Background: Carbohydrate catabolism provides the energy for nitrogen fixation in filamentous cyanobacteria.
Results: A nitrogen-regulated response regulator NrrA controlled glycogen catabolism in the filamentous cyanobacterium Anabaena sp. PCC 7120.
Conclusion: NrrA controls not only cellular differentiation but also carbohydrate metabolism.
Significance: A molecular network regulating glycogen metabolism in the nitrogen-fixing cyanobacteria is proposed.
Keywords: Bacterial Transcription, Carbohydrate Metabolism, Cell Differentiation, Cyanobacteria, Glycogenolysis, Nitrogen Fixation
Abstract
Anabaena sp. strain PCC 7120 is a filamentous cyanobacterium in which certain vegetative cells differentiate into heterocysts that are specialized cells for nitrogen fixation. Heterocysts are unable to carry out photosynthesis and depend on vegetative cells for carbohydrate to generate ATP and reductants required for nitrogen fixation. Thus, carbohydrate metabolism is very important for nitrogen fixation in the filamentous cyanobacteria; however, its regulatory mechanism remains unknown. In the present study, a nitrogen-regulated response regulator NrrA, which is a transcriptional regulator involved in heterocyst differentiation, was shown to control glycogen catabolism. The transcript levels of genes involved in glycogen catabolism, such as glgP1 and xfp-gap1-pyk1-talB operon, were decreased by the nrrA disruption. Moreover, glycogen accumulation and depression of nitrogenase activities were observed in this disruptant. NrrA bound specifically to the promoter region of glgP1, encoding a glycogen phosphorylase, and to the promoter region of sigE, encoding a group 2 σ factor of RNA polymerase. SigE activated expression of the xfp operon, encoding enzymes of glycolysis and the pentose phosphate pathway. It is concluded that NrrA controls not only heterocyst differentiation but also glycogen catabolism in Anabaena sp. strain PCC 7120.
Introduction
Cyanobacteria are a large group of eubacteria characterized by oxygen-evolving photosynthesis. Heterocysts are terminally differentiated cells of filamentous cyanobacteria specialized for nitrogen fixation. Upon limitation of combined nitrogen in the medium, particular vegetative cells differentiate into heterocysts with a regular spacing of 10–15 cells (1, 2). Differentiating cells undergo drastic metabolic and morphological change, namely O2-evolving photosystem II is inactivated, a rate of respiration is increased, and a thick envelope is formed outside of the cell wall, which limits oxygen diffusion into cells. Because heterocysts are unable to fix CO2 photosynthetically, vegetative cells supply carbohydrate to heterocysts, probably in the form of sucrose (3), and, in return, vegetative cells receive nitrogen fixation products from heterocysts (4). Thus, the heterocyst-forming cyanobacteria represent a unique case of a truly multicellular bacterium with two different cell types, which depend on each other and contribute to the operation of the filament as the organismic unit.
Cyanobacteria store carbon assimilated by photosynthesis in the form of glycogen. Glycogen is utilized as carbon and energy reserves to cope with transient starvation and stress conditions (5, 6). Moreover, glycogen is catabolized to provide reductants and ATP required for nitrogen fixation, particularly in the dark (7). Glycogen content in heterocysts is higher than that of vegetative cells (8). Interconnection between glycogen and sucrose metabolism has been observed in the nitrogen-fixing filaments (3). Inhibition of sucrose degradation in heterocysts alters the accumulation of glycogen and sucrose and impairs diazotrophic growth (9, 10). Although regulation of carbohydrate metabolism is very important for the nitrogen-fixing cyanobacteria, little is known about its molecular mechanisms.
In the filamentous cyanobacterium Anabaena sp. strain PCC 7120 (hereafter Anabaena PCC 7120), we found that the nrrA gene encoding a response regulator of the OmpR family is involved in the regulation of heterocyst differentiation (11). Expression of nrrA is up-regulated by nitrogen deprivation under the control of NtcA (12), which globally controls nitrogen metabolism in cyanobacteria (13). NrrA is required for the full induction of the hetR gene (11, 14), encoding a master regulator of heterocyst differentiation (15, 16). The sigE gene (alr4249; previously called sigF), encoding a group 2 σ factor of RNA polymerase (17), is up-regulated in heterocysts after nitrogen deprivation (18) and is required for normal expression of heterocyst-specific genes including nifH, fdxH, and hglE2 (19). NtcA binds to the upstream region of sigE, but it plays a partial role in the regulation of sigE expression (19). Another transcriptional regulator in the transcriptional regulation of sigE is suggested.
