Abstract
Introduction
Chronic pain has an impact on psychological and social factors. It is known that stress influences physiological and behavioral changes and affects several neurotransmitter and hormonal systems. It is also known that corticosterone is increased by stress. The role of chronic stress in sciatica in lumbar disc herniation (LDH) in rats has not been investigated. The aim of this study was to investigate the effect of the restraint stress (RS) on pain-related behavior induced by application of nucleus pulposus (NP) in rats.
Materials and methods
Adult female Sprague–Dawley rats were divided into six experimental groups (naive group; naive + RS; sham group; sham + RS; autologous nucleus pulposus [NP] applied on the left L5 nerve root [NP group]; and NP + RS group). Von Frey tests were used to test pain-related behavior. Concentrations of plasma corticosterone were measured to assess changes in levels of endogenous corticosterone caused by RS. Expression of ATF-3 in the left L5 DRG was examined by immunohistochemical analyses in each group.
Results
Mechanical withdrawal thresholds of the NP and NP + RS groups were significantly decreased after surgery compared with the naive group. Although the thresholds in the NP group recovered after 28 days, the thresholds in the NP + RS group were significantly decreased during the 42 days after surgery. RS increased the concentration of plasma corticosterone at 21 and 42 days after surgery. In the NP and the NP + RS groups, the expression of ATF-3 was significantly increased at 7 days after surgery. The expression of ATF-3 was sustained for 21 days by RS.
Conclusion
Concentrations of plasma corticosterone were increased in three groups that underwent RS. The pain-related behavior persisted for the long term in the LDH model. The expression of ATF-3 in DRG neurons increased for 21 days by RS. These results suggest that RS plays a role in the chronicity of pain-related behavior in the LDH rats.
Keywords: Repeated restraint stress, Corticosterone, Pain-related behavior, Nucleus pulposus, Lumbar disc herniation
Introduction
Lumbar disc herniation (LDH) is a major cause of sciatica. The herniated disc induces sciatica by both mechanical and chemical means [17, 18, 25, 32–34]. Mechanical factors include compression of the nerve root and dorsal root ganglion (DRG) by the herniated disc [44, 45, 49]. Chemical irritation is caused by inflammatory mediators such as nucleus pulposus (NP) [27, 36, 37, 39, 40, 42], interleukin-1β, interleukin-6, 5-hydroxytryptamine, and tumor necrosis factor-alpha induced by herniated NP [2, 14, 16, 23, 34, 38]. Pain after injury to the nervous system (neuropathic pain) is a major chronic condition that remains difficult to treat. Chronic pain affects both psychological and social factors [13, 31]. The pain threshold decreases as a result of stress [47]. Thus, pain stemming from illness can be mitigated by reducing psychological stress [12]. In addition, the concentration of glucocorticoids in the blood increases in patients with chronic pain [24, 28]. Because psychosocial stress is often endured with these conditions, and clinical observations suggest that stress increases susceptibility to developing pain and exacerbates existing pain, it is important to understand how stress affects the development and severity of neuropathic pain [6, 11, 31, 41, 46]. Clinical studies of the role of stress in the pathogenesis of chronic pain syndromes have implicated the hypothalamo-pituitary-adrenal axis [4, 29]. When an organism is exposed to stress, information about the stressful situation will reach an array of brain regions, including parts of the limbic system and areas involved in sensory processing. The output from these areas funnels through the nucleus paraventricularis of the hypothalamus, where it can give rise to activation of two hormone systems, that is, the rapid sympatho-adrenomedullar system and the slow-acting hypothalamo-pituitary-adrenal system. Activation of these systems leads to increased levels of adrenaline and corticosterone, respectively. Thus, increased plasma concentrations of corticosterone are a reflection of stress [15].
Although chronic stress might be regarded as an important factor of chronicity of pain in LDH, the influence of chronic stress in pain is unclear. The aim of this study was to investigate the effect of the restraint stress (RS) on pain-related behavior induced by the application of NP in rats. We also examined the release of endogenous corticosterone in plasma and the expression of activating transcription factor-3 (ATF-3) in DRG in a rat model of LDH.
