Background: Apolipoprotein A-I (apoA-I) is the major protein component of high density lipoprotein (HDL).
Results: C-terminal domain of apoA-I is the effector site providing the bactericidal activity.
Conclusion: ApoA-I contributes to the complement-mediated killing of a Gram-negative bacterium Yersinia enterocolitica.
Significance: The use of apoA-I mimetic peptides may be a therapeutic approach for the treatment of certain Gram-negative infections.
Keywords: Apolipoproteins, Bacteria, Complement, High Density Lipoprotein (HDL), Lipopolysaccharide (LPS), Innate Immunity
Abstract
Apolipoprotein A-I (apoA-I), the main protein component of high density lipoprotein (HDL), is well recognized for its antiatherogenic, antioxidant, and antiinflammatory properties. Here, we report a novel role for apoA-I as a host defense molecule that contributes to the complement-mediated killing of an important gastrointestinal pathogen, Gram-negative bacterium Yersinia enterocolitica. We specifically show that the C-terminal domain of apoA-I is the effector site providing the bactericidal activity. Although the presence of the lipopolysaccharide O-antigen on the bacterial surface is absolutely required for apoA-I to kill the bacteria, apoA-I does not interact with the bacteria directly. To the contrary, exposure of the bacteria by serum proteins triggers apoA-I deposition on the bacterial surface. As our data show that both purified lipid-free and HDL-associated apoA-I displays anti-bacterial potential, apoA-I mimetic peptides may be a promising therapeutic agent for the treatment of certain Gram-negative infections.
Introduction
High density lipoprotein (HDL) provides protection against atherosclerosis via several mechanisms from which promotion of reverse cholesterol transport from the periphery to the liver is perhaps physiologically the most important (1). However, in addition to these antiatherogenic functions, HDL is increasingly considered as a part of the innate immune system, the first line of host defense against invading pathogens. HDL displays broad antiviral activity by preventing virus penetration (2) and protects the host against trypanosomiasis (3) and Gram-negative bacteremia (4). In addition, HDL binds and neutralizes bacterial lipopolysaccharide (LPS) (5). The importance of HDL is also reflected by a dramatic decrease in serum levels of HDL and its major protein component, apolipoprotein A-I (apoA-I)2 during microbial infections (6). Accordingly, the risk of coronary artery disease is higher in patients suffering from any infection, even under conditions when the pathogen does not localize to the vessel wall (7).
The complement system is part of the primary line of human innate immunity (8). Pathogens, however, have developed a number of strategies to evade antimicrobial action of the complement system. One of them is acquisition of host complement regulators that protect host tissues against complement-mediated damage. This strategy provides microbes with the means to block complement activation on their own surfaces (8).
In addition to well characterized complement regulators, HDL has also been suggested to display a complement-inhibitory potential via interference with the final step in complement activation, assembly of the membrane-attack complex (C5b-9). HDL-associated lipoproteins, apoA-I and apoA-II, recognize the same binding site on C5b-9, bind to C9, and interfere with C9 polymerization or insertion of the membrane-attack complex into the membrane (9–11). Purified apoA-I and apoA-II, or delipidated HDL, however, display greater membrane-attack complex-inhibitory activity than intact HDL (10, 11).
Yersinia enterocolitica serotype O:3 is a food-borne enteropathogen that causes enterocolitis, mesenteric lymphadenitis, terminal ileitis, and reactive arthritis (12). Septicemia caused by Y. enterocolitica can occur in both normal and immunocompromised hosts. It may be associated with abscess formation in the liver and spleen, pneumonia, or lead to formation of an aneurysm (12). As expected on the basis of its pathogenicity, Y. enterocolitica resists bactericidal action of human serum (13). Interestingly, LPS of Y. enterocolitica (LPSYeO3), built of distal polysaccharide O-antigen, the core oligosaccharide and the membrane-anchoring lipid A (14–16), does not protect the bacteria against complement (13). However, outer membrane proteins YadA and Ail, expressed exclusively at 37 °C, confer serum resistance via binding of complement regulators (17–19).
Because Y. enterocolitica acquires host complement regulators to escape the complement attack, we investigated whether the bacteria could utilize the complement-regulatory potential of apoA-I. Surprisingly, we found that apoA-I mediates killing, not protection, of Y. enterocolitica. Here, we describe a new function for apo-AI as a pathogen-recognizing molecule that contributes to the killing of the bacteria in human serum.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, Bacteriophages, and Growth Conditions
Bacterial strains, plasmids, and bacteriophages used in this study are listed in supplemental Table 1. Bacteria were grown as described (13) (see also supplemental Methods).
Antibodies and Antisera
The antibodies used in this work are described in the supplemental Methods.
Human Plasma Lipoproteins
Human lipoproteins were isolated by sequential ultracentrifugation (20). The concentrations of lipoproteins used in the experiments are given in terms of their protein content. Protein concentration was determined as described (21).
Wild-type and Mutant ApoA-I Forms
Purified human apoA-I was provided by Dr. Peter Lerch (Swiss Red Cross, Bern, Switzerland). The mutant apoA-I Δ61–78, and Δ1–59 Δ185–243 forms were produced using baculovirus expression system (22). Adenoviruses expressing Δ185–243 and Δ220–243 were generated as described previously (23, 24). The adenoviruses expressing L218A/L219A/V221A/L222A and E223A/K226A were generated in a similar way (25).
Isolation of Lipopolysaccharide
LPSs from Y. enterocolitica strains were extracted as described in the supplemental Methods.
Normal Human Serum
Normal human serum (NHS) was obtained as described (13). Heat-inactivated serum (HIS) was generated by serum incubation at 56 °C for 30 min.
Serum Bactericidal Assay
Serum bactericidal assay was performed as described in the supplemental Methods.
Interaction of LPS with ApoA-I
Size exclusion chromatography and immunoprecipitation analyses were performed as described in the supplemental Methods.
Binding of ApoA-I to Y. enterocolitica
Immunoblotting and immunofluorescence analyses were performed as described in the supplemental Methods.