Several genes up-regulated by nitrogen deprivation in Anabaena PCC 7120 are involved in carbon metabolism, such as glycogen degradation, glgP1 (all1272); sucrose metabolism, invB (alr0819) and spsB (all4376); the oxidative pentose phosphate pathway, zwf (all4019), gnd (alr5275), talA (all4020), and talB (all2563); and glycolysis, gap1 (all2566) and pyk1 (all2564) (11). Expression of the spsB gene is shown to be regulated by NtcA (20). However, transcriptional regulation of the other genes has not been revealed. In this study, we investigated the regulation of genes involved in carbon metabolism that were up-regulated by nitrogen deprivation. It was found that NrrA and SigE were transcriptional regulators of glycogen catabolic genes. NrrA directly regulates expression of glgP1 and sigE. The sigE gene is required for the regulation of gap1, talB, and pyk1. It is concluded that NrrA controls not only heterocyst differentiation but also glycogen catabolism in collaboration with SigE.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Culture Conditions
Anabaena sp. strain PCC 7120 and its derivatives were grown in the BG-11 medium (containing NaNO3 as a nitrogen source) as described previously (11). For nitrogen deprivation experiments, cells grown in the BG-11 medium until they reached an OD750 of 0.4–0.5 were washed with the nitrogen-free medium (BG-110) and then resuspended in the BG-110 medium. Neomycin or spectinomycin was added to the medium at a final concentration of 30 μg/ml or 10 μg/ml, respectively, when required.
Mutant Construction
The sigE gene was inactivated as follows. DNA fragments upstream and downstream of the sigE gene were amplified by PCR using the primer pairs of sigE-5F and sigE-5R, and sigE-3F and sigE-3R, respectively (supplemental Table S1). The upstream fragment was cloned between the SacI and BamHI sites of pBluescript II KS+ (Agilent Technologies), and then the downstream fragment was cloned between the BamHI and XhoI sites. A neomycin resistance cassette excised by digestion with BamHI from the plasmid pRL161 (21) was inserted into the BamHI site between the upstream and downstream fragments. The SacI-XhoI fragment was excised from the resultant plasmid and cloned between the SacI and XhoI sites of pRL271 (22) to construct pRsigEK. pRsigEK was transferred by conjugation into Anabaena PCC 7120 or DR4312S (11) according to Elhai and Wolk (23) to construct the sigE mutant DRsigEK or the nrrA/sigE double mutant DR4312/sigEK, respectively.
Real-time Quantitative Reverse Transcription-PCR
Total RNA was extracted from whole filaments according to Pinto et al. (24) and was treated with DNase I (Takara Bio, Shiga, Japan). cDNAs were synthesized from 1 μg of total RNA with 1 μl of Anabaena-specific primer mix (11) and 1 pmol of a 16 S rRNA-specific reverse primer, RTrrn16SR2 (supplemental Table S1), using PrimeScript first strand cDNA synthesis kit (Takara Bio). qRT-PCR2 was performed with Thermal Cycler Dice Real Time System (Takara Bio) in a 10-μl reaction mixture containing 5 μl of SYBR Premix Ex Taq (Takara Bio), 0.2 μm each gene-specific forward and reverse primers (supplemental Table S1), and cDNA. Relative ratios were normalized with the value for 16 S rRNA and are represented as means of triplicate experiments.
Mapping of Transcription Initiation Sites by Rapid Amplification of cDNA Ends (RACE)-PCR
The transcription initiation sites of glgP1 were determined using the SMARTer RACE cDNA Amplification kit (Takara Bio). 5′-RACE-PCR was carried out with 1 μg of total RNA and gene-specific primers as recommended by the supplier (supplemental Table S1). A resulting PCR product was cloned into a pGEM-T Easy vector (Promega), and nine clones were sequenced.