Materials and methods
The experiment was carried out under the control of the Animal Care and Use Committee in accordance with the Guidelines for Animal Experiments of our institution and the Japanese Government Law Concerning the Protection and Control of Animals.
Animals and surgical procedure
A total of 210 adult female Sprague–Dawley rats (Japan SLC, Shizuoka, Japan) weighing 190–230 g were used. Animals were housed in plastic cages with free access to food and water. Rats were maintained under conditions of constant temperature (24 ± 2°C) and humidity (55 ± 15%) with a 12 h light–dark cycle (lights on at 7 h) for 7–8 days before starting the experiment.
Animals were anesthetized by intraperitoneal injection of 30 mg/kg sodium pentobarbital (Nembutal 50 mg/m; Abbott Laboratories, North Chicago, IL). Rats were placed in a prone position and an incision was made at the spinal midline at level L4–L6. Using a surgical microscope, the thoracolumbar fascia was incised along the left side of the supraspinous ligament for approximately 20 mm. The paraspinalis muscles were gently moved laterally to expose the left L5–L6 facet joint. The L5 nerve root, DRG, and spinal nerve were exposed by L5/6 partial laminectomy with great care taken to avoid trauma to tissue. In the NP group, autologous NP was harvested from the dorsal part of the tail. The harvested NP was 3 μL, and the mean weight of NP was 2.2 ± 0.3 mg (n = 5). NP was applied to the DRG and nerve root. Animals in the sham group underwent the same surgical procedure except for harvesting NP and application of autologous NP to the DRG.
The paraspinalis muscles of all rats were sutured and the skin was closed with metal clips. In the naive group, rats did not undergo surgery.
The surgical wound and physical condition of each animal was checked every day during the postoperative period. Postoperative analgesics were not used due to concerns about affecting the results.
Experimental groups
Rats were divided into six groups: NP group, NP + RS group, sham group, sham + RS group, naive group, and naive + RS group. RS commenced on the first day of the experiment. Rats receiving RS were placed individually into a wire mesh restrainer [26] (10 cm internal diameter, 20 cm length) for 6 h (08:00–14:00) daily from 1 day to 42 days after surgery. During RS, rats had the minimum amount of space in their restrainers to allow them to alter their posture. Rats that did not receive RS remained in the home cage.
Behavioral testing
Behavioral tests were performed in all groups (n = 11 in each group) during the day portion of the circadian cycle (16:00–18:00). All behavioral tests were performed by a technician who was unaware of the experimental groupings. Sensitivity to non-noxious mechanical stimuli was tested by the von Frey test. Baseline testing was performed 2 days before starting the experiment to accommodate animals with normal responses.
The hind paw withdrawal response to von Frey hair (North Coast Medical, Inc., Morgan Hill, CA) stimulation of the plantar surface of the footpads was determined at 1, 7, 14, 21, 28, 35, and 42 days after starting the experiment. The rats were placed individually into an acrylic cage with a mesh floor and allowed to acclimate for 15 min, until cage exploration and major grooming activities ceased. The lateral-plantar surface of the left hind paw, innervated by the L5 nerve [43], was stimulated with nine von Frey filaments (1.0, 1.4, 2.0, 4.0, 6.0, 8.0, 10.0, 15.0, and 26.0 g) threaded under the mesh floor. The grams for von Frey hairs were based on the manufacturer’s ratings. Stimulation was initiated with the 1.0-g filament. The filament was sequentially applied to the paw surface just until the filament bent, and was held for approximately 3 s. The response was considered positive if the hind limb indicated a lifting foot coupled with either licking or shaking of the foot as an escape response.
Determination of plasma corticosterone concentrations
Radioimmunoassay (RIA) measurements of plasma were performed in all groups at 21 and 42 days after surgery (n = 9 in each group). Because concentrations of plasma corticosterone have a circadian rhythm [9], blood samples were collected at 14:00–15:00 to avoid the influence of the circadian rhythm. Rats were anesthetized using 99% diethyl ether (Wako Pure Chemical Industries, Osaka, Japan). Before rats were killed by decapitation, blood samples (4.0 mL) were collected by heart puncture through a polyethylene tube (coated with heparin) and mixed with 1/10 volume of 1.5% disodium dihydrogen ethylenediamine tetracetate dehydrate (EDTA-2Na). To obtain platelet-poor plasma (PPP), samples were centrifuged at 3,000g for 10 min at room temperature. The supernatants (1,500 μL) were stored at −20°C until assayed. Corticosterone in PPP was measured by RIA. The lower detection limit of the method was 0.1 ng/mL.