Statistics
Statistical analyses were performed using the two-tailed t test; p < 0.05 was considered statistically significant.
RESULTS
ApoA-I Contributes to Complement-mediated Killing of Y. enterocolitica
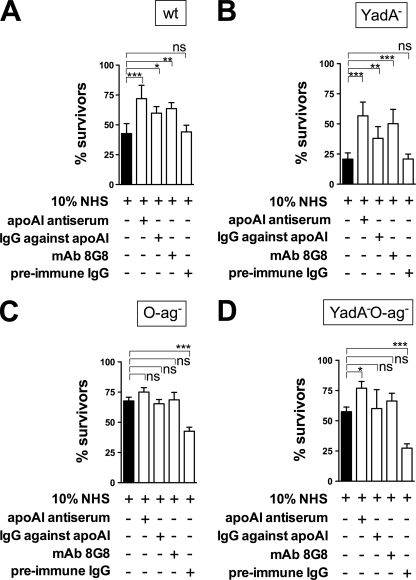
Because apoA-I belongs to negative complement regulators (9–11), we first examined whether Y. enterocolitica bacteria could protect themselves against complement-mediated killing by utilizing apoA-I. To this end, we blocked apoA-I in NHS by the addition of (i) rabbit antiserum against human apoA-I, (ii) IgG fraction of rabbit apoA-I antiserum, or (iii) mAb against apoA-I. As a control, the IgG fraction of preimmune rabbit serum was used. Bactericidal potential of the sera was tested against the wild-type Y. enterocolitica and its YadA-, O-antigen-, or YadA-O-antigen-negative derivatives. Contrary to our expectations, blocking of apoA-I in sera resulted in increased survival of smooth (O-antigen-expressing) bacteria (Fig. 1, A and B) whereas addition of the preimmune rabbit serum did not affect the bacterial survival. This suggested that the observed increase in bacterial survival was due to specific blocking of apoA-I in NHS. Interestingly, the rough (O-antigen-negative) bacteria did not benefit from apoA-I blocking in serum (Fig. 1, C and D), suggesting that apoA-I targets specifically O-antigen on the bacterial surface. In fact, the rough bacteria were more potently killed by the serum supplemented with the control preimmune IgG (Fig. 1, C and D). The absence of O-antigen on the bacterial surface could, however, expose antigenic Y. enterocolitica structures that cross-react with antibodies present in rabbit preimmune serum and, in consequence, activate the classical pathway of complement.
FIGURE 1.
Blocking of apoA-I in serum with specific antibodies and survival of Y. enterocolitica bacteria in 10% NHS. Wild-type Y. enterocolitica strain (YeO3; A) as well as its mutants lacking YadA (YeO3-O28; B), LPSYeO3 O-antigen (YeO3-R2; C), or both (YeO3-O28-R1; D) were incubated in NHS alone or supplemented with (i) rabbit antiserum against human apoA-I, (ii) IgG fraction of rabbit apoA-I antiserum (R283), (iii) mAb 8G8 against apoA-I, or (iv) control antibody represented by preimmune rabbit IgGs. Data represent means ± S.D. (error bars) from three separate experiments, each done in duplicate. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ns, not significant.
Because the smooth YeO3-O28 strain that does not express the main serum resistance protein YadA, benefitted the most from the apoA-I blocking (Fig. 1B), it was used as an indicator strain in all subsequent experiments. The survival of the indicator strain in serum increased with the increase of anti-apoA-I IgGs in a concentration-dependent manner (supplemental Fig. 1), demonstrating the specificity of the effect.
Interaction between ApoA-I and LPSYeO3
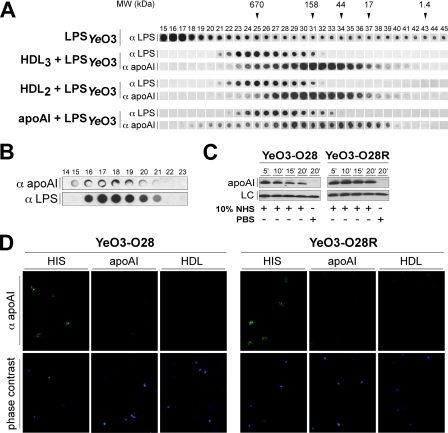
We next studied whether LPS could be the target for apoA-I. We incubated LPS purified from Y. enterocolitica grown at 10 °C with purified apoA-I, HDL2, or HDL3. Subsequently, the samples and LPSYeO3 alone were chromatographed on a high resolution Superose 6 column. The distribution of LPS and apoA-I in the eluted fractions was detected using the immuno-dot blotting with mAbs TomA6 and R297. Although LPS alone was widely distributed in the collected fractions, it emerged predominantly in the void volume (fractions 15–17, Fig. 2A). The pattern of LPSYeO3 distribution in apoA-I-, HDL2-, or HDL3-containing samples was dramatically different. In these samples LPSYeO3 co-eluted with apoA-I with a predominant distribution in those fractions containing large apoA-I-containing particles (Fig. 2A).
FIGURE 2.
