ambulatory ability and recovered urinary continence. The overall incidence of wound infection or breakdown was 6.8% and the local recurrence rate was 8.5%. There was a highly significant improvement in physical, role, cognitive and emotional functioning and global health status post-operatively. Greatest improvement in pain, function and overall quality of life occurred in the early post-operative period and was maintained until death or during the 12 month prospective follow-up period.
Conclusion
The potential for immediate and prolonged improvement in pain, function and quality of life in patients with symptomatic vertebral metastases should be considered during the decision-making process when selecting and counselling patients for surgery.
Keywords: Vertebral metastases, Cancer, Spinal tumour, Surgery, Quality of life
Introduction
The spine is the commonest site of skeletal metastatic disease and symptomatic spinal metastasis is the initial presentation of malignancy in up to 30% of patients, with breast, prostate and lung being the most common primary cancer sources [1–6]. The vertebral column has structural load-bearing as well as spinal cord and nerve-protecting functions; thus metastatic involvement often leads to one or a combination of severe pain, paralysis and urinary or faecal incontinence. This in turn adversely affects ambulatory ability, function and quality of life. The past decade has seen substantial improvements in the multidisciplinary diagnosis and management of patients with primary and metastatic disease. This has resulted in patients presenting earlier with metastases and with the potential to live longer [6, 7]. Furthermore, advances in surgical techniques and newer generation spinal instrumentation have resulted in surgery being more effective in circumferentially decompressing the spinal cord, with the ability to immediately reconstruct and stabilize the spine in patients with bony destruction and spinal cord, cauda equina or nerve root compression by metastatic cancer [8–13].
Conceding that surgery in spinal metastases cannot be curative, the goals of surgery are to provide symptomatic pain relief, restore structural stability to the spine and prevent or reverse neurological compromise without causing excessive morbidity. However, whether to undergo operative treatment still remains controversial, depending on several factors which influence the patient’s length of survival as well as their potential to benefit from surgery [14–18]. Surgery should be considered only if the anticipated improvement in pain, function and quality of life outweighs the risks of surgery. To date, there are few prospective studies on surgical outcomes and survival in patients with metastatic disease to the spine [11–13]. The magnitude and duration of effect of surgery on pain relief function and quality of life remains uncertain. Furthermore, post-operative quality of life outcomes are not currently considered in prognostic scoring systems in this group of patients [16–18]. For this reason, we conducted a prospective study investigating clinical, functional and quality of life outcomes and length of survival on a consecutive series of patients who underwent palliative surgery for vertebral metastases.
Methods
Patients
118 patients who had symptomatic vertebral metastases requiring surgery between September 2005 and November 2007 were prospectively enrolled in this study. There were 65 male and 53 female patients. Average age at time of surgery was 61.6 years (range 28–89 years). Primary lesions are summarized in Table 1. The commonest origin of the primary tumour was lung in 20%, followed by breast and renal in 17% of cases each. 77 patients had multimetastatic disease to visceral organs and/or other bones at time of surgery. 96% of patients had back pain, 58% had radicular pain and 44% had a motor or sensory deficit pre-operatively due to spinal cord, cauda equina or nerve root compression by tumour, 23% of patients had paraparesis or paraplegia, 20% had urinary sphincteric dysfunction and 22% of patients were non-ambulatory. The Frankel grading of motor and sensory neurological deficit pre-operatively was as follows: Frankel A 0% (complete paraplegia), B 2% (no motor function), C 20% (motor function useless), D 11% (slight motor function deficit) and E 67% (no motor deficit).
Table 1.
Primary tumour type
| Tumour | Number of patients | % |
|---|---|---|
| Lung | 23 | 20 |
| Breast | 20 | 17 |
| Renal | 20 | 17 |
| Prostate | 11 | 9 |
| Haematological | 11 | 9 |
| Gastrointestinal | 8 | 6 |
| Unknown | 10 | 8 |
| Other | 15 | 13 |
Surgery
Surgical indications were for intractable pain resistant to non-operative measures and pain and/or paralysis due to bony instability or spinal cord or cauda equina compression by tumour. Spinal instability was defined as kyphotic deformity and/or subluxation, pathologic fracture, or neurologic deficit. Goals of surgery were to provide pain relief, restore stability and reverse neurological compromise. Vertebroplasty or non-operative treatment was preferably performed in patients without bony instability and with metastases sensitive to chemotherapy, radiotherapy or hormone therapy and symptoms controlled by medication or in patients with poor general medical condition and life expectancy <3 months; these patients were not included in the present study. The selection of surgical approach was done on an individualized basis and generally depended on the topography of the metastasis. If the anterior column predominantly was involved, an anterior approach was usually performed with corpectomy and reconstruction using either poly-methyl methacrylate cement or a reconstruction cage and a supplementary anterior plate. If the posterior column predominantly was involved, posterior decompression and stabilization with pedicle screws and rods was usually performed. In patients with multiple metastases, limited life expectancy and unable to tolerate major surgery, limited posterior decompression usually with stabilization was performed for pain relief and ease of nursing.