Immunohistochemistry
Immunohistologic examinations were performed in all groups at 7, 14, 21, 28, and 42 days after starting the experiment (n = 5 at each time point for each group). Rats were anesthetized using 99% diethyl ether (Wako Pure Chemical Industries), and perfused with fresh 4% paraformaldehyde in 0.1% M phosphate-buffered saline (PBS), and the L5 DRG were removed. They were postfixed briefly in 4% paraformaldehyde and subsequently embedded in paraffin. Sections (6 μm) of DRGs were cut from each sample and placed on slides. Sections were deparaffinized with xylem and rehydrated with 100% ethanol. Nonspecific binding sites were blocked with 2% normal goat serum in PBS/Triton X-100 applied for 1 h at room temperature. Rabbit antibody to ATF-3 (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA) was applied for 2 h at room temperature. Sections were rinsed in PBS and incubated for 1 h at room temperature with goat anti-rabbit Alexa 488 (green) fluorescent antibody (1:200; Molecular Probes Inc., Eugene, OR). After rinsing, sections were mounted on microscope slides with VECTASHIELD® Mounting Medium with DAPI (H-1200, Vector, Burlingame, CA). DAPI (4′,6-diamidino-2-phenilindole) stains nuclei specifically, with little or no cytoplasmic labeling. Its blue fluorescence stands out in vivid contrast to green or red fluorescent probes of other structures. Fluorescent staining was analyzed using an Olympus Optical BX50 microscope equipped with imaging software (Axio Vision, Carl Zeiss, Gottingen, Germany). Two slices of each DRG were used to determine the numbers of ATF-3-immunoreactive (IR) neurons. The numbers of ATF-3- and DAPI-positive neurons were counted in each section. The percentage of ATF-3-positive cells in DAPI-positive neurons was calculated.
Statistical analysis
All data were reported as mean ± SD. Data of behavioral tests, comparisons of the plasma corticosterone levels, and immunohistologic examinations were analyzed using the Bonferroni test. P values less than 0.05 were considered significant.
Results
Behavioral tests
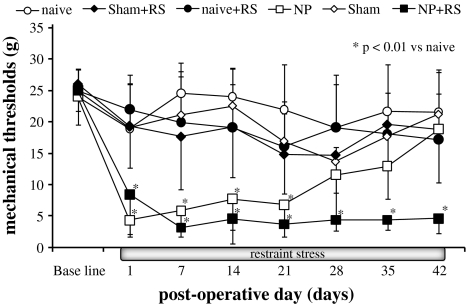
Rats in all groups showed stable conditions at baseline in response to mechanical stimulation. In the NP group, the mechanical withdrawal thresholds were significantly decreased for 21 days after surgery compared with the naive group (p < 0.01) (Fig. 1). In the NP + RS group, the mechanical withdrawal thresholds were significantly decreased for 42 days after surgery compared with the naive group (p < 0.01). There was a significant difference in withdrawal thresholds from day 28 to day 42 between the NP and NP + RS groups (p < 0.01). There were no significant differences of the thresholds among all groups except for the NP and NP + RS groups at 42 days.
Fig. 1.