Interaction between apoA-I and LPSYeO3. A, gel filtration analysis of the apoA-I-LPSYeO3 complex. Human apoA-I (1.5 mg/ml), HDL2 (1.5 mg/ml, as protein), or HDL3 (1.5 mg/ml, as protein) were preincubated with smooth LPS (50 μg/ml) and applied into a Superose 6 column (HR 10/30; Pharmacia Biotech). The control contained the corresponding amount of LPS incubated in the presence of PBS. The collected fractions were analyzed for the presence of apoA-I and LPSYeO3 using immuno-dot blotting with mAb TomA6 and R297 Abs. Arrows denote the elution positions of marker proteins: thyroglobulin, 670 kDa; bovine γ-globulin, 158 kDa; chicken ovalbumin, 44 kDa; equine myoglobin, 17 kDa; vitamin B12, 1.4 kDa. B, immunoprecipitation of the apoA-I-LPSYeO3 complex from the human serum. Protein G-Sepharose-coupled rabbit IgGs against apoA-I (R322) were used to immunoprecipitate apoA-I-complexes formed in 10% NHS that had been preincubated with LPSYeO3 (10 μg/ml) or PBS. The bound material was eluted (0.1 m glycine, pH 2.5) and loaded onto the Superose 12 column. Fractions were analyzed for the presence of apoA-I and LPSYeO3 using immuno-dot blotting. C, immunoblotting analysis of the apoA-I binding to Y. enterocolitica strains. YeO3-O28 and YeO3-O28R strains were incubated with of 50% NHS for 5, 10, 15, or 20 min at 37 °C. Bacteria incubated with PBS for 20 min at 37 °C served as control. After extensive washings, bound apoA-I was detected by immunoblotting with anti-apoA-I antibodies (R315). D, immunofluorescence analysis of apoA-I binding to Y. enterocolitica. YeO3-O28 and YeO3-O28R strains incubated with 10% HIS, apoA-I (0.15 mg/ml), HDL3 (0.15 mg/ml) for 40 min at 37 °C. Following three washes, the bacteria were incubated with 7.5 μg of R315 rabbit IgG against apoA-I. Bound antibodies were detected with Alexa Fluor 488 donkey anti-rabbit antibody.
To determine whether the interaction of apoA-I and LPSYeO3 also took place in human serum, we immunoprecipitated apoA-I-LPSYeO3 complexes using protein G-Sepharose-coupled anti-apoA-I IgG antibodies and fractionated the bound material. Immuno-dot blotting analyses using anti-LPSYeO3 and anti-apoA-I antibodies revealed the co-elution of apoA-I and LPSYeO3 in the collected fractions (Fig. 2B). This demonstrated that the apoA-I-LPSYeO3 interaction also took place in NHS.
We next examined whether apoA-I also binds to LPSYeO3 on the surface of Y. enterocolitica. We incubated the smooth or rough Y. enterocolitica strains with NHS for 5, 10, 15, or 20 min at 37 °C. Immunoblotting analyses revealed an immediate binding of apoA-I on both strains (Fig. 2C). ApoA-I deposition on the rough strain did not vary dramatically over time. On the contrary, the apoA-I deposition on the smooth Y. enterocolitica strain achieved the maximum at 5 min, after which the interaction decreased until 20 min (Fig. 2C). Importantly, at this time most of the bacteria were killed by the complement.
To examine whether the apoA-I deposition on the bacteria could also take place in the absence of complement activation, we incubated the smooth and rough Y. enterocolitica strains in HIS and analyzed the apoA-I binding by immunofluorescence. The staining revealed again the binding of apoA-I to Y. enterocolitica strains regardless of the presence of O-antigen (Fig. 2D). Because the O-antigen-negative strain is a spontaneous derivative of our indicator strain, the lack of O-antigen was confirmed by immunofluorescence (supplemental Fig. 2).
To examine whether apoA-I also binds to Y. enterocolitica in the absence of other serum components, we tested direct binding of purified apoA-I or HDL (subclass HDL3) to the smooth and rough strains by immunofluorescence microscopy. Surprisingly, apoA-I staining was negative for both strains (Fig. 2D), suggesting that the previously observed interaction between the purified LPSYeO3 and apoA-I (Fig. 2A) occurred most likely via the lipid A portion of LPS, as suggested earlier (26, 27). In summary, apoA-I interaction with Y. enterocolitica is not direct and seems to require preexposure of the bacteria to serum proteins.
Staining with the primary or conjugated antibodies alone in the immunofluorescence assays was negative (data not shown). No staining was seen with bacteria incubated in PBS instead of HIS (data not shown).
LPS O-antigen Is Required for ApoA-I Bactericidal Activity
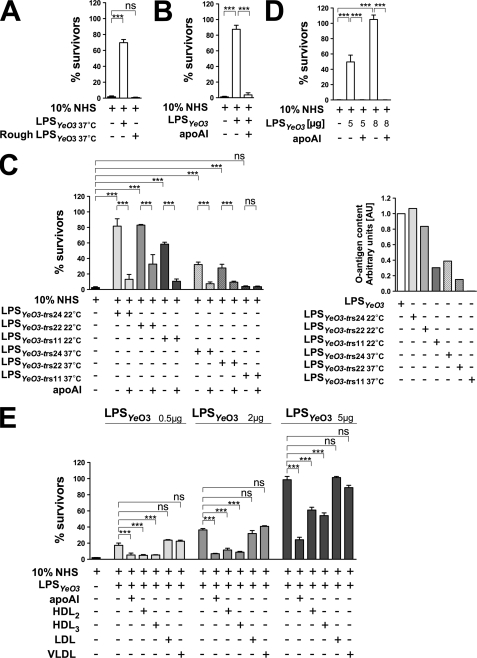
Given that LPSYeO3 interacts in serum with apoA-I (Fig. 2B), we aimed to block apoA-I in human serum using purified LPSYeO3. We preincubated NHS with 10 μg of smooth or rough LPSYeO3 isolated from the bacteria cultured at 37 °C. Subsequently, the sera were used to kill the indicator Y. enterocolitica strain. We demonstrated that the addition of smooth but not rough LPSYeO3 to human serum increased the survival of the indicator strain (Fig. 3A). Because the O-antigen expression in the stationary phase Y. enterocolitica is maximal below 30 °C (28), we performed the next experiment with 5 μg of the smooth LPSYeO3 isolated from the bacteria grown at 10 °C (Fig. 3B). Preincubation of NHS with this LPS caused a nearly 90% increase in bacterial survival (Fig. 3B). Thus, a higher survival rate was achieved with the use of 5 μg of 10 °C LPSYeO3 (Fig. 3B) compared with that obtained with 10 μg of 37 °C LPSYeO3 (Fig. 3A). Furthermore, the increased bacterial resistance depended on the blocking of apoA-I bactericidal function in serum, because the blocking was reversed by the addition of physiological amounts of exogenous lipid-free apoA-I into the 10 °C LPSYeO3-treated serum and was reflected by a drastic drop in bacterial survival (Fig. 3B).
