Background: Glucose is a fundamental metabolite that is sensed by the MondoA transcription complex. MondoA elevates transcription of thioredoxin-interacting protein to restrict glucose uptake.
Results: MondoA senses the phosphorylated forms of the non-glucose hexose sugars, allose, and 3-O-methylglucose and triggers an adaptive transcriptional response.
Conclusion: TXNIP is regulated by non-hexose sugars in a manner that requires their metabolism, and TXNIP is part of the hexose transport curb.
Significance: Sugar is a universal metabolite, so how cells respond to changes in glucose and the transcriptional level is an important question.
Keywords: Bioenergetics, Carbohydrate Metabolism, Glucose, Metabolism, Transcription, Mlx, MondoA, TXNIP, bHLHZip, Hexose
Abstract
Glucose is required for cell growth and proliferation. The MondoA·Mlx transcription factor is glucose-responsive and accumulates in the nucleus by sensing glucose 6-phosphate. One direct and glucose-induced target of MondoA·Mlx complexes is thioredoxin-interacting protein (TXNIP). TXNIP is a potent negative regulator of glucose uptake, and hence its regulation by MondoA·Mlx triggers a feedback loop that restricts glucose uptake. This feedback loop is similar to the “hexose transport curb” first described almost 30 years ago. We show here that MondoA responds to the non-glucose hexoses, allose, 3-O-methylglucose, and glucosamine by accumulating in the nucleus and activating TXNIP transcription. The metabolic inhibitor 3-bromopyruvate blocks the transcriptional response to allose and 3-O-methylglucose, indicating that their metabolism, or a parallel pathway, is required to stimulate MondoA activity. Our dissection of the hexosamine biosynthetic pathway suggests that in addition to sensing glucose 6-phosphate, MondoA can also sense glucosamine 6-phosphate. Analysis of glucose uptake in wild-type, MondoA-null, or TXNIP-null murine embryonic fibroblasts indicates a role for the MondoA-TXNIP regulatory circuit in the hexose transport curb, although other redundant pathways also contribute.
Introduction
Glucose is a fundamental nutrient required for the growth of all organisms. In higher eukaryotes, cells tightly control glucose uptake and glucose utilization through a variety of mechanisms that are understood with different levels of detail. It is clear that alterations in glucose homeostasis have pleiotropic effects in pathobiology. For example, it has been known for >80 years that tumor cells take up and utilize more glucose than their normal counterparts. Furthermore, a base-line change in glucose homeostasis in liver, adipose, and skeletal muscle is a common feature associated with insulin resistance and diabetes.
Knowing exactly how cells sense and adapt to changes in extracellular and/or intracellular glucose concentrations is one key to understanding glucose homeostasis. For example, pancreatic β-cells sense elevated intracellular glucose and rapidly secrete insulin into the bloodstream as a primary response. In this case, glucose sensing and response occur exclusively in the cytoplasmic compartment. By contrast, a family of transcriptional regulators has been characterized more recently that play an important role in glucose homeostasis by sensing glucose metabolism and triggering an adaptive transcriptional response (1). There are two members in this family of transcriptional regulators, MondoA and the carbohydrate response element-binding protein (ChREBP),3 also known as WBSCR14 or MondoB. MondoA and ChREBP are members of the basic region helix-loop-helix leucine zipper (bHLHZip) family of transcription factors. Both MondoA and ChREBP interact with another bHLHZip protein, Mlx. Following elevations in intracellular glucose-derived metabolites, MondoA·Mlx or ChREBP·Mlx complexes bind target promoters and primarily activate their transcription. MondoA and ChREBP are most highly expressed in skeletal muscle and liver, respectively, which is consistent with their function as glucose sensors (2). Further, each is a prominent regulator of glucose-induced transcription (3, 4). The activity and regulation of ChREBP have been recently reviewed (5, 6).
Our laboratory is focused on MondoA. MondoA does not respond to glucose directly; rather it monitors flux through glycolysis by sensing glucose 6-phosphate (Glc-6-P) (4). In the absence of glucose, MondoA shuttles between the nucleus and the cytoplasm, with the equilibrium strongly favoring a cytoplasmic localization (7). By contrast, in the presence of increasing glucose levels, MondoA accumulates in the nucleus and occupies the promoters of its targets (4, 7). One well characterized direct and glucose-induced target for MondoA is thioredoxin-interacting protein (TXNIP). TXNIP has several functions (8, 9), one of which is its potent negative regulation of glucose uptake (10, 11). Collectively, these data suggest a model where MondoA senses elevations in Glc-6-P and activates TXNIP transcription, thereby initiating a negative feedback mechanism that restricts further glucose uptake (1, 4, 7, 12).
This proposed negative feedback loop is conceptually similar to the hexose transport “curb” described in the literature nearly 30 years ago (13–16). The key features of this blockade of hexose uptake are that it can be triggered by a number of different sugars, e.g. glucose, allose, and glucosamine, and that it requires protein synthesis and oxidative phosphorylation (OXPHOS). Given these similarities, we examined whether MondoA could regulate TXNIP expression in response to different sugars and whether the MondoA-TXNIP regulatory circuit contributes to the previously described hexose transport curb.