Changes in mechanical withdrawal threshold of the footpad in rats. In the NP group, the mechanical withdrawal threshold was significantly decreased for 21 days after surgery compared with the naive group (p < 0.01). Significant differences between the NP + RS and naive groups can be observed for 42 days after surgery (p < 0.01). *p < 0.01 compared with the naive group
RIA analysis of plasma corticosterone
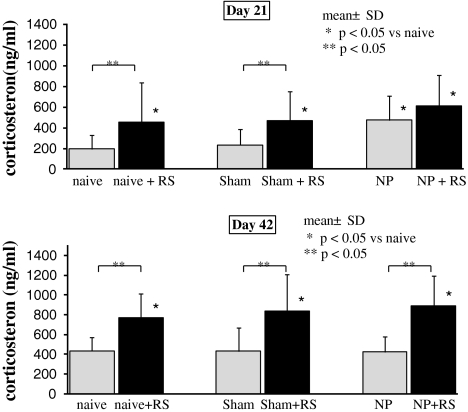
On day 21, concentrations of plasma corticosterone in the naive + RS, the sham + RS, the NP, and the NP + RS groups were significantly increased compared with the naive and sham groups (p < 0.05). There were no significant differences between the naive and sham groups (Fig. 2). Additionally, there were no significant differences between the NP and NP + RS groups. At 42 days after the start of the experiment, concentrations of plasma corticosterone in the three groups that underwent RS were significantly increased compared with the three groups that did not undergo RS (p < 0.05). Plasma corticosterone concentrations of the sham group and the NP group did not differ significantly from the naive group.
Fig. 2.
Plasma corticosterone concentrations. At 21 and 42 days after surgery, concentrations of plasma corticosterone in the three groups that underwent restraint stress (RS) were significantly increased compared with the naive group (p < 0.05). *p < 0.05 compared with the naive group. At 42 days, concentrations of plasma corticosterone in the three groups that underwent RS were significantly increased compared with the three groups that did not undergo RS (p < 0.05)
Immunohistochemical analysis of ATF-3
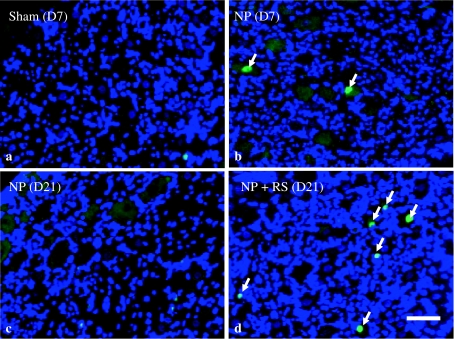
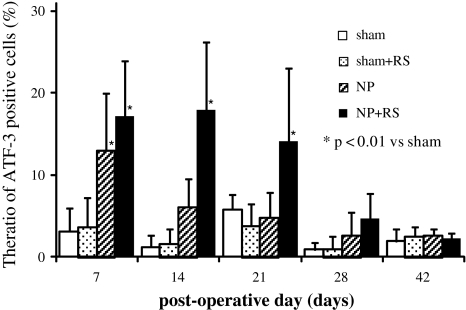
At 7 days after surgery, ATF-3-IR DRG neurons were observed in the NP and NP + RS groups. In contrast, there were few ATF-3-positive cells in the other four groups (Fig. 3). At 21 days after surgery, ATF-3-IR neurons were observed in the NP + RS group, but not in the NP group (Fig. 3). The ratio of ATF-3-IR neurons in the NP group was significantly increased compared with the sham and sham + RS groups at 7 days after surgery (p < 0.01). There were no differences of the ratio of ATF-3-IR cells among the NP, sham, and sham + RS groups from day 14 to day 42 (Fig. 4). In the NP + RS group, the ratio of ATF-3-IR neurons was significantly increased compared with the sham and sham + RS groups for 21 days after surgery (p < 0.01).
Fig. 3.
Immunofluorescence analysis of ATF-3 in the left L5 DRG. Arrows indicate ATF-3–positive DRG neurons (green). In the sham group, few ATF-3-immunoreactive cells were seen in the L5 DRG (a). In the NP group, some ATF-3-positive cells were seen in the L5 DRG at 7 days after surgery (b). At 21 days after surgery, there were few ATF-positive cells in the NP group (c), but some ATF-3-positive cells were seen in the NP + RS group (d). Scale bar 25 μm
Fig. 4.
The ratio of ATF-3-immunoreactive neurons to the total number of DRG neurons. In the NP + RS group, the ratio of ATF-3-positive cells was significantly increased during 21 days after surgery (p < 0.01). Data are mean ± SD (n = 5 for each group). *p < 0.01 compared with the sham group
Discussion
Rats subjected to RS alone showed no decrease in pain threshold, whereas NP rats subjected to RS showed a decrease in pain threshold that persisted for a long period. Plasma corticosterone concentrations increased in all groups that underwent RS. Plasma corticosterone concentrations also increased as a result of NP application, but this increase was temporary. In contrast, the increase in plasma corticosterone concentrations persisted for a long period with continual RS.