FIGURE 3.
ApoA-I targets the LPS O-antigen of Y. enterocolitica. NHS was used at a final concentration of 10%. A, survival of Y. enterocolitica indicator bacteria in the LPSYeO3-treated NHS. The bacteria were incubated in (i) NHS alone, (ii) NHS preincubated with 10 μg of 37 °C smooth or rough LPSYeO3, or (iii) HIS. B, LPSYeO3 treatment of NHS blocking apoA-I bactericidal activity. Y. enterocolitica indicator bacteria were incubated in (i) NHS alone, (ii) NHS pretreated with 5 μg of 10 °C smooth LPSYeO3, (iii) 10 °C LPSYeO3-treated NHS supplemented with a physiological amount of apoA-I (0.15 mg/ml), or (iv) HIS. C (right), abundance of the O-antigen in the LPSs isolated from the Y. enterocolitica outer core mutants (YeO3-trs24, YeO3-trs22, and YeO3-trs11) grown at 22 °C or 37 °C. The O-antigen content in the isolated LPSs was determined by the immuno-dot blotting using mAb TomA6 against the O-antigen. Quantitative analyses were performed using ImageJ software. C (left), apoA-I blocking potential of LPSs varying in the O-antigen content. The Y. enterocolitica indicator bacteria were incubated in (i) NHS alone, (ii) NHS pretreated with 5 μg of LPSs isolated from the Y. enterocolitica outer core mutants (YeO3-trs24, YeO3-trs22, and YeO3-trs11) grown at 22 °C or 37 °C, (iii) NHS pretreated with one of the above-mentioned LPSs supplemented with physiological amounts of apoA-I, or (iv) HIS. D, serotype O:3O-antigen flagging the Y. enterocolitica serotype O:8 bacteria for the apoA-I-mediated bactericidal attack. Y. enterocolitica serotype O:8 strain (8081-c-R2/pAY100:Tet) that expresses serotype O:3 O-antigen was incubated in (i) NHS, (ii) 10 °C LPSYeO3-treated NHS, (iii) 10 °C LPSYeO3-treated NHS supplemented with physiological amounts of exogenous apoA-I, or (iv) HIS. E, effects of HDL, LDL, and VLDL on Y. enterocolitica survival in human serum. The Y. enterocolitica indicator bacteria were incubated in (i) NHS, (ii) NHS pretreated with 0.5, 2, or 5 μg of 10 °C LPSYeO3, (iii) 10 °C LPSYeO3-treated NHS to which 0.15 mg/ml wild-type apoA-I, HDL2, or HDL3 was added, (iv) 10 °C LPSYeO3-treated NHS to which 0.11 mg/ml LDL or VLDL was added, or (v) HIS. Data represent means ± S.D. (error bars) from two separate experiments, each done in duplicate. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ns, not significant.
To show further that apoA-I bactericidal activity depends on the O-antigen content of LPSYeO3, we isolated LPS from Y. enterocolitica mutants that lack the LPS outer core (YeO3-trs24, YeO3-trs22, and YeO3-trs11) and vary in O-antigen expression levels (13, 29). The strains were grown at 37 °C or 22 °C, and the abundance of the O-antigen in the isolated LPS was determined by the immune-dot blotting and quantified using ImageJ software (National Institutes of Health) (Fig. 3C, right). LPSs isolated from the bacteria grown at 22 °C contained an amount of O-antigen comparable with 10 °C LPSYeO3. The only exception was the LPSYeO3-trs11 that contained reduced amount of O-antigen. As expected, 37 °C LPSs (LPSYeO3-trs24 and LPSYeO3-trs22) contained less O-antigen, and no O-antigen could be detected in the preparation of the LPSYeO3-trs11 (Fig. 3C, right).
Next, these LPSs were tested for their ability to block the activity of apoA-I in NHS. This would be measured as an increase in survival of the indicator Y. enterocolitica bacteria. In support of our hypothesis, the apoA-I blocking potential of the LPSs correlated to their O-antigen content (Fig. 3C). In general, the 22 °C LPSs were much more potent than their 37 °C counterparts (Fig. 3C, left). In both 22 °C and 37 °C sets, LPSYeO3-trs24 and LPSYeO3-trs22 were more potent than LPSYeO3-trs11 (Fig. 3C, left). Moreover, preincubation of NHS with the 37 °C LPSYeO3-trs11, being O-antigen-negative, did not compromise the serum killing potential at all (Fig. 3C, left). Furthermore, the increase in bacterial survival, resulting from the LPS-mediated blocking of apoA-I, could be reversed in each case by the supplementation of the LPS-treated sera with the physiological amounts of exogenous lipid-free apoA-I (Fig. 3C, left).
To confirm that the surface-expressed O-antigen of the serotype O:3 is required for apoA-I to exert its bactericidal activity, the LPSYeO3 O-antigen was expressed in the O-antigen-negative strain of Y. enterocolitica serotype O:8 (8081-c-R2) that did not respond to preincubation of serum with LPSYeO3 (data not shown). The resulting strain 8081-c-R2/pAY100:Tet was incubated with NHS preincubated with 5 or 8 μg of 10 °C LPSYeO3. Expression of LPSYeO3 O-antigen by 8081-c-R2/pAY100:Tet resulted in an increase in bacterial resistance in the serum pretreated with LPSYeO3, and the increase in resistance was LPSYeO3 concentration-dependent (Fig. 3D). Moreover, supplementation of the LPSYeO3-treated serum with the physiological amount of lipid-free apoA-I reduced the resistance of 8081-c-R2/pAY100:Tet to the basal level (Fig. 3D). To the contrary, no such effect was observed with the control strain 8081-c-R2/pBR322 (data not shown).
