Background: Integrin signaling causes FAK tyrosine phosphorylation upon cell attachment.
Results: Paxillin and not the closely related HIC5 protein supports tyrosine phosphorylation of FAK in the absence of cell attachment and augments transformation by activated RAS oncogenes.
Conclusion: FAK can be activated either by cell attachment or by paxillin association without cell attachment.
Significance: Paxillin and not HIC5 augments cell transformation through FAK activation.
Keywords: Actin, Cytoskeleton, Migration, Ras, Transformation, Tumor Viruses, FAK, Focal Adhesion Kinase
Abstract
Paxillin and HIC5 are closely related adapter proteins that regulate cell migration and are tyrosine-phosphorylated by focal adhesion kinase (FAK). Paxillin, HIC5, and FAK tyrosine phosphorylation increase upon cell attachment and decrease upon detachment from extracellular matrix. Unexpectedly, we found that although FAK tyrosine phosphorylation in attached cells did not require paxillin, in detached fibroblasts there was remaining FAK tyrosine phosphorylation that required expression of paxillin and was not supported by HIC5. The support of attachment-independent FAK tyrosine phosphorylation required the paxillin LIM domains and suggested that paxillin might facilitate oncogenic transformation. Paxillin but not HIC5 augmented anchorage-independent cell proliferation induced by RAS. Both anchorage-independent FAK tyrosine phosphorylation and RAS-induced colony formation required multiple docking sites on paxillin, including LD4 (docking sites for FAK-Src and GIT1/2-PIX-NCK-PAK complex), LD5, and all four carboxyl-terminal LIM domains (that bind tubulin and PTP-PEST). Analysis using paxillin mutants dissociated domains of paxillin that are required for regulation of cell migration from domains that are required for anchorage-independent cell proliferation and demonstrated essential functions of the paxillin LIM domains that are not found in HIC5 LIM domains. These results highlight the role of paxillin in facilitating attachment-independent signal transduction implicated in cancer.
Introduction
Many cells require attachment to extracellular matrix (ECM)3 for proliferation and survival. Integrins expressed on the cell surface contact ECM components and initiate a signal into the cell by clustering a complex of proteins collectively termed focal contacts and focal adhesions (reviewed in Ref. 1). Integrin adhesions signal that the cell is attached to the ECM, as well as provide a structural anchor for attaching actin filaments, thereby connecting the actin cytoskeleton to the ECM. If deprived of ECM contact, some epithelial cells undergo a form of apoptosis called anoikis, and one of the hallmarks of cellular transformation is the ability of transformed cells to form anchorage-independent colonies where cancer cells survive and continue to proliferate in the absence of contact with immobilized ECM (reviewed in Ref. 2).
Paxillin is the prototype of three related adapter proteins that include HIC5 and leupaxin that regulate cell migration (reviewed in Ref. 3), but the functional differences between the family members are not yet fully described. Paxillin regulates focal adhesion turnover (4, 5) and associates with focal adhesion proteins that are implicated in the regulation of cell attachment, spreading, and migration, including tyrosine kinases Src and focal adhesion kinase (FAK), the ARF-GAP adapters GIT1/GIT2, the serine-threonine kinase ILK and PAK, and the CRKL adapter (reviewed in Ref. 6). Paxillin tyrosine phosphorylation sites bind to SH2-containing proteins Src (at Tyr-31) and CRKL (at Tyr-118) and thereby to a RAC1 signaling complex activated by DOCK180 (7). Paxillin also contains five copies of a peptide motif termed LD1 through LD5 that serve as docking sites (consensus LXXLLXXL); LD1 interacts with actopaxin, vinculin, and the ILK serine/threonine kinase (8, 9); LD2 interacts with FAK and vinculin; both GIT1/GIT2 and FAK compete with each other for binding to LD4 (10); LD3 and LD5 interaction partners remain uncharacterized, although LD5 is functionally required to support FAK tyrosine phosphorylation in embryonic stem (ES) cells (11). LD motifs also serve as docking sites for the E6 oncoprotein of bovine papillomavirus type 1 (12, 13), whose binding to paxillin is closely associated with transformation by E6 (14). The carboxyl terminus of paxillin and HIC5 contain four LIM domains that serve to support the tyrosine phosphorylation of FAK in ES cells (LIM domains 1, 2, and 3) (11), localize paxillin to focal adhesions (LIMS 2 and 3) (11, 15), and associate with the tyrosine phosphatase PTP-PEST that regulates cell spreading and migration (16–18).