Corticosterone molecules reach all tissues, including the brain, readily penetrate the cell membrane, and interact with ubiquitous cytoplasmic/nuclear glucocorticoid receptors (GRs). Peripheral GRs play a significant role in the anti-inflammatory effects of corticosterone, which are mediated mainly through interactions between GRs and intracellular elements such as activating protein-1 at the site of tissue inflammation [30]. On the other hand, corticosterone could have neurotoxic effects contributing to neuronal damage [8]. Spinal neuronal GRs contribute to the development of neuropathic pain behaviors after chronic constriction injury [48]. It thus appears that rats experienced stress as a result of being restrained. In the present experiment, increased plasma corticosterone concentrations persisted and the pain threshold decreased during the same period as a result of RS in rats that received application of NP.
Stress-induced activation of the hypothalamo-pituitary-adrenal and sympathoadrenal axes exacerbates pain by enhancing the pronociceptive effects of immune mediators produced in peripheral tissue [19].
Recent studies have shown that corticosterone exacerbates neurogenic pain via GRs and NMDA receptors [1]. GRs appear to be intimately involved in the transmission of pain, as they are distributed extensively across the central nervous system and are expressed abundantly in the posterior horn of the spinal cord [3, 7]. In addition, GR expression increases with nerve damage [48, 50]. It is therefore possible that nerve damage increases the effect on nerve cells of corticosterone originating from stress.
In this study, expression of ATF-3, a marker of nerve damage, became prolonged as a result of RS. This finding suggests the possibility that nerve disorders originating from nerve damage are prolonged by corticosterone, although the mechanism of corticosterone involvement in the nerve disorder is not clear. Administration of high concentrations of corticosterone during the acute phase of nerve damage is beneficial because corticosterone has anti-inflammatory and antiedematous actions on nerves [22]; however, acute phase administration of high concentrations of corticosterone were not investigated in the present study. These results imply that continued high plasma corticosterone concentrations play a major role in maintaining neurogenic pain during the chronic phase.
It has been reported that the pain threshold is reduced by stress in normal rats [5, 10, 20, 21]. We found no reduction in the pain threshold as a result of RS in either naive or sham rats, which had no damage to the nerves. However, reduction in the pain threshold persisted in rats in which neurogenic pain accompanying nerve damage was provoked by NP application. These results imply that stress can be a primary factor in pain-related changes becoming chronic.
The withdrawal threshold lasted longer after ATF-3 expression decreased in the NP with RS group. The nerve injury might be process for recovering at day 28, this time lag of ATF-3 expression and the reduction of threshold might show that a decrease of ATF-3 expression does not reflect the recovery of nerve function. Further studies are needed to investigate the effects on pain-associated substances such as substance P, CGRP, and cytokines, with and without RS. In addition, pain thresholds were examined for only 42 days during RS; however, pain-related changes over a longer period and after stopping RS were not investigated in the present study. In addition, this study focused on NP application as a chemical factor. Another nucleus pulposus application model included disc herniation without compression did not show allodynia [35]. On the other hand, the NP model in the present study showed allodynia [27, 37, 38, 40, 41, 43]. This model applied a consistent volume of NP and covered the DRG in each animal. Therefore, the two models are different and the disc incision model might have less of an influence on the nerve. I the model of this study, the same volume of NP was applied to the DRG, and has been done in other studies [27, 37, 38, 40, 41, 43]. Therefore, we consider our model to be that which investigates a chemical factor associated with disc herniation. Future studies should compare whether the impact of RS is affected by pain-related changes induced by mechanical factors alone, chemical factors alone, or both as well as study the mechanism of corticosterone involvement in pain-related changes resulting from the application of NP. If the mechanism through which stress and corticosterone accompanying stress lead to chronicity were clarified, stress itself and corticosterone could possibly become targets for chronic pain therapy.