Bactericidal Properties of Human HDL
Because lipid-free apoA-I appeared to have bactericidal activity, we next examined whether apoA-I in spherical HDL2 and HDL3 particles displays the same potential. As a control, we used apoA-I-lacking particles, such as LDL and VLDL, containing apoB-100 as the major structural protein. Lipoproteins were added to the sera, treated with 0.5, 2, or 5 μg of 10 °C LPSYeO3, at equiprotein concentrations. We observed that similar to the lipid-free apoA-I, the addition of HDL2 and HDL3 to the LPSYeO3-treated serum significantly diminished the LPSYeO3 effect and, in consequence, survival of the indicator strain in serum was greatly reduced (Fig. 3E). In contrast, addition of LDL or VLDL, devoid of apoA-I, to the LPSYeO3-treated serum did not improve the serum killing potential (Fig. 3E). Taken together, the data suggest that HDL, but not LDL nor VLDL, displays host-protective antibacterial properties.
Mapping ApoA-I Domain Mediating Bactericidal Activity
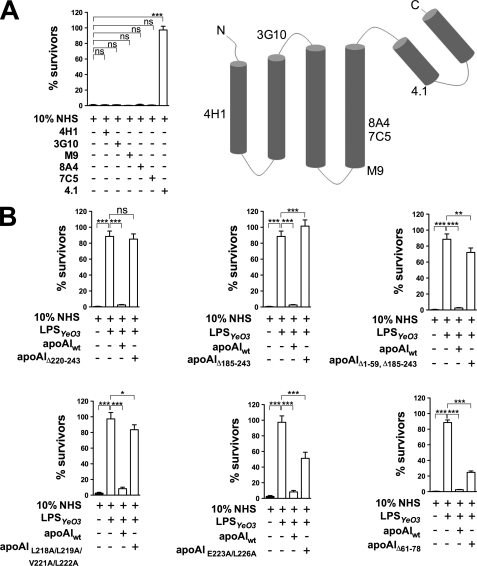
To map an apoA-I region that mediates bactericidal activity in human serum, we added mAbs specific for distinct domains of apoA-I (Fig. 4A, right) to NHS and subsequently determined the ability of the sera to kill Y. enterocolitica indicator bacteria. The only mAb that protected the bacteria was mAb 4.1, specific for the C-terminal domain of apoA-I (Fig. 4).
FIGURE 4.
Mapping the apoA-I domain necessary for the apoA-I-mediated bactericidal activity in 10% NHS. A (right), secondary structure of the lipid-free apoA-I-based computer modeling (43). Epitopes of mAbs specific for apoA-I (4H1, 3G10, M9, 8A4, 7C5, or 4.1) are indicated. A (left), mapping the apoA-I domain responsible for the bactericidal action in human serum using anti-apoA-I mAbs. The Y. enterocolitica indicator bacteria were incubated with (i) NHS, (ii) NHS to which 12 μg of mAb against apoA-I (4H1, 3G10, M9, 8A4, 7C5, or 4.1) was added, or (iii) HIS. B, bactericidal potential of the apoA-I mutants. The Y. enterocolitica indicator bacteria were incubated with (i) NHS, (ii) NHS pretreated with 5 μg of 10 °C LPSYeO3, (iii) 10 °C LPSYeO3-treated NHS to which 0.15 mg/ml wild-type or mutant apoA-I (Δ220–243, Δ185–243, Δ1–59, Δ185–243, L218A/L219A/V221A/L222A, and E223A/K226A) was added, or (iv) HIS. Data represent means ± S.D. (error bars) from three (A) or two (B) separate experiments, each done in duplicate. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ns, not significant.
To confirm that the C terminus of apoA-I has anti-bacterial activity, we used purified mutant apoA-I proteins with mutations in the C or N terminus or both. The mutant proteins were tested for their ability to reduce survival of the indicator bacteria in the 10 °C LPSYeO3-treated serum. In agreement with the antibody mapping results, mutant proteins having C-terminal deletions (Δ220–243, Δ185–243), C/N-terminal deletion (Δ1–59 Δ185–243), or having point mutations in the C-terminal portion of apoA-I (L218A/L219A/V221A/L222A) were not able to restore the serum killing potential as efficiently as the wild-type apoA-I (Fig. 4B). On the other hand, the mutant protein with point mutations E223A/K226A was able to reverse the LPSYeO3 effect partially whereas the mutant protein having N-terminal internal deletion (Δ61–78) was almost as potent as the wild type (Fig. 4B). These results, consistent with the data obtained with mAb 4.1, suggest strongly that the apoA-I region encompassing residues 218–222 is required for the bacterial killing.
Bactericidal Action of ApoA-I and Complement System Activation
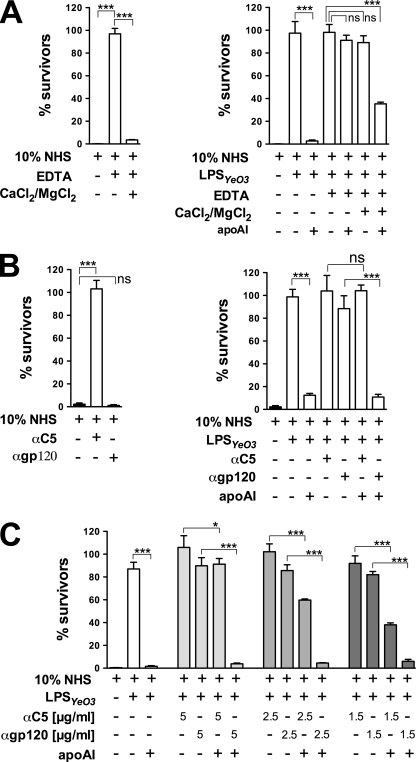
Because apoA-I alone is not able to kill Y. enterocolitica bacteria (data not shown), and it does not bind to the bacteria in the absence of serum (Fig. 2D), we investigated whether apoA-I action depends on the active complement system. The activation of complement requires both Mg2+ and Ca2+. We used 5 mm EDTA to chelate these ions and block all of the complement activation pathways. As shown in Fig. 5A, addition of EDTA to NHS efficiently blocked the complement activation, as reflected by 100% survival of the indicator strain. Moreover, reconstitution of EDTA-serum with Mg2+ and Ca2+ ions fully restored the complement activity and reduced the bacterial survival (Fig. 5A). We thus added EDTA to 10 °C LPSYeO3-treated sera and examined the ability of apoA-I to reduce bacterial resistance under these conditions. Interestingly, the apoA-I effect could only be seen in LPSYeO3-treated serum and EDTA-containing LPSYeO3-treated sera with reconstituted Mg2+ and Ca2+ ions (Fig. 5A, right).
