Summary
Background
The increasing prevalence of overweight and obesity needs effective approaches for weight loss in primary care and community settings. We compared weight loss with standard treatment in primary care with that achieved after referral by the primary care team to a commercial provider in the community.
Methods
In this parallel group, non-blinded, randomised controlled trial, 772 overweight and obese adults were recruited by primary care practices in Australia, Germany, and the UK. Participants were randomly assigned with a computer-generated simple randomisation sequence to receive either 12 months of standard care as defined by national treatment guidelines, or 12 months of free membership to a commercial programme (Weight Watchers), and followed up for 12 months. The primary outcome was weight change over 12 months. Analysis was by intention to treat (last observation carried forward [LOCF] and baseline observation carried forward [BOCF]) and in the population who completed the 12-month assessment. This trial is registered, number ISRCTN85485463.
Findings
377 participants were assigned to the commercial programme, of whom 230 (61%) completed the 12-month assessment; and 395 were assigned to standard care, of whom 214 (54%) completed the 12-month assessment. In all analyses, participants in the commercial programme group lost twice as much weight as did those in the standard care group. Mean weight change at 12 months was −5·06 kg (SE 0·31) for those in the commercial programme versus −2·25 kg (0·21) for those receiving standard care (adjusted difference −2·77 kg, 95% CI −3·50 to −2·03) with LOCF; −4·06 kg (0·31) versus −1·77 kg (0·19; adjusted difference −2·29 kg, −2·99 to −1·58) with BOCF; and −6·65 kg (0·43) versus −3·26 kg (0·33; adjusted difference −3·16 kg, −4·23 to −2·11) for those who completed the 12-month assessment. Participants reported no adverse events related to trial participation.
Interpretation
Referral by a primary health-care professional to a commercial weight loss programme that provides regular weighing, advice about diet and physical activity, motivation, and group support can offer a clinically useful early intervention for weight management in overweight and obese people that can be delivered at large scale.
Funding
Weight Watchers International, through a grant to the UK Medical Research Council.
Introduction
Obesity is a global health problem, with an estimated 1 billion people worldwide overweight and more than 300 million obese.1 Excess weight accounts for 44% of the global burden of diabetes, 23% of ischaemic heart disease, and 7–41% of some cancers.1 Weight loss of 5–10% is associated with clinically significant health benefits, including a reduction in risk factors for diabetes and cardiovascular disease.2,3 Several interventions result in weight loss of 5–10%,4 but few can be delivered on a large scale. Effective interventions to treat this problem in primary care or community settings are urgently needed.
Partnerships between primary care and commercial organisations have the potential to deliver weight management programmes on a large scale and at fairly low cost. Observational data lend support to the use of such an approach.5,6 However, few randomised controlled trials of commercial weight loss programmes have been done, and most assess self-selected participants or make comparisons with other self-help approaches.7–11 The efficacy of commercial weight loss programmes has not been assessed in direct comparison with standard care in a primary health-care setting, with participants identified by the primary care provider.
We compared the clinical efficacy of primary care referral to a commercial programme with standard care by examination of the change in weight and associated risk factors at 12 months in overweight and obese adults.
Methods
Study design and participants
We undertook a multicentre, randomised controlled trial with a parallel design. Participants were recruited from 39 primary care practices in Germany, 70 practices in Australia, and six practices in the UK between Sept 10, 2007, and Nov 28, 2008. People were screened for eligibility by a primary care provider in the UK, or first by the primary care provider and then by a member of the research team in Australia and Germany. Numbers of preliminary screenings in Germany were not recorded.
For more about the trial protocol see http://www.mrc-hnr.cam.ac.uk/communications/scienceunderthespotlight/primary-care-referral-protocol.html
Eligible participants were adults (aged ≥18 years) with a body-mass index (BMI) of 27–35 kg/m2 who had at least one additional risk factor for obesity-related disease. Risk factors included central adiposity (waist circumference >88 cm in women or >102 cm in men); type 2 diabetes without insulin treatment; family history of diabetes; previous gestational diabetes; impaired glucose tolerance or impaired fasting glycaemia, mild to moderate dyslipidaemia (defined by national guidelines), or treatment for dyslipidaemia; treatment for hypertension; polycystic ovarian syndrome or infertility without apparent cause other than weight; lower-limb osteoarthritis; or abdominal hernia. People were excluded if they met any of the following criteria: weight loss of 5 kg or more in the previous 3 months; history of a clinically diagnosed eating disorder; orthopaedic limitations preventing participation in regular physical activity; untreated thyroid disease or more than one change in thyroid treatment in the previous 6 months; receiving treatment with effects on weight or appetite; gastrointestinal disorders; previous surgical procedure for weight loss; major surgery in the previous 3 months; pregnancy or lactation; insulin-treated diabetes; diabetes diagnosis in the previous 6 months; glycated haemoglobin (HbA1c) of at least 75 mmol/mol (9·0%); heart problems in the previous 3 months; uncontrolled hypertension; new prescription drug for a chronic disorder in the previous 3 months or change in dose in the previous 1 month; history or presence of cancer, with the exception of completely resected basal or squamous cell carcinoma if treatment completed 6 months before enrolment or if treatment was stable; or participation in another clinical trial in the previous 30 days.