Conclusion
Increased plasma corticosterone concentrations persisted as a result of RS in naive, sham, and NP animals. In addition, a reduced pain threshold persisted as a result of RS in rats that received application of NP. These results suggest that chronic stress may prolong pain-related behavior.
Acknowledgments
The authors thank Dr. Nobuyuki Sasaki, Dr. Hiroshi Kobayashi, Dr. Youhei Matsuo, Dr. Katsuhiro Yoshida, Mr. Akira Sato, and Ms. Rie Shibuya for expert technical support.
Conflict of interest None.
References
- 1.Alexander JK, DeVries AC, Kigerl KA, et al. Stress exacerbates neuropathic pain via glucocorticoid and NMDA receptor activation. Brain Behav Immun. 2009;23:851–860. doi: 10.1016/j.bbi.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki Y, Rydevik B, Kikuchi S, et al. Local application of disc-related cytokines on spinal nerve roots. Spine. 2002;27:1614–1617. doi: 10.1097/00007632-200208010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Cintra A, Molander C, Fuxe K. Colocalization of Fos- and glucocorticoid receptor-immunoreactivities is present only in a very restricted population of dorsal horn neurons of the rat spinal cord after nociceptive stimulation. Brain Res. 1993;632:334–338. doi: 10.1016/0006-8993(93)91172-O. [DOI] [PubMed] [Google Scholar]
- 4.Crofford LJ, Engleberg NC, Demitrack MA. Neurohormonal perturbations in fibromyalgia. Baillieres Clin Rheumatol. 1996;10:365–378. doi: 10.1016/S0950-3579(96)80022-7. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Torres IL, Cucco SNS, Bassani M, et al. Long-lasting delayed hyperalgesia after chronic restraint stress in rats-effect of morphine administration. Neurosci Res (NY) 2003;45:277–283. doi: 10.1016/S0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- 6.DeLeo JA. Basic science of pain. J Bone Joint Surg Am. 2006;88:58–62. doi: 10.2106/JBJS.E.01286. [DOI] [PubMed] [Google Scholar]
- 7.Nicola AF, Moses DF, Gonzalez S, et al. Adrenocorticoid action in the spinal cord: some unique molecular properties of glucocorticoid receptors. Cell Mol Neurobiol. 1989;9:179–192. doi: 10.1007/BF00713027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuxe K, Diaz R, Cintra A, et al. On the role of glucocorticoid receptors in brain plasticity. Cell Mol Neurobiol. 1996;16:239–258. doi: 10.1007/BF02088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo PV, Weinberg J. Corticosterone rhythmicity in the rat: interactive effects of dietary restriction and schedule of feeding. J Nutr. 1981;111:208–218. doi: 10.1093/jn/111.2.208. [DOI] [PubMed] [Google Scholar]
- 10.Gamaro GD, Xavier MH, Denardin JD, et al. The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol Behav. 1998;63:693–697. doi: 10.1016/S0031-9384(97)00520-9. [DOI] [PubMed] [Google Scholar]
- 11.Gold SM, Mohr DC, Huitinga I, et al. The role of stress-response systems for the pathogenesis and progression of MS. Trends Immunol. 2005;26:644–652. doi: 10.1016/j.it.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum. 2004;51:625–634. doi: 10.1002/art.20533. [DOI] [PubMed] [Google Scholar]
- 13.Hasenbring M, Marienfeld G, Kuhlendahl D, et al. Risk factors of chronicity in lumbar disc patients. A prospective investigation of biologic, psychologic, and social predictors of therapy outcome. Spine. 1994;19:2759–2765. doi: 10.1097/00007632-199412150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi T, Kikuchi S, Shubayev V, et al. 2000 Volvo Award winner in basic science studies: exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology. Molecular, histologic, and behavioral comparisons in rats. Spine. 2000;25:2975–2980. doi: 10.1097/00007632-200012010-00003. [DOI] [PubMed] [Google Scholar]