The paxillin-related protein HIC5 is induced by TGF-β (19) and has been recently implicated in the regulation cell migration in response to TGF-β-induced epithelial to mesenchymal transition (20). HIC5 and paxillin share overall structure and close conservation within LD motifs and the LIM domains but differ in that some conserved tyrosine phosphorylation sites of paxillin (Tyr-31 and Tyr-118) are not found in HIC5. HIC5 and paxillin shuttle between the cytoplasm and nucleus (21); recent experiments demonstrate that HIC5 can serve as a transcriptional co-activator for androgen receptors (22).
Control of FAK phosphotyrosine is pivotal to regulating many of the signaling pathways that are associated with FAK. FAK autophosphorylates at Tyr-397 in response to growth factor and integrin stimulation, yet despite intensive study of FAK and the events that trigger the phosphorylation of Tyr-397, the direct mechanisms and the proteins involved in regulating the phosphorylation and dephosphorylation of Tyr-397 when cells are either forming or breaking down focal adhesions are largely unknown. Signaling through FAK has been shown to regulate focal adhesion dynamics as FAK-null cells show increased cell spreading, reduced motility, and decreased focal adhesion turnover, similar to the phenotype of paxillin-null cells (5, 23). FAK binds directly to paxillin at LD2 and LD4. Although FAK interaction with the LD motifs of paxillin might regulate FAK activity, direct evidence of this remains elusive. FAK autophosphorylation on Tyr-397 creates a binding site for several proteins including PI3K, p120RasGAP, and Src. Src binding to Tyr(P)-397 of FAK facilitates the further phosphorylation of FAK on tyrosines 576, 577, 861, and 925; these additional phosphorylation sites on FAK help to regulate FAK kinase activity and association with other proteins such as GRB2 and paxillin (reviewed in Refs. 24–26). Thus, the mechanisms that regulate FAK tyrosine phosphorylation are of high significance for the localization and activities of FAK.
Paxillin is required for FAK tyrosine phosphorylation in mouse ES cells (27). ES cells null for paxillin contain normal levels of FAK but markedly reduced overall FAK tyrosine phosphorylation and reduced FAK Tyr(P)-397. A fragment of paxillin containing only amino acids 302–502 (expressing the LD5-LIM3 region) is sufficient to support FAK tyrosine phosphorylation when expressed in paxillin-null ES cells, even though this fragment does not contain the LD2 and LD4 motifs that bind directly to FAK (11) or the LIM4 domain that interacts with PTP-PEST; thus, it is likely that a protein complex associated with the 302–502 region of paxillin supports FAK tyrosine phosphorylation in ES cells by an as yet undetermined mechanism. In contrast to paxillin-null ES cells, mouse embryo fibroblasts null for paxillin retain substantial FAK tyrosine phosphorylation when grown attached to tissue culture plates (28).