EXPERIMENTAL PROCEDURES
Cell Culture
Cells were maintained at 37 °C in 5% CO2 in DMEM containing penicillin/streptomycin, glutamine, and 10% FBS (HyClone) unless otherwise indicated. HA1ER cells (gift of William Hahn, Dana-Farber Cancer Institute) are human embryonic kidney epithelial cells expressing hTERT, the early region from simian virus 40 and H-RasG12V (17). Wild-type CCL39 and phosphoglucose isomerase-defective DS7 cells (gift of Danièle Roux, Université de Nice Sophia-Antipolis) are Chinese hamster lung fibroblast cells (18). 143B cells (gift of Navdeep Chandel, Northwestern University) are a human osteosarcoma cell line. MondoA-deficient murine embryonic fibroblast (MEF) cells were created by harvesting cells from a MondoAloxP/loxP embryo at day 14 of gestation and transducing the cells in vitro with retroviral pBabePuro-Cre to delete both alleles of MondoA. MondoAloxP/loxP and MondoA−/− MEFs were passaged on a 3T3 protocol to create the immortalized cell lines used here. Additionally, immortalized MondoA−/− cells were transduced with pWZL retroviral vector encoding full-length human MondoA.
Plasmids
Plasmids expressing MondoA-V5 and Mlx-FLAG have been described (19). Wild-type and carbohydrate response element (ChoRE) point mutant TXNIP luciferase reporter constructs have also been described (12). Plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen).
Hexose Treatments
Cells were incubated overnight in glucose-free DMEM containing 2% serum, followed by a 3-h treatment in the same medium supplemented with a 20 mm concentration, unless otherwise specified, of one of the following hexoses: glucose, 2-deoxy-d-glucose (2DG), 6-deoxy-d-glucose (6DG), 3-O-methylglucose (3MG), or allose. Glucosamine and N-acetylglucosamine were used at 5 mm or 10 mm as indicated in the figure legends.
Glucose Uptake Assay
Cells at subconfluent densities in 35-mm plates were washed with Krebs-Ringer-HEPES (KRH) buffer and then incubated for 10 min in KRH containing 1 mm 2DG and 1 mCi of 2-[3H(G)]DG (specific activity 5–10 Ci/mmol; PerkinElmer Life Sciences). Cells were washed with ice-cold KRH to terminate glucose uptake and were then solubilized with 0.5% Nonidet P-40 + 0.5 m NaOH in H2O, and an aliquot of the lysate assessed by scintillation counting for radiolabel incorporation. Assay results were normalized by determining radiolabel incorporation in control cells pretreated with cytochalasin B (10 mm; Sigma) as well as protein content for each sample.
Western Blotting
Primary antibodies were used at the following dilutions: anti-MondoA 1:500 (20); anti-VDUP1 (TXNIP) 1:1,000 (Medical and Biological Laboratories); anti-tubulin 1:10,000 (Sigma). Secondary antibodies were used at 1:5,000 (Amersham Biosciences). Western Lightning Plus-ECL (PerkinElmer Life Sciences) was used for detection.
Immunofluorescence and Microscopy
Cells were fixed on glass coverslips using PBS containing 3.7% formaldehyde for 10 min and then labeled by standard indirect immunofluorescence procedures. Mouse anti-V5 (Invitrogen) was used at 1:1,000, Alexa Fluor 594 goat anti-mouse IgG (Molecular Probes) was used at 1:500, and Hoechst 33342 (Molecular Probes) was used at 2 mg/ml to label nuclei. Subcellular immunolocalization of MondoA-V5 protein within individual cells was scored as described previously (4). Data are presented as the average ± S.D. resulting from cell counts in five random fields per sample, performed in triplicate.
Gene Silencing and Glycolysis Inhibition
Nonspecific or MondoA-specific shRNA were introduced into the HA1ER cells as described previously (4). The glycolysis inhibitor, 3-bromopyruvate (3BrPA) (Aldrich), was used concurrently with hexose treatment in glucose-free DMEM.
Mass Spectrometry Detection of Hexoses
Cells were incubated overnight in glucose-free medium and then treated with glucose, 3MG or allose. Cells were washed once with PBS and scraped into 100 μl of PBS and snap frozen. Metabolites were extracted from cell extracts as described (21). Dried and protein-free pellets were suspended in 40 μl of O-methyl hydroxylamine hydrochloride (Sigma-Aldrich) in pyridine (40 mg/ml), sonicated for 5 min to fully dissolve the dried sample followed by 1 h of incubation at 34 °C. After incubation the solid debris was removed by centrifugation for 3 min at 10,000 × g. Samples were loaded onto a MPS 2 autosampler (Gerstel, Linthicum, MD) where a second derivatization step was programmed. To each sample was added 10 μl of N-methy-N-(trimethylsilyl) trifluoroacetamide (Pierce) followed by a 30-min incubation with shaking at 37 °C. After incubation, 1 μl was injected to an Agilent split/splitless injector at a 20:1 split ratio. Injector temperature was 250 °C. An Agilent 6890 (Agilent, Santa Clara, CA) gas chromatograph was employed with the initial temperature held at 95 °C for 2 min followed by a 40 °C/min ramp to 110 °C. A second 5 °C/min ramp was employed to a temperature of 250 °C followed by a final 25 °C/min ramp to 350 °C. This final temperature was held for 3 min. A Restek (Bellefonte, PA) 30-m RTX-5MS column fitted with a 5-m guard column was employed for metabolite separation. A Waters (Beverly, MA) GCT Premier TOF mass spectrometer was used for metabolite detection.
Statistical Methods
For the experiments examining the hexose curb in the different MEF populations, two-way ANOVA models with interaction were fit to log-transformed data. Two-sided Wald p values for the interaction term are reported.