This study received ethics approval from Nottingham Research Ethics Committee (UK), the ethical committee of the Faculty of Medicine of the Technische Universität München (Germany), and the ethics review committee (Royal Prince Alfred Hospital zone) of the Sydney South West Area Health Service (Australia). All patients provided written informed consent.
Randomisation and masking
The randomisation sequence was computer generated with Stata (version 9.0) by APM and built into the database by the data manager, who was independent from the study team, and was stratified by country, sex, and diabetes status, with an upper limit of 50% of participants with diabetes. Participants were allocated in a 1:1 ratio to receive 12 months of free access to a commercial programme or 12 months of standard care, as defined by national treatment guidelines in the three participating countries. Treatment allocation was concealed by use of an online database (Filemaker Pro 9, version 3). Because of the nature of the intervention and the primary care setting, participants and people assessing outcome measures were not masked to treatment assignments.
Procedures
Participants in the commercial programme group received free access to weekly community-based Weight Watchers meetings for 12 months. They were requested not to mention their participation in the study to the group leader or other attendees. This commercial programme promotes a hypoenergetic, balanced diet based on healthy-eating principles, increased physical activity, and group support. Weight loss goals are self-selected with input from the group leader, and participants are encouraged to attend weekly meetings for a weigh-in and group discussion, behavioural counselling, and motivation. Participants were able to access internet-based systems to monitor their food intake, activity, and weight change; to participate in community discussion boards; and to access a library of information, recipes, and meal ideas.
Participants in the standard care group received weight loss advice from a primary care professional at their local general practitioner (GP) practice. Professionals delivering this intervention were provided with, and encouraged to use, Australian, German, and UK national clinical guidelines for treatment, and were made aware of information providing advice about weight loss.
For Australian guidelines for obesity treatment see http://www.health.gov.au/internet/main/publishing.nsf/Content/obesityguidelines-index.htm
For German guidelines for obesity treatment see http://www.dge.de
For UK guidelines for obesity treatment see http://www.nice.org.uk/CG043
Bodyweight, height, fat mass, waist circumference, and blood pressure were measured at baseline, and at 2, 4, 6, 9, and 12 months. Clinical measurements were recorded at GP practices in the UK and at the research centre in Australia. In Germany, all measurements were recorded at GP practices except for fat mass, which was measured at the research centre.
In the UK and Australia, bodyweight (in light clothes without shoes) and fat mass were measured with a Tanita BC-418 segmental body composition analyser (Tanita Corporation of America, Arlington Heights, IL, USA). In Germany, weight was measured in GP practices with standard scales, and fat mass was measured at the research centre with the Tanita BC-418. Systolic and diastolic blood pressures were measured according to local standard operating procedures. At each assessment appointment, participants self-reported the number of appointments with their health-care provider or the number of commercial programme meetings that they had attended since the last assessment.
Fasting blood samples were taken to measure glucose, insulin, and lipid profile at baseline, and at 6 and 12 months at either a research centre (Australia), GP practice (Germany), or biochemistry department at a local hospital (UK). HbA1c was measured at baseline; in patients with diabetes and impaired glucose tolerance, HbA1c was also measured at 6 and 12 months. Biochemical analyses were done locally in each country according to standardised methods (webappendix p 1).
The primary outcome was weight change from baseline to 12 months. Secondary outcomes were changes in fat mass, waist circumference, blood pressure, and biomarkers of cardiovascular risk. Self-reported data were recorded for dietary intake (4-day food diary), eating behaviour (three factor eating questionnaire R-2112), physical activity (7-day pedometer record, international physical activity questionnaire13), and quality of life (impact of weight on quality of life-lite14), which will be analysed and published separately. Self-reported data for drug use will be used with additional data sources (including session attendance) in future cost-effectiveness analyses. DNA samples were obtained for genetic analyses, and these data are being analysed to examine the genetic variations that could modify the extent of weight loss and improvements in cardiovascular risk factors. The protocol for this study was amended to include follow-up assessments at 18 and 24 months, and results will be published after data analysis.
Statistical analysis
We calculated that 804 patients needed to be recruited in the three countries to have 90% power at a 5% significance level to detect a difference of 1·9 kg in the primary outcome between the treatment groups, assuming an SD of 8 kg. With allowance for a drop-out of 50% of patients, this sample size would be sufficient to detect a difference in weight change of 2·6 kg in an analysis of only patients who completed the study, with 90% power at a 5% significance level.
The primary outcome was analysed by intention to treat, including all randomised participants, with last observation carried forward (LOCF) for missing data. Weight change at 12 months was analysed by use of linear regression with fixed effects for continuous normal data; intervention group (commercial programme vs standard care), country (Australia, Germany, and the UK), and baseline measurement were used as the fixed effects. No country-by-treatment interactions were identified (p>0·10), so we have reported findings for the pooled analysis. Mean changes are presented as mean (SE), with 95% CIs unless otherwise specified.
