Abstract
Several models of scoliosis were developed in the past 10 years. In most of them, deformations are induced in old animals and required long time observation period and a chest wall ligation ± resection. The purpose of the study was to create a scoliosis model with a size similar to an early onset scoliosis and an important growth potential without chest wall injuring. An original offset implant was fixed posteriorly and connected with a cable in seven (6 + 1 control) one-month-old Landrace pigs. The mean initial spinal length (T1-S1) was 25 cm and the mean weight was 9 kg. After 2 months observation, spinal deformities were assessed with a three dimension stereographic analysis. In four animals, the cable was sectioned and the deformities followed-up for next 2 months. No post-operative complication was observed. Mean weight growth was 10 kg/month and mean spine lengthening (T1-S1) was 7 cm/month. In 2 months, we obtained structural scoliotic curves with vertebral and disk wedging which were maximal at the apex of the curve. Mean frontal and sagittal Cobb angles was 45°. Chest wall associated deformities were similar to those observed in scoliotic deformities and were correlated to spinal deformities (p = 0.03). The cable section resulted in a partial curve regression influenced by disk elasticity and could probably be influenced by gravity loads (Decrease of the Cobb angle of 30% in the sagittal plane and 45% in the frontal plane). According to the results, the model creates a structural scoliosis and chest wall deformity that is similar to an early onset scoliosis. The spinal deformities were obtained quickly, and were consistent between animals in term of amount and characteristic.
Keywords: Early onset scoliosis, Experimental scoliosis, Animal models, Growth modulation, Thorax growth
Introduction
Idiopathic scoliosis is a three dimensional deformity of the spine, which occurs during spinal growth. The deformities are well characterised in humans and associate a coronal curvature, a hypokyphosis in the sagittal plane and a vertebral rotation in the axial plane [1].
Currently used conservative or surgical treatments lead to important locomotive and respiratory morbidity [2–4] in children. The conservative treatments are noninvasive, preserve growth and have minimal risks compared to surgery, but they are not as effective to control important curves and lead to deformed and deformed rib cages over time [5]. In contrast, spinal fusions provide a better deformity correction but eliminate spinal growth and motion and are tied to long term concerns with junctional failure and sagittal plane misalignments. Moreover, the spine and the chest wall growth are interrelated with early deformities limiting pulmonary development [6, 7]. Significant spinal deformities combined with the negative consequences of current treatments results in diminished pulmonary function leading to shorten life expectancy [8, 9]. Fusionless techniques are promising and have received increasing attention over the past several years. Different treatments based on growing rods techniques and VEPTR principles have been proposed to spare the function of the spine and to preserve chest wall growth [10]. Trying to obtain supporting data on their efficiency is difficult to achieve for a host of reasons: acquiring a uniform treatment or observation group is hampered by the heterogeneity and the rarity of the aetiologies; pulmonary functions are difficult to assess in young patients… Animal models have been developed for these reasons.
Spinal deformity creation procedures were initially reported on small animals and more recently on larger animals (goats, minipigs, yorkshire pigs). Conventional large animal scoliosis models are based on asymmetric spinal tethering with ribs ligation and/or resection [11–17]. Methods with minimal violation of the spinal elements have recently been developed, however, they still violate the thoracic cavity and require a long observation period to obtain the deformity [16–19]. The length and size of the spine obtained after deformity creation is also taller than in human early onset scoliosis. Although these models mimic idiopathic scoliosis deformities, violation of chest wall elements are required, thus making this model suboptimal for implant testing and pulmonary parameters analysis. For models with posterior tethering, the need to perform a thoracic tethering has also many drawbacks: Early high mortality of the animals; intervention tends to be done when the animals are slightly bigger thus missing the exponential growth period resulting in long study period waiting for deformities. On note, the effect of the chest wall procedure on spinal deformity is also difficult to predict and can modulate variably the deformity. Also the thoracic scars with extensive paraspinal fibrosis may limit our ability to test new methods of correction.