RESULTS
MondoA Is Required for Hexose-induced TXNIP Expression
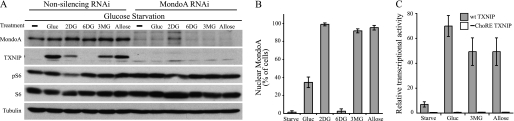
MondoA·Mlx complexes accumulate in the nucleus and activate TXNIP expression directly in cells treated with either glucose or its nonmetabolizable analog, 2DG (4, 20). Previous experiments suggested that nuclear accumulation of MondoA requires the enzymatic activity of hexokinase (4). Consistent with our previous findings in different cell types (4), TXNIP was induced in the HA1ER kidney epithelial cells that were starved for glucose overnight and then treated with either glucose or 2DG for 3 h (Fig. 1A). The nonphosphorylatable glucose analog 6DG did not trigger the nuclear accumulation of MondoA·Mlx complexes and failed to induce TXNIP protein expression (Fig. 1, A and B). Thus, the regulation of TXNIP in HA1ER cells is identical to that observed in other cell lines representing a variety of cell lineages. Thus, the regulation of MondoA by glucose and 2DG is likely a ubiquitous adaptation to changing glucose concentration.
FIGURE 1.
MondoA broadly regulates hexose-induced TXNIP expression. A, HA1ER cells expressing nonsilencing control or MondoA-specific RNAi were glucose (Gluc)-starved overnight and then treated with the indicated hexoses (20 mm; 3h). Western blot analysis of MondoA, TXNIP, ribosomal S6 protein, phospho-S6, and tubulin was performed following treatment. B, control HA1ER cells were transfected with MondoA-V5 and FLAG-Mlx prior to glucose starvation and hexose treatment. Percentage of cells displaying nuclear immunolocalization of MondoA-V5 protein is shown. Error bars, S.D. C, activities of the wild-type (wt) and ChoRE point mutant (−ChoRE) TXNIP luciferase reporters were measured in HA1ER cells following glucose starvation and hexose treatment.
3MG and the rare sugar epimer of glucose, d-allose, up-regulate TXNIP in insulinoma and hepatocellular carcinoma cells, respectively (22, 23), but MondoA has not been implicated in this regulation. Consistent with these previous reports, TXNIP protein was elevated in both 3MG- and allose-treated control HA1ER cells (Fig. 1A). However, none of the hexoses tested induced TXNIP in cells with reduced MondoA. Thus, TXNIP can be induced by multiple hexoses and in every case its induction was strongly dependent on MondoA.
Hexose-dependent Induction of TXNIP Requires ChoRE
In response to glucose or 2DG, MondoA·Mlx complexes accumulate in the nucleus and occupy the ChoRE of the TXNIP promoter (4, 7). This ChoRE consists of a pair of CACGAG E-box sequences separated by 5 bp and is essential for glucose- or 2DG-dependent activation of TXNIP transcription (22). Consistent with their induction of TXNIP, 3MG and allose, but not 6DG, drove significant nuclear accumulation of epitope-tagged MondoA in HA1ER cells (Fig. 1B). As with glucose, 3MG and allose strongly increased transcriptional activity of the TXNIP luciferase reporter, and all transcriptional activity was eliminated by mutation of the first E-box sequence of the ChoRE (Fig. 1C). Consistent with MondoA regulating TXNIP directly, glucose, 2DG (4, 7) and allose (data not shown) induced MondoA binding to a region in the TXNIP promoter that contains the ChoREs.
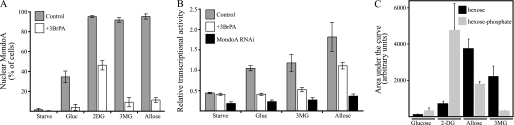
MondoA nuclear accumulation requires the phosphorylation of glucose or 2DG by hexokinases, which is the first enzymatic step in the glycolytic pathway (4). To investigate further the role of hexose metabolism in MondoA transcriptional activity, we used the glycolytic inhibitor 3-BrPA. Treatment with 3-BrPA significantly reduced the nuclear accumulation of MondoA in cells treated with the different hexoses (Fig. 2A). 3-BrPA also inhibited transcriptional activity from the TXNIP promoter following treatment with each hexose (Fig. 2B). Finally, the hexose-dependent transcriptional activity of the TXNIP luciferase reporter was reduced in MondoA knockdown cells (Fig. 2B), confirming its role in the hexose-dependent activation of TXNIP. Together, these data demonstrate that the hexose-dependent activation of TXNIP requires MondoA, an activity blocked by 3-BrPA, and an intact ChoRE in the TXNIP promoter.
FIGURE 2.
MondoA-activated TXNIP expression requires hexokinase activity. A, HA1ER cells transfected with MondoA-V5 and FLAG-Mlx were glucose (Gluc)-starved overnight and then treated with the indicated hexoses (20 mm; 3h) ± 3BrPA (20 μm). Percentage of cells displaying nuclear immunolocalization of MondoA-V5 is shown. B, activity of TXNIP luciferase reporter was determined following hexose treatment of HA1ER cells expressing MondoA RNAi or control RNAi ± 3-BrPA. C, levels of the indicated hexoses in MEFs were determined by mass spectrometry. Error bars, S.D.