To maximise the comparability of our data with that in other studies, weight change was also analysed by use of baseline observation carried forward (BOCF), and in the population who completed the 12-month assessment (completers only). These analyses used the same fixed effects model as in the LOCF analysis. 12-month changes in biomarkers of cardiovascular risk were analysed by the same regression-based methods. We also calculated the percentage of randomised participants and those completing the 12-month assessment who achieved at least 5% and at least 10% weight loss. Odds ratios for percentage weight change were analysed with logistic regression. All analyses were done with STATA (version 11.0).
This trial is registered, number ISRCTN85485463.
Role of the funding source
The commercial programme intervention was delivered by an employee of the sponsor, but the sponsor had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication.
Results
Figure 1 shows the trial profile. 1010 participants were screened for eligibility, of whom 134 were not eligible, 12 were screened after recruitment had ended, and 92 chose not to participate. The remaining 772 participants (268 Germany, 268 Australia, 236 UK) entered the trial and completed a baseline assessment (figure 1). Demographic and clinical characteristics of participants were well balanced across treatment groups (table 1), and country was the only factor used in adjusted analyses.
Figure 1.
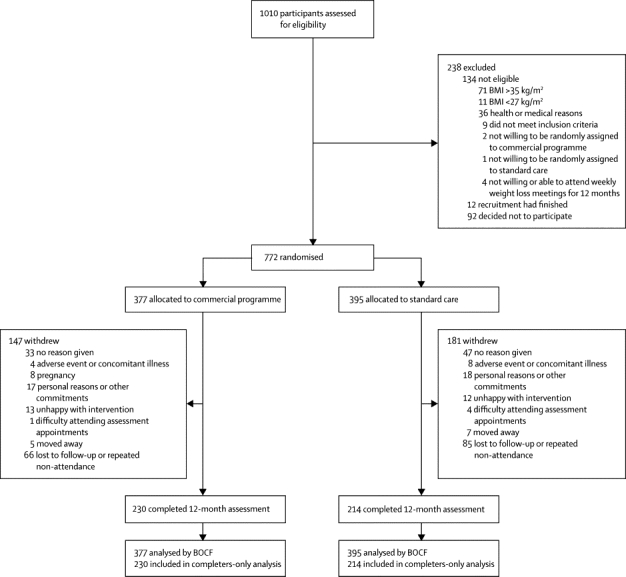
Trial profile
BMI=body-mass index. BOCF=baseline observation carried forward.
Table 1.
Baseline characteristics of participants according to treatment group
| Commercial programme (n=377) | Standard care (n=395) | ||
|---|---|---|---|
| Sex | |||
| Women | 330 (88%) | 338 (86%) | |
| Men | 47 (12%) | 57 (14%) | |
| Age (years) | 46·5 (13·5) | 48·2 (12·2) | |
| Weight (kg) | 86·9 (11·6) | 86·5 (11·5) | |
| Height (m) | 1·66 (0·1) | 1·66 (0·1) | |
| BMI (kg/m2) | 31·5 (2·6) | 31·3 (2·6) | |
| Fat mass (kg) | 33·3 (7·0) | 32·9 (7·4) | |
| Waist circumference (cm) | 100 (9·2) | 99·9 (9·3) | |
| Systolic blood pressure (mm Hg) | 124·7 (17·1) | 124·2 (14·7) | |
| Diastolic blood pressure (mm Hg) | 78·2 (9·8) | 79·1 (9·0) | |
| Type 2 diabetes* | 24 (6%) | 27 (7%) | |
Data are number (%) or mean (SD). BMI=body-mass index.
Defined by national guidelines.
In all analyses, both treatment groups lost weight but mean 12-month weight loss was significantly greater for participants in the commercial programme than for those in the standard care group (table 2). Figure 2 shows the weight trajectories of the two treatment groups with all measured weights at each timepoint.
Table 2.