The purpose of this study was to create a reliable model of early onset scoliosis without chest wall insult, by using an original offset device at early stage of development of the animal during its exponential growing period.
Materials and methods
The study protocol was approved by the ethical committee and followed national guidelines.
Pilot study
In regards to spinal growth, a preparatory study was undertaken during 6 months in 2 landrace pigs with bimonthly AP and lateral radiographic measurements. The T1-S1 length increased regularly by 10 cm/month from 25 cm at 1 month to 75 cm at 6 months. We concluded the relative spinal growth decreases exponentially and the tether system had to be applied the early as possible. Based on this pilot study, we found that individual vertebral body height contribution to the overall spine length is independent and stable during growth. Hence overall spine length can be predicted by calculating growth of a single vertebra not included in the deformity and extrapolated to the balance of the spine. We empirically chose the L4 vertebra of the pigs as the internal controls for growth. The theoretical spine length across the instrumented segment could be calculated by using the percentage of growth observed across the individual growth of L4.
We analysed the importance of an “offset implant” in the amount of deformity creation. We have simulated different types of constructs (offset ± aligned fixation). The finite elements method was used to simulate vertebrae and disk form and elasticity. For an identical applied load (300 N), the translation of the apex vertebrae was reported in different constructs (Fig. 1).
Fig. 1.
Representation of the curve lateralisation in accordance to the offset amount for an identical applied load (300 N) in several constructs. Resistance of the disk and of the vertebral body were simulated with finite element method
Eight pigs were involved in the pilot study to obtain the definitive protocol. Three generations of offset implants were designed and tested to obtain the final offset device (Fig. 2). Pedicle screws fixation was also a concern in these mainly cartilaginous and immature skeletons. We observed a screw ploughing when a pretension (100 N) was applied initially on the cable. This concern was cleared up with applying no initial tension on the cable. The corresponding spinous process was folded laterally and the laminae irritated to create somewhat of a box of bone around the screws of implant to enhance fixation. The screws were inserted with the intent to go beyond the anterior aspect of the vertebral body by 15 mm to minimise the risk of screw pull-out during vertebral body growth.
Fig. 2.
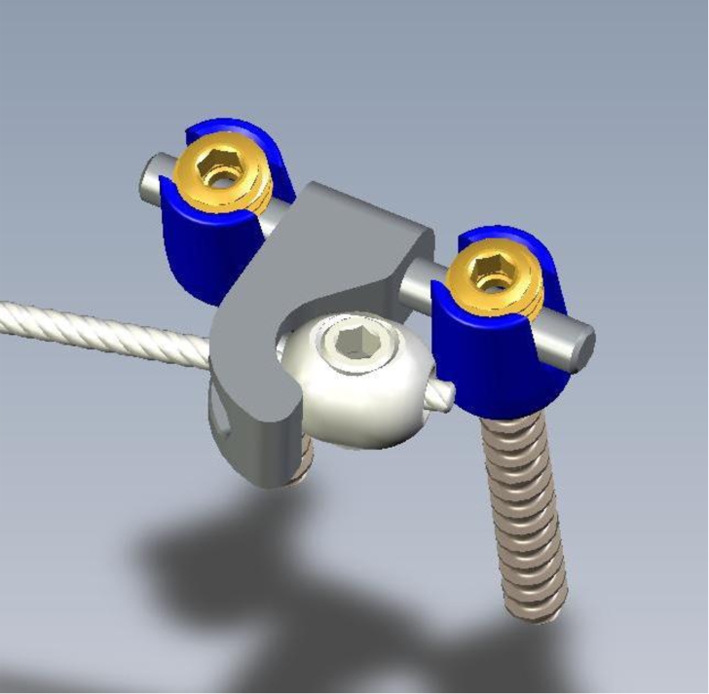
Definitive offset design used with the pedicle screws, the cable with the olive fixation
Surgical procedure
Seven female landrace pigs (age 4 weeks, mean weight 9 kg) were used (6 study pigs + 1 control pig). A posterior left flexible asymmetric tether was used to create scoliosis. Pigs were premedicated with xylazine (0.1 mg/kg), ketamine (10 mg/kg) and morphine (0.2 mg/kg) IM. A bolus dose of thiopental (not exceeding 12 mg/kg IV) was used on induction. The anaesthesia was maintained with isoflurane (2%) and oxygen after tracheal intubation. During the procedure, there was a 20% increase from baseline in heart rate, a bolus of morphine (0.1 mg/kg, IV) was given to control pain. Intravenous Ringer lactate solution was administered at 10 ml/kg/h throughout anaesthesia. Post-operative pain management consisted of morphine (0.1 mg/kg IM) immediately after extubation. A fentanyl patch was applied during the first 3 days and subcutaneous administrations of carprofen (4 mg/kg/day) over the next 8 days. Peri-operatively prophylactic antibiotics were also given. The antibiotic used was cephalexin (30 mg/kg), which was given intravenously on induction and carried out by intramuscular injections (20 mg/kg/day) during 7 days following surgery.
