Abstract
Ultrashort UV pulses at 258 nm with repetition rate of 10 kHz have been used to irradiate buffer solution of antibody. The tryptophan residues strongly absorb this radiation thus becoming capable to disrupt the disulfide bridges located next to them. Due to their high reactivity the opened bridges can anchor a gold plate more efficiently than other sites of the macromolecule giving rise to preferential orientations of the variable part of the antibody. UV irradiation has been applied to anchor antiIgG antibody to the electrode of a Quartz Crystal Microbalance (QCM) that lends itself as a sensor, the antibody acting as the bio-receptor. An increase of the QCM sensitivity and of the linear range has been measured when the antibody is irradiated with UV laser pulses. The photo-induced reactions leading to disulfide bridge breakage have been analyzed by means of a chemical assay that confirms our explanation. The control of disulfide bridges by UV light paves the way to important applications for sensing purpose since cysteine in combination with tryptophan can act as a hook to link refractory bio-receptors to surfaces.
OCIS codes: (280.1415) Biological sensing and sensors, (350.3450) Laser-induced chemistry, (170.0170) Medical optics and biotechnology
1. Introduction
The quest for label free biosensors, in which no tag is applied to molecule to be detected, is underpinning the development of quartz-crystal-microbalance (QCM) based sensors [1]. Based on the measurement of the mass of the analyte, they also offer high versatility for different applications and are relatively low-priced and high sensitive, with the main constraint being the need of a specific binding partner (e.g. polyclonal antibody) for the molecule to be detected. Although this might appear a severe limitation for QCMs, the actual availability of antibodies for a wide range of analytes as well as their performances in terms of detection limit make these sensors very appealing for bio-sensing (an extensive review about the features of different types of biosensors can be found in ref [2].). As opposed to optical biosensors, QCMs are able to operate directly in complex liquids without the need for purification and their fast response and reliability have been proved even in the virus detection [3]. In applications like DNA assembly and hybridization no significant difference was found in comparison to a widespread technique such as Surface Plasmon Resonance with regard to linear range as well as limit of detection [4].
In QCMs the analyte is recognized by bio-receptors immobilized on the sensor surface: the larger the density of active bio-receptors, the higher the effectiveness of the biosensor. The strategies to anchor bio-receptors on the gold surface of the QCMs can be gathered into two basic categories: passive adsorption or covalent immobilization. The former relies upon non-specific interaction between the bio-receptor (e.g. antibody) and the gold surface. This procedure is fast and simple, but as a consequence of the nonspecific interaction it leads to relatively unstable anchoring as well as randomly oriented bio-receptors thus affecting the sensing effectiveness. On the opposite the covalent immobilization is based upon specific (covalent) interaction between the functional residues linked on the surface and the bio-receptors. This gives rise to specific and stable bonds, but the surface functionalization is generally achieved by time consuming procedures requiring qualified expertise and quite advanced technology [5,6]. Alternative tools to surface activation are of general interest in biosensing research since it is a non-avoidable step in biosensors based on fiber optics [7], surface plasmon resonance or interferometry [8].
As a method to overcome such drawbacks it has been proposed the use of UV light to break the disulfide bridges in protein upon absorption by nearby aromatic amino acids [9]. In fact, the bridge opening leads to the formation of reactive thiol groups that are very effective in bonding many surfaces, gold made plate among the other. Moreover, since the anchor link is determined by the position of the thiol group, the orientation of the bio-receptor on the plate can be controlled to a large extent. This is especially true when antibody is used as bio-receptor, since if the antibody were anchored to the plate through its variable part (the so-called Fab, Fragment Antigen Binding) the recognition would be hampered [10]. The disulfide bond breaking can be realized through the interaction with excited endogenous tryptophan residue [11], the latter being easily accomplished exploiting the high UV absorption by tryptophan [12].