Changes in clinical outcomes (mean; SE) between baseline and 12 months by treatment group (with analysis by LOCF, BOCF, and in those who completed the 12-month assessment)
| N | Commercial programme | Standard care | Adjusted difference (95% CI)* | p value | |
|---|---|---|---|---|---|
| Bodyweight (kg) | |||||
| LOCF | 772 | −5·06 (0·31) | −2·25 (0·21) | −2·77 (−3·50 to −2·03) | <0·0001 |
| BOCF | 772 | −4·06 (0·31) | −1·77 (0·19) | −2·29 (−2·99 to −1·58) | <0·0001 |
| Completers | 444 | −6·65 (0·43) | −3·26 (0·33) | −3·16 (−4·23 to −2·11) | <0·0001 |
| Waist circumference (cm) | |||||
| LOCF | 760 | −5·60 (0·37) | −3·16 (0·28) | −2·39 (−3·28 to −1·51) | <0·0001 |
| BOCF | 760 | −4·05 (0·35) | −2·34 (0·26) | −1·72 (−2·56 to −0·88) | 0·0001 |
| Completers | 429 | −6·86 (0·50) | −4·34 (0·43) | −2·36 (−3·65 to −1·08) | 0·0004 |
| Fat mass (kg) | |||||
| LOCF | 695 | −4·23 (0·28) | −1·85 (0·19) | −2·32 (−2·96 to −1·68) | <0·0001 |
| BOCF | 695 | −3·21 (0·27) | −1·34 (0·17) | −1·84 (−2·45 to −1·23) | <0·0001 |
| Completers | 397 | −5·36 (0·38) | −2·54 (0·30) | −2·52 (−3·45 to −1·60) | <0·0001 |
| Systolic blood pressure (mm Hg) | |||||
| LOCF | 771 | −2·37 (0·67) | −1·50 (0·64) | −0·71 (−2·35 to 0·92) | 0·39 |
| BOCF | 771 | −2·04 (0·56) | −0·96 (0·53) | −0·96 (−2·37 to 0·44) | 0·18 |
| Completers | 441 | −3·38 (0·92) | −1·77 (0·96) | −1·46 (−3·82 to 0·89) | 0·22 |
| Diastolic blood pressure (mm Hg) | |||||
| LOCF | 771 | −1·61 (0·44) | −1·29 (0·41) | −0·64 (−1·73 to 0·45) | 0·25 |
| BOCF | 771 | −1·41 (0·36) | −0·77 (0·35) | −0·88 (−1·82 to 0·05) | 0·07 |
| Completers | 441 | −2·34 (0·59) | −1·42 (0·65) | −1·40 (−2·95 to 0·15) | 0·08 |
LOCF=last observation carried forward. BOCF=baseline observation carried forward.
Adjusted for baseline observation and country.
Figure 2.
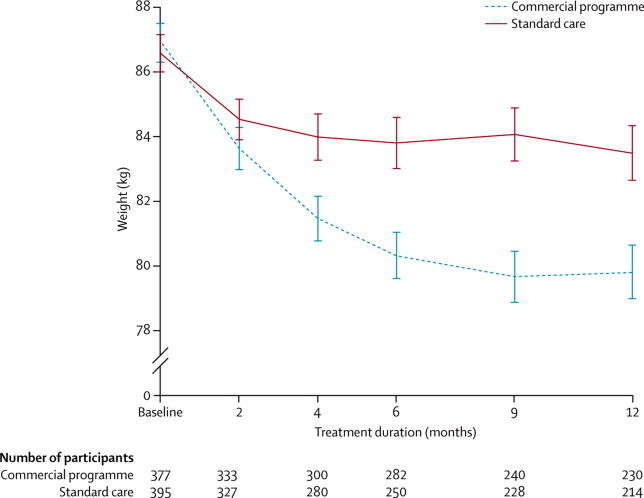
Change in weight during 12 months of treatment
Data are mean (SE). All measured weights are included at each timepoint.
Participants assigned to the commercial programme had increased odds of losing 5% or more (odds ratio [OR] 3·0, 95% CI 2·0–4·4) and 10% or more (3·2, 2·0–5·3) initial weight at 12 months than did those assigned to standard care. Similarly, participants who completed the 12-month assessment and were assigned to the commercial programme also had increased odds of losing 5% or more (2·9, 2·1–3·9) and 10% or more (3·5, 2·3–5·4) initial weight at 12 months than did those assigned to standard care. Figure 3 shows the percentages of participants who lost 5% or more and 10% or more bodyweight.
Figure 3.
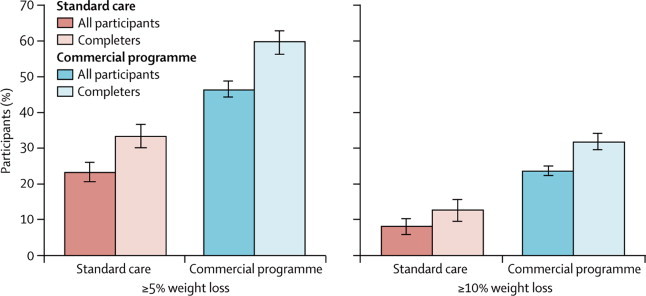
Proportion of participants who lost at least 5% and at least 10% of their initial weight at the 12-month assessment
Error bars show SE.
The greater weight loss in participants assigned to the commercial programme than in those assigned to standard care was accompanied by larger reductions in waist circumference and fat mass in all analyses (table 2). Table 3 shows changes in insulin, glucose, HbA1c, triglycerides, total cholesterol, HDL and LDL cholesterol, and the ratio of total to HDL cholesterol over 12 months. Participants in the commercial programme had significantly greater improvements in insulin and ratio of total to HDL cholesterol than did those assigned to standard care. We noted weak evidence of improvements in glucose, and HDL and LDL cholesterol in the commercial programme group, although these differences did not reach significance for all analyses at the 5% level (table 3).