- 15.Joels M, Krugers HJ. LTP after stress: up or down? Neural Plast. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato K, Kikuchi S, Konno S, Sekiguchi M. Participation of 5-hydroxytryptamine in pain-related behavior induced by nucleus pulposus applied on the nerve root in rats. Spine. 2008;3(12):1330–1336. doi: 10.1097/BRS.0b013e318173298b. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami M, Tamaki T, Weinstein JN, et al. Pathomechanism of pain-related behavior produced by allografts of intervertebral disc in the rat. Spine. 1996;21:2101–2107. doi: 10.1097/00007632-199609150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kayama S, Konno S, Olmarker K, et al. Incision of the anulus fibrosus induces nerve root morphologic, vascular, and functional changes. An experimental study. Spine. 1996;21:2539–2543. doi: 10.1097/00007632-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 19.Khasar SG, Burkham J, Dina OA, et al. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28:5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasar SG, Dina OA, Green PG, et al. Sound stress-induced long-term enhancement of mechanical hyperalgesia in rats is maintained by sympathoadrenal catecholamines. J Pain. 2009;10:1073–1077. doi: 10.1016/j.jpain.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khasar SG, Green PG, Levine JD. Repeated sound stress enhances inflammatory pain in the rat. Pain. 2005;116:79–86. doi: 10.1016/j.pain.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Kingery WS, Guo T, Agashe GS, et al. Glucocorticoid inhibition of neuropathic limb edema and cutaneous neurogenic extravasation. Brain Res. 2001;913:140–148. doi: 10.1016/S0006-8993(01)02763-9. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Kato K, Kikuchi S, Konno S, Sekiguchi M. Interactions of 5-hydroxytryptamine and tumor necrosis factor-α to pain-related behavior by mucleus pulposus applied on the nerve root in rats. Spine. 2011;36(3):210–218. doi: 10.1097/BRS.0b013e3181fea618. [DOI] [PubMed] [Google Scholar]
- 24.Lephart ED, Galindo E, Bu LH. Stress (hypothalamic-pituitary-adrenal axis) and pain response in male rats exposed lifelong to high versus low phytoestrogen diets. Neurosci Lett. 2003;342:65–68. doi: 10.1016/S0304-3940(03)00262-3. [DOI] [PubMed] [Google Scholar]
- 25.McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low-back pain. Spine. 1987;12:760–764. doi: 10.1097/00007632-198710000-00009. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin KJ, Gomez JL, Baran SE, et al. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyoshi S, Sekiguchi M, Konno S, Kikuchi S, Kanaya F. Increased expression of vascular endothelial growth factor protein in dorsal root ganglion exposed to nucleus pulposus on the nerve root in rats. Spine. 2011;36(1):E1–E6. doi: 10.1097/BRS.0b013e31820240d8. [DOI] [PubMed] [Google Scholar]
- 28.Moore RA, Evans PJ, Smith RF, et al. Increased cortisol excretion in chronic pain. Anaesthesia. 1983;38:788–791. doi: 10.1111/j.1365-2044.1983.tb13947.x. [DOI] [PubMed] [Google Scholar]
- 29.Neeck G, Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am. 2000;26:989–1002. doi: 10.1016/S0889-857X(05)70180-0. [DOI] [PubMed] [Google Scholar]
- 30.Neeck G, Renkawitz R, Eggert M. Molecular aspects of glucocorticoid hormone action in rheumatoid arthritis. Cytokines Cell Mol Ther. 2002;7:61–69. doi: 10.1080/13684730412331302081. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson K, Martelli MF. The problem of pain. J Head Trauma Rehabil. 2004;19:2–9. doi: 10.1097/00001199-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Olmarker K, Brisby H, Yabuki S, et al. The effects of normal, frozen, and hyaluronidase-digested nucleus pulposus on nerve root structure and function. Spine. 1997;22:471–475. doi: 10.1097/00007632-199703010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Olmarker K, Rydevik B, Nordborg C. Autologous nucleus pulposus induces neurophysiologic and histologic changes in porcine cauda equina nerve roots. Spine. 1993;18:1425–1432. [PubMed] [Google Scholar]