FIGURE 5.
Complement system and the bactericidal action of apoA-I in 10% serum. A (left), efficacy of EDTA to block the complement activation. The Y. enterocolitica indicator bacteria were incubated with (i) NHS, (ii) NHS treated with 0.5 mm EDTA, (iii) EDTA-treated NHS supplemented with 2 mm CaCl2 and 0.5 mm MgCl2, or (iv) HIS. A (right), EDTA-blocked complement activation and apoA-I activity. The Y. enterocolitica indicator bacteria were incubated with (i) NHS, (ii) NHS pretreated with 5 μg of 10 °C LPSYeO3, (ii) 10 °C LPSYeO3-treated NHS supplemented with physiological amounts of apoA-I, (iii) 10 °C LPSYeO3-treated NHS to which 0.5 mm EDTA was added, (iv) apoA-I-supplemented, 10 °C LPSYeO3-treated NHS containing only EDTA, or EDTA supplemented with 2 mm CaCl2 and 0.5 mm MgCl2, (v) HIS. B (left), efficacy of the anti-C5 antibody to block the complement activity. The Y. enterocolitica indicator strain was incubated with (i) NHS, (ii) NHS supplemented with the mAb against C5 (5 μg/ml) or its isotype-matched control, anti-HIV1 gp120 antibody (5 μg/ml), or (iii) HIS. B (right) and C, apoA-I activity in anti-C5 antibody-treated serum. The Y. enterocolitica indicator strain was incubated with (i) NHS, (ii) NHS pretreated with 5 μg of 10 °C LPSYeO3, (ii) 10 °C LPSYeO3-treated NHS supplemented with physiological amounts of apoA-I, (iii) 10 °C LPSYeO3-treated NHS to which anti-C5 (5 μg/ml, B; 1.5, 2.5, or 5 μg/ml, C) or anti-gp120 was added (5 μg/ml, B; 1.5, 2.5, or 5 μg/ml, C), (iv) apoA-I-supplemented, 10 °C LPSYeO3-treated, NHS containing anti-C5 or anti-gp120 antibodies, (v) HIS. Data represent means ± S.D. (error bars) from two separate experiments, each done in duplicate. *, p < 0.05; **, p < 0.005; ***, p < 0.0005; ns, not significant.
To demonstrate that the impaired apoA-I activity in EDTA-serum was due to the complement inhibition rather than to the effect of the chemical on apoA-I, we blocked the three complement activation pathways in 10 °C LPSYeO3-treated serum using a mAb against the complement component C5. A mAb against HIV gp120 was used as an isotype-matched control. As shown in Fig. 5B (left), addition of anti-C5 mAb to NHS completely blocked the complement activity as reflected by the 100% survival of the indicator strain. In contrast, the serum supplemented with the control antibody was as efficient in killing the bacteria as NHS alone (Fig. 5B, left). The antibodies were thus added to 10 °C LPSYeO3-treated sera, and the ability of apoA-I to reduce bacterial resistance under these conditions was tested. Blocking of complement with anti-C5 mAb totally abolished the apoA-I effect in LPSYeO3-treated serum, as the apoA-I activity could only be observed in sera to which no antibody or the control antibody was added (Fig. 5B, right). Furthermore, decreasing anti-C5 mAb concentration in LPSYeO3-treated serum increased apoA-I activity in dose-dependent manner (Fig. 5C).
In summary, these findings strongly suggest that the active complement is the absolute requirement for the bactericidal function of apoA-I in human serum.
DISCUSSION
In this study we have investigated the role of HDL in host defense against bacterial pathogens. Using Y. enterocolitica we demonstrated that both purified apoA-I and HDL display anti-bacterial activity that is mediated via the complement activation. We mapped the domain of apoA-I required for its anti-bacterial activity and obtained data suggesting that expression of O-antigen sensitizes the bacterial cell for the apoA-I-mediated destruction.
Y. enterocolitica are usually acquired by ingestion of contaminated food or water (30). They pass through the acidic content of the stomach and reach the small intestine. There, they are shuttled across the intestinal epithelium by the M cells of Peyer's patches and gain access to subepithelial space (31–33). Subsequently, the bacteria reach the mesenteric lymph nodes and in some cases spread further to the liver and spleen. It is likely that HDL, along with the complement proteins, would leak into the inflamed tissue area from the dilated capillaries. In fact, HDL has been shown to infiltrate the synovial inflamed tissue (34). In addition to lymphatics, however, Yersiniae may disseminate from the Peyer's patches via blood vessels (31). Therefore, the bacteria could encounter apoA-I and complement also directly in the bloodstream.
As Y. enterocolitica binds inhibitors of the alternative and classical pathway (17, 19, 35), we first examined whether the bacteria could also use apoA-I in interference with the terminal complement pathway (11). Surprisingly, we found that apoA-I contributes to the bacterial killing in serum and that its complement inhibitory properties could not be used by the bacteria. Interestingly, apoA-I deposition on the bacterial surface was detected exclusively in the presence of serum. Thus, bacterial opsonization by serum proteins seems to be a prerequisite for apoA-I to bind to the bacterial surface. Similarly, binding of apoA-I to endothelial cells exposed to complement is 10-fold higher compared with untreated cells (9).
We showed that the active complement system was critical for the action of apoA-I. Interestingly, studies with endothelial cells indicate that apoA-I may interfere with either insertion of C9 into a target membrane or with C9 polymerization at C5b-8 sites (11). Indeed, HDL has been shown to interfere with insertion of nascent fluid phase C5b-7 complexes to cell membranes (36). Thus, HDL may provide alternative hydrophobic sites that could compete with membranes for the binding of fluid phase complement attack complexes thereby protecting bystander cells against complement. Alternatively, HDL could function as a carrier of membranophilic complement complexes and transport them to sites when they are needed to fight against an intruder. In fact, recent proteomic analyses of HDL composition revealed the presence of most of the complement components, including those of the membranophilic complexes (37, 38). Interestingly, although LDL can also protect cell membranes against insertion of fluid phase C5b-7 (36, 39), we did not find evidence for the involvement of LDL or VLDL in the killing of Y. enterocolitica.