Using two mini-invasive posterior approaches, a custom offset implant was fixed on two vertebral adjacent levels (T5-T6 and L1-L2) with unilateral pedicle screw (Vertex® multiaxial screws, 4 mm diameter, Medtronic Sofamor Danek, Memphis, TN). The proximal and distal offsets implants (Fig. 1) were connected with a 2.5 mm flexible stainless steel cable inserted in a subcutaneous fashion without any tension in 6 of the animals. The seventh animal (control) had the offset implants inserted without the cable.
Animals were clinically followed during a 2 months period. They were fed with a pre-established fat controlled diet and were allowed ad lib activity. Spinal deformities course were assessed by a CT-scan (Hispeed NXIpro General Electric) carried out under general at 1 and 2 months. In four animals, the cable was surgically severed after 2 months and deformities course were assessed by a CT-scan during next 2 months.
Spinal and vertebral deformity analysis
A 3D reconstruction of the entire spine was generated by combining a stereoradiographic technique and a geometric and statistic analysis [20]. This technique uses the axial CT-scan slices to generate a calibrated 3D image of the spine in space (ENSAM-LBM Patent, Paris, 2007). With the help of this 3D reconstruction, we were able to quantify the orientation and the position of the different vertebrae in the space. For the vertebral numeration, the thoraco-lumbar junction was chosen as reference (T12-L1). Relevant morphological parameters were then quantified to accurately define the location and extent of the induced spinal deformities. These parameters mimicked the clinically relevant parameters recommended by the Scoliosis Research Society when evaluating global spinal deformities: i.e. frontal and sagittal Cobb angles, lateral tilt and rotation of each vertebra. The vertebral wedging was calculated with the angle between the cranial and the sagittal end plate (Advantage windows workstation, General Electric, Milwaukee, WI). For each noninstrumented vertebrae involved in the spinal deformity, length and orientation of the pedicles and length and orientation of the dorsal arch was in a local coordinate system (for each vertebra) using a 3D reconstruction of the spine thanks to the IMOD software (IMOD® Boulder laboratory for 3-dimensional Electron Microscopy of Cells and the Regents of the University of Colorado). In respect to evaluate spinal growth modulation, the theoretical spinal length for each specimen was then compared to the actual measured length off of the 3D spine model of each specimen. Hence, we were able to directly measure the impact the tether had on spinal growth.
Thorax and lung assessment
The thoracic parameters were calculated by analysing a CT-scan slice at the level of the apex vertebra of each specimen with a software package (Advantage windows workstation, General Electric, Milwaukee, WI). The thorax deformity was analysed with spinal penetration index, thoracic rotation and posterior hemithoracic symmetry ratio calculation [21]. The lungs were identified as the structure of interest by changing the threshold levels until the lungs were isolated from the rest of the thorax cavity and content [22]. This corresponded to a threshold of 992–198 Hounsfield units. The software performed a 3D reconstruction of the voxels. Right, left and complete lung volumes were calculated. Left to right lung volume ratios were calculated on the post-op CT-scan for each specimen immediately post-op and after 2 months.