- 34.Olmarker K, Larsson K. Tumor necrosis factor alpha and nucleus-pulposus-induced nerve root injury. Spine. 1998;23:2538–2544. doi: 10.1097/00007632-199812010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Otani K, Arai I, Mao GP, et al. Experimental disc herniation: evaluation of the natural course. Spine. 1997;22:2894–2899. doi: 10.1097/00007632-199712150-00012. [DOI] [PubMed] [Google Scholar]
- 36.Otoshi K, Kikuchi S, Konno S, Sekiguchi M (2011) Anti-HIMGB1 nurtralization antibody improves pain-related behavior induced by application of anutologous nucleus pulposus onto nerve roots in rats. Spine [Epub ahead of print] [DOI] [PubMed]
- 37.Otoshi K, Kikuchi S, Konno S, Sekiguchi M. The reactions of glial cells and endoneurial macrophages in the dorsal root. Ganglion and their contribution to pain-related behavior after application of nucleus pulposus onto the nerve root in rats. Spine. 2010;35(1):10–17. doi: 10.1097/BRS.0b013e3181c67f1e. [DOI] [PubMed] [Google Scholar]
- 38.Rand N, Reichert F, Floman Y, et al. Murine nucleus pulposus-derived cells secrete interleukins-1-beta, -6, and -10 and granulocyte-macrophage colony-stimulating factor in cell culture. Spine. 1997;22:2598–2601. doi: 10.1097/00007632-199711150-00002. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki N, Sekiguchi M, Kilichi S, Konno S. Effects of asialo erhythropoietin on pain-related behavior and expression of phosphorylated p38 MAP kinase and tumor necrosis factor-alpha induced by application of aurologous nuxleus pulposus on nerve root in rat. Spine. 2011;36(2):E86–E94. doi: 10.1097/BRS.0b013e3181f137a8. [DOI] [PubMed] [Google Scholar]
- 40.Sekiguchi M, Otoshi K, Kikuchi S, and Konno S (2011) Analgesic effects of prostaglandin E2 receptor subtype EP1 receptor antagonis––experimental study of application of nucleus pulposus. Spine [Epub ahead of print] [DOI] [PubMed]
- 41.Strang P. Cancer pain––a provoker of emotional, social and existential distress. Acta Oncol. 1998;37:641–644. doi: 10.1080/028418698429973. [DOI] [PubMed] [Google Scholar]
- 42.Tachihara H, Sekiguchi M, Kikuchi S, Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation? Spine. 2008;33(7):743–747. doi: 10.1097/BRS.0b013e3181696132. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 44.Takayama B, Sekiguchi M, Yabuki S, Kikuchi S, Konno S. Localization and function of insulin-like growth factor 1 in dorsal root ganglia in a rat disc herniation model. Spine. 2011;36(2):E75–E79. doi: 10.1097/BRS.0b013e3181d56208. [DOI] [PubMed] [Google Scholar]
- 45.Takayama B, Sekiguchi M, yabuki S, Fujita I, Shimada H, Kikuchi S. Gene expression changes in dorsal root ganglion of rat experimental lumber disc herniation models. Spine. 2008;33(17):1829–1835. doi: 10.1097/BRS.0b013e3181801d9a. [DOI] [PubMed] [Google Scholar]
- 46.Turner JA, Jensen MP, Warms CA, et al. Catastrophizing is associated with pain intensity, psychological distress, and pain-related disability among individuals with chronic pain after spinal cord injury. Pain. 2002;98:127–134. doi: 10.1016/S0304-3959(02)00045-3. [DOI] [PubMed] [Google Scholar]
- 47.Vershinina EA. Pain sensitivity in chronic psychoemotional stress in humans. Neurosci Behav Physiol. 1999;29:333–337. doi: 10.1007/BF02465346. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Lim G, Zeng Q, et al. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe K, Konno S, Sekiguchi M, Sasaki N, Honda Y, Kikuchi S. Increase of 200-kDa nuerofilament-immunoreactive afferents in the substantia gelatinosa in allodynia rats induced by compression of the dorsal root ganglion. Spine. 2007;32:1265–1271. doi: 10.1097/BRS.0b013e318059aef8. [DOI] [PubMed] [Google Scholar]
- 50.Yan P, Xu J, Li Q, et al. Glucocorticoid receptor expression in the spinal cord after traumatic injury in adult rats. J Neurosci. 1999;19:9355–9363. doi: 10.1523/JNEUROSCI.19-21-09355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]