Follow-up
Patients were prospectively followed up for 1 year or until death. The post-operative follow-up was at 1, 3, 6 months and 1 year. Pre- and post-operative data were obtained on pain, radiculopathy, neurological status, walking ability, Frankel grade, urinary sphincter function, Karnofsky functional status and the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire. The EORTC QLQ-C30 questionnaire is a validated cancer-specific, self-administered, structured questionnaire designed for use in clinical trials to assess cancer patients’ physical, psychological and social functioning [19, 20]. It contains 30 questions which examine functional, symptom and global quality of life domains. Of these, we examined the following multi-item scales: physical functioning, role functioning, social functioning, cognitive functioning, emotional functioning, pain and global health status. Data from the EORTC QLQ-C30 questionnaire were obtained at all follow-up appointments, scored and linearly transformed according to the EORTC scoring manual to yield scores from 0 to 100 [21]. A high score for the functional scales represents a better level of functioning whereas a high score for the pain symptom scale represents a high level of symptomatology.
Statistical analysis
The distribution of the variables is given as the mean, standard deviation and range. The 2-tailed independent t test was used to compare parametric data and the Kruskal–Wallis test used to compare non-parametric data. The Chi-square test was used to compare categorical variables. Survival rate was analysed according to Kaplan–Meier method. The date of surgery was considered as the starting date, and death or the 12-month follow-up appointment was the end point. The Cox regression analysis model was used to analyse univariate and multivariate predictors of survival. Variables significant at a probability value <0.05 in the univariate analysis were tested through a backward stepwise selection process for their independent impact on survival. Data were analyzed with SAS Statistical software (version 8.1, SAS Institute Inc., Carey, NC, USA). A P value of <0.05 was considered as being statistically significant.
Results
The location and approach of surgery is summarized in Table 2. The metastases requiring operative intervention were most commonly located in the thoracic spine, followed by the lumbar, then cervical spine. Sixteen patients (13.6%) received pre-operative radiotherapy and 25 patients (21.2%) had pre-operative chemotherapy. 82 patients underwent posterior decompression and stabilization. 32 patients underwent anterior decompression with reconstruction of the anterior column. 4 patients underwent a two-stage posterior followed by anterior procedure. 53% of operations took <2 h, 42% took between 2 and 3 h and 5% took between 3 and 4 h. Average blood loss was 718 ml (range 0–4,945 ml). 47% of patients received an intra-operative blood transfusion. There was no significant difference in blood loss or operation duration between anterior versus posterior surgery. Patients were discharged from hospital after an average of 9.7 days (range 2–44 days).
Table 2.
Location and approach of surgery
| Location of surgery | Posterior | Anterior | Posterior then anterior | Total |
|---|---|---|---|---|
| Cervical | 6 | 8 | – | 14 |
| Cervical + thoracic | 1 | 5 | 1 | 7 |
| Thoracic | 39 | 16 | 2 | 57 |
| Thoracic + lumbar | 4 | – | – | 4 |
| Lumbar | 21 | 3 | 1 | 25 |
| Lumbar + sacral | 11 | – | – | 11 |
| Total | 82 | 32 | 4 | 118 |
Following surgery, at the time of discharge from hospital all patients except one had either improvement or no change in back pain and in the patients with radiculopathy, all except one had either improved or unchanged radicular pain. The early results of surgery (at the time of discharge from hospital) are summarized in Table 3. 53 patients had complete resolution of their radicular symptoms and 22 out of 49 (45%) patients with a neurological deficit pre-operatively fully recovered post-operatively. 13 out of 22 (59%) patients who were incontinent pre-operatively recovered urinary sphincter function; however, three patients became incontinent. 12 out of 26 (46%) patients with paralysis or paraparesis recovered, although four patients became paraplegic or paraparetic. 96% of patients had either better Frankel grades or showed no signs of deterioration after surgery. Post-operatively, 90% of all patients had functionally useful Frankel Grade D or E compared with 78% pre-operatively. 62% of patients who were not already Frankel grade E improved by one or more grades.
Table 3.