Glucose and 2DG are well known substrates for hexokinases; however, the previous results suggest that allose and 3MG are also metabolized to trigger MondoA nuclear accumulation and its activation of TXNIP. Allose phosphate is easily detectable in cell lysates (24), whereas 3MG is generally assumed to be nonmetabolizable. However, several papers provide direct evidence that 3MG can be phosphorylated (25, 26). To address this point, we determined the level of each hexose and the corresponding phosphorylated counterpart in MEFs by mass spectrometry (Fig. 2C). Consistent with their rapid flux through glycolysis, glucose and glucose phosphate accumulated to low levels, By contrast, 2DG phosphate, which is not further metabolized by glycolysis, accumulated to high levels. Allose phosphate and 3MG phosphate were also easily detectable in this assay, indicating that they are also substrates for hexokinases. The levels of glucose phosphate and 3MG phosphate were comparable, suggesting 3MG phosphate accumulated to a sufficient level to drive MondoA activation of TXNIP.
MondoA Senses Glycolytic Flux
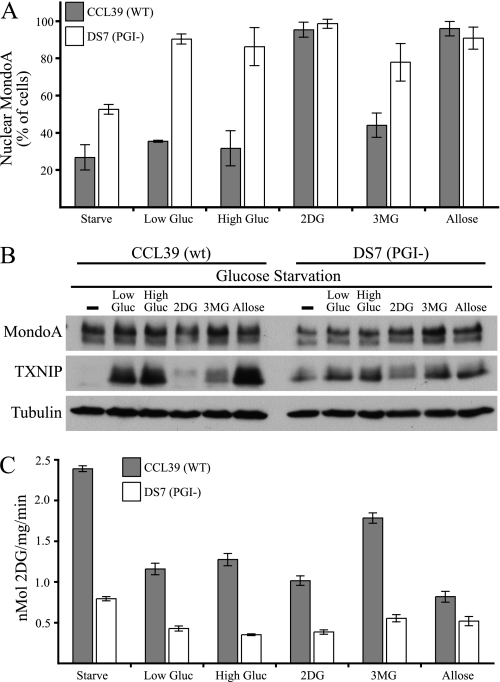
2DG drives MondoA nuclear accumulation and activity at the TXNIP promoter more effectively than does glucose (4). We hypothesized that this differential effect reflects an accumulation of 2DG 6-phosphate, whereas Glc-6-P is rapidly shunted into other metabolic pathways (Fig. 2C) (1, 4). We used a mutant cell line highly defective in phosphoglucose isomerase (PGI−) and its wild-type counterpart to investigate more thoroughly the role of hexose phosphates in regulating MondoA activity (18, 27). PGI converts Glc-6-P to fructose 6-phosphate; thus, the PGI-null cells accumulate higher levels of Glc-6-P than the wild-type controls.
The wild-type parental cell line, CCL39, displayed marked nuclear MondoA accumulation following treatment with 2DG and allose, but not with low or high glucose or 3MG. This finding is consistent with the higher accumulation of 2DG phosphate and allose phosphate in MEFs (Fig. 2C). In contrast, the PGI− DS7 cells displayed strong nuclear accumulation of MondoA in the presence of all of the hexoses (Fig. 3A). Thus, loss of PGI allows glucose and 3MG to drive nuclear accumulation of MondoA to a similar level as that observed with 2DG and allose, a finding consistent with MondoA sensing a phosphorylated hexose.
FIGURE 3.
Modulation of MondoA activity by PGI. A, CCL39 (wild type) or DS7 (PGI−) cells transfected with MondoA-V5 and FLAG-Mlx were glucose (Gluc)-starved overnight and then treated with the indicated hexoses (low gluc = 5 mm, all others = 20 mm; 3 h). Percentage of cells displaying nuclear immunolocalization of MondoA-V5 protein is shown. B, Western blot analysis of endogenous MondoA, TXNIP, and tubulin in CCL39 and PGI-DS7 cells following glucose starvation and the indicated hexose treatment was performed. C, glucose uptake in CCL39 and PGI-DS7 cells following glucose starvation and the indicated hexose treatment was measured. Error bars, S.D.
Each hexose induced TXNIP expression in the parental CCL39 cells (Fig. 3B), similar to the induction observed in the HA1ER cells (Fig. 1A). The low level of TXNIP in 2DG-treated cells likely results from reduced translational efficiency because 2DG inhibits mTOR kinase, as evidenced by a reduction in phosphorylated S6 in HA1ER cells (Fig. 1A) and as reported in the literature (28). The PGI− DS7 cells showed a similar pattern of TXNIP induction with the different hexoses, although TXNIP levels were generally lower in the mutant cell line. This result is not surprising because the nuclear levels of MondoA and its activity at the TXNIP promoter can be uncoupled (7). In both cell lines, each sugar induced a hexose transport curb and reduced glucose uptake by about 50% (Fig. 3C). Thus, TXNIP induction generally correlated with the hexose transport curb. Paradoxically, glucose uptake in the PGI− DS7 cells was lower under each condition compared with those observed in the CCL39 cells (Fig. 3C). Together, these data suggest a role for TXNIP in the hexose transport curb, but other TXNIP-independent mechanisms altered in the PGI mutant cells must govern basal glucose uptake.
