Abstract
Most viruses are naturally immunogenic and can be engineered to express tumor antigen transgenes. Moreover, many types of recombinant viruses have been shown to infect professional antigen-presenting cells, specifically dendritic cells, and express their transgenes. This enhanced presentation of tumor antigens to the immune system has led to an increase in the frequency and avidity of cytotoxic T-lymphocytes that target tumor cells expressing the tumor antigen(s) encoded in the vaccine vector. Logistically, recombinant viruses can be produced, administered and quality controlled more easily compared to other immunotherapy strategies. The intrinsic properties of each virus have distinct advantages and disadvantages, which can determine their applicability in a particular therapeutic setting. The disadvantage of some vectors is the development of host-induced neutralizing antibodies to the vector itself, thus limiting its continued use. The “off-the-shelf” nature of viral vaccine platforms renders them exceptionally suitable for multicenter randomized trials. This review will describe and discuss the strategies employed and results using viral-based vaccines, with emphasis on phase II and III clinical trials. Future directions will involve the evaluation of viral-based vaccines in the adjuvant and neo-adjuvant settings, in patients with low burden metastatic disease, and in combination with other forms of therapy including immunotherapy.
Keywords: cancer vaccines, viral vaccines, immunotherapy, MVA vaccines, TRICOM vaccines, prime-boost vaccination, adenovirus, poxvirus vaccines
Introduction
There are numerous reasons why recombinant viral vectors are potentially useful vaccine vehicles for cancer therapy. The intrinsic properties of each virus have distinct advantages and disadvantages, which can determine their applicability in a particular therapeutic setting. The safety and anti-tumor activity of viral-based vaccines in preclinical models has led to clinical trials to evaluate the immunologic and clinical efficacy of this treatment modality. In this review we will discuss the strategies employed and results using viral-based vaccines, with emphasis on phase II and III clinical trials completed to date.
I. Viral Delivery Systems
Most viruses are naturally immunogenic and can be engineered to express tumor antigen transgenes. Moreover, many types of recombinant viruses have been shown to infect professional antigen-presenting cells (APCs), specifically dendritic cells (DCs), and express their transgenes.1–6 This enhanced presentation of tumor antigens to the immune system has led to an increase in the frequency and avidity of cytotoxic T-lymphocytes that target tumor cells expressing the tumor antigen(s) encoded in the vaccine vector.5–6 Several studies have shown that transgenes expressed by a viral vector are more immunogenic than antigen administered with adjuvant.7–8 This observation is attributed to the pro-inflammatory environment produced by the expression of viral proteins. Logistically, recombinant viruses can be produced more easily compared to other immunotherapy strategies, such as whole tumor cell and dendritic cell vaccines. The latter may require complex, time-consuming and sometimes costly methods to produce because they are customized treatments. Recombinant viruses, on the other hand, are a more acceptable “off the shelf” means of vaccine vehicle, given the relative ease of production, purification, and storage. It is thus also more feasible to conduct multi-center clinical trials given the relatively low cost of viral vaccine production. The disadvantage of some vectors is the development of host-induced neutralizing antibodies to the vector itself, thus limiting its continued use.
II. Distinguishing Viral Vaccines from Viruses Used for Gene Therapy and Oncolysis
Viruses can also be used as vectors for gene delivery (Table 1). For example, adenoviral vectors have been engineered to express suicide genes, such as herpes simplex virus-1 (HSV-1) thymidine kinase (TK),9 which renders target cells expressing the transgene susceptible to treatment with gancyclovir.10 Viruses have also been used as oncolytic agents. Measles virus,11–12 herpes simplex virus (HSV),13 and vesicular stomatitis virus (VSV),14 among others (Table 1), have been shown to preferentially infect and propagate in tumor cells. These viruses undergo a lytic life cycle, killing the tumor cell and then spreading to uninfected tumor cells.15 Thus, these viruses not only have a direct cytopathic effect on tumor cells but they also have been shown to enhance immune-mediated killing of tumor cells, likely through the release of tumor antigens. However, some oncolytic viruses are unlikely to be used as a cancer vaccine vector because they have a short duration of transgene expression in infected cells given the onset of lysis and limited tropism for DCs; this might limit their ability to generate a robust immune response against the transgene. These therapeutic strategies are summarized in excellent reviews.12, 16 This article will focus on recombinant viruses used primarily as cancer vaccines in clinical development.
Table 1.
Viral Vectors and Cancer Immunotherapy
| Viral Vector | Advantages | Disadvantages |
|---|---|---|
Mammalian Poxviruses
|
|
|
Avian Poxvirus
|
|
|
| Adenovirus (Ad) |
|
|
| Alphavirus |
|
|
| Measles Virus (MV) |
|
|
| Herpes Simplex virus (HSV) |
|
|
| Vesicular stomatitis virus |
|
|
DC – Dendritic cells
CAR – Coxsackie and adenovirus receptor
III.Viral Vector Types
Each viral vector has its own potential advantages and disadvantages (Table 1). The most comprehensively studied viral vectors are from the poxviridae family. They include derivatives of vaccinia virus, from the orthopoxvirus genera, and members of the avipoxvirus genera, such as fowlpox and canarypox (ALVAC). Poxviruses have a long and successful history in vaccination programs. Most notably, vaccinia virus was used to vaccinate over 1 billion people against smallpox, leading to the eradication of this disease in 1978.17 Poxviruses are a double-stranded DNA virus with a linear genome. They have the ability to accept large inserts of foreign DNA, and thus can accommodate multiple genes. Vaccinia virus has a genome of ~190 kbp, encoding ~250 genes.18 Fowlpox virus has a ~260–309 kbp genome and has ~260 putative genes.19 Attenuated canarypox strain ALVAC has a ~330 kbp genome and has ~320 putative genes.20
Poxviral vectors have wide host range, stable recombinants, accurate replication, and efficient post-translational processing of inserted genes. Intracellular expression of the transgene(s) allows for processing of the tumor antigen by both class I and II MHC pathways, leading to activation of both CD4+ and CD8+ T cells.21 Induction of a humoral response against the tumor antigen can also be seen. Viral replication and transcription of the poxvirus genome is restricted to the cytoplasm of the host cell; this extranuclear replication removes the risk of insertional mutagenesis, the random insertion of viral genetic sequences into host cell genomic DNA.22 Vaccinia virus infects mammalian cells, and expresses recombinant genes for about 7 days before the infected cell is cleared by the immune system.1 Avipox viruses infect mammalian cells and express their transgenes for 14–21 days.23–24
Despite the desirable features of poxviruses, replication competent viruses like vaccinia should not be administered to severely immunocompromised patients. To address this problem, an attenuated vaccinia virus called modified vaccinia virus Ankara (MVA) was developed for high-risk individuals. MVA was generated by over 500 serial passages of a smallpox vaccine from Ankara, Turkey, in chicken embryo fibroblasts, resulting in over 15% loss of the vaccinia virus genome.25 MVA can infect mammalian cells and express transgenes, but it cannot produce infective viral particles. Similarly, canarypox and fowlpox, which are pathogenic in some avian species, are unable to productively infect humans because they cannot complete their life cycle and form an infectious particle.26 As a result, mammalian poxviruses generate a more robust immune response, compared to avipox viruses. Unfortunately, MVA and vaccinia virus vectors can only be given once or twice to vaccinia immune or vaccinia naïve patients due to the development of host neutralizing antibodies against these vectors.27 Neutralizing antibodies are not developed against the avipoxvirus vectors, allowing them to be administered multiple times to patients as booster vaccinations.28
Numerous phase I trials have been conducted using viruses other than poxviruses as the immunotherapy delivery system (Table 1). However, the only other vaccine that has reached phase II development is an alphavirus vector.29 Alphavirus is a positive single strand RNA virus of about 11–12 kbp in length that includes members of the Venezuelan Equine Encephalitis virus, Semiliki Forest virus, and Sindbis virus.30 The alphavirus RNA vector can be delivered in viral particles containing the RNA vector or as a naked RNA or cDNA vector.31 Alphaviruses have high levels of replication leading to high expression of transgenes. Transgenes are expressed for a short time given the induction of apoptosis by the propagating nature of the replicon-competent vector.30 Lysis of infected tumor cells leads to release of tumor antigens that can further enhance the anti-tumor immune response. In addition, viral RNA is naturally immunogenic and has been shown to induce an IFN-α I immune response.32 Alphaviruses, like avipox viruses, are also desirable vectors because infected hosts do not develop neutralizing antibodies to the vectors, allowing for multiple injections.