Gel Mobility Shift Assay
The promoter region of glgP1 (P1272), corresponding to the region from −293 to +12 with respect to the translation start site of the glgP1 gene, was amplified by PCR using the primer pair P1272-F and P1272-R (supplemental Table S1) and was cloned into the HincII site of the pBluescript II SK+ (Agilent Technologies). A digoxigenin-labeled T7promoter primer (supplemental Table S1) was used to prepare a digoxigenin-labeled probe in combination with P1272-R. DNA fragments, PhetR1 and PhetR2, corresponding to the region from −876 to −659 and from −440 to −182 with respect to the translation start site of the hetR gene, respectively, were prepared as described previously (14). Fragments P1272D and PhetR1D were generated by deleting the conserved sequence AA(A/T)GTCA using PrimeSTAR mutagenesis basal kit (Takara Bio) with primer pairs P1272D2-F and P1272D2-R, and PhetRD-F and PhetRD-R, respectively (supplemental Table S1). Probes PsigE, corresponding to the region from −258 to +34 with respect to the translation start site of the sigE gene, and P2806, corresponding to the region from −394 to −10 with respect to the translation start site of all2806, were prepared by PCR using the primer pair sigE-F and sigE-5R, and P2806-F and P2806-R (supplemental Table S1).
His-NrrA protein was overproduced and purified from Escherichia coli by Ni-affinity chromatography as described previously (14). Gel mobility shift assays were carried out using 1 fmol of the digoxigenin-labeled probes as described previously (14).
Glycogen Determination
The glycogen content of whole filaments was determined according to Forchhammer and Tandeau de Marsac (25). Filaments were suspended in 200 μl of 2.5% (v/v) sulfuric acid solution and boiled for 40 min. Glucose in the hydrolysate was quantified by a colorimetric assay using the o-toluidine solution (Sigma). Protein concentrations were determined with a protein assay kit (Bio-Rad) by using bovine serum albumin as the standard.
Acetylene Reduction Assays
The nitrogenase activity was determined by the acetylene reduction assays. Portions (1 ml) of the cell suspension (approximately 3–7 μg of Chl a/ml) were transferred to 7.8-ml vials, and acetylene was added to a final concentration of 12% (v/v) in air. After 0.5–1 h of incubation under illumination, the concentration of ethylene in the headspace was measured by gas chromatography (GC-8A; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a Porapak T-packed column (80/100 mesh, 3 mm × 1 m). The rates of acetylene reduction to ethylene were normalized to OD750.
RESULTS
NrrA Positively Regulates Expression of the glgP1 Gene
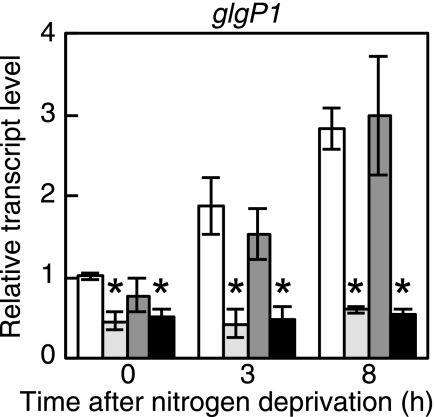
In the previous study (11), effects of the nrrA gene disruption on whole gene expression of Anabaena PCC 7120 under nitrogen-deprived conditions were investigated using a DNA microarray. Expression of the glgP1 gene, encoding a glycogen phosphorylase, was affected by the nrrA disruption (11). Differential expression of the glgP1 gene was confirmed by qRT-PCR analysis. In the wild-type strain, the glgP1 gene was up-regulated within 3 h after nitrogen deprivation, with increases in the transcript level of about 3-fold after 8 h (Fig. 1). In the nrrA disruptant, the glgP1 transcript level was reduced to a half of the wild-type level in the presence of nitrate, and no induction of glgP1 by nitrogen deprivation was observed (Fig. 1). It is indicated that NrrA positively regulates the expression of glgP1.
FIGURE 1.
Changes in the transcript level of glgP1 after nitrogen deprivation. The relative transcript levels of glgP1 before (0 h) and 3 or 8 h after nitrogen deprivation were determined by qRT-PCR in the wild-type strain (white bars), the nrrA disruptant (light gray bars), the sigE disruptant (dark gray bars), and the nrrA/sigE double mutant (black bars). The transcript levels were determined in duplicate using three independently grown cultures. The transcript level at 0 h of the wild-type strain was taken as 1. Data that represent a significant difference (p < 0.05) by applying the t test between the wild-type strain and each mutant strain are marked with asterisks.