Statistical analysis
Statistical analyses were performed with R software. Spearman’s rank correlation was used to determine the association between thoracic measures and Cobb angle. Comparisons of two means were analysed using Wilcoxon signed rank test. All p values were two sided with p < 0.05.
Results
No animals developed post-operative complications. The operations lasted an average of 60 min. Proximal fixation was located on T5-T6 five out of the seven pigs (T3-T4 and T6-T7 once) and the distal fixation was on L1-L2 with the exception of T12-L1 in one case. Post-operative CT-scan showed a single mal-positioned thoracic pedicle screw, which encroached the spinal canal (pig 53). It was repositioned with no consequence. No post-operative complications or neurological deficits were observed. An average of six (5–7) vertebrae was spanned by the instrumentation. Mean weight gain of the animals was 10 kg/month.
All six tethered spines developed a severe progressive structural lordoscoliotic deformity associated with chest wall deformity during the two-month observation period (Table 1, Fig. 3). The mean Cobb angle was 42 ± 4° in the frontal plane and 42 ± 4° in the sagittal plane. One specimen (#55) had a less important deformity due to a precocious screw pullout and dislodgement of the first anchorage screw of the proximal fixation. The 3D regional analysis is shown in Figs. 4, 5. In the frontal plane, we observed a smooth and symmetrical progressive rotational deformity as the vertebrae approached the apex. Mean rotation was 22° (16–25°) at the apex vertebra. The vertebrae, which had the tether were the most tilted and the least rotated. In contrast, the apical vertebrae were the least tilted yet the most rotated and translated. This smooth symmetrical deformity pattern was found in four animals and was nicely demonstrated when plotted in graph form. The two other animals had different curve patterns. The tethered spine had a relatively straight segment followed by an acute bend and sharp rotational deformity located at the junction between the one-third and the two-third of the curve. One animal had this deformity at the proximal 1/3 (#43) while the other at the distal 1/3 (#54). Specific morphological local analysis revelled characteristic wedging of the vertebrae and disk (Fig. 2). The most wedged vertebrae were located at the apex of the curve. Length and orientation of left and right pedicles were not statistically different. Nevertheless, at the apex vertebra, a trend towards a smaller pedicle on the concave side of the deformity was observed (19.5 mm ± 1.5 vs. 22.3 ± 0.9). Length of the posterior arch was similar on each side, on the contrary of its orientation: in the transverse plane, the arch on the concave side of the deformity tended to be less vertical than on the other side. Analysis of the chest wall deformity reported a mean thoracic rotation of 40° and a mean hemithoracic posterior ratio of 3. The thorax rotation angle, representing the windswept deformity of the thorax, was statistically correlated with Cobb angle (p = 0.03) and the hemiposterior asymmetric ratio (p = 0.003). The mean spinal penetration index was 9%. Mean left/right volume ratio was 57% initially and evolved to 66% with a significant difference (p = 0.03).
Table 1.
Spinal, thoracic and lung parameters of the different specimen
| Animal | Cobb frontal (°) | Cobb sagittal (°) | Spinal penetration index (%) | Thoracic rotation | Post hemithoracic ratio | Lung Vol. | Right | Left | Ratio L/R |
|---|---|---|---|---|---|---|---|---|---|
| 43 | 55 | 50 | 8 | 49 | 3.3 | 1,340 | 924 | 416 | 0.69 |
| 51 | 46 | 58 | 5 | 40 | 3.7 | 1,292 | 796 | 495 | 0.62 |
| 52 | 53 | 53 | 11 | 50 | 3.9 | 1,603 | 924 | 679 | 0.73 |
| 53 | 42 | 38 | 7.5 | 42 | 3.1 | 1,440 | 861 | 579 | 0.67 |
| 54 | 35 | 53 | 13 | 37 | 3 | 1,311 | 795 | 515 | 0.64 |
| 55 | 21 | 15 | 6 | 23 | 2.9 | 971 | 585 | 386 | 0.65 |
Fig. 3.