Glucosamine 6-Phosphate Regulates MondoA Activity
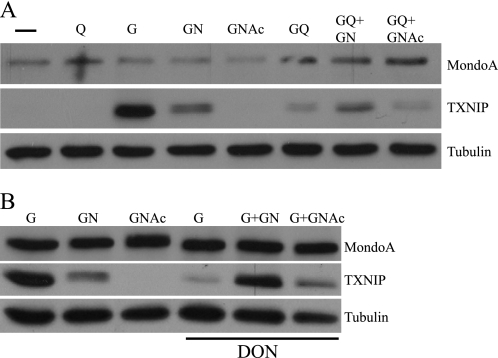
The hexosamine biosynthetic pathway is implicated in blocking glucose uptake and inducing insulin resistance (29). Further, glucosamine, which enters the hexosamine biosynthetic pathway, is sufficient to trigger a hexose transport curb (15). Based on these findings, we thought that glucosamine might also regulate MondoA transcriptional activity. Glucosamine 6-phosphate is generated through the donation of the amine from glutamine to fructose 6-phosphate in the first step of the hexosamine biosynthetic pathway. Consistent with our previous reports in other cell lines (4, 7, 12, 30), we found that glucose stimulated TXNIP expression in the 143B osteosarcoma cell line and that glutamine repressed the glucose-dependent activation (Fig. 4A). Glucosamine stimulated TXNIP expression in the complete absence of glucose. Glucosamine also stimulated TXNIP expression in BxPC3 cells, indicating that its effect is not restricted to 143B cells (data not shown)
FIGURE 4.
MondoA senses glucosamine 6-phosphate. Western blot analysis of endogenous MondoA, TXNIP, and tubulin from the osteosarcoma cell line 143B treated with the different nutrient combinations is shown. Q, glutamine (2 mm); G, glucose (25 mm); GN, glucosamine (10 mm); GNAc, N-acetylglucosamine (10 mm); DON, 6-diazo-5-oxonorleucine (20 μm); −, no glucose or glutamine.
Two mechanisms may account for the glucosamine-dependent activation of TXNIP: (i) MondoA may sense glucosamine 6-phosphate directly, or (ii) glucosamine 6-phosphate may be processed by the hexosamine biosynthetic pathway and MondoA may sense a downstream metabolite. N-Acetylglucosamine, which enters the hexosamine pathway just below glucosamine 6-phosphate did not drive TXNIP expression (Fig. 4A). This result suggests that MondoA does not sense hexosamine biosynthetic pathway intermediates downstream of glucosamine 6-phosphate, but may sense glucosamine 6-phosphate directly.
To test whether MondoA can sense glucose flux into glucosamine 6-phosphate we blocked the conversion of fructose 6-phosphate to glucosamine 6-phosphate via glutamine:fructose-6-phosphate amidotransferase using the glutamine:fructose-6-phosphate amidotransferase inhibitor 6-diazo-5-oxonorleucine (DON). DON potently inhibited the glucose-dependent induction of TXNIP (Fig. 4B), suggesting that intermediates downstream of fructose 6-phosphate and glutamine:fructose-6-phosphate amidotransferase contribute to TXNIP induction. Glucosamine completely rescued the DON blockade, whereas N-acetylglucosamine had a minimal effect. Thus, these data suggest that glucosamine 6-phosphate, but not other downstream components of the hexosamine biosynthetic pathway, regulate TXNIP expression. Furthermore, we conclude that flux into the hexosamine pathway and not just flux through glycolysis can stimulate TXNIP expression.
TXNIP Functions Downstream of MondoA
To determine the roles of MondoA and TXNIP in the hexose transport curb, we investigated the curb in wild-type, MondoA knock-out or TXNIP knock-out MEFs (7). We also investigated the role of TXNIP as a MondoA effector by expressing a TXNIP-mCherry chimera in MondoA knock-out cells. TXNIP protein was strongly induced by allose in wild-type MEFs, but was completely absent in MondoA knock-out cells under all growth conditions (Fig. 5A). TXNIP-mCherry expression in MondoA knock-out cells was unresponsive to changes in hexose status. Identical to our previous results (7), MondoA knock-out MEFs had elevated glucose uptake compared with wild-type controls in normal growth medium and following an overnight period in glucose-free medium (Fig. 5B and data not shown). Addition of allose to wild-type MEF cells reduced glucose uptake 2.2-fold. In contrast, allose reduced glucose uptake just 1.6-fold in MondoA knock-out MEFs. Thus, MondoA contributes to the hexose transport curb.
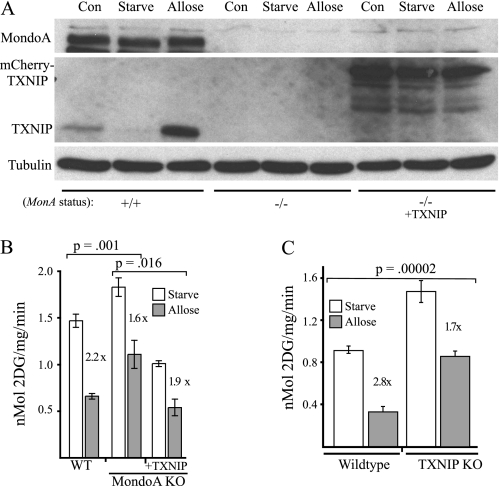
FIGURE 5.
TXNIP is a MondoA effector. A, Western blot analysis of MondoA, TXNIP and tubulin in wild-type (WT) or MondoA knock-out (KO) MEFs, with or without ectopic TXNIP-mCherry. Cells were maintained under normal growth conditions (Con) or glucose-starved overnight and then treated with allose (20 mm; 3 h). B, glucose uptake in WT and MondoA KO MEFs, with or without TXNIP-mCherry, following the indicated treatments. C, glucose uptake in wild-type or TXNIP knock-out MEFs following the indicated treatments. Error bars, S.D.