Table 3.
Changes in biomarkers of cardiovascular disease risk (mean; SE) between baseline and 12 months by treatment group (with analysis by LOCF, BOCF, and in those who completed the 12-month assessment)
| N | Commercial programme | Standard care | Adjusted difference (95% CI)* | p value | |
|---|---|---|---|---|---|
| Insulin (pmol/L) | |||||
| LOCF | 749 | −3·89 (0·97) | −0·65 (0·95) | −2·89 (−5·47 to −0·31) | 0·0284 |
| BOCF | 749 | −3·66 (0·87) | −0·45 (0·89) | −3·04 (−5·44 to −0·64) | 0·0132 |
| Completers | 423 | −6·15 (1·44) | −0·84 (1·67) | −5·74 (−9·86 to −1·61) | 0·0065 |
| Glucose (mmol/L) | |||||
| LOCF | 760 | −0·06 (0·02) | 0·01 (0·03) | −0·07 (−0·15 to 0·00) | 0·0440 |
| BOCF | 760 | −0·06 (0·02) | −0·01 (0·03) | −0·05 (−0·11 to 0·01) | 0·07 |
| Completers | 428 | −0·10 (0·03) | −0·02 (0·05) | −0·09 (−0·19 to 0·01) | 0·08 |
| HbA1C(%) | |||||
| LOCF | 757 | −0·08 (0·01) | −0·05 (0·01) | −0·02 (−0·05 to 0·01) | 0·14 |
| BOCF | 757 | −0·07 (0·01) | −0·05 (0·01) | −0·02 (−0·04 to 0·01) | 0·28 |
| Completers | 248 | −0·18 (0·03) | −0·16 (0·03) | 0·00 (−0·08 to 0·07) | 0·96 |
| Triglycerides (mmol/L) | |||||
| LOCF | 760 | −0·09 (0·03) | −0·06 (0·03) | −0·03 (−0·10 to 0·04) | 0·41 |
| BOCF | 760 | −0·05 (0·02) | −0·05 (0·03) | 0·00 (−0·07 to 0·06) | 0·92 |
| Completers | 429 | −0·09 (0·04) | −0·10 (0·05) | −0·01 (−0·12 to 0·09) | 0·80 |
| Cholesterol (mmol/L) | |||||
| LOCF | 760 | 0·00 (0·04) | 0·03 (0·03) | −0·04 (−0·13 to 0·05) | 0·40 |
| BOCF | 760 | 0·01 (0·03) | 0·07 (0·03) | −0·07 (−0·14 to 0·01) | 0·09 |
| Completers | 430 | 0·01 (0·05) | 0·13 (0·05) | −0·11 (−0·24 to 0·02) | 0·09 |
| LDL cholesterol (mmol/L) | |||||
| LOCF | 758 | −0·01 (0·03) | 0·02 (0·03) | −0·05 (−0·13 to 0·03) | 0·22 |
| BOCF | 758 | −0·01 (0·03) | 0·06 (0·02) | −0·09 (−0·15 to −0·02) | 0·0100 |
| Completers | 428 | −0·02 (0·04) | 0·11 (0·04) | −0·13 (−0·24 to −0·02) | 0·0222 |
| HDL cholesterol (mmol/L) | |||||
| LOCF | 760 | 0·06 (0·01) | 0·04 (0·01) | 0·03 (0·00 to 0·06) | 0·08 |
| BOCF | 760 | 0·07 (0·01) | 0·04 (0·01) | 0·03 (0·01 to 0·06) | 0·0051 |
| Completers | 428 | 0·12 (0·02) | 0·07 (0·02) | 0·05 (0·01 to 0·09) | 0·0148 |
| Total cholesterol:HDL | |||||
| LOCF | 759 | −0·18 (0·03) | −0·11 (0·03) | −0·09 (−0·17 to −0·01) | 0·0270 |
| BOCF | 759 | −0·17 (0·03) | −0·07 (0·02) | −0·12 (−0·18 to −0·05) | 0·0008 |
| Completers | 428 | −0·29 (0·04) | −0·13 (0·05) | −0·17 (−0·28 to −0·06) | 0·0021 |
LOCF=last observation carried forward. BOCF=baseline observation carried forward. HbA1c=glycated haemoglobin.
Adjusted for baseline observation and country.
We recorded small reductions in blood pressure in both treatment groups; however, the reduction did not differ significantly between groups (table 2). 96 participants in the commercial programme and 99 assigned to standard care were receiving an antihypertensive drug at baseline. During the course of treatment, two participants in the commercial programme stopped antihypertensive treatment and two started, whereas one stopped and six started in the standard care group.
At 12 months, 328 (42%) participants had withdrawn from the trial (figure 1). More completed the final assessment in the commercial programme group (230 [61%]) than in standard care group (214 [54%]), but this difference was not significant (p=0·06). Attrition differed significantly between countries (p<0·0001), with the number of participants not completing higher in the UK (150 [64%]) than in Australia (111 [41%]) and Germany (67 [25%]). Despite differences in completion rates, the weight loss in the commercial programme group was significantly greater than in the standard care group in each country in all analyses (data not shown).