a Frontal view of a CT-scan with three dimensional reconstruction of the spinal and thoracic deformity. b Sagittal reconstruction of the thoracic deformity and thoracic parameters calculation. Posterior hemithoracic symmetry ratio: a line is drawn across the posterior aspect of the thorax on the basis of the location of the anterior tip of the rib heads articulating with the spine. The distance from the spine at each rib head to the inner border of the hemithorax is measured, and a ratio is derived by dividing the larger value by the smaller value. In a normal thorax, the ratio is one, but as a rib hump develops, the ratio increases, reflecting the onset of early windswept thorax. Thoracic ratio: it is represented by the angle between a line bisecting the thorax and the sagittal plane of the vertebra
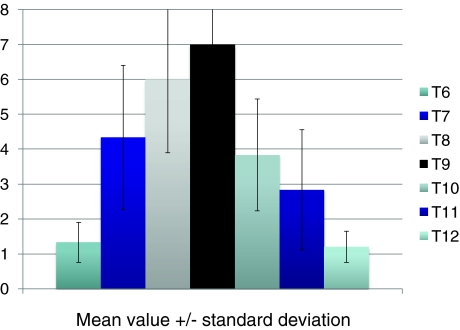
Fig. 4.
Mean value with standard deviation (black vertical segment) of the vertebral wedging. Vertical axis: Vertebral wedging in degrees
Fig. 5.
Lateral tilt (dark grey line) and rotation of individual vertebrae (light grey line) of each specimens. The tethered segments are in between the vertical dotted lines
Mean spinal length was 40 cm (39–41 cm) at 2 month follow-up. Analysis of spinal growth pointed out that the difference between the expected spinal length and the actual measured spinal length never exceeded 3%. This variation is within the measurement error, hence spinal growth was not affected by the tether and a true spinal growth modulation occurred.
The Cobb angle of the four specimens, which had their cable sectioned decreased by 30% in the frontal and in the sagittal plane during the first month. We have only observed a 30% decreased of the Cobb angle in the frontal plane during the second month. In the sagittal plane, where the gravity forces apply in quadrupeds, no curve correction was observed. (Table 2).
Table 2.
Evolution of the Cobb angle post cable-cutting (in degree)
| Animal | 1 months | 2 months | ||
|---|---|---|---|---|
| Cobb frontal | Cobb sagittal | Cobb frontal | Cobb sagittal | |
| 43 | 40 | 37 | 30 | 36 |
| 51 | 32 | 38 | 23 | 38 |
| 52 | 38 | 37 | 28 | 39 |
| 53 | 29 | 26 | 22 | 25 |
Discussion
A variety of large animals have been employed in different scoliosis models ([23] for review). Landrace pigs were chosen for the following reasons. They are sociable, hardy, easy to handle and cheap animals. They are available all year-round (no cyclical breeding) and early weaning is possible at 3 weeks. This allows the experiments to run during the exponential growth of the first months of life. From an anatomic point of view, the vertebrae and thorax shape of the landrace pigs are close to human morphology. Their thorax are more cubical than in goats and the ribs have a rounded shape as in human. In sheep, the lumbar vertebrae have also long and large transverse process and wedged posterior joint facets limiting axial rotation [24].
Our model is based only on a single posterior spinal constrain, which requires a solid fixation of the device onto the spinal elements. To obtain a solid fixation of the offset device in this immature spine, only limited forces had to be applied initially and secondary, the pedicle screw fixation enhanced with the local fusion around the offsets is required. The fusion between the two adjacent vertebrae has no responsibility in the whole deformity of the spine. From a technical point of view the procedure was straight forward with one exception. The selection of the proximal fixation point was more difficult due to the variable location of the scapula and the difficulty to palpate the spinous process at this level. This was not an issue in selection of the distal fixation point. Despite these minor misplacements there was no change in the amount of the final deformity.