While predominately used as a vehicle for gene therapy, adenovirus (Ad) has also been developed to generate an anti-tumor immune response. Adenovirus is a non-enveloped double-stranded DNA virus. The 36 kbp genome accepts cDNA sequences up to 7.5 kbp. Replication of the genome occurs in the nucleus but remains extrachromosomal, minimizing the risk of insertional mutagenesis with this vector. Adenovirus can infect proliferating and resting cells and express transgenes with high efficacy. The majority of adenoviral vectors are replication-incompetent, following deletion of E1 and E3 viral genes. This limits their pathogenicty, while still allowing for the generation of a humoral and cellular response to transgenes. Adenoviral vectors are stable and easily propagated in laboratory settings, which allow researchers to easily modify the vector; this includes retargeting the virus’s tropism to enhance infection of DCs and target cells lacking the common Ad receptor, which is critical for infection. Adenovirus induces neutralizing antibodies in infected hosts, thus limiting the number of vaccinations.33
IV.Innovative Strategies Using Viral-Vector Vaccines
Diversified Prime and Boost
Tumor antigens are more immunogenic when delivered as transgenes in a viral vector, compared to employing tumor antigens used as a peptide or protein vaccine.8, 34 This is a result of the mammalian immune system’s ability to recognize the viruses, through toll-like-receptors, as foreign to avoid replicating infections and subsequent illness. While virtually all of the viruses used as vectors for vaccine have had pathogenic genes deleted, they have retained their immunogenicity. This same property, however, is also the reason that most viral-based vaccines can be given only once due to host-neutralizing immunity of subsequent vaccinations. This is exemplified in the use of most adenoviruses and in the use of mammalian poxviruses such as vaccinia and MVA. Preclinical studies employing vaccinia and MVA have shown that in a vaccinia-naive murine host, recombinant vaccinia and MVA can be used for two vaccinations to enhance immunity to the tumor antigen transgene. In the vaccinia-immune host, however, these agents can only be given once; no further immunity to the tumor antigen transgene is induced following the second vaccination. Similar results were seen in clinical trials, which found that vaccinia-immune patients, who were vaccinated with a recombinant vaccina virus expressing carcinoembryonic antigen (CEA) or prostate-specific antigen (PSA), were able to mount an immune response to the transgene after the first vaccination but not to the second.35–36 These preclinical and clinical studies have led to a diversified prime-boost approach in which recombinant vaccinia is used for the prime vaccination and recombinant avipox (i.e., fowlpox) is used for multiple booster vaccinations. Multiple injections of fowlpox have been shown to induce antibodies to fowlpox in both mice and humans, but these antibodies are non-neutralizing.37–38 This is due to the fact that only early viral proteins are made in mammalian cells and thus the late viral proteins, which would normally induce neutralizing antibodies, are absent. Initial clinical trials were carried out using a prime-boost strategy employing recombinant vaccinia expressing PSA (rV-PSA) and multiple boostings with recombinant avipox (fowlpox) rF-PSA. A randomized trial in patients with prostate cancer demonstrated the advantage of priming with rV-PSA and boosting with rF-PSA vs. the reciprocal, or giving multiple vaccinations of only rF-PSA.39 More recent studies using the diversified prime and boost technique have employed vectors containing transgenes for a TAA and a triad of T-cell costimulatory molecules consisting of B7.1, ICAM-1, LFA-3, and designated TRICOM. Numerous preclinical studies demonstrated the advantage of using vectors containing the TRICOM transgenes in terms of induction of increased numbers of tumor-specific T cells, higher avidity of those T cells, and enhanced tumor activity. Clinical studies using the TRICOM vaccine will be discussed below. Whereas many cancer vaccine clinical studies have used the vaccinia prime/avipox boost approach, some trials in cancer and in HIV vaccinations are employing a diversified vaccination approach employing vaccinia, MVA or avipox as one mode of vaccination and DNA as the second.
Route and Mode of Administration
Viral-based vaccines also provide the flexibility for different routes of administration. While most clinical studies have been conducted with either the subcutaneous or intradermal vaccination, recent studies have shown the feasibility and safety of intratumoral vaccination. A clinical study employing direct vaccination of metastatic melanoma lesions with rF-TRICOM demonstrated some evidence of clinical benefit.40 Preclinical studies have also shown the anti-tumor activity of intratumoral rF-CEA-TRICOM vaccination in CEA positive tumors, which was amplified by the concurrent subcutaneous vaccination of rV-CEA-TRICOM prime and rF-CEA-TRICOM boost. These studies formed the rationale for a recent clinical trial in patients with locally recurrent prostate cancer in which patients were given multiple intratumoral rF-PSA-TRICOM with concurrent subcutaneous rV-PSA-TRICOM prime and rF-PSA-TRICOM boost. This trial demonstrated the safety and feasibility of this approach. Moreover, this vaccination strategy showed a strong immune infiltrate in post-vaccination biopsies of tumor, along with a clear prolongation of PSA doubling time.
Ex-Vivo Viral Infection of Antigen-Presenting Cells
TRICOM infection of murine or human B cells enhanced the expression of each of the three costimulatory molecule transgenes (B7-1, ICAM-1, LFA-3) on their cell surface and enhanced their antigen-presenting capability.41–42 It has been shown, moreover, that the T cells generated have higher avidity than peptide-pulsed control dendritic cells or dendritic cells infected with wild-type vector.43 These findings have led to a clinical trial employing CEA-MUC1-TRICOM (PANVAC) ex vivo infection of human dendritic cells employed as a vaccine in metastatic colorectal cancer patients who have undergone mastectomy,44–45 as will be discussed below.
Immune Stimulants
Viral vectors can also be employed as vehicles for the delivery of immune stimulant transgenes such as cytokines. Preclinical studies have shown that insertion of cytokines such as GM-CSF, IL-2 and IL-15 can be inserted into viral vectors to be used in combination viral-based vaccines to enhance their efficacy.46–50 Clinical studies have been completed and are ongoing employing this approach.51–52
V. Clinical Trials
MVA Vector-based Trials
Several clinical trials in metastatic renal cell carcinoma (Table 2) have been conducted with the TroVax vaccine, which consists of a recombinant MVA expressing the 5T4 tumor-associated antigen (TAA). Initial trials demonstrated some objective clinical responses, stable disease and both antibody and T-cell responses to 5T4. A phase III randomized, placebo-controlled study employed MVA 5T4 with and without cytokines and sunitinib in patients (n=733) with metastatic renal cell cancer (Table 2). Treatment arms were well-balanced. There was, however, no significant difference in overall survival between the two treatment arms. The magnitude of the 5T4-specific antibody response post-vaccination was associated with increased patient survival, as was seen in previous trials. A second vaccine has also been analyzed in patients with metastatic renal cell carcinoma. The TG4010 vaccine consists of MVA expressing recombinant MUC-1 and IL-2 transgenes. The vaccine was used either alone or in combination with exogenous IFN-α and IL-2. No objective responses were noted. Eighteen percent of patients had stable disease for > 6 months with vaccine alone, and 30% had stable disease for > 6 months with vaccine and cytokine. Median overall survival was 19.3 months for all patients and 22.4 months for patients receiving vaccine and cytokine.
Table 2.
MVA Vaccine Trials: Renal Caner
| Vaccine Therapy (superscript number denotes reference) | Phase (No. of pts) | Immune Response to Vaccine Antigen | Clinical Outcome | |
|---|---|---|---|---|
| TROVAXa (MVA-5T4) | + IFN-α as 1st or 2nd line therapy77 | I/II (11) | 11/11 anti-5T4 antibodies, 5/11 T-cell response | No objective tumor response Median TTP 9 mo (2.1–18+ mo) |
| ± IFN-α78 | II (23) | 21/25 anti-5T4 Ab, 7/21 T-cell response Median TTP: T-cell responders, 10.2 mo; non-responders, 4.5 mo (p=0.04); Greater median OS in pts with > median fold increase in 5T4 antibodies at week 7 (p=0.01) | 1/15 PR SD: 7/15 TroVax + IFN-α; 7/13 TroVax | |
| + high dose IL-279 | I/II (25) | 23/23 anti-5T4 Ab; 13/23 increase in T-cell response | 3/25 NED post-surgery; 12/25 SD (1–18 mo) | |
| + low dose IL-280 | II (25) | 21/25 anti-5T4 Ab, 5/11 T-cell response Pts who developed greater than median anti-5T4 antibody titers had a greater TTP and better OS (p<0.05) | 2 CR (>24 mo), 1 PR (>13 mo), 6 SD (6–21 mo) Median OS >12.87 (1.9–24.76) Median TTP >3.37 (1.5–24.76) | |
| + IL-2, IFN-α, or Sunitnib vs. placebo + IL-2, IFN-α, or Sunitnib; if PD: vaccine ± sorafenib81 | III (733) | 56% developed anti-5T4 Ab More pts developed anti-5T4 Abs in MVA-5T4 + IFN-α group than in vaccine + IL-2 or vaccine + sunitinib group High 5T4 antibody responders had a favorable survival compared with placebo-treated pts |
Terminated given little prospect of showing survival benefit Pts with good prognosis receiving IL2 + vaccine vs. IL-2 alone had improved OS |
|
| TG4010 (MVA- MUC1-IL2) | Vaccine, followed by addition of IFN- α + IL-2 if PD82 | II (37) | 7/37 CD8+ T cell response; 6/28 CD4+ T-cell response MUC1-specific CD8+ T cell response associated with longer OS | Median OS 19.3 mo overall, 22.4 mo for combination therapy SD > 6 mo: 5/27 TG4010 (median SD 5.9 mo), 6/20 TG4010 + cytokines (median SD 8.4 mo) |
TroVax – modified vaccinia ankara (MVA) virus expressing 5T4
TG4010 – modified vaccinia ankara virus expressing MUC-1 and IL-2 transgenes.