Although the mechanism of apoA-I bactericidal activity mediated by the activation of the complement system requires further study, one could speculate that apoA-I might enhance the deposition of the terminal complement complexes on the bacterial membrane. In this regard, apoA-I appears to act as an immune surveillance molecule that protects the host cells against the complement attack and contributes to the complement-mediated killing of Y. enterocolitica. It becomes obvious that to perform both functions, apoA-I needs to discriminate betweens self and non-self. Pathogen-associated molecular pattern recognized by apoA-I on Y. enterocolitica surface could be O-antigen.
We have shown that only those bacteria that expressed O-antigen were targeted by apoA-I and that the amount of the O-antigen conditioned the extent of apoA-I activity. Importantly, earlier studies had shown that rough bacteria were more serum-resistant than their smooth derivatives expressing the main serum resistance proteins YadA and Ail (13). In light of the results presented in this report, one could suggest that the increased resistance of the O-antigen-lacking bacteria could result from inability of apoA-I to destruct these bacteria. Accordingly, we found that the expression of LPSYeO3 O-antigen in the rough Y. enterocolitica serotype O:8 strain, that normally did not respond to apoA-I, transformed this strain to the apoA-I responder. LPSYeO3 O-antigen is a homopolymer of 6-deoxy-l-altrose (40), a sugar that is an extremely rare component of bacterial polysaccharides and cannot be found in mammals. Interestingly, we did not detect apoA-I-mediated bactericidal action against Y. enterocolitica O:8 nor O:9 (data not shown). The O-antigens of these strains, however, dramatically differ from that of serotype O:3 (41, 42). In the future, it will be important to determine how broad is a range of LPS O-antigens that apoA-I could recognize.
Human apoA-I is a 243-residue polypeptide that consists of amphipathic α-helices (43). The C-terminal residues 220–241 play a major role in binding of apoA-I to phospholipids (44, 45). Moreover, the C-terminal portion of apoA-I has recently been reported to interact with LPS (26). Our results pointed to an involvement of specific residues, within the 218–222 region of the C-terminal domain, in the apoA-I bactericidal activity. A smaller but substantial inhibition of the bactericidal activity was also observed by substitution of Glu-223 and Lys-226 by alanine. The role of the 218–226 region of apoA-I in bacterial killing needs to be studied further with short apoA-I mimetic peptides.
New strains of bacterial pathogens resistant to antibiotics have emerged in recent years. Detailed characterization of the role of HDL and apoA-I in host innate defense mechanisms could be of great importance in the combat against bacterial infections. Further studies are thus warranted to establish how broad is the spectrum of Gram-negative species responding to the bactericidal activity of apoA-I. In clinical context low HDL cholesterol level indicates an unfavorable prognosis in human sepsis (46) and is accompanied by extensive changes in HDL apolipoprotein composition, such as loss of apoA-I (47). Our results insinuate a new possibility to exploit HDL and apoA-I in the treatment of certain Gram-negative infections.
Supplementary Material
Acknowledgments
We thank Dr. Angelika Chroni for purification of some of the apoA-I mutants, Katharina Gründler for help in characterization of apoAI mutant E223A/K226A, and Sari Nuutinen and Anita Liljegren for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant HL48739 (to V. Z.). This work was also supported by the Finnish Cultural Foundation (to M. B.-S.), the Paulo Foundation (to M. B.-S.), the Aarne Koskelon Foundation (to M. B.-S.), Orion Farmos (to M. B.-S.), the Alfred Kordelin Foundation (to M. B.-S.), the Research Council for Biosciences and Environment, Academy of Finland Grant 114075 (to M. S.), the Finnish Foundation for Cardiovascular Research (to M. J.), and the Research Council for Health, Academy of Finland, Grant 132629 (to M. J.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2, Table 1, Methods, and additional references.
- apoA-I
- apolipoprotein A-I
- HIS
- heat-inactivated serum
- NHS
- normal human serum.