In this paper we report on the antibody anchoring to a QCM gold electrode for sensing applications. Since the aromatic aminoacid absorption is the springboard for the bond breakage, high UV mean power is needed. Moreover, if the dissociation must be kept low, femtosecond rather than nanosecond pulses are appropriate in view of the resonant one photon excitation occurring in aromatic aminoacid in the UV range [13,14]. Thus, we have used the fourth harmonic of a 1030 nm femtosecond laser pulse delivered at 10 kHz repetition rate to irradiate the antiIgG from goat to detect IgG from mouse observing an increase in the detection efficiency explained in terms of improved orientation of antibody on the electrode. This antibody has 12 disulfide bridges at a distance from tryptophan not longer than approximately 5 Å [15,16]. These are located in such a way that their interaction with the gold surface prevents the antibody from anchoring upside down, which would impede the antigen recognition.
The mechanism underlying the light induced antibody anchoring is described in Fig. 1 . The tryptophan residues absorb in the UV range and relax by transferring the absorbed energy to its neighbors. The cysteines are close enough to the tryptophans so that the photon energy is virtually absorbed by the former. The resulting free thiol groups are highly reactive so that they can efficiently link the gold surfaces of a QCM.
Fig. 1.
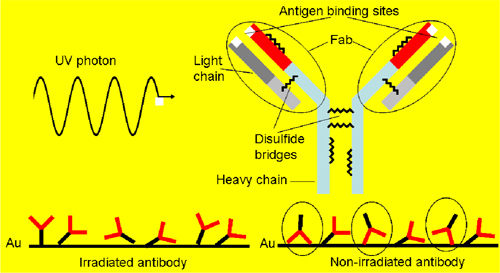
(a) One UV photon is absorbed by the antibody and the disulfide bridge is opened thereby forming thiol groups. Their interaction with the gold surface leads to oriented Fab region so that the upside down position (circled in the right side of the picture) is hampered and the antigen binding is more effective.
2. QCM based biosensors and experimental procedure
A quartz crystal microbalance (QCM, μLibra, Technobiochip, Italy) with a fundamental frequency f0 of 10 MHz and gold electrodes was used in the experiments. The frequency shift Δf of the quartz crystal after bio-receptor adsorption was measured at the first overtone order, i.e.10 MHz. The relation between the frequency change and the mass deposition Δm is given by the Sauerbrey equation [17] from which we have Δf = -K(Δm), K being a constant depending on several experimental parameters (resonance frequency, piezoelectrically active crystal active area, quartz density and shear modulus for AT-cut crystal).
Figure 2 shows the pipeline system we have used to convey the solutions to the gold plate. The solution is drawn from a cuvette by peristaltic pump allowing a laminar flow onto the plate. To irradiate the antibody the laser was shined into the cuvette for 5′ before the pump was switched on and was kept on while the antibody was flowing into the pipeline circuit. The UV light was delivered by a femtosecond laser system (Pharos, Light Conversion, http://www.lightcon.com/) operating at 10 kHz repetition rate. The energy of the fourth harmonic (λ = 258 nm) was 30 μJ resulting in 0.3 W average power laser brought to the sample with no further focusing.
Fig. 2.
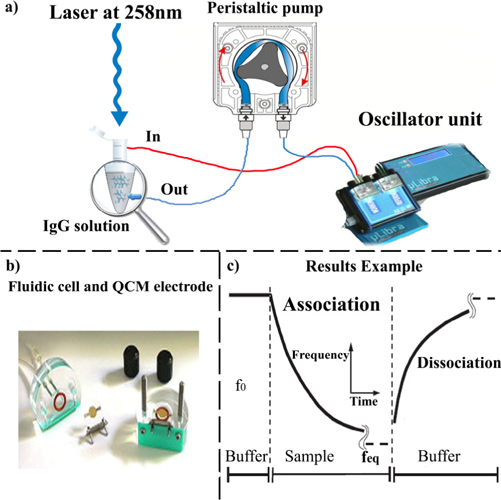
(a) Experimental setup to convey the molecules to the electrode. (b) QCM cell for fluidic applications with gold electrodes. (c) Typical output showing the decrease of the frequency due to the association (anchoring) and the frequency rise produced by the dissociation (unanchoring).