TTP – time to progression; OS – overall survival; PR – partial response; NED – no evaluable disease; SD – stable disease
The TroVax vaccine has also been evaluated in four small single-arm trials in patients with metastatic colorectal cancer (Table 3). Antibody and T-cell responses, as well as stable disease, were noted along with some complete responses and partial responses in the various trials. In a trial involving the combination of TroVax with FOLFIRI chemotherapy, 11/17 patients were considered evaluable for immunologic evaluation; of these, six had complete or partial responses as well as T-cell or antibody responses to vaccine. These immune responses correlated with clinical benefit.
Table 3.
MVA Vaccine Trials: Colon Cancer/ Prostate Cancer/ NSCLC
| Vaccine Therapy Tumor Type (superscript number denotes reference) | Phase (No. of pts) | Immune Response to Vaccine Antigen | Clinical Outcome | |
|---|---|---|---|---|
| TROVAXa (MVA-5T4) | Single agent Colon cancer83 | I/II (22) | 14/17 anti-5T4 antibodies; 16/18 T-cell response Serum CA-242/CEA levels correlated with tumor burden in 21/22 | 5/22 SD (3–18 mo) 5T4-specific antibody titer predicted improved patient survival and TTP (p<0.05) |
| Pre- and Post- surgery Colon cancer84 | II (20) | 18/19 anti-5T4 antibodies; 18/18 T-cell response; 3/9 generated T-cell response using isolated TILs | Higher levels of anti-5T4 antibodies, T-cell proliferation, and/or CD3 density in tumor correlated with improved survival (p<0.05) Lower than median Treg:CD4 ratio in tumor associated with improved survival | |
| + FOLFOXb Colon cancer85 | II (17) | 10/11 anti-5T4 antibodies; 10/11 T-cell response 7/11 had >50% reduction in CEA occurring during therapy or after | 1 CR, 5 PR, 1 SD, 4 PD, at 14 week follow-up post- chemotherapy Median OS: 68 wks in 17 ITT pts, 118 wks in 11 evaluable pts 5T4-specific cellular responses positively correlates with RECIST response at follow-up (p<0.05) | |
| + FOLFIRIc Colon cancer86 | II (19) | 10/12 anti-5T4 antibodies; 11/12 T cell response 6/8 >50% decline in CEA, 1/6 stable levels following chemotherapy | 1 CR, 6 PR, 5 SD (34 wks), at 8 week follow-up post chemotherapy 5T4 cellular response correlates with tumor response (p<0.05) | |
| ± GM-CSF Prostate Cancer87 | II (27) | 24/24 + anti-5T4 antibodies; 9/24 T cell response (6 in vaccine + GM-CSF) T cell responders vs. non-responders had a greater TTP (5.6 vs. 2.3 mo, respectively) (P<0.05) |
No clinical/ immunological differences seen with + GM-CSF No objective clinical response | |
| TG4010d (MVA-MUC1-IL2) | Prostate Cancer88 | II (40) | 18/34 pre-existing T-cell response 7/34 de novo T-cell response: 6/7 > median PSA-DT fold increase |
Primary endpoint of 50% decline in baseline PSA not reached 13/40 pts had a more than two fold improvement in PSA-DT 10/40 pts had PSA stabilized > 8 months |
| ± Cisplatin/ Vinorelbine, if PD on vaccine: Cisplatin/ Vinorelbine added NSCLC89 | II (65) | 8/31 T-cell response | 13/37 PR in combination therapy; 2/ SD (>6 mo) with vaccine alone 1/14 CR, 1/14 PR in vaccine with subsequent chemotherapy Median OS 12.7 mo combination therapy; 14.9 mo for vaccine alone 1-year survival rate was 53% and 60% for combination therapy and vaccine alone group, respectively | |
| TG1031e Breast Cancer90 | I/II (9) | 0/9 anti-MUC1 antibodies; 2/9 T-cell response 1/2 T-cell responders had decrease in CEA and SD (10 wks) | No objective tumor responses | |
TroVax – modified vaccinia ankara (MVA) virus expressing 5T4
FOLFOX – 5-Fluorouracil, leukovorin, and oxaliplatin
FOLFIRI – 5-Fluorouracil, leukovorin, and irinotecan
TG4010 – modified vaccinia ankara virus expressing MUC-1 and IL-2 transgenes
TG1031- vaccinia virus expressing two transgenes: MUC1 and IL-2 (VV-MUC1-IL-2)
NSCLC – non-small cell lung cancer; OS – overall survival; TTP – time to progression; TIL – tumor-infiltrating lymphocyte; CR – complete response; Treg – regulatory T cell; PR – partial response; SD – stable disease; PD – progressive disease; ITT – intention to treat; RECIST – Response Evaluation Criteria in Solid Tumors; GM-CSF – granulocyte macrophage colony-stimulating factor; PSA-DT – prostate-specific antigen doubling time; NSCLC – non-small cell lung cancer
The TG4010 vaccine was also evaluated in prostate cancer patients (n=40) with biochemical failure (Table 3). Thirteen of 40 patients had >2-fold improvement in PSA doubling time, and 10 patients had their PSA stabilized over 18 months. This vaccine has also been evaluated in patients (n=65) with stage 3/4 non-small cell lung cancer. In this randomized phase II study, patients in arm 1 received vaccine in combination with chemotherapy; in arm 2 patients received vaccine monotherapy until disease progression, followed by vaccine plus the same chemotherapy. The median overall survival in arm 1 was 12.7 months vs. 14.9 months for arm 2. One-year overall survival rate was 53% for arm 1 and 60% for arm 2.
Vaccinia or Avipox Vector Trials
Several trials have employed either vaccinia alone or avipox (Alvac) alone (Table 4). In patients with metastatic melanoma, a recombinant vaccinia containing transgenes for multiple melanoma antigens and CD80 and CD86 costimulatory molecules was used as a prime followed by peptide boosting. T-cell responses to the melanoma antigens were observed with three of 17 patients showing some mixed responses and seven of 17 patients with stable disease. Alvac-GP-100 vaccine as a prime was given with GP-100 peptide boost in metastatic melanoma patients. Eight of 18 patients were shown to develop immunologic responses to the vaccine. This was not enhanced by the addition of tetanus toxoid.
Table 4.