NrrA Binds to the Promoter Region of the glgP1 Gene
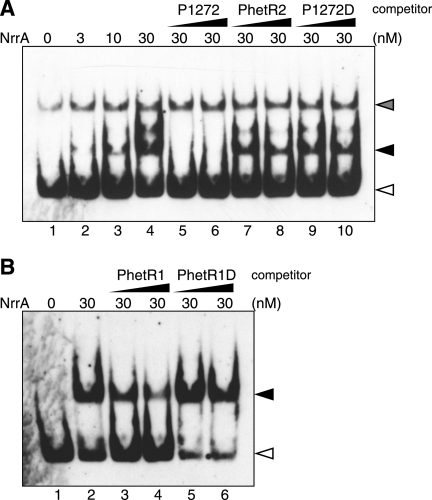
Gel mobility shift assays were carried out with purified His-NrrA and DNA probe for the upstream region of the glgP1 gene (Fig. 2A). His-NrrA reduced the electrophoretic mobility of probe P1272 including the glgP1 promoter, and the amount of the NrrA-P1272 complex increased in proportion to the concentration of His-NrrA (Fig. 2A, lanes 1–4). The band intensity of the NrrA-P1272 complex was decreased upon addition of a nonlabeled P1272 fragment (Fig. 2, lanes 5 and 6). The addition of a fragment PhetR2, which does not interact with His-NrrA (14), did not affect the amount of the NrrA-P1272 complex (Fig. 2A, lanes 7 and 8). These results show that His-NrrA binds to the region upstream of the glgP1 gene in a sequence-specific manner.
FIGURE 2.
Gel mobility shift assays with His-NrrA and the promoter regions of the glgP1 and hetR genes. A, digoxigenin-labeled probe P1272 (50 pm) including the glgP1 promoter region was mixed with His-NrrA in the amounts indicated above each lane, and then the mixtures were subjected to electrophoresis. Nonlabeled fragments of P1272 (lanes 5 and 6), PhetR2 (lanes 7 and 8), and P1272D (lanes 9 and 10) were added at a final concentration of 0.5 nm (lanes 5, 7, and 9) or 1.5 nm (lanes 6, 8, and 10). B, binding of His-NrrA to probe PhetR1 (50 pm) was examined. His-NrrA was added in the amount indicated above each lane. Nonlabeled fragments of PhetR1 (lanes 3 and 4) and PhetR1D (lanes 5 and 6) were added at a final concentration of 5 nm (lanes 3 and 5) and 15 nm (lanes 4 and 6). Filled arrows, complexes of His-NrrA and P1272 or PhetR1; open arrows, probe alone; gray arrow, a nonspecific fragment amplified by PCR.
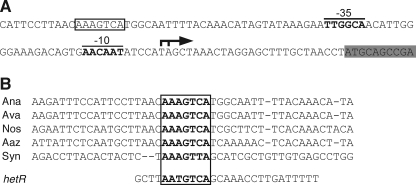
The transcription initiation sites of glgP1 were determined by RACE-PCR experiments. Two 5′ ends of the glgP1 transcripts were detected corresponding to nucleotides −26 and −27 with respect to the translation start site (Fig. 3A). Putative −10 and −35 promoter regions were found upstream of the transcription initiation sites (Fig. 3A). Sequence analyses of upstream regions of the glgP1 orthologs from Anabaena spp. and Nostoc spp. identified a conserved sequence (AAAGTCA) (Fig. 3B). This conserved sequence was also found within the promoter region of the glgP gene (slr1367) of Synechocystis sp. PCC 6803 (Fig. 3B), which is up-regulated by nitrogen deprivation (26). The NrrA binding region of the hetR promoter has been determined by DNase I footprinting assays (14), and a similar sequence (AATGTCA) was found within the region (Fig. 3B). Interaction between NrrA and the conserved sequence was elucidated by competition assays (Fig. 2). A fragment P1272D, in which the conserved sequence was deleted, did not compete with P1272 (Fig. 2A, lanes 9 and 10), indicating that the conserved sequence is indispensable for the NrrA-P1272 interaction. NrrA bound to the hetR promoter region, including the conserved sequence (Fig. 2B, lanes 1–4), as reported previously (14). Deletion of the conserved sequence from PhetR1 (PhetR1D) abolished the interaction between NrrA and PhetR1 (Fig. 2B, lanes 5 and 6).
FIGURE 3.