In large animal, successful methods to create scoliosis deformity have required more or less extensive vertebral and costal asymmetric posterior tethering. The techniques have been minimised in order to respect growth structures. Braun [15] was the first to report a consistent model. A mean increase of 20° of the initial curves (41–60°) was obtained in a mean 12 weeks in sheep. In a second study [25], greater deformities were observed (55–74°) with the use of a flexible tether. Viguier [26] also has described the possibility of generating (granted inconstantly) a scoliosis deformity of the spine (up to 30°), with associated rotation and lordosis up to 25° after the insertion of a posterior asymmetrical ligamentoplasty in 4-and 8-week-old sheep. The maximum of the deformity appeared during the peak growth period between 2 and 4 months. Schwab et al. [16] have also generated significant curves with a flexible tethering in a porcine model and more recently Zhang et al. [27] in goats with a less invasive procedure on the rib cage. In these models, a pre-tensioning of the cable is mandatory to obtain a deformity due to the older age of the animals and some animal species with a less important growth potential (sheep, goat). The extend of the thoracic procedure is associated with an increase of the post-operative morbidity (up to 30%) [27].
We acknowledge marked limitations of pilot data. We generated a 45° lordoscoliosis thoracic deformity within 2 months. At the end of the study, the model provides a scoliosis spine the size and length of a 9-to 10-year-old child with vertebrae big enough that allows implantation of standard human implants (30 kg weight and 42 cm T1-S1 length) with an important growth remaining. Limited data were available for the size and the growth potential in the reported models and no comparison were done with control animals [15–19, 22, 27].
No previous study analysed segmental spinal deformities in animal models. We had two types of curve expression: four animals had a regular large curve and two animals had acute and sharp deformity. Nevertheless, the typical AIS deformity with and inverse relation between spinal rotation and vertebral tilt was preserved. The spinal length analysis revealed that the tether generated a real modulation of the vertebral growth rather than an asymmetrical growth arrest. This data may be very useful in further studies to evaluate the real effect of correcting methods on spinal growth.
We observed a strong correlation between the spinal and chest wall deformity confirming previous reports on chest wall and spine interrelation [6]. The thorax and pulmonary volume (= 1,300 cc) is also similar to human scoliosis with a concave side lung volume comparatively more affected (decreased) than convex side lung volume.
The objective of this research field is to study the possibility to restore growth in the curve concavity to correct deformity. The first results reported the difficulty to obtain a reverse process in the concavity [28–31]. The precocity, the length of time the tether is in place and the vertebral dysplasia are surely involved in the deformity persistence. The threshold values of this “vicious cycle” are not determined yet. Further studies will be necessary to study the potential of growth modulation and to improve our knowledge in the structures responsible for growth and spinal mobility. Factors such as time of initial constrain; muscular actions and gravity loads; amount of disk and vertebral wedging; histological and biochemical changes will need to be studied to determine the “functional activity zone” of these structures. The fibrous thoracic healing process could be also a limiting factor for correction. Granted our results are based only upon four specimens, however, the results are promising. Initial curve regression during the first month is probably mainly explained by the disk viscoelasticity and corresponded to the ratio between vertebral wedging and disk wedging. After the first month, the curve correction could be modulated by the gravity loads: i.e. when gravity forces are acting (on the anterior-posterior plane in quadrupeds), the possibility of correction is minimised and the curve is maintained. The curve correction observed is quiet regular, continuous and decreases with time. The more important vertebral wedging were less corrected. The information extracted from this preliminary study could be the basis of early stage treatment in children.
Conclusion
This surgical procedure using only a spinal tether creates a structural spine and chest wall deformity with a minimal morbidity and tissue alteration that is similar to a juvenile scoliosis in term of local and regional 3D deformity and size. Useful spinal and chest information in scoliosis process could be extracted from this model. This model, obtained only with a temporary spinal constrain, could be an interesting tool to study the growth process in scoliosis deformity.