Clinical features of patients before and after surgery (at time of discharge)
| Symptom/feature | Pre-op (%) | Post-op (%) |
|---|---|---|
| Back pain | 96 | 57 |
| Radiculopathy | 56 | 11 |
| Neurological deficit | 44 | 27 |
| Urinary incontinence | 20 | 11 |
| Paraparesis or paraplegia | 24 | 18 |
| Ambulatory | 78 | 89 |
| Frankel grade E | 68 | 79 |
| Frankel grade D | 10 | 12 |
| Frankel grade C | 20 | 8 |
| Frankel grade B | 2 | 1 |
| Frankel grade A | 0 | 0 |
| Karnofsky score 80–100 | 17 | 17 |
| Karnofsky 50–70 | 66 | 78 |
| Karnofsky 10–40 | 17 | 5 |
From a functional standpoint, 17 out of 25 (68%) patients who were unable to walk pre-operatively regained mobility. Of those patients who were ambulant pre-operatively, 93% maintained mobility. The median Karnofsky score changed from to 60 pre-operatively to 70 post-operatively. 41% of patients functionally improved by one or more Karnofsky functional scale; 21% remained the same and 28% were worse.
Complications
A total of 45 complications were recorded in 31 (26%) of the 118 patients (Table 4). Of these, 14 patients developed 19 complications during their inpatient admission, and 18 patients reported 26 delayed complications during the outpatient follow-up period, at an average of 2 months post-surgery. The overall incidence of wound infection or breakdown was 6.8%. 8 out of 19 patients (42%) who had pre-operative radiotherapy to the metastasis developed complications, with wound infection or breakdown occurring in three patients (15.8%). All cases of wound infection or breakdown occurred in posterior surgery. 33% of patients who had a posterior procedure developed complications, compared with 13% of patients who underwent an anterior procedure. The incidence of complications was otherwise not influenced by age, sex, presence of medical co-morbidities, smoking, Karnofsky performance status, or operative location or duration. The 30-day mortality rate was 7.6% (9 patients out of 118), which included three patients who died as inpatients. Six patients (5%) required further operative intervention within the 12-month follow-up period; three for washout or debridement of haematoma or infection and three because of tumour recurrence. The overall local recurrence rate was 8.5%, with three of the ten cases causing paraplegia. The local recurrence rate for posterior surgery was 9.8% and slightly higher than the 6.3% recurrence rate for anterior surgery. The 12-month mortality rate was higher in patients who developed complications compared with those who did not (67 vs. 41%, P = 0.02).
Table 4.
Complications that occurred during the 12-month follow-up period
| Complication | Total no. |
|---|---|
| Dural tear | 2 |
| Haematoma/collection | 10 |
| Wound infection/breakdown | 8 |
| Neurological aggravation (acute) | 4 |
| Respiratory | 3 |
| Cardiovascular | 2 |
| Local tumour recurrence | 7 |
| Local recurrence causing paraplegia | 3 |
| Instrumentation failure | 1 |
| Neurological deterioration (delayed) | 5 |
| Total | 45 |
Survival and follow-up
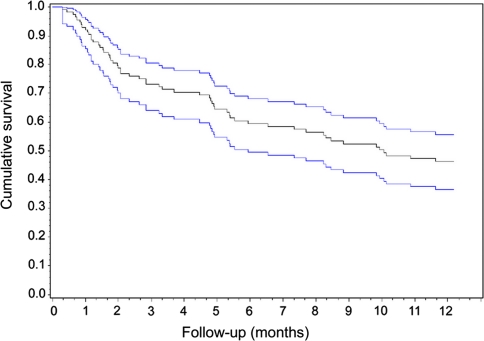
Of the 118 patients, 57 (48.3%) died within 1 year of surgery. Fourteen (11.9%) patients were lost to follow-up after an average of 2 months post-surgery (range 0–9 months). 47 (39.8%) patients were alive at 1 year post-surgery. The rate of survival up to 12 months is shown in Fig. 1. On univariate analysis, the lung cancer as the primary tumour (P = 0.03), vascular disease as a co-morbidity (P = 0.03), lower ASA score (P = 0.04), receiving chemotherapy before surgery (P = 0.03), weight loss (P = 0.0004), lower Tokuhashi score (P = 0.005) and lower Karnofsky performance status (P = 0.001) were associated with lower survival rates. Sex (P = 0.2), age (P = 0.3), neurological deficit (P = 0.2), sphincteric dysfunction (P = 0.9) and number of vertebrae affected (P = 0.4) did not affect survival. On subsequent multivariate regression analysis, lung cancer as the primary tumour, lower Karnofsky performance status, associated vascular disease and receiving chemotherapy before surgery were confirmed as significant negative survival prognostic factors (Table 5).
Fig. 1.
Kaplan-Meier survival curve showing overall survival of all patients following surgery, with 95% confidence intervals
Table 5.