Vaccinia or Avipox Vector Vaccine Trials
| Vaccine Therapy Tumor Type (superscript number denotes reference) | Phase (No Pts) | Immune Response to Vaccine Antigen | Clinical Outcome | |
|---|---|---|---|---|
| rVVmel-B7a (Prime), antigen peptide (Boost) + GM-CSF Melanoma91 | I/II (20) | 15/18 T-cell response | 3/17 MXR; 7/17 SD; 7/17 PD | |
| Intratumoral rV-TRICOMb Melanoma40 | I (13) | 30.7% objective response rate 1/13 CR (>22mo); 1/13 PR; 3/13 SD | ||
| rV-PSA ± GM-CSF Prostate Cancer35 | I (33) | T-cell response: 5/7 to PSA | 14/33 no PSA progression (>6mo), 9/14 stable PSA (11–25 mo), 6 stable PSA >25 mo | |
| ALVAC(2)-gp100c (prime), gp100 peptides (boost) ± low or high dose tetanus toxoid or gp100 peptides alone Melanoma92 | I/II (42) | T-cell response: 8/18 vaccine, 0/6 peptide, 1/11 prime- boost + toxoid group; greater CTL responses generated by intranodal vs. subcutaneous injections of vaccine | Stage II: NED Stage III: 2/12 PD, 10/12 NED post-surgery Stage IV: 11/15 PD, 3/15 SD, 1/15 NED post-surgery |
|
| ALVAC-CEA-B7.1 | ± GM-CSF Colon Cancer54 | I/II (60) | GM-CSF did not increase T-cell responses # of prior chemotherapy regimens negatively correlated with generation of T-cell response; positive correlation between # of mo from the last chemotherapy regimen and T-cell response | SD after 4 injections: 11/25 vaccine + GM-CSF, 6/22 vaccine alone |
| + FOLFIRI ±Tetanus toxoid Colon Cancer93 | II (180) | T-cell response generated in majority of evaluated pts (n=46) | 2 CR, 40 PR, 42 SD out of total evaluable pts (n=104) No difference in immunological or clinical responses between groups | |
rVVmel-B7 – UV-inactivated recombinant vaccinia virus expressing 5 transgenes: three HLA-A0201-restricted epitopes (Melan-A/MART-127–35, gp100280–288, and tyrosinase1–9 ) together with two costimulatory molecules (CD80 and CD86)
Intratumoral rV-TRICOM – intratumoral injections of superficial melanoma metastases with a recombinant vaccinia virus expressing three costimulatory molecules (B7.1, ICAM-1, LFA-3)
ALVAC(2)-gp100m- recombinant canarypox virus expressing full-length gp100 containing 2 modified epitopes and two modified gp100 epitope peptides
MXR – mixed response; GM-CSF – granulocyte macrophage colony-stimulating factor; MXR – mixed response; SD – stable disease; PD – progressive disease; PR – partial response; PSA – prostate-specific antigen; CTL – cytotoxic T-lymphocyte; NED – no evaluable disease
A trial was also completed employing intratumoral injection of vaccinia virus containing three different costimulatory molecules (rV-TRICOM) in patients (n=12 evaluable) with metastatic melanoma (Table 4). There was a 30.7% objective clinical response rate, with one patient achieving a complete response of more than 22 months.
rV-PSA has also been administered to 33 men with rising PSA or metastatic disease (Table 4). Patients in the highest dose cohort also received GM-CSF. Fourteen of 33 vaccinated patients were stable for at least 6 months; nine patients remained stable for 11–25 months and six of these patients remained progression free with stable PSA levels. Immunologic studies demonstrated specific T-cell responses directed against PSA. Certain patients remained without evidence of clinical progression for at least 21 months.
Alvac-CEA-B7 vaccine has also been evaluated in carcinoma patients.53–54 After an initial dose escalation phase, the vaccine was administered to patients (n=60) with advanced CEA-expressing tumors. All of the patients had evidence of leukocytic infiltration and CEA expression in vaccine biopsy sites. In patients receiving GM-CSF along with the vaccine (n=30), leukocyte infiltrates were enhanced. The addition of GM-CSF, however, did not statistically increase CEA-specific T cells in peripheral blood compared with vaccine alone. The number of prior chemotherapy regimens was negatively correlated with the generation of T-cell responses, whereas there was a positive correlation between the number of months from the last chemotherapy regimen and T-cell response. Patients receiving GM-CSF with vaccine also had a greater degree of disease stabilization for up to 13 months.
Alphavirus Trial
An alphavirus vector has also been evaluated in a phase I/II clinical trial. The cancer vaccine, designated AVX701 (CEA(6D)-VRP), is a recombinant alphavirus expressing CEA, packaged in virus-like replicon particles (VRP). The vaccine was given to patients (n=28) with metastatic cancer expressing the tumor antigen CEA. A majority of patients were able to develop CEA-specific T-cell and antibody responses. Despite the development of neutralizing antibodies to VRP, anti-tumor immunity was enhanced with each booster vaccination, but reached a maximum following the fourth vaccination. There was one complete response, two patients with stable disease, and two patients, who did not have evidence of metastatsis prior to immunization, remained disease free. Patients with CEA-specific T-cell responses had longer overall survival.29
Heterologous Prime-Boost: Vaccinia/Fowlpox
Prostate Cancer Trials
A clinical trial was conducted using rV- prime and rF- boost both with tyrosinase as a transgene, in combination with low- or high-dose IL-2, in patients with metastatic melanoma. T-cell responses to tyrosinase were observed only in a minority of patients and there was no significant difference in clinical benefit of vaccine plus IL-2 vs. IL-2 alone. A randomized multi-center phase II trial in patients with prostate cancer was evaluated employing different combinations of rV-PSA and rF-PSA in prime-boost regimens. No antibodies to PSA were observed, but all 30 patients evaluated demonstrated T-cell response to PSA peptide. Twenty-nine of 64 patients demonstrated no evidence of PSA progression at 19 months. Median time to progression was 9.2 months in patients receiving only rF-PSA, vs. 9.1 months in patients receiving rF-PSA prime and rV-PSA boost, vs. 18.2 months in patients receiving rV-PSA prime and rF-PSA boost. These findings demonstrating the superiority of rV- prime followed by rF-boost were also observed in a 50-month follow-up.
Another series of trials was conducted using an admix of recombinant vaccinias containing transgenes for PSA and for B7.1 followed by boosting with rF-PSA (Table 5). Using this vaccine regimen in patients with metastatic prostate cancer, T-cell responses were similar in patients receiving vaccine alone vs. vaccine in combination with docetaxel and steroids (dexamethasone). These studies thus demonstrated that neither the chemotherapy nor the steroids inhibited immune responses to the vaccine. Progression-free survival was increased when docetaxel was given following vaccine. In another study, patients with non-metastatic castrate-resistant prostate cancer (CRPC) were randomized to receive either vaccine or the hormone nilutamide and the combination therapy at progression (cross-over for each arm). The median survival at 4.4 years showed a trend in improvement in survival of patients who were initially randomized to the vaccine arm. A trend toward further improved overall survival was observed in patients who received vaccine before nilutamide vs. vaccine after nilutamide. In a third trial using this vaccine regimen, patients with localized prostate cancer received either vaccine plus radiotherapy or radiotherapy alone. No detectable increases in PSA-specific T-cell responses were seen in the radiotherapy arm only, while 13 of 17 patients in the vaccine plus radiotherapy arm demonstrated PSA-specific T-cell responses. There was also evidence of de novo generation of T cells to other prostate-associated antigens not found in the vaccine, providing indirect evidence of immune-mediated tumor killing.
Table 5.