Libra microbalance uses 10 MHz AT-cut quartzes with gold electrodes on chromium layer. An alternating voltage applied to the electrodes causes the quartz to resonate at a particular frequency, and resonant frequency difference is directly proportional to the mass change. A simple model system, IgG mouse as antigen and anti-mouse IgG as antibody, has been used to evaluate the effect of the light assisted antibody immobilization on the biosensor.
Briefly, the experimental protocol was
-
1)
Initial QCM wash with 1x Phosphate Buffer (PBS) pH 7.4 for basal resonant frequency stabilization.
-
2)
Light- assisted adsorption or passive adsorption (as control) of anti-mouse IgG (Sigma, Milan).
-
3)
Wash with PBS 1x to eliminate the excess of anti-mouse IgG from goat.
-
4)
Blocking with Bovin Serum Albumin (BSA) solution (100 μg/mL) to avoid nonspecific-binding. In fact, the QCM gold surface used have a high affinity for proteins. Therefore, after the anti-mouse IgG immobilization, it is important to block the remaining gold surface to prevent non-specific binding of the detection antibodies during subsequent steps.
-
5)
Wash with PBS 1x to eliminate BSA in excess.
-
6)
Flowing of mouse-IgG (Sigma, Milan) to allow the specific antigen-antibody complex formation.
-
7)
Final wash with PBS 1x to eliminate weakly bonded mouse IgG .
The experiment were performed in triplicate in both fluidic cells (working and reference) showing a good intra-assay accuracy and reproducibility between channels. The QCM response, i.e. Δf versus time, is shown in Fig. 3 for each of the seven protocol steps, for 5µg/mL of anti IgG and 1µg/mL of mouse IgG: The solid and dashed lines refer to the non-irradiated and irradiated antibody, respectively. The comparison between the two curves evidences a larger amount of detected antigen when the antibody is irradiated, while no significant change in the anchored antibody is observed. This is consistent with the mechanism reported in Fig. 1, according to which the bond breakage facilitates the right orientation of the antibody on the plate, rather than increasing the number of anchored antibodies.
Fig. 3.
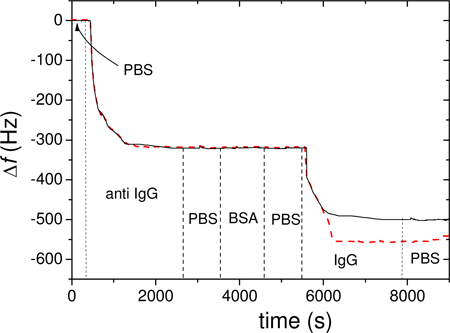
QCM output obtained with with 5µg/mL of anti IgG) and 1µg/mL of mouse IgG of non-irradiated (black solid line) and irradiated antibody (red dashed line). The vertical dashed lines show the steps described in the text.
3. Results
3.1 Effect of antibody irradiation on QCM performances
In Fig. 4 we report the frequency shifts as a function of antigen concentration flowing onto the plate. We have analyzed two conditions: in the first the antibodies were adsorbed without any previous interaction with the UV light (black squares) whereas in the latter the antibodies were irradiated before and during the adsorption (see previous section) by the gold electrode of QCM (red circles). The clear enhancement of detector response is observed when the antibodies are previously irradiated with the UV light which can be interpreted by taking into account the steric effect induced by the disulfide bridge breaking when the antibody is irradiated. More specifically, the otherwise random antibody orientation on the gold plate is influenced by the opened disulfide bond in such a way that the Fab fragments are more exposed to the antigen [9,11]. The free sulphur favors the “right” antibody orientation and, hence, the antigen capture probability increases as well as the total amount of linkable antigen. In this view, the functional form of the curves in Fig. 4 and its dependence on the relevant experimental parameters can be deduced by the following argument.
Fig. 4.