Participants who completed the 12-month assessment were significantly older at baseline (mean 50·2 years [SD 12·5]) than were those who did not (43·6 years [12·4]; p<0·0001). We recorded no significant effect of sex, baseline weight, or diabetes status on whether individuals completed the 12-month assessment (data not shown).
Participants attending assessment visits for standard care reported a mean of one appointment per month with their health-care provider, whereas those in the commercial programme attended a mean of three meetings per month in the UK and Australia and two meetings per month in Germany (webappendix p 1).
No participant reported any serious adverse events attributable to weight loss or trial participation during the study.
Discussion
This trial provides important data to inform weight management interventions in primary care. Participants referred to a community-based commercial provider lost more than twice as much weight during 12 months as did those who received standard care, even in the most conservative analyses (BOCF). The similar weight losses achieved in Australia, Germany, and the UK imply that this commercial programme, in partnership with primary care providers, is a robust intervention that is generalisable to other economically developed countries. These results are broadly similar to previous investigations of the commercial programme compared with other community-based programmes or self-help treatments.7,8
The greater weight loss in participants assigned to the commercial programme was accompanied by greater reductions in waist circumference and fat mass than in participants assigned to standard care, which would be expected to lead to a reduction in the risk for type 2 diabetes and cardiovascular disease.15,16 We also detected a suggestion of greater improvements in glucose and lipid metabolism in participants in the commercial programme group, although these differences were not significant in all analyses. Measured changes in blood pressure were small, although a quarter of participants were receiving antihypertensive drugs at the beginning of the study, which could have masked any improvements, and the remainder had normal blood pressure at baseline. Reductions in blood pressure during weight loss are mainly recorded in patients with hypertension, and less so in participants with normal blood pressure.17–19 These modest changes relative to other clinical trials of weight loss interventions are probably indicative of the lower BMI entry criteria of this study and the lower prevalence of comorbid risk factors.7,8
Although mean weight loss in the standard care group was lower than that in commercial programme participants, a quarter of all patients randomly assigned to standard care lost 5% or more bodyweight during 12 months. This finding shows the capability of primary care professionals to deliver advice and support to enable patients to lose weight over 1 year. The delivery of standard care programmes differed between countries but was based on similar principles, as expounded in national treatment guidelines. The specifics of standard care also differed both between and within countries, although participants in standard care and attending assessment visits reported on average one session per month with a primary care provider. This level of contact in participating practices is probably higher than in standard care nationally,20 and could be indicative of either the effect of the trial on weight management services in these practices or a characteristic of participants who remained in the study.
Weight loss in the standard care group in this trial can be compared with that from an audit of the Counterweight programme.21 The Counterweight programme provides intensive training and support for staff delivering weight loss treatment in primary care and recommends at least six appointments or group sessions in the first 3 months, with follow-up appointments every 3 months. After 12 months, mean weight loss was 3·0 kg (SE 0·3) in people who completed the Counterweight programme compared with 3·3 kg (0·3) in those who completed our study. Drop-out rates were also similar, with 45% completing the Counterweight programme and 54% completing standard care in our study. Further research is needed to identify whether weight loss is directly attributable to any specific weight management support provided, a result of personal actions arising from increased awareness of obesity and motivation to lose weight, or the accountability provided by regular follow-up.
By contrast with previous studies of commercial weight loss programmes in which participants were self-referred, this study examined a partnership model, with participants who were likely to benefit from early intervention for weight loss identified and referred by a health professional. This study used one example of a commercial provider of weight loss treatment. A trial that compared a range of self-help programmes (meal replacements, commercial weight loss groups, and diet books) showed that over 6 months all diets resulted in clinically meaningful weight loss (4·9–7·3%) compared with no diet (0·6%), with no significant differences between diets.8 Findings from two randomised controlled trials in the USA showed that self-selected participants randomly assigned to free participation in the Jenny Craig programme and free-of-charge prepackaged foods lost significantly more weight than did those referred to usual care over 12 months (mean weight loss 6·6 kg [SE 1·8] vs 0·7 kg [0·9]; p<0·01)9 and 24 months (mean 7·4 kg [95% CI 6·1–8·7] vs 2·0 kg [1·2–3·6]; p<0·001).11 These studies suggest that other commercial providers working in partnership with primary care providers could also offer effective treatment options, but these programmes need to be assessed in a primary health-care context.