Footnotes
The animal study was supported by a grant of Medtronic International, Switzerland.
References
- 1.Bridwell KH. Surgical treatment of idiopathic adolescent scoliosis. Spine. 1999;24:2607–2616. doi: 10.1097/00007632-199912150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Cochran T, Irstam L, Nachemson A. Long-term anatomic and functional changes in patients with adolescent idiopathic scoliosis treated by Harrington rod fusion. Spine. 1983;8:576–584. doi: 10.1097/00007632-198309000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Dickson R. Early-onset idiopathic scoliosis. In: Weinstein S, editor. The pediatric spine: principles and practice. New York: Raven Press; 1994. [Google Scholar]
- 4.Roach J. Adolescent idiopathic scoliosis: non surgical treatment. In: Weinstein S, editor. The pediatric spine: principles and practice. New York: Raven Press; 1994. [Google Scholar]
- 5.Danielsson AJ, Wiklund I, Pehrsson K, et al. Health-related quality of life in patients with adolescent idiopathic scoliosis: a matched follow-up at least 20 years after treatment with brace or surgery. Eur Spine J. 2001;10:278–288. doi: 10.1007/s005860100309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canavese F, Dimeglio A, Volpatti D, et al. Dorsal arthrodesis of thoracic spine and effects on thorax growth in prepubertal New Zealand white rabbits. Spine. 2007;32:E443–E450. doi: 10.1097/BRS.0b013e3180bc2340. [DOI] [PubMed] [Google Scholar]
- 7.Charles YP, Dimeglio A, Marcoul M, et al. Influence of idiopathic scoliosis on three-dimensional thoracic growth. Spine. 2008;33:1209–1218. doi: 10.1097/BRS.0b013e3181715272. [DOI] [PubMed] [Google Scholar]
- 8.Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis. A study of mortality, causes of death, and symptoms. Spine. 1992;17:1091–1096. doi: 10.1097/00007632-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Thompson GH, Lenke LG, Akbarnia BA, et al. Early onset scoliosis: future directions. J Bone Joint Surg Am. 2007;89(Suppl 1):163–166. doi: 10.2106/JBJS.F.01513. [DOI] [PubMed] [Google Scholar]
- 10.Nachlas IW, Borden JN. The cure of experimental scoliosis by directed growth control. J Bone Joint Surg Am. 1951;33:24–34. [PubMed] [Google Scholar]
- 11.Langenskiold, Michelsson JE. Experimental progressive scoliosis in the rabbit. J Bone Joint Surg Br. 1961;43-B:116–120. doi: 10.1302/0301-620X.43B1.116. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S, Dave PK. Experimental scoliosis in monkeys. Acta Orthop Scand. 1985;56:43–46. doi: 10.3109/17453678508992978. [DOI] [PubMed] [Google Scholar]
- 13.Pal GP, Bhatt RH, Patel VS. Mechanism of production of experimental scoliosis in rabbits. Spine. 1991;16:137–142. [PubMed] [Google Scholar]
- 14.Sevastikoglou JA, Aaro S, Lindholm TS, et al (1978) Experimental scoliosis in growing rabbits by operations on the rib cage. Clin Orthop Relat Res:282-6 [PubMed]
- 15.Braun JT, Ogilvie JW, Akyuz E, et al. Experimental scoliosis in an immature goat model: a method that creates idiopathic-type deformity with minimal violation of the spinal elements along the curve. Spine. 2003;28:2198–2203. doi: 10.1097/01.BRS.0000085095.37311.46. [DOI] [PubMed] [Google Scholar]
- 16.Schwab F, Patel A, Lafage V, Patel A, Farcy JP. A porcine model for progressive thoracic scoliosis. Spine. 2009;34(11):E397–E404. doi: 10.1097/BRS.0b013e3181a27156. [DOI] [PubMed] [Google Scholar]