Multivariate analysis of survival prognostic factors
| Variables | Hazards ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Primary tumour | |||
| Breast | 0.009 | ||
| Lung | 3.84 | 1.48–10.00 | |
| Renal | 3.94 | 1.39–11.18 | |
| Other | 1.76 | 0.70–4.43 | |
| Karnofsky performance status (score from 0 to 100) | 0.97 | 0.95–0.99 | 0.0004 |
| Associated vascular disease | |||
| Absent | 2.56 | 1.34–4.89 | 0.005 |
| Present | |||
| Chemotherapy before surgery | |||
| Absent | 2.39 | 1.27–4.47 | 0.007 |
| Present | |||
Functional outcomes and quality of life
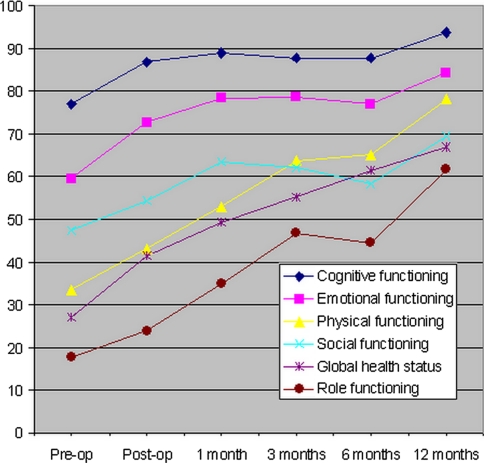
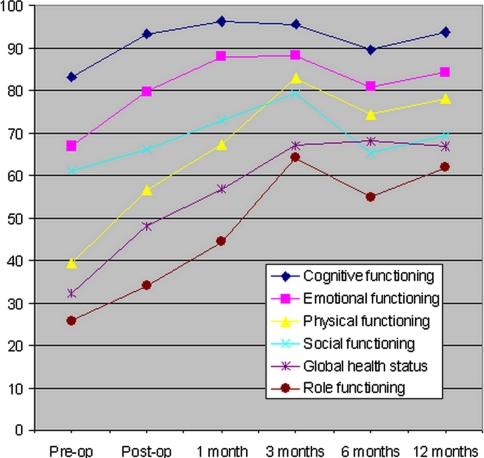
From pre- to post-operatively at time of discharge from hospital, there was a statistically significant improvement in physical functioning (P = 0.01), role functioning (P = 0.02), cognitive functioning (P = 0.002) and emotional functioning (P = 0.0003) scales (Fig. 2). This improvement was maintained during the 12-month follow-up period and there was a trend for further improvement with increasing survival. Each of these functional scales was significantly improved compared with pre-operative averages at the 1, 3, 6 and 12 month follow-up periods. There was no significant improvement in social functioning from pre- to post-operatively (P = 0.17), but there was significant improvement at 12 months (P = 0.006). There was a highly significant improvement in the global health status/quality of life scale from the pre-operative to all post-operative time points (P < 0.0001 for all time points). The pre-operative baseline functional, quality of life and pain scores were all slightly superior in the one-year survivors compared with the rest of the patients; however, only the role, social and cognitive functional scales were significantly different. When patients who survived 12 months were analyzed separately, there was a statistically significant difference from pre- to post-operatively in emotional functioning (P = 0.01), physical functioning (P = 0.03) and global health status (P = 0.001), but not cognitive functioning (P = 0.06), role functioning (P = 0.15) or social functioning (P = 0.56). By 12 months there was a significant difference in cognitive (P = 0.02) and role (P < 0.0001) functioning (Fig. 3).
Fig. 2.
Average EORTC QLQ-C30 scores of all patients for global health status and cognitive, emotional, physical, social and role functioning are illustrated at pre-operative, 1-, 3-, 6- and 12-month post-operative time points
Fig. 3.