Heterologous Prime-Boost: Vaccinia/Avipox Virus
| Vaccine Therapy Tumor Type (superscript number denotes reference) | Phase (No. of pts) | Immune Response to Vaccine Antigen | Clinical Outcome | |
|---|---|---|---|---|
| rV-/rF-TYRa (Prime-Boost) ± low or high dose IL-2 Melanoma94 | II (64) | 3/49 anti-tyrosinase antibodies T-cell response: 3/23 to tyrosinase peptides, 4/16 to full-length tyrosinase | Clinical benefit of vaccine + IL-2 is not significant vs. IL-2 alone | |
| PROSTATE CANCER | ||||
| rF-PSA (Arm A) vs. rF-PSA (prime), rV-PSA (boost) (Arm B) vs. rV-PSA (prime), rF-PSA (boost)39 | II (64) | 0/64 anti-PSA antibodies T-cell response: 30/30 to PSA peptide | 29/64 no PSA progression at 19.1 mo; 78.1% PFS at 19.1 mo Median TTP (rise in PSA): 9.2 (Arm A), 9.1 (Arm B), 18.2 (Arm C) 50 mo follow-up: 80% Arm A/B and 90% Arm C free of PD |
|
| rV-PSA/ rV-B7.1b(prime) rF-PSA (boost) + GM-CSF | ± Docetaxel, if PD on vaccine, Docetaxel given95 | II (28) | 0/28 anti-PSA antibodies 3.33 median fold increase in PSA-specific T cells in both treatment groups | 3/14 PSA decline in vaccine alone group: 0/3 >50% decline 6/14 PSA decline in vaccine + docetaxel group: 3/6 >75% decline |
| + IL-2 ± EBRT96 | II (30) | 13/17 had increase in PSA-specific T cells of at least 3-fold in combination therapy vs. no increase in radiotherapy-only arm | 2/17 vaccine + EBRT had biochemical failure at 20 mo follow-up 2/9 EBRT alone had biochemical failure at 25 mo follow-up |
|
| ± Nilutamide, if PD cross-over treatment group97–98 | II (42) | Improved OS when vaccine is given before nilutamide vs. after | ||
rV-/rF-TYR – recombinant vaccinia virus (rV-)/recombinant fowlpox virus (rF-) expressing full-length tyrosinase protein
rV-PSA/rV-B7.1 – admixture of two recombinant vaccinia viruses, one expressing transgene for PSA, the other expressing transgene for costimulatory molecule B7.1
CR – complete response; SD – stable disease; PSA – prostate-specific antigen; TTP – time to progression; PD – progressive disease; EBRT – external beam radiation therapy; OS overall survival
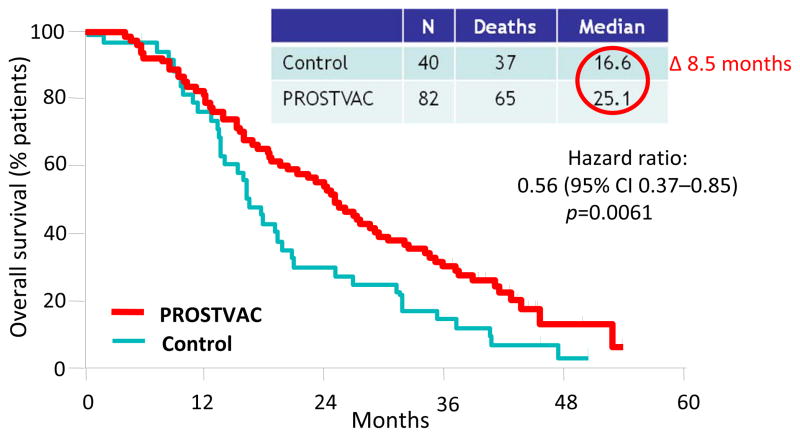
Several studies55–57 have now been conducted with rV- prime and multiple rF- boosts with rV-PSA-TRICOM (designated PROSTVAC) (Table 6). A 43-center randomized placebo (empty vector) controlled trial was carried out in patients (n=125) with metastatic castrate-resistant prostate cancer. GM-CSF was also employed locally with vaccine. There was no difference in either arm in progression-free survival. However, at 3 years post-study, PROSTVAC patients had a better overall survival (30%) vs. 17% for the control group (Figure 1). Patients in the vaccine arm had a longer median survival by 8.5 months (25.1 months vs. 16.6 months for control), with an estimated hazard ratio of 0.56 and stratified log rank P=0.0061.57 Vaccination with this off-the-shelf vaccine thus fared well compared to the FDA-approved Sipuleucel-T vaccine. A concurrent trial using PROSTVAC (Table 6) in patients with metastatic CRPC was also carried out.56 Twelve of 32 patients showed declines in serum PSA post-vaccination and two of 12 showed decreases in index lesions. Median overall survival was similar to that of the randomized trial and was 26.6 months (the predicted median overall survival for these patients employing the Halabi nomogram was 17.4 months). Patients with greater PSA-specific T-cell responses showed a trend (p=0.055) toward enhanced survival. Patients with a Halabi predicted survival of ≥18 months (median predicted survival 20.9 months) demonstrated a median overall survival of at least 37.3 months with 12 of 15 patients living longer than predicted. Regulatory T cell (Treg) suppressive function was shown to decrease following vaccine in patients surviving longer than predicted and to increase in patients surviving less than predicted. This hypothesis-generating study provided evidence that patients with more indolent metastatic prostate cancer may benefit more from vaccine therapy compared to similar patients receiving docetaxel chemotherapy. Recent clinical studies have also shown a potential for the use of the anti-CTLA4 antibody ipilimumab in combination with PROSTVAC to enhance survival. However, a randomized study must be conducted to validate this finding.
Table 6.
TRICOM-based Vaccines
| Vaccine Therapy Tumor Type (superscript number denotes reference) | Phase (No. of pts) | Immune Response to Vaccine Antigen | Clinical Outcome |
|---|---|---|---|
| PROSTATE CANCER: PROSTVAC-VFa | |||
| ± GM-CSF mCRPC56 | II (32) | 0/32 anti-PSA antibodies No immunological differences seen with addition of GM-CSF | 12/32 declines in PSA, 2/12 had decreases in index lesions Median OS was 26.6 mo (Halabi predicted OS-17.4 mo); 12/15 pts with Halabi predicted OS ≥18 mo are living longer than predicted (p = 0.035) |
| + GM-CSF vs. control vector mCRPC57 | II (125) | 0/125 anti-PSA antibodies | Vaccine group had longer median OS by 8.5 mo compared to pts receiving placebo, with 25/82 alive in vaccine group and 7/40 alive in placebo group, 3 years post-study |
| + GM-CSF Stage D0.5b Prostate Cancer55 | II (50) | Not yet available | 66% of pts had > 6 mo progression-free rate and increase in PSA-DT |
| Flutamide ± vaccine Non-Metastatic CRCP61 | II (26) | Ongoing | Median TTP: 223 days Flutamide + vaccine vs. 85 days Flutamide |
| + Ipilimumab mCRPC99 | I (30) | T-cell response to PSA detected | Median TTP (radiographic) 5.9 mo; Median OS 31.8 mo (Median Halabi predicted OS 18.5 mo); 74% survival probability at 24 mo |
| PAN-CARCINOMA: CEA-TRICOM and PANVAC-VFc | |||
| rF-CEA-TRICOM vs. rV-/rF-CEA-TRICOMd (prime-boost) ± GM-CSF Metastatic CEA+ tumors58 | I (58) | T-cell response: 10/13 to CEA peptide 6/33 increase in anti-CEA antibodies 11/58 decrease in CEA | 1/58 CR 23/58 SD (4mo), 14/23 prolonged SD (>6mo) |
| + GM-CSF Metastatic Breast and Ovarian Cancere |
I (26) | 6/14 increase in Teffector to Treg ratio | 1 CR, 4 SD 2/5 decrease in CEA levels; 2/14 decrease in CA-125 levels |
| Docetaxel ± PANVAC-VF + GM-CSF Metastatic Breast Cancer100 | II (15) | ongoing | Vaccine only:1/11 PR, 2/11 pts had measurable decrease in metastatic lesions Vaccine + Docetaxel: 1/3 PR, 2/3 pts had measurable clinical response |
| PANVAC-VF + GM-CSF Second-line Metastatic Pancreatic Cancer101 | III (255) | Did not meet primary endpoint of overall survival | |
| PANVAC s.c. vs. DC infected with PANVAC post-CRC metastectomy Lung and Liver Mets44–45 | II (72) | A trend for RFS with T-cell responses | 90% overall survival at 40 months vs. 58% in contemporary control group |
PROSTVAC-VF – heterologous prime-boost vaccine regimen using recombinant vaccinia (PROSTVAC-V, prime) and fowlpox virus (PROSTVAC-F, boost) expressing four transgenes: PSA (prostate-specific antigen) and three costimulatory molecules (B7.1, ICAM-1, LFA-3)
Stage D0.5 – Patients who has a rising PSA following local therapy for prostate cancer with no radiographic evidence of metastatic disease
PANVAC-VF – heterologous prime-boost vaccine regimen using recombinant vaccinia (PANVAC-V, prime) and fowlpox virus (PANVAC-F, boost) expressing five transgenes: CEA, MUC1 and three costimulatory molecules (B7.1, ICAM-1, LFA-3)
rV-/rF-CEA-TRICOM – recombinant vaccinia virus (rV-)/recombinant fowlpox virus (rF-) expressing 4 transgenes: tumor antigen CEA, and three costimulatory molecules (B7.1, ICAM-1, LFA-3)
Mohebtash et al., A pilot study of MUC-1/CEA/TRICOM poxviral-based vaccine in patients with metastatic breast and ovarian cancer (submitted manuscript)
GM-CSF – granulocyte macrophage colony-stimulating factor ; mCRPC – metastatic castrate-resistant prostate cancer; OS – overall survival; PSA-DT – PSA doubling time ; TTP – time to progression;, CR – complete response; SD – stable disease; PR – partial response; RECIST – Response Evaluation Criteria in Solid Tumors; RFS – relapse free survival; CRC – colorectal cancer
Figure 1. Overall survival advantage using PROSTVAC.
Overall survival of a randomized, placebo (empty vector) controlled 43-center trial of PROSTVAC (rV-, rF-PSA-TRICOM) vaccine in patients with metastatic castrate-resistant prostate cancer (mCRPC). There was an overall survival advantage of 8.5 months (p=0.006) and a 44% reduction in death in the vaccine arm. Adapted from Kantoff, et al.57
It is interesting to note that in both the Sipuleucel-T vaccine and the PROSTVAC vaccine trials in patients with metastatic prostate cancer, there was minimal objective response, no improvement in time to progression, but a statistically significant increase in overall survival. It has now been shown that in contrast to treatment with chemotherapy, tumor growth rate following vaccine therapy can be slowed. It thus appears that this reduction in growth rate is manifested in improved overall survival, and may be further influenced by subsequent additional therapy.