Nucleotide sequence of the glgP1 promoter region. A, The coding region of the glgP1 gene is shaded in gray. A bent arrow and boldface letters indicate the identified transcription initiation sites and the −10 and −35 promoter regions, respectively. A NrrA-binding site is enclosed in a box. B, alignment of the regions upstream of glgP1 orthologs from Anabaena PCC 7120 (Ana), Anabaena variabilis ATCC 29413 (Ava), Nostoc punctiforme PCC 73102 (Nos), Anabaena azollae 0708 (Aaz), and Synechocystis PCC 6803 (Syn). The NrrA-binding region within the hetR promoter (14) is shown under the alignment. The conserved sequences are shown by boldface letters.
NrrA Activates Glycogen Catabolism
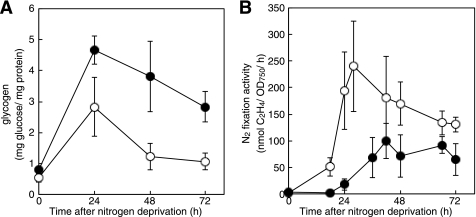
Fig. 4A shows glycogen contents in the wild-type strain and the nrrA disruptant after nitrogen deprivation. The glycogen content in the wild-type strain increased to about 5-fold during the first 24 h and then decreased to a level twice as much as that in the nitrate-grown filaments. In the nrrA disruptant, the glycogen content drastically increased by nitrogen deprivation and then decreased to a level approximately 3-fold higher than that in the wild type 72 h after nitrogen deprivation. It is suggested that NrrA activates glycogen degradation.
FIGURE 4.
Glycogen content (A) and nitrogenase activities (B) of the wild-type strain (open circles) and the nrrA disruptant (closed circles) during diazotrophic growth. Filaments grown with nitrate were transferred to the nitrogen-free medium at time 0. Amounts of glycogen and nitrogenase activities were determined at the indicated times. Means ± S.D. (error bars) of three independent experiments are shown.
To see the correlation between glycogen catabolism and nitrogen fixation, nitrogenase activities were determined (Fig. 4B). Nitrogenase activity was highest 28 h after nitrogen deprivation in the wild-type strain, whereas that in the nrrA disruptant reached a maximum level 42 h after nitrogen deprivation, which was consistent with the delay in heterocyst differentiation of the nrrA disruptant (11). In addition, nitrogenase activities in the nrrA disruptant were lower than that in the wild type even after maturation of heterocysts (Fig. 4B). These results support the idea that NrrA positively regulates nitrogenase activity by stimulating glycogen catabolism.
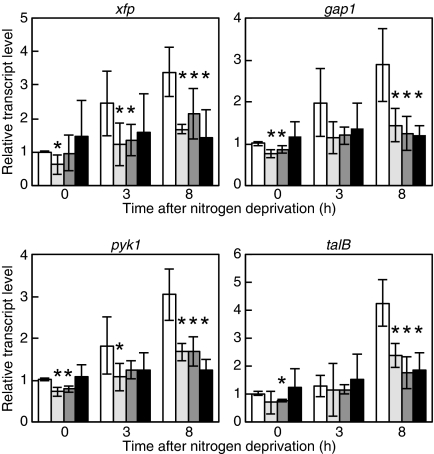
SigE Is a Positive Regulator of the xfp Operon
The rre37 gene, an ortholog of nrrA in Synechocystis sp. PCC 6803, is involved in regulation of glycolytic genes, gap1 and pfkA, under nitrogen-depleted conditions (27). In Anabaena PCC 7120, glycolytic genes, gap1 (all2566) encoding a glyceraldehyde-3-phosphate dehydrogenase and pyk1 (all2564) encoding a pyruvate kinase, were up-regulated by nitrogen deprivation (11). These genes were transcribed with the xfp gene (all2567) encoding a phosphoketolase and the talB gene (all2563) encoding a transaldolase as an operon, and the putative promoters were found upstream of xfp and talB (data not shown). Disruption of nrrA prevented the induction of these genes by nitrogen deprivation (Fig. 5). However, gel mobility shift assays have failed to show any interaction between NrrA and fragments upstream of xfp and talB (data not shown). Thus, effects of the nrrA disruption on expression of the xfp operon may be indirect and mediated by other proteins whose activities or expression are affected by nrrA.
FIGURE 5.
Changes in the transcript levels of the xfp operon after nitrogen deprivation. The relative transcript levels of xfp, gap1, pyk1, and talB were determined by qRT-PCR as described in Fig. 1.