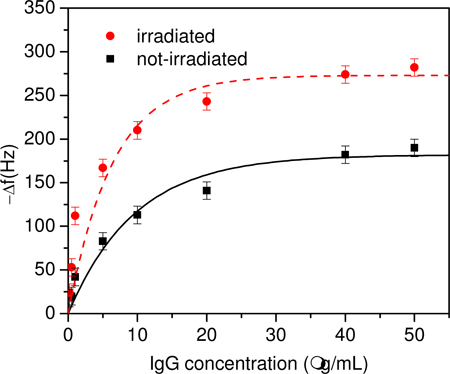
Frequency shift measured with (red circle) irradiation and without (black square) antibody UV irradiation as a function of the antigen mass concentration. The best fit obtained with Eq. (3) provides (Δf)max = 182 Hz and [M]0 = 9.6 μg/mL for the non-irradiated sample. For the irradiated sample the dashed curve is Eq. (3) with (Δf)max,IRR = 273 Hz and [M]0,IRR = 6.4 μg/mL.
The link between the antibody and the antigen occurs with a probability p, i.e. p is the probability for an antigen to be captured while passing onto the plate when all the N antibodies are free. As a consequence of the reduction of the available sites for antigen binding, the second antigen will have a probability to be fixed on the plate, whereas for the third the probability will be and so on. The occupancy rate r of the N antibodies will be
| (1) |
where the summation is extended to M antigens interacting with N antibodies. As M→∞ and p→0 we have
| (2) |
The proportionality between Δf and the antigen mass deposited on the plate, which in turn is proportional to the concentration flowing in the fluidic circuit, leads to
| (3) |
where [M] is the mass concentration, [M]0 a “characteristic mass concentration” whose value is an estimation of the linear range of the antigen sensor and a fit parameter. The best fit of the data obtained when the sample is not irradiated provides (Δf)max = 182 Hz and [M]0 = 9.6 μg/mL and the resulting curve being in good agreement with the experimental results (black squares).
The experimental data obtained when the antibody is irradiated show an increase in the slope as well as in the saturation level. This can be explained by considering that the irradiation brings on oriented deposition of antibodies with their variable part facing up. As a consequence, more antibodies are effective for antigen linking thus increasing the probability p for an antigen to be linked during the flow. Since [M]0∝p−1 [Eq. (2)] the characteristic concentration decreases and the slope increases. The increase in the number of effective linking sites also accounts in a natural way for the larger saturation value for which (Δf)max∝p is expected. This means that (Δf)max and [M]0 should scale by the same factor when the effective antibody density is changed, i.e.
| (4) |
In Eq. (4) the subscript IRR refers to the values of the parameters when the antibody is irradiated. The fair fit of the experimental data (red dashed line in Fig. 4) has been obtained with that yields (Δf)max,IRR = 273 Hz and [M]0,IRR = 6.4 μg/mL in Eq. (3). Assuming a linear dependence of the binding probability on the number of effective antibodies, Eq. (4) states that when irradiated approximately 50% more antiIgG are available to bind the antigen. Not only more bio-receptors on the electrode means more detectable antigens, thereby extending the linear range, but also larger antigen binding probability and, hence, higher sensitivity. With the help of Eq. (4), the latter can be easily worked out to be
| (5) |
SIRR and S being the sensitivity with and without the irradiation procedure. Thus, in our case the antibody irradiation more than doubles the sensor sensitivity.
3.2 Ellman assay
To verify the occurrence of disulfide bridge breaking we have carried out the Ellman assay based on a reagent [5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB] able to quantify the concentration of thiol groups in a sample [18]. The reaction of the thiol with DTNB gives the mixed disulfide and 2-nitro-5-thiobenzoic acid (TNB) that is quantified by the absorbance of the anion (TNB2-) at 412nm. The reagent has been widely used for the quantification of thiols in peptides and proteins and it has also been used to assay the disulfides present after blocking any free thiols and reducing the disulfides prior to reaction with the reagent [18].
The amount of free thiol groups as a function of the irradiation time and average irradiation power are reported in Figs. 5 and 6 .
Fig. 5.
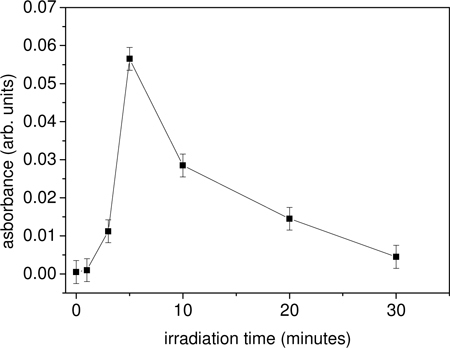
Absorbance of antibody at 412 nm as a function of the irradiation time. The average laser power is 0.3 W. The reagent DTNB is added immediately after the laser exposure providing a measurement of the opened disulfide bridges.