Pan Carcinoma Trials
The first clinical trial to employ CEA-TRICOM vaccine consisted of rV-CEA-TRICOM (designated “V”) and avipox (fowlpox) rF-CEA-TRICOM (“A”). Fifty-nine patients with advanced CEA positive progressing cancers were accrued (Table 6). Cohorts received AAAA alone, VAAA, or VAAA plus GM-CSF. Vaccines were administered every month for six doses and then every 3 months. Most patients had GI cancers and were heavily pretreated. There were no dose-limiting toxicities and no evidence of autoimmunity. Forty percent of patients had stable disease for more than 4 months, with one pathologic complete response; seven of these patients had been stable for more than 12 months. CEA-specific immune responses were observed in all HLA-A2 patients tested. Survival of patients in the VAAA groups receiving GM-CSF was greater than in the other groups and progression-free survival was related to CEA-specific T-cell responses.58 Of great interest, 12 patients who were stable on six monthly vaccines went on to receive vaccine every 3 months and all progressed; however, 6/12 of these patients restabilized after returning to monthly vaccinations.
To potentially overcome problems of antigenic heterogeneity or antigenic drift, TRICOM vaccines (rV- and rF-) containing transgenes for both CEA and MUC-1 were developed. This vaccine platform has been designated PANVAC. The first in-human study of this vaccine59 showed that immune responses to both CEA and MUC-1 could be simultaneously generated (Table 6). In addition, several patients had better than expected clinical responses. A patient with metastatic clear cell ovarian cancer with rapidly progressive disease had large symptomatic ascites that completely disappeared, and a CA-125 that went from 281 U/mL on study to sustained normal values (≤20 U/mL) for 18 months.60
A series of hypothesis-generating randomized phase II trials at NCI are comparing standard-of-care hormonal therapy, radiation therapy and chemotherapy alone, and in tandem with a poxviral TRICOM-based vaccine. In patients with non-metastatic CRPC, interval data favor patients receiving flutamide (an androgen receptor antagonist used as a second-line hormonal therapy) with PSA-TRICOM, compared to flutamide alone. With half of the patients enrolled on this 62-patient trial, median time to progression with flutamide alone is 85 days vs. 233 days employing flutamide with PROSTVAC.61 For patients with advanced metastatic castrate-resistant prostate cancer (mCRPC) who have already progressed on doctaxel, cohorts are randomized to Quadramet62 (chelated Samarium-153, a radiopharmaceutical that delivers localized radiation to bone metastasis and is FDA approved for palliation) vs. Quadramet plus PROSTVAC. In this trial now enrolling at three centers, there is a clear trend favoring Samarium-153 with PROSTVAC vs. Samarium-153 alone.63 In an ongoing trial, patients with metastatic breast cancer are randomized to either standard chemotherapy (docetaxel) alone vs. docetaxel plus PANVAC (rV, rF-CEA-MUC1-TRICOM), with time to progression as the endpoint.64 The hypothesis is that the combination therapy will take advantage of both modalities in tumor control. At this time there is again an interval trend in time to progression favoring the vaccine combination arm.
There are many anecdotal reports and several publications implying that patients who have received vaccine therapy and then progressed undergo unexpected clinical responses with the administration of subsequent therapies.65–68 This phenomenon, however, has not been validated prospectively to date. A randomized Eastern Cooperative Oncology Group, NCI (ECOG) multi-center clinical trial has recently been initiated in patients with metastatic prostate cancer to prospectively evaluate this phenomenon.69 Patients will receive either (a) docetaxel or (b) 2 months of PROSTVAC vaccine followed by docetaxel; survival will be the primary endpoint.
VI.Clinical Trial Design
A classic example of the distinction between a vaccine’s potential efficacy and a poor clinical trial design was evidenced by an ill-conceived corporate phase III trial in which PANVAC vaccine (Therion Biologics, Cambridge, MA, U.S.A.) was administered to patients with metastatic pancreatic cancer who had already failed prior gemcitabine therapy.70 Poor clinical trial design was clearly illustrated by (a) the median overall survival of less than 3 months in this patient population; (b) the fact that only one drug combination has been approved by the FDA for the therapy of pancreatic cancer (gemcitabine plus erlotinib), which extended survival by 0.4 months; and (c) in one phase III study, median second-line survival was 4.8 months for treatment with oxaliplatin, folinic acid and 5-FU vs. 2.3 months with best supportive care;71 numerous randomized trials of various FDA-approved drug combinations have failed to extend survival in this patient population of second-line pancreatic cancer.
PANVAC trials in other patient populations have provided evidence of patient benefit. Numerous preclinical studies have demonstrated that once a tumor reaches a certain volume, vaccine monotherapy will have limited effectiveness. In a multicenter trial led by M. Morse and K. Lyerly,44 patients (n=74) with no evidence of disease after resection of colorectal cancer (CRC) metastases to the liver and lung and completion of their physician-determined peri-operative chemotherapy were randomized 1:1 to four vaccinations with PANVAC, or PANVAC-modified dendritic cells. Data from a prospectively registered, comparable, contemporary control group of CRC patients at Duke who had undergone metastectomy were also available. The two vaccine arms and the contemporary controls were well balanced. The 2-year relapse-free survival (RFS) was similar in all groups: 50% for the DC-PANVAC group, 56% for the PANVAC group, and 55% for the contemporary control group. However, at a follow-up at approximately 40 months,44 there were 2/37 deaths in the DC-PANVAC group and 5/37 in the PANVAC alone group, for a total of approximately 10% deaths (7/74, i.e., a 90% overall survival at 40 months); this is in contrast to approximately 58% deaths in the contemporary control group, and with 3-year survival data of CRC patients post-metastectomy from five other trials.72–76 It is emphasized that this must be considered preliminary data in that the vaccine arms were not randomized to the contemporary control arm and randomized phase III study is warranted to confirm these results. It is of interest, however, that this is still another example of a vaccine trial showing little or no evidence of RFS, but with an apparent benefit in overall survival.
Concluding Comments
The TRICOM vaccine platforms (PROSTVAC and PANVAC) and other viral vector–based vaccines described here have demonstrated minimal toxicity in a wide range of tumor types, different stages of disease, and in combination with radiation, chemotherapy and hormone therapy. Future directions will involve their evaluation in the adjuvant and neo-adjuvant settings, as well as in patients with low burden metastatic disease. These viral-based vaccines will undoubtedly also be used in combination with other forms of immunotherapy including checkpoint inhibitors, cytokines and other immune stimulants, adoptive T-cell transfer, and other vaccine types.
The “off-the-shelf” nature of viral vaccine platforms renders them exceptionally suitable for multicenter randomized trials. While evidence of patient benefit has been seen in randomized phase II studies, only phase III trials in the appropriate patient populations and with the appropriate clinical endpoints for therapeutic vaccines will define if these viral-based vaccine platforms will have a place in cancer management either as a monotherapy or in combination therapies.
Acknowledgments
We thank Debra Weingarten for her excellent editorial assistance in the preparation of this manuscript.
Funding disclosure: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH; Howard Hughes Medical Institute (HHMI)
Footnotes
The authors have no conflicts of interest to declare.