Using DNA microarrays, we found that the xfp operon was down-regulated by disruption of the sigE gene.3 The effect of the sigE disruption on expression of genes involved in glycogen catabolism was determined by qRT-PCR. Expression of glgP1 was not affected by disruption of sigE (Fig. 1), whereas induction of the xfp operon by nitrogen deprivation was abolished in the sigE disruptant (Fig. 5). Thus, SigE is a transcriptional activator of the xfp operon. The genetic relationship between NrrA and SigE was investigated with an nrrA/sigE double mutant. Disruption of the nrrA gene again reduced the glgP1 transcript level and abolished the induction of glgP1 by nitrogen deprivation in the sigE mutant background (Fig. 1). In contrast, expression patterns of the xfp operon in the double mutant were comparable with the single mutants (Fig. 5). Because NrrA does not bind to the promoter regions of the xfp operon, NrrA is likely to control the expression of the xfp operon via SigE.
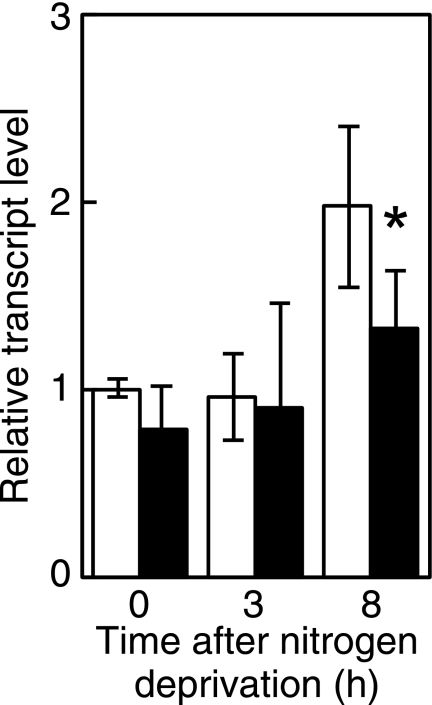
NrrA Directly Regulates the sigE Expression
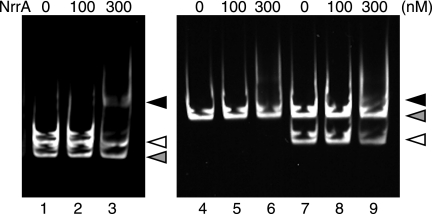
The sigE transcript is shown to increase 12 h after nitrogen deprivation in Anabaena PCC 7120 (19). Changes in the transcript level of sigE were examined at earlier stages of nitrogen deprivation. Expression of sigE was increased 8 h after nitrogen deprivation (Fig. 6). The transcript level of sigE at 8 h was decreased by disruption of nrrA (Fig. 6). Interaction of NrrA with the region upstream of the sigE gene (PsigE), in which the promoter region necessary for the sigE expression was included (19), was determined by gel mobility shift assays (Fig. 7). In the presence of both PsigE and PhetR2, NrrA specifically bound to PsigE (lanes 1–3). The specific binding of NrrA to PsigE was investigated further using P2806 as a competitor. NrrA did not interact with P2806 (lanes 4–6). The specific interaction between NrrA and PsigE was observed in the presence of P2806 (lanes 7–9). These results indicate that NrrA directly regulates the sigE expression.
FIGURE 6.
Changes in the transcript levels of sigE after nitrogen deprivation. The relative transcript levels of sigE were determined by qRT-PCR in the wild-type strain (white bars) and the nrrA disruptant (black bars) as described Fig. 1.
FIGURE 7.
Gel mobility shift assays with His-NrrA and the sigE promoter region. His-NrrA was mixed with 10 nm PsigE (lanes 1–3 and 7–9) or 10 nm P2806 (lanes 4–6) in the amounts indicated above each lane. Probe PhetR2 (lanes 1–3) or P2806 (lanes 7–9) was added in a final concentration of 10 nm to demonstrate the specific interaction between NrrA and PsigE. Black arrow, complex with NrrA and PsigE; open arrow, PsigE alone; gray arrow, PhetR2 or P2806 alone.
DISCUSSION
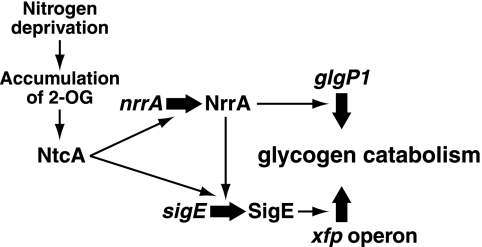
In the present study, we demonstrated that NrrA controlled glycogen catabolism in Anabaena PCC 7120 (Fig. 8). Disruption of the nrrA gene resulted in reduced expression of the genes involved in glycogen degradation and glycolysis (Figs. 1 and 5) and in accumulation of glycogen (Fig. 4). NrrA not only regulates the expression of the glgP1 gene directly (Fig. 2), but also regulates the expression of the xfp operon indirectly via SigE (Figs. 1 and 5). NrrA plays a pivotal role in the regulation of carbon metabolism in collaboration with SigE.