Introduction of TXNIP-mCherry into MondoA knock-out MEFs reduced glucose uptake to levels below those observed in wild-type cells in control and glucose-starved cells (Fig. 5B and data not shown). Addition of allose to the complemented cells resulted in an intermediate reduction of 1.9-fold in glucose uptake compared with wild-type or MondoA knock-out MEFs (Fig. 5B). Thus, TXNIP is sufficient to function as a MondoA effector in the hexose transport curb.
As a final test of the contribution of the MondoA-TXNIP circuit to the hexose transport curb, we determined glucose uptake in wild-type and TXNIP-null MEFs. As expected, genetic deletion of TXNIP resulted in increased glucose uptake (Fig. 5C). Similar to the effect of MondoA deletion, TXNIP deletion reduced the magnitude of the allose-induced hexose curb. In this experiment, allose reduced glucose uptake 2.8-fold of control levels in wild-type MEFs, but only reduced glucose uptake 1.7-fold in TXNIP knock-out cells. Thus, like MondoA, TXNIP contributes to the hexose transport curb.
2,4-Dinitrophenol (DNP) Regulates TXNIP Expression
In addition to hexose treatment, the hexose transport curb also depends on oxidative phosphorylation (31). We investigated the effects of the proton gradient uncoupler DNP on TXNIP expression and glucose uptake in MEFs. DNP treatment almost completely prevented the robust allose-dependent up-regulation of TXNIP (Fig. 6A). Consistent with the changes in TXNIP expression, glucose uptake was significantly lower in allose-treated MEFs (Fig. 6B), and treatment with DNP almost completely eliminated the allose-induced glucose uptake curb.
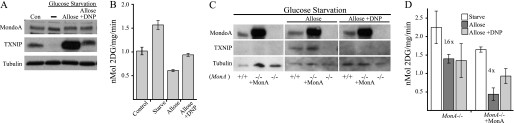
FIGURE 6.
OXPHOS is required for the hexose transport curb and TXNIP expression. A, Western blot analysis of MondoA, TXNIP, and tubulin in wild-type MEFs maintained under normal growth conditions (Con) or glucose-starved overnight and then treated with allose (20 mm; 3 h) with or without DNP (0.2 mm). B, glucose uptake in wild-type MEFs following the indicated treatments. C, Western blot analysis of MondoA, TXNIP, and tubulin in wild-type (MonA+/+) or MondoA knock-out (MonA−/−) MEFs, with or without ectopic MondoA. Different cell populations were glucose-starved overnight and then treated with allose (20 mm; 3 h) with or without DNP (0.2 mm). D, glucose uptake in MonA−/− MEFs, with or without ectopic MondoA, following the indicated treatments. The difference in the allose-driven hexose transport curb between the MondoA knock-out cells (MonA−/−) and the complemented cells (MonA−/− + MonA) is statistically significant (p = 0.0018). Error bars, S.D.
To investigate the role of MondoA in mediating the effects of DNP, we examined MondoA-null MEFs and MondoA-null MEFs complemented with wild-type MondoA. As expected, following allose treatment, TXNIP expression was absent in the MondoA-null cells, but completely restored in the rescued MEFs (Fig. 6C). Allose + DNP co-treatment blocked TXNIP expression in the complemented MEFs. Similar to findings shown in Fig. 5B, MondoA-null MEFs responded partially to allose treatment and reduced glucose uptake 1.6-fold (Fig. 6D). Complemented MEFs showed a more dramatic 4-fold allose-triggered reduction in glucose uptake. DNP did not reverse the hexose transport curb in MondoA-null MEFs, but the DNP effect was restored in the complemented cells (Fig. 6D). Thus, MondoA is required for DNP to block the hexose transport curb.
DISCUSSION
Cells sense and respond to glucose in an effort to maintain the delicate balance of glucose homeostasis. The MondoA and ChREBP transcription factors are predominant glucose sensors in mammalian cells (1, 5, 6). Here, we show that MondoA also responds to other hexoses and is required for full elaboration of the previously described hexose transport curb. TXNIP contributes as a MondoA effector of the hexose transport curb, but other factors also play a role. Finally, we show that uncoupling of the electron transport chain abrogates TXNIP expression, reduces the hexose transport curb, and increases glucose uptake. These data are consistent with MondoA transcriptional activation at the TXNIP promoter being controlled by flux through glycolysis and mitochondrial activity. Thus, MondoA can interpret and integrate intracellular bioenergetic cues.
Previous studies showed that 3MG and allose induce TXNIP expression (23, 32). These two sugars drive MondoA nuclear accumulation and activate TXNIP transcription in a manner that is entirely dependent on the ChoRE in the TXNIP promoter. Induction of TXNIP by 3MG and allose is severely impaired in MondoA knockdown cells (Fig. 1A). Furthermore, allose fails to induce TXNIP in cells with a genetic deletion of MondoA (Figs. 5A and 6C), yet this induction is restored when the knock-out cells are complemented with wild-type MondoA. Collectively, these data suggest that the induction of TXNIP by 3MG and allose results from their stimulation of MondoA transcriptional activity at the TXNIP promoter. Interestingly, the glucose epimers psicose, altrose, mannose, and galactose do not induce TXNIP (data not shown), demonstrating that not all hexoses are capable of regulating MondoA function.