Contributor Information
Cecilia Larocca, Howard Hughes Medical Institute, Research Scholars’ Program at the NIH; Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute, Bethesda, MD
Jeffrey Schlom, Chief, Laboratory of Tumor Immunology and Biology Center for Cancer Research, National Cancer Institute, Bethesda, MD
References
- 1.Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci U S A. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown M, Davies DH, Skinner MA, et al. Antigen gene transfer to cultured human dendritic cells using recombinant avipoxvirus vectors. Cancer Gene Ther. 1999;6:238–245. doi: 10.1038/sj.cgt.7700014. [DOI] [PubMed] [Google Scholar]
- 3.Drillien R, Spehner D, Bohbot A, et al. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology. 2000;268:471–481. doi: 10.1006/viro.2000.0203. [DOI] [PubMed] [Google Scholar]
- 4.Bonini C, Lee SP, Riddell SR, et al. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells. J Immunol. 2001;166:5250–5257. doi: 10.4049/jimmunol.166.8.5250. [DOI] [PubMed] [Google Scholar]
- 5.Hodge JW, Chakraborty M, Kudo-Saito C, et al. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang S, Tsang KY, Schlom J. Induction of higher-avidity human CTLs by vector-mediated enhanced costimulation of antigen-presenting cells. Clin Cancer Res. 2005;11:5603–5615. doi: 10.1158/1078-0432.CCR-05-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantor J, Irvine K, Abrams S, et al. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084–1091. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 8.Kass E, Schlom J, Thompson J, et al. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676–683. [PubMed] [Google Scholar]
- 9.Li N, Zhou J, Weng D, et al. Adjuvant adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of liver transplantation in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2007;13:5847–5854. doi: 10.1158/1078-0432.CCR-07-0499. [DOI] [PubMed] [Google Scholar]
- 10.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8:12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 11.Myers R, Greiner S, Harvey M, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- 12.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou E. Oncolytic herpes viruses as a potential mechanism for cancer therapy. Acta Oncol. 2003;42:660–671. doi: 10.1080/0284186031000518. [DOI] [PubMed] [Google Scholar]
- 14.Bridle BW, Boudreau JE, Lichty BD, et al. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol Ther. 2009;17:1814–1821. doi: 10.1038/mt.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanis E. Therapeutic potential of oncolytic measles virus: promises and challenges. Clin Pharmacol Ther. 2010;88:620–625. doi: 10.1038/clpt.2010.211. [DOI] [PubMed] [Google Scholar]
- 16.Lech PJ, Russell SJ. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev Vaccines. 2010;9:1275–1302. doi: 10.1586/erv.10.124. [DOI] [PubMed] [Google Scholar]
- 17.Fenner F, Henderson D, Arita I. Smallpox and its eradication. Geneva: World Health Organization; 1988. [Google Scholar]
- 18.Jacobs BL, Langland JO, Kibler KV, et al. Vaccinia virus vaccines: past, present and future. Antiviral Res. 2009;84:1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afonso CL, Tulman ER, Lu Z, et al. The genome of fowlpox virus. J Virol. 2000;74:3815–3831. doi: 10.1128/jvi.74.8.3815-3831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tulman ER, Afonso CL, Lu Z, et al. The genome of canarypox virus. J Virol. 2004;78:353–366. doi: 10.1128/JVI.78.1.353-366.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsang KY, Zaremba S, Nieroda CA, et al. Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KL, Smith GL. Vaccinia virus morphogenesis and dissemination. Trends Microbiol. 2008;16:472–479. doi: 10.1016/j.tim.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Somogyi P, Frazier J, Skinner MA. Fowlpox virus host range restriction: gene expression, DNA replication, and morphogenesis in nonpermissive mammalian cells. Virology. 1993;197:439–444. doi: 10.1006/viro.1993.1608. [DOI] [PubMed] [Google Scholar]
- 24.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–5777. [PubMed] [Google Scholar]
- 25.Sutter G, Staib C. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr Drug Targets Infect Disord. 2003;3:263–271. doi: 10.2174/1568005033481123. [DOI] [PubMed] [Google Scholar]
- 26.Pastoret PP, Vanderplasschen A. Poxviruses as vaccine vectors. Comp Immunol Microbiol Infect Dis. 2003;26:343–355. doi: 10.1016/S0147-9571(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 27.Kundig TM, Kalberer CP, Hengartner H, et al. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine. 1993;11:1154–1158. doi: 10.1016/0264-410x(93)90079-d. [DOI] [PubMed] [Google Scholar]
- 28.Taylor J, Paoletti E. Fowlpox virus as a vector in non-avian species. Vaccine. 1988;6:466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- 29.Morse MA, Hobeika AC, Osada T, et al. An alphavirus vector overcomes the presence of neutralizing antibodies and elevated numbers of Tregs to induce immune responses in humans with advanced cancer. J Clin Invest. 2010;120:3234–3241. doi: 10.1172/JCI42672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlesinger S. Alphavirus vectors: development and potential therapeutic applications. Expert Opin Biol Ther. 2001;1:177–191. doi: 10.1517/14712598.1.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Kelly BJ, Fleeton MN, Atkins GJ. Potential of alphavirus vectors in the treatment of advanced solid tumors. Recent Pat Anticancer Drug Discov. 2007;2:159–166. doi: 10.2174/157489207780832432. [DOI] [PubMed] [Google Scholar]
- 32.Quetglas JI, Ruiz-Guillen M, Aranda A, et al. Alphavirus vectors for cancer therapy. Virus Res. 2010;153:179–196. doi: 10.1016/j.virusres.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 33.Dharmapuri S, Peruzzi D, Aurisicchio L. Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin Biol Ther. 2009;9:1279–1287. doi: 10.1517/14712590903187053. [DOI] [PubMed] [Google Scholar]
- 34.Arlen PM, Kaufman HL, DiPaola RS. Pox viral vaccine approaches. Semin Oncol. 2005;32:549–555. doi: 10.1053/j.seminoncol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 36.Marshall JL, Hoyer RJ, Toomey MA, et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 37.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 38.Grosenbach DW, Feldman J, Schlom J, et al. Recombinant viral and bacterial vaccines. In: Kaufman H, Wolchok JD, editors. General Principles of Tumor Immunotherapy: Basic and CLinical Applications of Tumor Immunology. The Netherlands: Springer; 2008. pp. 217–250. [Google Scholar]
- 39.Kaufman HL, Wang W, Manola J, et al. Phase II prime/boost vaccination using poxviruses expressing PSA in hormone dependent prostate cancer: follow-up clincial results from ECOG 7897. J Clin Oncol; ASCO Annual Meeting.2005. p. 16S.p. abstr 4501. [Google Scholar]
- 40.Kaufman HL, Cohen S, Cheung K, et al. Local delivery of vaccinia virus expressing multiple costimulatory molecules for the treatment of established tumors. Hum Gene Ther. 2006;17:239–244. doi: 10.1089/hum.2006.17.239. [DOI] [PubMed] [Google Scholar]
- 41.Palena C, Zhu M, Schlom J, et al. Human B cells that hyperexpress a triad of costimulatory molecules via avipox-vector infection: an alternative source of efficient antigen-presenting cells. Blood. 2004;104:192–199. doi: 10.1182/blood-2003-09-3211. [DOI] [PubMed] [Google Scholar]
- 42.Litzinger MT, Foon KA, Sabzevari H, et al. Chronic lymphocytic leukemia (CLL) cells genetically modified to express B7-1, ICAM-1, and LFA-3 confer APC capacity to T cells from CLL patients. Cancer Immunol Immunother. 2009;58:955–965. doi: 10.1007/s00262-008-0611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh S, Hodge JW, Ahlers JD, et al. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003;170:2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 44.Lyerly HK, Hobeika A, Niedzwiecki D, et al. A dendritic cell-based vaccine effects on T-cell responses compared with a viral vector vaccine when administered to patients following resection of colorectal metastases in a randomized phase II study. J Clin Oncol; 2011; ASCO Annual Meeting.2011. p. abstr 2533. [Google Scholar]
- 45.Morse M, Niedzwiecki D, Marshall J, et al. Survival rates among patients vaccinated following resection of colorectal cancer metastases in a phase II randomized study compared with contemporary controls. J Clin Oncol; 2011; ASCO Annual Meeting.2011. p. abstr 3557. [Google Scholar]
- 46.Kass E, Panicali DL, Mazzara G, et al. Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res. 2001;61:206–214. [PubMed] [Google Scholar]
- 47.Reali E, Canter D, Zeytin H, et al. Comparative studies of Avipox-GM-CSF versus recombinant GM-CSF protein as immune adjuvants with different vaccine platforms. Vaccine. 2005;23:2909–2921. doi: 10.1016/j.vaccine.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 48.Kudo-Saito C, Garnett CT, Wansley EK, et al. Intratumoral delivery of vector mediated IL-2 in combination with vaccine results in enhanced T cell avidity and anti-tumor activity. Cancer Immunol Immunother. 2007;56:1897–1910. doi: 10.1007/s00262-007-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudo-Saito C, Wansley EK, Gruys ME, et al. Combination therapy of an orthotopic renal cell carcinoma model using intratumoral vector-mediated costimulation and systemic interleukin-2. Clin Cancer Res. 2007;13:1936–1946. doi: 10.1158/1078-0432.CCR-06-2398. [DOI] [PubMed] [Google Scholar]
- 50.Perera LP, Waldmann TA, Mosca JD, et al. Development of smallpox vaccine candidates with integrated interleukin-15 that demonstrate superior immunogenicity, efficacy, and safety in mice. J Virol. 2007;81:8774–8783. doi: 10.1128/JVI.00538-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Wang S, Shan B, et al. Advances in viral-vector systemic cytokine gene therapy against cancer. Vaccine. 2010;28:3883–3887. doi: 10.1016/j.vaccine.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 52.Heery CR, Pinto PA, Schlom J, et al. Intraprostatic PSA-TRICOM vaccine administration in patients with locally recurrent prostate cancer. J Clin Oncol; 2011; ASCO Annual Meeting.2011. p. abstr 2530. [Google Scholar]
- 53.von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res. 2000;6:2219–2228. [PubMed] [Google Scholar]
- 54.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed] [Google Scholar]
- 55.DiPaola RS, Chen Y, Bubley GJ, et al. A Phase II study of PROSTVAC-V (vaccinia)/TRICOM and PROSTVAC-F (fowlpox)/TRICOM with GM-CSF in patients with PSA progression after local therapy for prostate cancer: results of ECOG. ASCO Genitourinary Cancers Symposium.2009. p. abstr 108. [Google Scholar]
- 56.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59:663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 59.Gulley JL, Arlen PM, Tsang KY, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Cancer Institute Clinical Trials (PDQ) [Accessed: October 2007]; http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=389439&version=HealthProfessional&protocolsearchid=2897251.