Fig. 6.
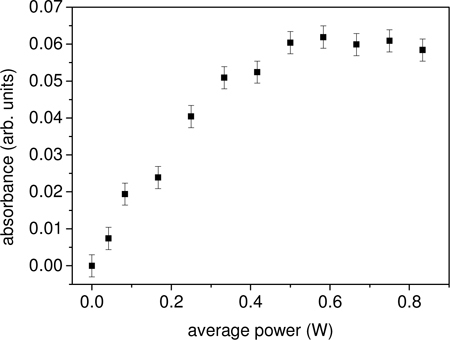
Absorbance of antibody at 412 nm as a function of the average laser power. The irradiation time is 5′.
The existence of both an optimal irradiation time as well as a saturation effect when the energy per pulse (i.e. the average power) is increased suggests that the disulfide bridges have two loss channels after they have been opened by a UV photon: they can close again being available for a further opening or, alternatively, they can be “destroyed”, e.g. by linking each other as a consequence of their high reactivity, thus giving rise to a net loss of molecule available for absorption. However, the Ellman assay demonstrates that the experimental conditions we have used really maximize the production of free thiol groups thereby supporting our description of the microscopic process.
4. Conclusions
Our work has demonstrated the effectiveness of ultrashort UV laser pulse in breaking the disulfide bridge in antibody thereby producing highly reactive free sulphurs that allow the right orientation of the antibody variable part (the so-called Fab region). This technique offers a powerful tool to steer the antibody orientation thus leading to more effective “hooks” available on the electrode of a QCM. The sensor performances in terms of linear range and sensitivity turn out to be significantly improved. Although we tested our method using antiIgG from goat to detect IgG from mouse, it is expected to observe similar behavior for any antibody since the presence of disulfide bridges is a common feature of all the antibodies. This opens interesting perspectives in the sensor research since it is possible to design peptide molecules that bind to a specific target molecule (aptamers) [19] and immobilize it on a QCM electrode by adding cysteines at one terminal. The ultrashort UV laser pulses would then be used to reduce the cysteines making them prone to fast binding to gold surface without any need for surface functionalization.
The photon assisted immobilization technique is competitive against alternative methods to anchor protein on to gold electrodes even if ultrafast UV pulses are to be used. This is because the functionalization is usually achieved by means of expensive toxic chemical reagents and requires long chemical procedures in well-equipped laboratories. On the contrary, femtosecond sources like that used in the present work are user friendly and can be safely run by non-expert personnel.
Since the high chemical reactivity of the opened bridges can lead them to different pathway other than surface binding (e.g. they can bind each other in solution), the optimal irradiation conditions have been found with the help of a chemical assay that allowed us to measure the amount of thiol groups in solution. Finally, the frequency shift measured by QCM has been interpreted by a simple statistical model that accounts for the experimental findings providing a physical insight of the microscopic phenomena involved in the UV light interaction with the antibody and its subsequent binding to gold electrode surface.