Average EORTC QLQ-C30 scores of the 47 patients who were known 12-month survivors at the end of the prospective study period for global health status and cognitive, emotional, physical, social and role functioning are illustrated at pre-operative, 1-, 3-, 6- and 12-month post-operative time points. Baseline pre-operative scores were all slightly higher than the average scores for all patients but the magnitude and maintenance of improvement was similar to that seen in Fig. 2
Pain
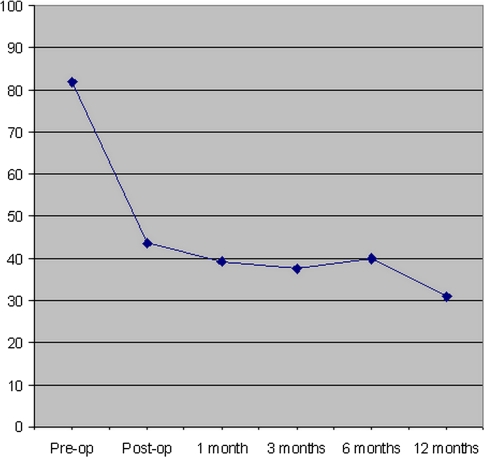
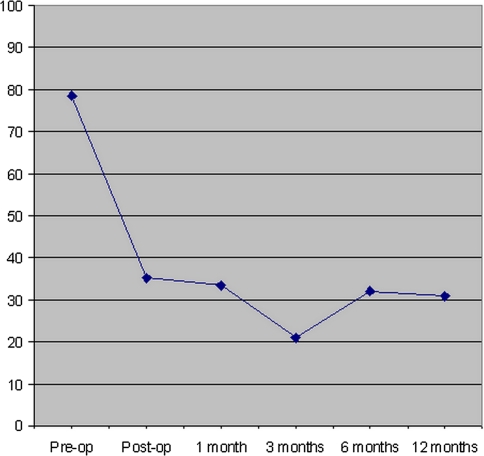
The QLQ-C30 pain symptom scale measures pain based on two questions. It is scored from 0 to 100 with larger numbers having increased pain. There was marked improvement in the pain symptom scale from pre- to post-operatively and at all follow-up time points (Fig. 4, P < 0.0001 for all time points). When the 47 patients who survived 12 months were analyzed separately, there was a similar magnitude and maintenance of pain relief post-operatively (Fig. 5).
Fig. 4.
Average EORTC QLQ-C30 score for pain for all patients are illustrated at pre-operative, 1-, 3-, 6- and 12-month post-operative time points
Fig. 5.
Average EORTC QLQ-C30 score for pain for the 47 patients who were known 12-month survivors at the end of the prospective study period are illustrated at pre-operative, 1-, 3-, 6- and 12-month post-operative time points. The magnitude and maintenance of improvement was similar to the average scores for all patients as illustrated in Fig. 4
Discussion
There are few prospective studies on treatment indications and surgical outcomes in patients with metastatic disease to the spine. The majority of the current literature is based on retrospective data, which suggest that in well-selected patients, surgery can provide significant improvement in overall quality of life and function [15, 17, 22–31]. However, patient selection for surgery is still controversial and depends on balancing the patient’s estimated survival with the anticipated risks and benefits of surgery. Furthermore, it remains uncertain as to how long the beneficial effect of surgery lasts. In the present study of a consecutive series of patients who underwent palliative surgery for vertebral metastases, surgery was effective in achieving rapid overall improvement in axial and radicular pain, neurological deficit and ambulatory status, with acceptable complication rates and morbidity. Importantly, this major improvement in pain, function and overall quality of life occurred in the early post-operative period prior to discharge from hospital and was maintained for the 12-month prospective follow-up period or until death.
Post-operatively, by the time of discharge from hospital all patients except one had either improved or unchanged back and/or radicular pain, and >30% of patients had complete resolution of their pain. The neurological and functional improvement in our patients following surgery was similar to those previously reported in the literature [8, 11–13, 15, 17, 22–33]. That is, almost half of all patients who had a neurological deficit or who were paraplegic or paraparetic recovered. More than 50% of patients who were immobile regained mobility, and a similar proportion of patients who had urinary sphincteric dysfunction regained continence. 62% of patients who were not Frankel grades E pre-operatively improved by one or more grades and ambulatory status was maintained in 93% of patients. We did not compare our results of surgery with non-operative management and radiotherapy. However, a meta-analysis of the literature performed in 2005 showed that surgery was superior to radiotherapy at relieving pain, preserving and regaining ambulatory ability and recovering sphincteric function [32]. Patchell et al. [10] performed the only prospective randomized trial to date investigating this topic and found that in patients presenting with incomplete or progressive paraplegia of <48-h duration, decompressive surgery and stabilization plus radiotherapy resulted in regaining and retention of ambulatory function more than radiotherapy alone, with no increased hospitalization time or morbidity or mortality rates. The beneficial effects following surgery observed in this study were similar to ours; ambulatory status was maintained in 94% of patients who underwent surgery compared with 74% of patients who underwent radiotherapy alone, and 62% of patients regained the ability to walk following surgery compared with 19% in the radiotherapy-only treated group.