- 17.Pomero V, Mitton D, Laporte S, et al. Fast accurate stereoradiographic 3D-reconstruction of the spine using a combined geometric and statistic model. Clin Biomech. 2004;19:240–247. doi: 10.1016/j.clinbiomech.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Newton PO, Faro FD, Farnsworth CL, Shapiro GS, Mohamad F, Parent S, Fricka K. Multilevel spinal growth modulation with an anterolateral flexible tether in an immature bovine model. Spine. 2005;30:2608–2613. doi: 10.1097/01.brs.0000188267.66847.bf. [DOI] [PubMed] [Google Scholar]
- 19.Newton PO, Upsani VV, et al. Spinal growth modulationwith the use of a tether in an immature porcine model. J Bone Joint Surg Am. 2008;90(12):2695–2706. doi: 10.2106/JBJS.G.01424. [DOI] [PubMed] [Google Scholar]
- 20.Campbell RM,, Jr, Smith MD, Hell-Vocke AK. Expansion thoracoplasty: the surgical technique of opening-wedge thoracostomy. Surgical technique. J Bone Joint Surg Am. 2004;86-A(Suppl 1):51–64. [PubMed] [Google Scholar]
- 21.Gollogly S, Smith JT, Campbell RM. Determining lung volume with three-dimensional reconstructions of CT scan data: A pilot study to evaluate the effects of expansion thoracoplasty on children with severe spinal deformities. J Pediatr Orthop. 2004;24:323–328. doi: 10.1097/01241398-200405000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Betz RR, Kim J, D’Andrea LP, et al. An innovative technique of vertebral body stapling for the treatment of patients with adolescent idiopathic scoliosis: a feasibility, safety, and utility study. Spine. 2003;28:S255–S265. doi: 10.1097/01.BRS.0000092484.31316.32. [DOI] [PubMed] [Google Scholar]
- 23.Braun JT, Akyuz E, Ogilvie JW. The use of animal models in fusionless scoliosis investigations. Spine. 2005;30:S35–S45. doi: 10.1097/01.brs.0000175187.61474.9a. [DOI] [PubMed] [Google Scholar]
- 24.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disk disorders/degeneration? Eur Spine J. 2008;17(1):2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun JT, Ogilvie JW, Akyuz E, et al. Fusionless scoliosis correction using a shape memory alloy staple in the anterior thoracic spine of the immature goat. Spine. 2004;29:1980–1989. doi: 10.1097/01.brs.0000138278.41431.72. [DOI] [PubMed] [Google Scholar]
- 26.Viguier E. Création d’un modèle de scoliose chez le mouton utilisant un système de ligamentoplastie asymétrique. Congrès Fondation Avenir. Paris: Personal communication; 2002. [Google Scholar]
- 27.Zhang Y, Zheng G, Wang Y, et al. Scoliosis model created by pedicle scerw tethering in immature goats: the feasability, reliability and complications. Spine. 2009;34(21):2305–2310. doi: 10.1097/BRS.0b013e3181b1fdd0. [DOI] [PubMed] [Google Scholar]
- 28.Patel A, Schwab F, Lafage R, Lafage V, Farcy JP (2011) Does removing the spinal tether in a porcine model result in persistent deformity?. Clin Orthop Relat Res s11999-010-170-5 [DOI] [PMC free article] [PubMed]
- 29.Newton PO, Farnsworth C, Upasani V, et al. Effects of intraoperative tensioning of an anterolateral spinal tether on spinal growth modulation in a porcine model. Spine. 2011;36(2):109–117. doi: 10.1097/BRS.0b013e3181cc8fce. [DOI] [PubMed] [Google Scholar]
- 30.Odent T, Cachon T, Peultier B et al. (2008) Porcine scoliosis model based on animal growth created with minimal invasive off-set tethering 43rd Scoliosis Research Society Annual meeting Salt lake City Utah USA
- 31.Stokes IA. Analysis and simulation of progressive adolescent scoliosis by biomechanical growth modulation Eur. Spine J. 2007;16:1621–1628. doi: 10.1007/s00586-007-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]