FIGURE 8.
Regulatory network model of genes involved in glycogen catabolism. Thick black arrows represent up-regulation of gene expression. For details, see the text.
In the nrrA disruptant, the transcription of glgP1 was suppressed, and its induction by nitrogen deprivation was abolished (Fig. 1). It was shown that NrrA bound to the glgP1 promoter region, and the sequence AAAGTCA within the glgP1 promoter was required for their interaction (Fig. 2A). A similar sequence (AATGTCA) found within the NrrA-binding site of the hetR promoter (14) was also indispensable for the binding of NrrA to the hetR promoter (Fig. 2B). Thus, the sequence AA(A/T)GTCA is a candidate for the NrrA recognition sequence.
It was noted that SigE was a transcriptional activator of the xfp operon (Fig. 5). Expression of sigE is shown to be up-regulated in differentiating cells at late stage of heterocyst differentiation using the sigE-gfp transcriptional reporter fusion (18), and SigE is involved in regulation of heterocyst-specific genes, such as nifH, fdxH, and hglE2 (19). In the present study, we showed that the transcription of sigE was up-regulated within 8 h after nitrogen deprivation, and NrrA regulated the sigE expression (Fig. 6). Binding of NtcA to the region approximately 700 bp upstream of sigE has been reported; however, the NtcA-binding site is dispensable for the regulation of sigE expression (19). The 260-bp region upstream of the translation start site of sigE has been shown to be necessary for its transcriptional regulation (19). NrrA bound to the DNA fragment including the 260-bp upstream region of sigE (Fig. 7). A putative NrrA-binding site (AAAGACA) is found within the 260-bp upstream region of sigE. It is likely that the expression of the sigE gene is regulated by NrrA and partly by NtcA (Fig. 8).
Up-regulation of the xfp operon preceded that of the sigE gene (Figs. 5 and 6). The activity of SigE is regulated by the anti-σ factor ChlH in Synechocystis PCC 6803 (28). It is supposed that SigE is post-translationally activated first, and then the transcription of the sigE gene is activated to further induce the xfp operon.
The nrrA disruptant exhibited a high abundance of glycogen during diazotrophic growth (Fig. 4A), indicating the reduced expression of the glgP1 gene accompanied with decreased degradation of glycogen. Glycogen is utilized to provide reductants and ATP for nitrogen fixation (29). The depressed nitrogenase activity in the nrrA disruptant may be due to the decreased degradation of glycogen (Fig. 4B). Alternatively, down-regulation of the sigE gene in the nrrA disruptant might reduce expression of the nifHDK operon encoding the nitrogenase subunits (19). However, the transcript levels of nifHDK in heterocysts of the nrrA disruptant were higher than that in the wild-type strain (data not shown). Hence, it is unlikely that the down-regulation of sigE is responsible for the depressed nitrogenase activity.
In Synechocystis PCC 6803, rre37, an ortholog of nrrA, and sigE are involved in regulation of sugar catabolism (26, 27). Rre37 and SigE independently regulate sugar catabolism under the control of NtcA in contrast to Anabaena PCC 7120, and transcriptional regulation of sigE is exclusively dependent on NtcA (30). In Anabaena PCC 7120, expression of genes involved in sugar metabolism is subjected to developmental regulation. Because ntcA, nrrA, and sigE are up-regulated in differentiating cells (11, 18, 31), a regulatory cascade consisting of NtcA, NrrA, and SigE could regulate reorganization of the carbon metabolic system in the process of heterocyst development.
Supplementary Material
This work was supported by the Adaptable and Seamless Technology Transfer Program from the Japan Science and Technology Agency (to S. E.), Grant-in-aid for Scientific Research (B) 23603005 from the Japan Society for the Promotion of Science (to S. E.), and the Sasakawa Scientific Research Grant from the Japan Science Society (to S. E.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
S. Ehira and M. Ohmori, unpublished data.
- qRT-PCR
- quantitative reverse transcription PCR
- RACE
- rapid amplification of cDNA ends.