Weight loss reported with more intensive lifestyle interventions, such as the Diabetes Prevention Program3 (5·6 kg over an average of 2·8 years) and the Look Ahead Study22 (mean 8·6 kg [SE 0·2] in the first year), was greater than that recorded in our study. However, the effectiveness of such programmes when delivered in routine clinical practice, at large scale, and with limited resources has not been assessed. Some commercial programmes, such as that used in this study, use many of the techniques that are used in more intensive behavioural treatments delivered by health professionals, such as self-monitoring, goal setting, nutritional advice (reduced energy, low-fat diets) and exercise education, problem solving, stimulus control, and relapse prevention.23 Although both commercial programme and usual care interventions are based on individual-level behaviour change, commercial approaches are delivered in larger groups by community members and are likely to be less expensive than usual care. The large group and standardised format of commercial programmes could dilute treatment effects that are recorded in one-to-one and small closed group interventions, but could be beneficial in primary care because this format offers opportunities for frequent contact and regular weighing (standard care participants attended one appointment per month on average, and participants in the commercial programme attended three meetings per month in Australia and the UK and two meetings in Germany). The peer-support element of group treatment might also be beneficial for some people,24 although for interventions delivered by a range of health-care professionals, individual therapy produced greater weight loss than did group-based treatment.25 Further research will examine the relative cost-effectiveness of different treatment approaches.
By contrast with most other weight loss trials we included only individuals with overweight and moderate obesity (BMI 27–35 kg/m2), to study a population with a limited severity of comorbidities and at low risk of treatment complications, who are suited to a commercial weight loss programme setting. The results clearly show that in these selected participants, referral to a commercial weight loss programme provider is effective and safe. Most participants were women. This gender bias is common in studies of weight loss in primary care,21,26,27 and further research should consider whether it results from differences in willingness to participate in weight loss interventions, gender differences in general attendance at the GP surgery, or differences in referral practices.
As with many other clinical obesity trials, the drop-out rate was high; however, this rate was anticipated in the sample size calculations. Moreover, the finding of greater weight loss in participants referred to the commercial programme was consistent across both conservative and liberal analysis protocols, and across countries with different attrition rates. Drop-out was particularly high in the UK, perhaps because of difficulties in scheduling of follow-up appointments in routine clinical practice, which did not occur in the specialist research facilities. Additionally, the diverse sites, both within and between countries, made introduction of a consistent model of standard care impractical. The variability recorded in weight loss in the standard care group was correspondingly greater than in the commercial programme group. However, this finding probably indicates the diversity of routine weight management practices in different health-care settings and systems. Participation in the trial and the five follow-up assessments, at which participants were weighed, might have increased the total weight loss recorded in both groups, by enhancing motivation to lose weight. However, participation would not affect the difference between the treatments in this randomised controlled trial.
Obesity and its associated comorbidities demand early intervention, but the high and rising prevalence of obesity puts pressure on scarce health-care resources. Data from our study suggest that referral of selected participants by a primary health-care professional to a commercial weight loss programme that provides regular weighing, advice about diet and physical activity, motivation, and group support can offer a clinically useful early intervention for weight management in overweight and obese people that can be delivered at large scale (panel). Further research is needed to examine long-term weight loss maintenance, together with a formal analysis of cost-effectiveness.
Panel. Research in context.
Systematic review
We searched PubMed and Scopus and identified only a few of randomised controlled trials that had assessed commercial weight loss programmes. Most of these programmes were in the USA, all included self-selected participants, and none compared the commercial programme to weight management provision in primary care. Furthermore, no randomised controlled trials have assessed the effectiveness of a commercial programme operating in partnership with health-care professionals in a primary care setting.
Interpretation
This trial assessed the clinical effectiveness of referral to a commercial weight management programme that offers regular weighing, and promotes a hypoenergetic balanced diet based on healthy-eating principles, increased physical activity, and group support, compared with provision of standard weight loss treatment by primary care providers. In three countries, over 12 months, participants referred to the commercial weight loss programme lost twice as much weight, and were three times more likely to lose more than 5% of initial weight, than those receiving standard care.
Acknowledgments
Acknowledgments
This trial was funded by Weight Watchers International, through a grant to the UK Medical Research Council. We thank participants and the staff of participating primary care practices for their contribution to the trial.
Contributors
SAJ, LMA, AES, SP, UA-G, APM, HH, and IDC contributed to study conception and design. SAJ, LMA, AES, UA-G, APM, HH, and IDC obtained funding. SAJ, ALA, LMA, CH, JS, UA-G, AES, NRF, SP, NSL, HH, and IDC contributed to acquisition of data. SAJ, ALA, ADO, APM, HH, and IDC analysed and interpreted data. SAJ, ALA, ADO, APM, HH, and IDC drafted the report, and all authors contributed to revision of the report.
Conflicts of interest
All authors declare financial support to their institutions for the submitted work from Weight Watchers. SAJ has received research grants for other clinical trials from Sanofi-Aventis and Coca Cola. IDC, NSL, AES, and NRF have received research grants for other clinical trials funded by Sanofi-Aventis, Allergan, Roche products, MSD, and GlaxoSmithKline. NRF has received conference travel expenses from Allergan. HH has received a travel grant from Roche. SAJ is a member of the Tanita Medical Advisory Board and has received payment for nutrition articles and lectures for Rosemary Conley Enterprises. HH is on the Advisory Board for Weight Watchers International and has received payment for lectures from Sara Lee, Lilly, Novartis, Sanofi-Aventis, and Bristol-Myers Squibb. IDC was a board member for the SCOUT trial and has received payment for lectures from iNova Pharmaceuticals, Eisai Pharmaceuticals, Pfizer Australia, and Servier Laboratories (Australia).