We originally proposed that MondoA senses Glc-6-P (4) and our experiments here using the PGI-defective cells provides further evidence to support that conclusion (Fig. 3). Glucosamine can also activate TXNIP expression. Glucosamine feeds the hexosamine biosynthetic pathway, which is implicated in insulin resistance and diabetes (29). Thus, glucosamine may contribute to insulin resistance by up-regulating TXNIP. The glutamine:fructose-6-phosphate amidotransferase inhibitor, DON, reduces the glucose-dependent induction of TXNIP, and this blockade can be overcome with glucosamine, but not N-acetylglucosamine (Fig. 4). The lack of rescue by N-acetylglucosamine suggests that MondoA is not regulated by flux through the hexosamine biosynthetic pathway and makes it unlikely that MondoA itself is sugar-modified in a way that controls its transcriptional activity. Interestingly, a recent paper demonstrates that ChREBP is β-N-acetylglucosamine-modified which increases its transcriptional activity (33). Apparently, MondoA and ChREBP are functionally divergent in this regard. Glucosamine induced nuclear accumulation of MondoA (data not shown), suggesting that it functions similarly to glucose. This is not surprising as Glc-6-P and glucosamine 6-phosphate only differ at the 2 position. We propose that the glucose-induced transcriptional activation of TXNIP reflects the combined effects of Glc-6-P, its flux into the hexosamine biosynthetic pathway, and production of glucosamine 6-phosphate.
TXNIP is also activated by allose and 3MG, and this activation requires MondoA. Allose phosphate has been detected in mammalian cells, but the kinase responsible has not been identified (24). By contrast, 3MG is generally considered to be nonmetabolizable; however, several publications challenge this convention (25, 26). We detected allose phosphate and 3MG phosphate using mass spectrometry approaches, and their activation of TXNIP is blocked by the metabolic inhibitor 3-BrPA. The level of 3MG phosphate is low likely because it is a poor substrate for hexokinases. However, it does accumulate to levels similar to Glc-6-P, suggesting that even at low levels 3MG phosphate is sufficient to drive the nuclear accumulation of MondoA·Mlx complexes. 3MG phosphate accumulates to a level below that of 2DG phosphate, yet both hexoses induce TXNIP to a similar level. This finding suggests that MondoA·Mlx transcriptional activity is limited by mechanisms other than the absolute level of hexose phosphate. Thus our data are consistent with MondoA nuclear activity being regulated by the phosphorylated forms of allose and 3MG.
The hexose transport curb is not a passive response of cells to accumulated Glc-6-P, but rather requires initial feeding with a hexose, an intact oxidative phosphorylation pathway, and protein synthesis (13, 14, 27, 31, 34). Our data suggest that the MondoA-TXNIP negative feedback circuit is a required component of the hexose transport curb. Multiple experiments support this contention. First, the hexose transport curb is reduced in MondoA- or TXNIP-null MEFs (Fig. 5, B and C), directly implicating both proteins in the curb. Second, the hexose transport curb is restored in MondoA-null MEFs complemented with wild-type MondoA (Fig. 6D), indicating that the defect in the curb in MondoA knock-out cells can be attributed to MondoA. Third, the hexose transport curb is also restored in MondoA-null MEFs by complementation with TXNIP, indicating an important role for TXNIP as a MondoA effector. Finally, both the hexose transport curb and TXNIP protein synthesis are extinguished by DNP (Fig. 6, A and B), demonstrating a strict requirement for mitochondrial function in regulating TXNIP protein levels and maintaining the curb. Thus, we propose that the hexose-and MondoA-dependent regulation of TXNIP is required for the full elaboration of the hexose transport curb.
The curb is only partially reduced in MEFs lacking MondoA or TXNIP, indicating contributions by other factors. The nuclear function of ChREBP is also glucose-regulated (5, 6), thus it is possible that ChREBP functions redundantly with MondoA in the hexose transport curb. Similarly, the TXNIP paralog, ARRDC4, is also up-regulated by glucose and allose in a MondoA-dependent manner (data not shown). Like TXNIP, ARRDC4 is a potent negative regulator of glucose uptake (11). Thus, it is likely that TXNIP and ARRDC4 function together downstream of MondoA, and possibly ChREBP, to drive the majority of the hexose transport curb. Our work here provides the foundation to address this important question.
DNP effectively blocked the allose-dependent and MondoA-dependent induction of TXNIP, suggesting that MondoA requires a signal generated by OXPHOS to activate TXNIP transcription. Furthermore, consistent with previous reports (34), DNP reversed the hexose transport curb (Fig. 6B). We are currently working to identify this signal. Given that MondoA senses phosphorylated hexoses, it is possible that OXPHOS-derived ATP is required for MondoA nuclear activity. Although precise mechanism(s) by which OXPHOS controls MondoA activity and TXNIP expression remains to be determined, our studies highlight cross-talk between mitochondrial activity and glycolytic flux in regulating MondoA and the hexose transport curb.
Acknowledgments
We thank the Ayer laboratory for reviewing the manuscript and W. Chutkow for the TXNIP+/+ and TXNIP−/− MEFs.
This work was supported, in whole or in part, by National Institutes of Health Grants GM55668 and GM60387 (to D. E. A.). This work was also supported by the Huntsman Cancer Foundation. DNA sequencing and oligonucleotide synthesis were supported by the Cancer Center Support Grant 2P30 CA42014.
- ChREBP
- carbohydrate response element-binding protein
- 3-BrPA
- 3-bromopyruvate
- ChoRE
- carbohydrate response element
- 2DG
- 2-deoxy-d-glucose
- 6DG
- deoxy-d-glucose
- DNP
- 2,4-dinitrophenol
- DON
- 6-diazo-5-oxonorleucine
- MEF
- murine embryonic fibroblast
- 3MG
- 3-O-methylglucose
- OXPHOS
- oxidative phosphorylation
- PGI
- phosphoglucose isomerase
- TXNIP
- thioredoxin-interacting protein.