REFERENCES
- 1. Flores E., Herrero A. (2010) Nat. Rev. Microbiol. 8, 39–50 [DOI] [PubMed] [Google Scholar]
- 2. Kumar K., Mella-Herrera R. A., Golden J. W. (2010) Cold Spring Harb. Perspect. Biol. 2, a000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cumino A. C., Marcozzi C., Barreiro R., Salerno G. L. (2007) Plant Physiol. 143, 1385–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolk C. P., Ernst A., Elhai J. (1994) in The Molecular Biology of Cyanobacteria (Bryant D. A. ed) pp. 769–823, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 5. Ballicora M. A., Iglesias A. A., Preiss J. (2003) Microbiol. Mol. Biol. Rev. 67, 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suzuki E., Ohkawa H., Moriya K., Matsubara T., Nagaike Y., Iwasaki I., Fujiwara S., Tsuzuki M., Nakamura Y. (2010) Appl. Environ. Microbiol. 76, 3153–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fay P. (1976) Appl. Environ. Microbiol. 31, 376–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valladares A., Maldener I., Muro-Pastor A. M., Flores E., Herrero A. (2007) J. Bacteriol. 189, 4425–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López-Igual R., Flores E., Herrero A. (2010) J. Bacteriol. 192, 5526–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vargas W. A., Nishi C. N., Giarrocco L. E., Salerno G. L. (2011) Planta 233, 153–162 [DOI] [PubMed] [Google Scholar]
- 11. Ehira S., Ohmori M. (2006) Mol. Microbiol. 59, 1692–1703 [DOI] [PubMed] [Google Scholar]
- 12. Muro-Pastor A. M., Olmedo-Verd E., Flores E. (2006) FEMS Microbiol. Lett. 256, 171–177 [DOI] [PubMed] [Google Scholar]
- 13. Herrero A., Muro-Pastor A. M., Valladares A., Flores E. (2004) FEMS Microbiol. Rev. 28, 469–487 [DOI] [PubMed] [Google Scholar]
- 14. Ehira S., Ohmori M. (2006) J. Bacteriol. 188, 8520–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buikema W. J., Haselkorn R. (1991) Genes Dev. 5, 321–330 [DOI] [PubMed] [Google Scholar]
- 16. Buikema W. J., Haselkorn R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2729–2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimura H., Okamoto S., Tsumuraya Y., Ohmori M. (2007) DNA Res. 14, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aldea M. R., Mella-Herrera R. A., Golden J. W. (2007) J. Bacteriol. 189, 8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mella-Herrera R. A., Neunuebel M. R., Kumar K., Saha S. K., Golden J. W. (2011) J. Bacteriol. 193, 1823–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcozzi C., Cumino A. C., Salerno G. L. (2009) Arch. Microbiol. 191, 255–263 [DOI] [PubMed] [Google Scholar]
- 21. Elhai J., Wolk C. P. (1988) Gene 68, 119–138 [DOI] [PubMed] [Google Scholar]
- 22. Black T. A., Cai Y., Wolk C. P. (1993) Mol. Microbiol. 9, 77–84 [DOI] [PubMed] [Google Scholar]
- 23. Elhai J., Wolk C. P. (1988) Methods Enzymol. 167, 747–754 [DOI] [PubMed] [Google Scholar]
- 24. Pinto F. L., Thapper A., Sontheim W., Lindblad P. (2009) BMC Mol. Biol. 10, 79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forchhammer K., Tandeau de Marsac N. (1995) J. Bacteriol. 177, 2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Osanai T., Imamura S., Asayama M., Shirai M., Suzuki I., Murata N., Tanaka K. (2006) DNA Res. 13, 185–195 [DOI] [PubMed] [Google Scholar]
- 27. Azuma M., Osanai T., Hirai M. Y., Tanaka K. (2011) Plant Cell Physiol. 52, 404–412 [DOI] [PubMed] [Google Scholar]
- 28. Osanai T., Imashimizu M., Seki A., Sato S., Tabata S., Imamura S., Asayama M., Ikeuchi M., Tanaka K. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6860–6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ernst A., Reich S., Böger P. (1990) J. Bacteriol. 172, 748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muro-Pastor A. M., Herrero A., Flores E. (2001) J. Bacteriol. 183, 1090–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Olmedo-Verd E., Muro-Pastor A. M., Flores E., Herrero A. (2006) J. Bacteriol. 188, 6694–6699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.