The potential beneficial effect of surgery on pain and neurological and functional outcome must be weighed against surgery-related morbidity and survival prognosis. The overall rate of complications from surgical procedures for metastatic spine disease has been reported as being as high as 20–30%, with wound infection being the most frequent complication and ranging from 5 to 30% [8, 13, 22, 25, 27, 28, 31, 33–38]. The complication rate in our series was 26%, with the wound infection or breakdown rate being 7% and all occurring in posterior procedures. Like others, we observed a threefold increase in wound infection in patients who had received pre-operative radiotherapy compared with those who had not [24, 34, 39]. 58% of complications occurred after discharge from hospital, at an average of 2 months post-surgery. Local tumour recurrence was the most common problem, with an overall incidence of 8.5%. 30% of patients who had local recurrence developed paraplegia and 30% required revision surgery. The development of complications was higher in posterior compared with anterior procedures, but otherwise not influenced by age, presence of medical co-morbidities, smoking, or duration or location of surgery. However, the 12-month mortality rate was significantly higher in patients who developed complications compared with those who did not (67 vs. 41%). The 30-day mortality rate was 7.6%, which is within the 4–13% range reported in the literature [8, 25, 32, 37, 38]. The 12-month survival rate of 40–50% in the present study is also consistent with that observed by others [11, 22, 24, 25, 32, 36, 40]. In univariate and multivariate regression analysis, a statistically significant prognostic influence was observed for primary tumour type, vascular disease as a co-morbidity, pre-operative chemotherapy and Karnofsky performance status. This supports the findings of others that tumour type and the patient’s general medical condition influence survival prognosis in patients undergoing surgery for vertebral metastases [41–43].
Physical, role, cognitive, emotional and social functional improvement is important to the palliative patient [44]. These factors should also be considered when selecting patients with vertebral metastases for surgery, rather than basing decisions solely on various apparent survival prognostic factors which comprise current scoring systems. Using the EORTC-QLQ-C30 questionnaire, these multidimensional functional domains were assessed and monitored prospectively at regular pre- and post-operative intervals. Importantly, we observed a significant improvement in all functional domains; physical, role, cognitive, social and emotional functioning, as well in global health status and quality of life following surgery (Fig. 2). The degree of improvement was greatest between the immediate pre- to post-operative time points. There was a trend for continual improvement in role and physical functioning and quality of life in patients who continued to survive during the prospective 12-month follow-up period. Interestingly, of all the functional scales the largest improvement after surgery was in the emotional functioning. According to the questions which evaluate the emotional functioning domain, surgery significantly alleviates tension, worry, irritability and depression, perhaps because it gives these patients hope for the future. The pain symptom scale also showed a considerable improvement in pain between the pre- and post-operative time points (Fig. 4). This improvement was maintained throughout the 12-month follow-up period. Our findings not only confirm the short-term efficacy of surgery in improving pain, function and quality of life in patients with symptomatic vertebral metastases, but also suggest that the beneficial effect of surgery is maintained for at least 12 months. Furthermore, the trend for continual improvement in all scales with time suggests that long survivors (patients surviving for more than 12 months following surgery) will continue to benefit from surgery, highlighting the importance of patient selection and the ability to estimate favourable survival prognosis.
Limitations of this paper include the total patient number of 118 and the heterogeneity of tumour types, resulting in low patient numbers of each tumour type. This precludes valid analysis of individual patient survival. Furthermore, advances in chemo- immuno- and radiotherapies may greatly influence the prognosis and outcome of patients with specific tumours. However, non-operative measures have limited impact on intractable pain or paralysis due to bony instability, pathological fracture or metastatic epidural spinal cord compression, which were the criteria for patient selection for surgery in the present study, since they cannot restore stability to the weakened spine or rapidly relieve neural compression. Moreover, to our knowledge this is currently the largest single institution consecutive series of patients undergoing surgery for spinal metastases to have function and quality of life outcomes assessed and prospectively followed up for 1 year. A prospective multicentre study with strict patient inclusion criteria and defined surgical indications is required in order to determine definitive treatment guidelines for patients with symptomatic spinal metastases from specific cancer types.
Conclusion
In this single-institution, prospective study of a consecutive series of patients who underwent palliative surgery for vertebral metastases, surgery was effective in achieving rapid improvement in axial and radicular pain, neurological deficit and ambulatory status, with acceptable complication rates and morbidity. Greatest improvement in pain, function and overall quality of life occurred in the early post-operative period and was maintained until death or during the 12-month prospective follow-up period. The potential for immediate and prolonged improvement in pain, function and quality of life in patients with symptomatic vertebral metastases should be considered during the decision-making process when selecting patients for surgery.
Acknowledgments
This study was part of an approved French National Research and Clinical Hospital Project funded in 2003 by a public national grant. Dr. Quan was supported by a Post-Doctoral Fellowship from the National Health and Medical Research Council of Australia. The authors would like to acknowledge the assistance of the Centre de Recherche Epidémiologie et Biostatistique PU-PH, Service d’information médicale, CHU de Bordeaux Institut de Santé Publique, d’Epidémiologie et de Développement (ISPED) Université de Bordeaux, France.