- 61.Bilusic M, Gulley J, Heery C, et al. A randomized phase II study of flutamide with or without PSA-TRICOM in nonmetastatic castration-resistant prostate cancer. J Clin Oncol; 2011; ASCO Genitourinary Cancer Symposium.2011. p. abstr 163. [Google Scholar]
- 62.Anderson P, Nunez R. Samarium lexidronam (153Sm-EDTMP): skeletal radiation for osteoblastic bone metastases and osteosarcoma. Expert Rev Anticancer Ther. 2007;7:1517–1527. doi: 10.1586/14737140.7.11.1517. [DOI] [PubMed] [Google Scholar]
- 63.153Sm-EDTMP With or Without a PSA/TRICOM Vaccine To Treat Men With Androgen-Insensitive Prostate Cancer. [Accessed April 11, 2011]; http://www.clinicaltrials.gov/ct2/show/NCT00450619?term=Samarium+vaccine&rank=1.
- 64. [Accessed April 11, 2011];Docetaxel alone or in combination with vaccine to treat breast cancer. http://www.clinicaltrials.gov/ct2/show/NCT00179309?term=gulley&rank=9.
- 65.Petrylak D. Defining the optimal role of immunotherapy and chemotherapy: Advanced prostate cancer patients who receive sipuleucel-T (PROVENGE) followed by docetaxel derive greatest survival benefit. 14th Annual Meeting of the Chemotherapy Foundation Symposium; New York, NY. November 8–11, 2006. [Google Scholar]
- 66.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 67.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 68.Arlen PM, Pazdur M, Skarupa L, et al. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clin Breast Cancer. 2006;7:176–179. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 69.Docetaxel and prednisone with or without vaccine therapy in treating patients with metastatic hormone-resistant prostate cancer. [Accessed April 11, 2011]; http://www.clinicaltrials.gov/ct2/show/NCT01145508?term=McNeel&rank=5.
- 70.Madan RA, Arlen PM, Gulley JL. PANVAC-VF: poxviral-based vaccine therapy targeting CEA and MUC1 in carcinoma. Expert Opin Biol Ther. 2007;7:543–554. doi: 10.1517/14712598.7.4.543. [DOI] [PubMed] [Google Scholar]
- 71.Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676–1681. doi: 10.1016/j.ejca.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. discussion 722–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andres A, Majno PE, Morel P, et al. Improved long-term outcome of surgery for advanced colorectal liver metastases: reasons and implications for management on the basis of a severity score. Ann Surg Oncol. 2008;15:134–143. doi: 10.1245/s10434-007-9607-1. [DOI] [PubMed] [Google Scholar]
- 75.Arru M, Aldrighetti L, Castoldi R, et al. Analysis of prognostic factors influencing long- term survival after hepatic resection for metastatic colorectal cancer. World J Surg. 2008;32:93–103. doi: 10.1007/s00268-007-9285-y. [DOI] [PubMed] [Google Scholar]
- 76.House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 77.Hawkins RE, Macdermott C, Shablak A, et al. Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother. 2009;32:424–429. doi: 10.1097/CJI.0b013e31819d297e. [DOI] [PubMed] [Google Scholar]
- 78.Amato RJ, Shingler W, Goonewardena M, et al. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32:765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 79.Kaufman HL, Taback B, Sherman W, et al. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma (Published online 2009 January 7. doi: 10.1186/1479-5876-7-2) J Transl Med. 2009:7. doi: 10.1186/1479-5876-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Amato RJ, Shingler W, Naylor S, et al. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res. 2008;14:7504–7510. doi: 10.1158/1078-0432.CCR-08-0668. [DOI] [PubMed] [Google Scholar]
- 81.Amato RJ, Hawkins RE, Kaufman HL, et al. Vaccination of metastatic renal cancer patients with MVA-5T4: a randomized, double-blind, placebo-controlled phase III study. Clin Cancer Res. 2010;16:5539–5547. doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 82.Oudard S, Rixe O, Beuselinck B, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother. 2011;60:261–271. doi: 10.1007/s00262-010-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harrop R, Connolly N, Redchenko I, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 84.Elkord E, Dangoor A, Drury NL, et al. An MVA-based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother. 2008;31:820–829. doi: 10.1097/CJI.0b013e3181876ab3. [DOI] [PubMed] [Google Scholar]
- 85.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin and irinotecan) is safe and induces potent immune responses. Cancer Immunol Immunother. 2008;57:977–986. doi: 10.1007/s00262-007-0428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Harrop R, Drury N, Shingler W, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res. 2007;13:4487–4494. doi: 10.1158/1078-0432.CCR-07-0704. [DOI] [PubMed] [Google Scholar]
- 87.Amato RJ, Drury N, Naylor S, et al. Vaccination of prostate cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother. 2008;31:577–585. doi: 10.1097/CJI.0b013e31817deafd. [DOI] [PubMed] [Google Scholar]
- 88.Dreicer R, Stadler WM, Ahmann FR, et al. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs. 2009;27:379–386. doi: 10.1007/s10637-008-9187-3. [DOI] [PubMed] [Google Scholar]
- 89.Ramlau R, Quoix E, Rolski J, et al. A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008;3:735–744. doi: 10.1097/JTO.0b013e31817c6b4f. [DOI] [PubMed] [Google Scholar]
- 90.Scholl SM, Balloul JM, Le Goc G, et al. Recombinant vaccinia virus encoding human MUC1 and IL2 as immunotherapy in patients with breast cancer. J Immunother. 2000;23:570–580. doi: 10.1097/00002371-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 91.Zajac P, Oertli D, Marti W, et al. Phase I/II clinical trial of a nonreplicative vaccinia virus expressing multiple HLA-A0201-restricted tumor-associated epitopes and costimulatory molecules in metastatic melanoma patients. Hum Gene Ther. 2003;14:1497–1510. doi: 10.1089/104303403322495016. [DOI] [PubMed] [Google Scholar]
- 92.Spaner DE, Astsaturov I, Vogel T, et al. Enhanced viral and tumor immunity with intranodal injection of canary pox viruses expressing the melanoma antigen, gp100. Cancer. 2006;106:890–899. doi: 10.1002/cncr.21669. [DOI] [PubMed] [Google Scholar]
- 93.Kaufman HL, Lenz HJ, Marshall J, et al. Combination chemotherapy and ALVAC- CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res. 2008;14:4843–4849. doi: 10.1158/1078-0432.CCR-08-0276. [DOI] [PubMed] [Google Scholar]
- 94.Lindsey KR, Gritz L, Sherry R, et al. Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma. Clin Cancer Res. 2006;12:2526–2537. doi: 10.1158/1078-0432.CCR-05-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arlen PM, Gulley JL, Parker C, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gulley JL, Arlen PM, Bastian A, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 97.Arlen PM, Gulley JL, Todd N, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 98.Madan RA, Gulley JL, Schlom J, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14:4526–4531. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Madan RA, Mohebtash M, Arlen PM, et al. Overall survival analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab in the treatment of metastatic castration-resistant prostate cancer. J Clin Oncol; 2010; ASCO Annual Meeting.2010. p. 15s.p. abstr 2550. [Google Scholar]
- 100.Mohebtash M, Madan RA, Gulley JL, et al. PANVAC vaccine alone or with docetaxel for patients with metastatic breast cancer. J Clin Oncol; 2008; ASCO Annual Meeting.2008. May, p. abstr 3035. [Google Scholar]
- 101.Therion Biologics Announces Conclusion of PANVAC-VF Phase 3 Trial. 2006 http://www.medicalnewstoday.com/medicalnews.php?newsid=46137.