References and links
- 1.Cooper M. A., Singleton V. T., “A survey of the 2001 to 2005 quartz crystal microbalance biosensor literature: applications of acoustic physics to the analysis of biomolecular interactions,” J. Mol. Recognit. 20(3), 154–184 (2007). 10.1002/jmr.826 [DOI] [PubMed] [Google Scholar]
- 2.Arlett J. L., Myers E. B., Roukes M. L., “Comparative advantages of mechanical biosensors,” Nat. Nanotechnol. 6(4), 203–215 (2011). 10.1038/nnano.2011.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y. G., Chang K. S., “Application of a flow type quartz crystal microbalance immunosensor for real time determination of cattle bovine ephemeral fever virus in liquid,” Talanta 65(5), 1335–1342 (2005). 10.1016/j.talanta.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 4.Su X., Wu Y. J., Knoll W., “Comparison of surface plasmon resonance spectroscopy and quartz crystal microbalance techniques for studying DNA assembly and hybridization,” Biosens. Bioelectron. 21(5), 719–726 (2005). 10.1016/j.bios.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 5.Ostuni E., Yan L., Whitesides G. M., “The interaction of proteins with self-assembled monolayers of alkanethiolates on gold and silver,” Colloids Surf. 13, 3–30 (1999). [Google Scholar]
- 6.Ruan Y., Foo T. C., Warren-Smith S., Hoffmann P., Moore R. C., Ebendorff-Heidepriem H., Monro T. M., “Antibody immobilization within glass microstructured fibers: a route to sensitive and selective biosensors,” Opt. Express 16(22), 18514–18523 (2008). 10.1364/OE.16.018514 [DOI] [PubMed] [Google Scholar]
- 7.Ott J. R., Heuck M., Agger C., Rasmussen P. D., Bang O., “Label-free and selective nonlinear fiber-optical biosensing,” Opt. Express 16(25), 20834–20847 (2008). 10.1364/OE.16.020834 [DOI] [PubMed] [Google Scholar]
- 8.Daghestani H. N., Day B. W., “Theory and applications of surface plasmon resonance, resonant mirror, resonant waveguide grating, and dual polarization interferometry biosensors,” Sensors (Basel Switzerland) 10(11), 9630–9646 (2010). 10.3390/s101109630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neves-Petersen M. T., Snabe T., Klitgaard S., Duroux M., Petersen S. B., “Photonic activation of disulfide bridges achieves oriented protein immobilization on biosensor surfaces,” Protein Sci. 15(2), 343–351 (2006). 10.1110/ps.051885306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.C. A. Janeway, Jr., P. Travers, M. Walport, and M. J. Shlomchiket, Immunobiology (Garland Science, 2001). [Google Scholar]
- 11.Neves-Petersen M. T., Gryczynski Z., Lakowicz J., Fojan P., Pedersen S., Petersen E., Bjørn Petersen S., “High probability of disrupting a disulphide bridge mediated by an endogenous excited tryptophan residue,” Protein Sci. 11(3), 588–600 (2002). 10.1110/ps.06002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.J. R. Lakowicz, Principles of Fluorescence Spectroscopy (Springer, 2006). [Google Scholar]
- 13.Weinkauf R., Aicher P., Wesley G., Grotemeyer J., Schlag E. W., “Femtosecond versus nanosecond multiphoton ionization and dissociation of large molecules,” J. Phys. Chem. 98(34), 8381–8391 (1994). 10.1021/j100085a019 [DOI] [Google Scholar]
- 14.Gu B., Lou K., Wang H. T., Ji W., “Dynamics of two-photon-induced three-photon absorption in nanosecond, picosecond, and femtosecond regimes,” Opt. Lett. 35(3), 417–419 (2010). 10.1364/OL.35.000417 [DOI] [PubMed] [Google Scholar]
- 15.DataBank RCSB PDB, IgG structure (http://www.rcsb.org/pdb/explore/explore.do?structureId=3I75).
- 16.Ioerger T. R., Du C., Linthicum D. S., “Conservation of cys-cys trp structural triads and their geometry in the protein domains of immunoglobulin superfamily members,” Mol. Immunol. 36(6), 373–386 (1999). 10.1016/S0161-5890(99)00032-2 [DOI] [PubMed] [Google Scholar]
- 17.Sauerbrey G. Z., “Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung,” Z. Phys. 155(2), 206–222 (1959). 10.1007/BF01337937 [DOI] [Google Scholar]
- 18.Ellman G. L., “Tissue sulfhydryl groups,” Arch. Biochem. Biophys. 82(1), 70–77 (1959). 10.1016/0003-9861(59)90090-6 [DOI] [PubMed] [Google Scholar]
- 19.Yao C., Zhu T., Qi Y., Zhao Y., Xia H., Fu W., “Development of a quartz crystal microbalance biosensor with aptamer as bio-recognition element,” Sensors (Basel Switzerland) 10(6), 5859–5871 (2010). 10.3390/s100605859 [DOI] [PMC free article] [PubMed] [Google Scholar]