Conflict of interest None.
Contributor Information
Gerald M. Y. Quan, Email: gerald.quan@austin.org.au
Vincent Pointillart, Phone: +33-5-56795528, FAX: +33-5-56796043, Email: vincent.pointillart@chu-bordeaux.fr.
References
- 1.Harrington K. Metastatic tumors of the spine: diagnosis and treatment. J Am Acad Orthop Surg. 1993;1:76–86. doi: 10.5435/00124635-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bohm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84:521–529. doi: 10.1302/0301-620X.84B4.12495. [DOI] [PubMed] [Google Scholar]
- 3.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15:1–4. doi: 10.1097/00007632-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9:188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 5.Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol. 2006;2:87–94. doi: 10.1038/ncpneuro0116. [DOI] [PubMed] [Google Scholar]
- 6.Bartels RH, Linden YM, Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58:245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 8.Jansson K, Bauer HC. Survival, complications and outcome in 282 patients operated for neurological deficit due to thoracic or lumbar spinal metastases. Eur Spine J. 2006;15:196–202. doi: 10.1007/s00586-004-0870-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aebi M. Spinal metastasis in the elderly. Eur Spine J. 2003;12:S202–S213. doi: 10.1007/s00586-003-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Hrysio RJ, Mohiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;66:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim A, Crockard A, Antonietti P, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. J Neurosurg Spine. 2008;8:271–278. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 12.Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine. 2008;34:69–73. doi: 10.1097/BRS.0b013e3181913f19. [DOI] [PubMed] [Google Scholar]
- 13.Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF. Impact of surgical intervention on quality of life in patients with spinal metastases. Spine. 2006;31:2849–2856. doi: 10.1097/01.brs.0000245838.37817.40. [DOI] [PubMed] [Google Scholar]
- 14.Chi JH, Gokaslan Z, McCormick P, Tibbs PA, Krysio RJ, Patchell RA. Selecting treatment for patients with malignant epidural spinal cord compression—does age matter? Results from a randomized clinical trial. Spine. 2009;34:431–435. doi: 10.1097/BRS.0b013e318193a25b. [DOI] [PubMed] [Google Scholar]
- 15.Vrionis FD, Small J. Surgical management of metastatic spinal neoplasms. Neurosurg Focus. 2003;15:1–8. doi: 10.3171/foc.2003.15.5.12. [DOI] [PubMed] [Google Scholar]
- 16.Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005. [DOI] [PubMed] [Google Scholar]
- 17.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 18.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for properative evaluation of metastatic spine tumor prognosis. Spine. 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 19.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 20.Fayers P, Bottomley A. Quality of life research within the EORTC–the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38:S125–S133. doi: 10.1016/S0959-8049(01)00448-8. [DOI] [PubMed] [Google Scholar]
- 21.Fayers P, Aaronson NK, Bjordal K, Curran D, Groenvold M (2001) On behalf of the EORTC Quality of Life Study Group. The EORTC QLQ-C30 scoring manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
- 22.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Muller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Spine. 1999;24:2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hatrick NC, Lucas JD, Timothy AR, Smith MA. The surgical treatment of metastatic disease of the spine. Rad Onc. 2000;56:335–339. doi: 10.1016/S0167-8140(00)00199-7. [DOI] [PubMed] [Google Scholar]
- 24.Fourney DR, Abi-Said D, Lang FF, McCutcheon IE, Gokaslan ZL. Use of pedicle screw fixation in the management of malignant spinal disease: experience in 100 consecutive procedures. J Neurosurg Sp. 2001;94:25–37. doi: 10.3171/spi.2001.94.1.0025. [DOI] [PubMed] [Google Scholar]
- 25.Hirabayashi H, Ebara S, Kinoshita T, Yuzawa Y, Nakamura I, Takahashi J, Kamimura M, Ohtsuka K, Takaoka K. Clinical outcome and survival after palliative surgery for spinal metastases. Cancer. 2003;97:476–484. doi: 10.1002/cncr.11039. [DOI] [PubMed] [Google Scholar]