Web Extra Material
References
- 1.WHO . Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization; Geneva: 2009. [Google Scholar]
- 2.Klein S, Burke LE, Bray GA. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franz MJ, VanWormer JJ, Crain AL. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Ahern A, Olson A, Aston L, Jebb S. Weight Watchers on prescription: an observational study of weight change among adults referred to Weight Watchers by the NHS. BMC Public Health. 2011;11:434. doi: 10.1186/1471-2458-11-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavin JH, Avery A, Whitehead SM. Feasibility and benefits of implementing a slimming on referral service in primary care using a commercial weight management partner. Public Health. 2006;120:872–881. doi: 10.1016/j.puhe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Heshka S, Anderson JW, Atkinson RL. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA. 2003;289:1792–1798. doi: 10.1001/jama.289.14.1792. [DOI] [PubMed] [Google Scholar]
- 8.Truby H, Baic S, deLooy A. Randomised controlled trial of four commercial weight loss programmes in the UK: initial findings from the BBC “diet trials”. BMJ. 2006;332:1309–1314. doi: 10.1136/bmj.38833.411204.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity (Silver Spring) 2007;15:939–949. doi: 10.1038/oby.2007.614. [DOI] [PubMed] [Google Scholar]
- 10.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56–66. doi: 10.7326/0003-4819-142-1-200501040-00012. [DOI] [PubMed] [Google Scholar]
- 11.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304:1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 12.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 13.Craig CL, Marshall AL, Sjostrom M. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 14.Kolotkin RL, Crosby RD, Kosloski KD, Williams GR. Development of a brief measure to assess quality of life in obesity. Obes Res. 2001;9:102–111. doi: 10.1038/oby.2001.13. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs EJ, Newton CC, Wang Y. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 16.Miyazaki Y, DeFronzo RA. Visceral fat dominant distribution in male type 2 diabetic patients is closely related to hepatic insulin resistance, irrespective of body type. Cardiovasc Diabetol. 2009;8:44. doi: 10.1186/1475-2840-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauner H, Meier M, Wendland G, Kurscheid T, Lauterbach K. Weight reduction by sibutramine in obese subjects in primary care medicine: the SAT Study. Exp Clin Endocrinol Diabetes. 2004;112:201–207. doi: 10.1055/s-2004-817934. [DOI] [PubMed] [Google Scholar]
- 18.Jordan J, Scholze J, Matiba B, Wirth A, Hauner H, Sharma AM. Influence of sibutramine on blood pressure: evidence from placebo-controlled trials. Int J Obes (Lond) 2005;29:509–516. doi: 10.1038/sj.ijo.0802887. [DOI] [PubMed] [Google Scholar]
- 19.Ebrahim S, Smith GD. Lowering blood pressure: a systematic review of sustained effects of non-pharmacological interventions. J Public Health Med. 1998;20:441–448. doi: 10.1093/oxfordjournals.pubmed.a024800. [DOI] [PubMed] [Google Scholar]
- 20.Flodgren G, Deane K, Dickinson HO. Interventions to change the behaviour of health professionals and the organisation of care to promote weight reduction in overweight and obese people. Cochrane Database Syst Rev. 2010;3 doi: 10.1002/14651858.CD000984.pub2. CD000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Counterweight Project Team Evaluation of the counterweight programme for obesity management in primary care: a starting point for continuous improvement. Br J Gen Pract. 2008;58:548–554. doi: 10.3399/bjgp08X319710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadden TA, West DS, Neiberg RH. One-year weight losses in the Look AHEAD study: factors associated with success. Obesity (Silver Spring) 2009;17:713–722. doi: 10.1038/oby.2008.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark VL, Pamnani D, Wadden TA. Behavioural treatment of obesity. In: Kopelman PG, Caterson ID, Dietz WH, editors. Clinical obesity in adults and children. 3rd edn. Blackwell; Chichester: 2010. [Google Scholar]
- 24.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. J Consult Clin Psychol. 2001;69:717–721. [PubMed] [Google Scholar]
- 25.Steinbeck K, Droulers AM, Caterson ID. The effect of an individual versus group program of weight loss. Asia Pacific J Clin Nutr. 1997;6:119–121. [PubMed] [Google Scholar]
- 26.Nanchahal K, Townsend J, Letley L, Haslam D, Wellings K, Haines A. Weight-management interventions in primary care: a pilot randomised controlled trial. Br J Gen Pract. 2009;59:e157–e166. doi: 10.3399/bjgp09X420617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity (Silver Spring) 2010;18:1614–1618. doi: 10.1038/oby.2009.457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