REFERENCES
- 1. Kaadige M. R., Elgort M. G., Ayer D. E. (2010) Transcription 1, 36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billin A. N., Ayer D. E. (2006) in The Myc/Max/Mad Transcription Factor Network (Eisenman R. N. ed) pp. 255–278, Springer, Heidelberg [Google Scholar]
- 3. Ma L., Robinson L. N., Towle H. C. (2006) J. Biol. Chem. 281, 28721–28730 [DOI] [PubMed] [Google Scholar]
- 4. Stoltzman C. A., Peterson C. W., Breen K. T., Muoio D. M., Billin A. N., Ayer D. E. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 6912–6917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Postic C., Dentin R., Denechaud P. D., Girard J. (2007) Annu. Rev. Nutr. 27, 179–192 [DOI] [PubMed] [Google Scholar]
- 6. Uyeda K., Repa J. J. (2006) Cell Metab. 4, 107–110 [DOI] [PubMed] [Google Scholar]
- 7. Peterson C. W., Stoltzman C. A., Sighinolfi M. P., Han K. S., Ayer D. E. (2010) Mol. Cell. Biol. 30, 2887–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S. Y., Suh H. W., Chung J. W., Yoon S. R., Choi I. (2007) Cell. Mol. Immunol. 4, 345–351 [PubMed] [Google Scholar]
- 9. Muoio D. M. (2007) Cell Metab. 5, 412–414 [DOI] [PubMed] [Google Scholar]
- 10. Parikh H., Carlsson E., Chutkow W. A., Johansson L. E., Storgaard H., Poulsen P., Saxena R., Ladd C., Schulze P. C., Mazzini M. J., Jensen C. B., Krook A., Björnholm M., Tornqvist H., Zierath J. R., Ridderstråle M., Altshuler D., Lee R. T., Vaag A., Groop L. C., Mootha V. K. (2007) PLoS Med. 4, e158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patwari P., Chutkow W. A., Cummings K., Verstraeten V. L., Lammerding J., Schreiter E. R., Lee R. T. (2009) J. Biol. Chem. 284, 24996–25003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaadige M. R., Looper R. E., Kamalanaadhan S., Ayer D. E. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14878–14883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kalckar H. M., Ullrey D. B. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 1126–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ullrey D. B., Kalckar H. M. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 5858–5860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ullrey D. B., Kalckar H. M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 3678–3680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ullrey D. B., Kalckar H. M. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 4350–4351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hahn W. C., Dessain S. K., Brooks M. W., King J. E., Elenbaas B., Sabatini D. M., DeCaprio J. A., Weinberg R. A. (2002) Mol. Cell. Biol. 22, 2111–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pouysségur J., Franchi A., Salomon J. C., Silvestre P. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 2698–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Billin A. N., Eilers A. L., Coulter K. L., Logan J. S., Ayer D. E. (2000) Mol. Cell. Biol. 20, 8845–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sans C. L., Satterwhite D. J., Stoltzman C. A., Breen K. T., Ayer D. E. (2006) Mol. Cell. Biol. 26, 4863–4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ai J., Trygg J., Gullberg J., Johansson A. I., Jonsson P., Antti H., Marklund S. L., Moritz T. (2005) Anal. Chem. 77, 8086–8094 [DOI] [PubMed] [Google Scholar]
- 22. Minn A. H., Hafele C., Shalev A. (2005) Endocrinology 146, 2397–2405 [DOI] [PubMed] [Google Scholar]
- 23. Yamaguchi F., Takata M., Kamitori K., Nonaka M., Dong Y., Sui L., Tokuda M. (2008) Int. J. Oncol. 32, 377–385 [PubMed] [Google Scholar]
- 24. Ullrey D. B., Kalckar H. M. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 1504–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gatley S. J., Holden J. E., Halama J. R., DeGrado T. R., Bernstein D. R., Ng C. K. (1984) Biochem. Biophys. Res. Commun. 119, 1008–1014 [DOI] [PubMed] [Google Scholar]
- 26. Cortès S., Gromova M., Evrard A., Roby C., Heyraud A., Rolin D. B., Raymond P., Brouquisse R. M. (2003) Plant Physiol. 131, 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pouysségur J., Franchi A., Silvestre P. (1980) Nature 287, 445–447 [DOI] [PubMed] [Google Scholar]
- 28. Corradetti M. N., Inoki K., Bardeesy N., DePinho R. A., Guan K. L. (2004) Genes Dev. 18, 1533–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClain D. A. (2002) J. Diabetes Complications 16, 72–80 [DOI] [PubMed] [Google Scholar]
- 30. Elgort M. G., O'Shea J. M., Jiang Y., Ayer D. E. (2010) Genes Cancer 1, 893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalckar H. M., Christopher C. W., Ullrey D. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 6453–6455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minn A. H., Couto F. M., Shalev A. (2006) Biochemistry 45, 11047–11051 [DOI] [PubMed] [Google Scholar]
- 33. Guinez C., Filhoulaud G., Rayah-Benhamed F., Marmier S., Dubuquoy C., Dentin R., Moldes M., Burnol A. F., Yang X., Lefebvre T., Girard J., Postic C. (2011) Diabetes 60, 1399–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ullrey D. B., Franchi A., Pouyssegur J., Kalckar H. M. (1982) Proc. Natl. Acad. Sci. U.S.A. 79, 3777–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]