- 26.Wai EK, Finkelstein JA, Tangente RP, Holden L, Chow E, Ford M, Yee A. Quality of life in surgical treatment of metastatic spine disease. Spine. 2003;28:508–512. doi: 10.1097/01.BRS.0000048646.26222.FA. [DOI] [PubMed] [Google Scholar]
- 27.Holman PJ, Suki D, McCutcheon I, Wolinsky JP, Rhines L, Gokaslan ZL. Surgical management of metastatic disease of the lumbar spine; experience with 139 patients. J Neurosurg Sp. 2005;2:550–563. doi: 10.3171/spi.2005.2.5.0550. [DOI] [PubMed] [Google Scholar]
- 28.Villavicencio AT, Oskouian RJ, Roberson C, Stokes J, Park J, Shaffrey C, Johnson JP. Thoracolumbar vertebral reconstruction after surgery for metastatic spinal tumors: long-term outcomes. Neurosurg Focus. 2005;19:1–8. doi: 10.3171/foc.2005.19.3.9. [DOI] [PubMed] [Google Scholar]
- 29.Kondo T, Hozumi T, Goto T, Seichi A, Nakamura K. Intraoperative radiotherapy combined with posterior decompression and stabilization for non-ambulant paralytic patients due to spinal metastasis. Spine. 2008;33:1898–1904. doi: 10.1097/BRS.0b013e31817c0410. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita T, Aota Y, Kushida K, Murayama H, Hiruma T, Takeyama M, Iwamura Y, Saito T. Changes in physical function after palliative surgery for metastatic spinal tumor: association of the Revised Tokuhashi Score with neurologic recovery. Spine. 2008;33:2341–2346. doi: 10.1097/BRS.0b013e3181878733. [DOI] [PubMed] [Google Scholar]
- 31.Hessler C, Burkhardt T, Raimund F, Regelsberger J, Vettorazzi E, Madert J, Eggers C. Dynamics of neurological deficit after surgical decompression of symptomatic vertebral metastases. Spine. 2009;34:566–571. doi: 10.1097/BRS.0b013e31819a825d. [DOI] [PubMed] [Google Scholar]
- 32.Klimo P, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.North RB, Larocca VR, Schwartz J, North CA, Zahurak M, Davis RF, McAfee PC. Surgical management of spinal metastases: analysis of prognostic factors during a 10-year experience. J Neurosurg Sp. 2005;2:564–573. doi: 10.3171/spi.2005.2.5.0564. [DOI] [PubMed] [Google Scholar]
- 34.Pascal-Moussellard H, Broc G, Pointillart V, Simeon F, Vital JM, Senegas J. Complications of vertebral metastasis surgery. Eur Spine J. 1998;7:438–444. doi: 10.1007/s005860050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rompe JD, Hopf CG, Eysel P. Outcome after palliative posterior surgery for metastatic disease of the spine—evaluation of 106 consecutive patients after decompression and stabilization with the Cotrel-Dubousset instrumentation. Arch Orthop Trauma Surg. 1999;119:394–400. doi: 10.1007/s004020050008. [DOI] [PubMed] [Google Scholar]
- 36.Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine. 1999;24:1943–1951. doi: 10.1097/00007632-199909150-00014. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein JA, Zaveri G, Wai E, Vidmar M, Kreder H, Chow E. A population-based study of surgery for spinal metastases. Survival rates and complications. J Bone Joint Surg Br. 2003;85:1045–1050. doi: 10.1302/0301-620X.85B7.14201. [DOI] [PubMed] [Google Scholar]
- 38.Patil CG, Lad SP, Santarelli J, Boakye M. National inpatient complications and outcomes after surgery for spinal metastasis from 1993–2002. Cancer. 2007;110:625–630. doi: 10.1002/cncr.22819. [DOI] [PubMed] [Google Scholar]
- 39.Ghogwala Z, Mansfield FL, Borges LF. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001;26:818–824. doi: 10.1097/00007632-200104010-00025. [DOI] [PubMed] [Google Scholar]
- 40.Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer. 1995;76:1453–1459. doi: 10.1002/1097-0142(19951015)76:8<1453::AID-CNCR2820760824>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 41.Van der Linden YM, Dijkstra SP, Vonk EJ, et al. Prediction of survival in patients with metastases in the spinal column. Results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 42.Bartels RH, Feuth T, Maazen R, Verbeek AL, Kappelle AC, Grotenhuis JA, Leer JW. Development of a model with which to predict the life expectancy of patients with spinal epidural metastases. Cancer. 2007;110:2042–2049. doi: 10.1002/cncr.23002. [DOI] [PubMed] [Google Scholar]
- 43.Chow E, Abdolell M, Panzarella T, Harris K, Bezjak A, Warde P, Tannock I. Validation of a predictive model for survival in metastatic cancer patients attending an outpatient palliative radiotherapy clinic. Int J Rad Onc Biol Phys. 2009;73:280–287. doi: 10.1016/j.ijrobp.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 44.Cella DF. Quality of life outcomes: measurement and validation. Oncology. 1996;10:S233–S245. [PubMed] [Google Scholar]