REFERENCES
- 1. Rader D. J. (2006) J. Clin. Invest. 116, 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh I. P., Chopra A. K., Coppenhaver D. H., Ananatharamaiah G. M., Baron S. (1999) Antiviral Res. 42, 211–218 [DOI] [PubMed] [Google Scholar]
- 3. Pérez-Morga D., Vanhollebeke B., Paturiaux-Hanocq F., Nolan D. P., Lins L., Homblé F., Vanhamme L., Tebabi P., Pays A., Poelvoorde P., Jacquet A., Brasseur R., Pays E. (2005) Science 309, 469–472 [DOI] [PubMed] [Google Scholar]
- 4. Hubsch A. P., Casas A. T., Doran J. E. (1995) J. Lab. Clin. Med. 126, 548–558 [PubMed] [Google Scholar]
- 5. Ulevitch R. J., Johnston A. R. (1978) J. Clin. Invest. 62, 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sammalkorpi K., Valtonen V., Kerttula Y., Nikkilä E., Taskinen M. R. (1988) Metabolism 37, 859–865 [DOI] [PubMed] [Google Scholar]
- 7. Mendall M. A., Goggin P. M., Molineaux N., Levy J., Toosy T., Strachan D., Camm A. J., Northfield T. C. (1994) Br. Heart J. 71, 437–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambris J. D., Ricklin D., Geisbrecht B. V. (2008) Nat. Rev. Microbiol. 6, 132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton K. K., Sims P. J. (1991) J. Clin. Invest. 88, 1833–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packman C. H., Rosenfeld S. I., Leddy J. P. (1985) Biochim. Biophys. Acta 812, 107–115 [DOI] [PubMed] [Google Scholar]
- 11. Rosenfeld S. I., Packman C. H., Leddy J. P. (1983) J. Clin. Invest. 71, 795–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottone E. J. (1997) Clin. Microbiol. Rev. 10, 257–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biedzka-Sarek M., Venho R., Skurnik M. (2005) Infect. Immun. 73, 2232–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caroff M., Karibian D. (2003) Carbohydr. Res. 338, 2431–2447 [DOI] [PubMed] [Google Scholar]
- 15. Lüderitz O., Galanos C., Rietschel E. T. (1981) Pharmacol. Ther. 15, 383–402 [DOI] [PubMed] [Google Scholar]
- 16. Rietschel E. T., Brade H. (1992) Sci. Am. 267, 54–61 [DOI] [PubMed] [Google Scholar]
- 17. Biedzka-Sarek M., Jarva H., Hyytiäinen H., Meri S., Skurnik M. (2008) Infect. Immun. 76, 4100–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biedzka-Sarek M., Salmenlinna S., Gruber M., Lupas A. N., Meri S., Skurnik M. (2008) Infect. Immun. 76, 5016–5027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirjavainen V., Jarva H., Biedzka-Sarek M., Blom A. M., Skurnik M., Meri S. (2008) PLoS Pathog. 4, e1000140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havel R. J., Eder H. A., Bragdon J. H. (1955) J. Clin. Invest. 34, 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 22. Liadaki K. N., Liu T., Xu S., Ishida B. Y., Duchateaux P. N., Krieger J. P., Kane J., Krieger M., Zannis V. I. (2000) J. Biol. Chem. 275, 21262–21271 [DOI] [PubMed] [Google Scholar]
- 23. Chroni A., Koukos G., Duka A., Zannis V. I. (2007) Biochemistry 46, 5697–5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chroni A., Liu T., Gorshkova I., Kan H. Y., Uehara Y., Von Eckardstein A., Zannis V. I. (2003) J. Biol. Chem. 278, 6719–6730 [DOI] [PubMed] [Google Scholar]
- 25. Koukos G., Chroni A., Duka A., Kardassis D., Zannis V. I. (2007) Biochem. J. 406, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henning M. F., Herlax V., Bakás L. (2011) Innate Immun. 17, 327–337 [DOI] [PubMed] [Google Scholar]
- 27. Levine D. M., Parker T. S., Donnelly T. M., Walsh A., Rubin A. L. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 12040–12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lahtinen P., Brzezinska A., Skurnik M. (2003) in The Genus Yersinia: Entering the Functional Genomic Era (Skurnik M., Granfors K., Bengoechea J. A. eds), Kluwer Academic Publishers, Norwell, MA [Google Scholar]
- 29. Muszynski A. (2004) Characterization of Lipopolysaccharides from Mutants of Yersinia enterocolitica O:3 Cultivated at Different Temperatures. Ph.D. thesis, University of Silesia, Katowice, Poland [Google Scholar]
- 30. Naktin J., Beavis K. G. (1999) Clin. Lab. Med. 19, 523–536 [PubMed] [Google Scholar]
- 31. Autenrieth I. B., Firsching R. (1996) J. Med. Microbiol. 44, 285–294 [DOI] [PubMed] [Google Scholar]
- 32. Marra A., Isberg R. R. (1997) Infect. Immun. 65, 3412–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simonet M., Richard S., Berche P. (1990) Infect. Immun. 58, 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bresnihan B., Gogarty M., Fitzgerald O., Dayer J. M., Burger D. (2004) Arthritis Res. Ther. 6, R563–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. China B., N'Guyen B. T., de Bruyere M., Cornelis G. R. (1994) Infect. Immun. 62, 1275–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lint T. F., Behrends C. L., Gewurz H. (1977) J. Immunol. 119, 883–888 [PubMed] [Google Scholar]
- 37. Collins L. A., Olivier M. (2010) Proteome Sci. 8, 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaisar T., Pennathur S., Green P. S., Gharib S. A., Hoofnagle A. N., Cheung M. C., Byun J., Vuletic S., Kassim S., Singh P., Chea H., Knopp R. H., Brunzell J., Geary R., Chait A., Zhao X. Q., Elkon K., Marcovina S., Ridker P., Oram J. F., Heinecke J. W. (2007) J. Clin. Invest. 117, 746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Podack E. R., Kolb W. P., Müller-Eberhard H. J. (1978) J. Immunol. 120, 1841–1848 [PubMed] [Google Scholar]
- 40. Hoffman J., Lindberg B., Brubaker R. R. (1980) Carbohydr. Res. 78, 212–214 [DOI] [PubMed] [Google Scholar]
- 41. Caroff M., Bundle D. R., Perry M. B. (1984) Eur. J. Biochem. 139, 195–200 [DOI] [PubMed] [Google Scholar]
- 42. Tomshich S. V., Gorshkova R. P., Ovodov Y. S. (1987) Khim. Prirod. Soed. 657–664 [Google Scholar]
- 43. Nolte R. T., Atkinson D. (1992) Biophys. J. 63, 1221–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palgunachari M. N., Mishra V. K., Lund-Katz S., Phillips M. C., Adeyeye S. O., Alluri S., Anantharamaiah G. M., Segrest J. P. (1996) Arterioscler. Thromb. Vasc. Biol. 16, 328–338 [DOI] [PubMed] [Google Scholar]
- 45. Laccotripe M., Makrides S. C., Jonas A., Zannis V. I. (1997) J. Biol. Chem. 272, 17511–17522 [DOI] [PubMed] [Google Scholar]
- 46. Chien J. Y., Jerng J. S., Yu C. J., Yang P. C. (2005) Crit. Care Med. 33, 1688–1693 [DOI] [PubMed] [Google Scholar]
- 47. Barlage S., Fröhlich D., Böttcher A., Jauhiainen M., Müller H. P., Noetzel F., Rothe G., Schütt C., Linke R. P., Lackner K. J., Ehnholm C., Schmitz G. (2001) J. Lipid Res. 42, 281–290 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.