In this study, we demonstrate through the use of paxillin-null fibroblasts that multiple mechanisms exist to control FAK tyrosine phosphorylation. In stably attached fibroblasts, FAK tyrosine phosphorylation is independent of either HIC5 or paxillin expression. When fibroblasts are detached, FAK tyrosine phosphorylation declines but is not lost; in detached cells, remaining FAK tyrosine phosphorylation requires paxillin expression. This is in contrast to embryonic stem cells that require paxillin for FAK phosphorylation whether the cells are attached or detached from the ECM. Interestingly, we show that additional domains of paxillin are necessary to support FAK tyrosine phosphorylation in detached fibroblasts beyond those that are required in ES cells and that these domains of paxillin enhance oncogene-induced attachment-independent cell proliferation. We find a close correlation between domains of paxillin that are required to support transformation by ras and domains that are required to support attachment-independent tyrosine phosphorylation of FAK. Paxillin mutants and shRNA to FAK indicate that paxillin interactions with FAK are required to support anchorage-independent cell proliferation, indicating that complex multimeric and competing interactions on paxillin are required to augment anchorage-independent cell proliferation.
MATERIALS AND METHODS
Antibodies and Reagents
Mouse monoclonal antibodies to FAK (clone A2 from Upstate and 77 from Transduction Labs) HIC5, (Transduction Labs); Flag, Tubulin, and anti-phosphotyrosine clones 4G10 (Sigma), GIT1 (NeuroMab), and GFP (Chemicon) were obtained commercially. Rabbit antibody specific to phosphorylated tyrosine 397 of FAK was from BioSource International, and rabbit polyclonal anti-paxillin is previously described (11).
Cells and Cell Culture
Paxillin-null ES cells and T17 fibroblasts were generated and cultured as described (27). T17 cells were cloned by limiting dilution in the presence of mitomycin C-treated excess T17 feeder cells and were obtained at a dilution of less than one cell/well to derive T17 clones A and B. Immortalized paxillin-null mouse embryo fibroblasts were a gift of James Casanova (University of Virginia), are immortalized, and were originally supplied by Sheila Thomas (28). Transient transfections were performed using either Lipofectamine 2000 (Invitrogen) or electroporation (for T17 cells) using a Bio-Rad Gene Pulser and a 4-mm gap cuvette. Anchorage-independent growth assays were performed in 0.3% agarose as previously described (29). Replication defective retroviruses and lentiviruses were packaged by transient transfection of Phoenix cells (a gift of Gary Nolan, Stanford University) or co-transfection of 293T cells with packaging plasmids.
Plasmids
Paxillin, paxillin mutants, and HIC5 were stably expressed by retroviral transduction. Paxillin LD4 point mutants were generated using the QuikChange site-directed mutagenesis system (Stratagene). Mutants were fully sequenced. The PXN/HIC5 chimera fused amino acids 1–316 of paxillin to amino acids 219–461 of HIC5 with a KL linker in-between, and the HIC5/PXN chimera fused amino acids. 1–216 of HIC5 with amino acids 316–559 of PXN with a LK linker in-between. All other paxillin mutants were previously described (11). Human H-RAS with an amino-terminal Myc epitope tag and activating mutations were the gift of Alan Hall (Sloan Kettering, New York), avian v-Src was the gift of Tom Roberts and Jean Zhao (Dana Farber, Boston), and human K-RAS with a G12V activating mutation and an amino-terminal FLAG tag was the gift of Andrei Khokhlatchev (University of Virginia); all were subcloned into the murine leukemia virus-blasticidin retrovirus plasmid pWZL-Blast (a kind gift of Jay Morgenstern). shRNA plasmids and sequences are listed in the supplemental data.
FAK Tyrosine Phosphorylation Assay
Identical 10-cm plates of cells were grown to 80% confluence, and then one set of plates of was trypsinized, quenched with 4 ml of FBS media, and put in a 15-ml conical screw top tube, incubated with gentle rocking at 37 °C for 1 h, then centrifuged, washed with ice-cold PBS, and lysed in 400 μl of 0.5× Nonidet P-40 lysis buffer on ice (1× = 150 mm NaCl, 50 mm Tris-HCl, pH 7.5, 50 mm NaF, 5 mm sodium pyrophosphate, 1% Nonidet P-40, 0.01% PMSF, 1 mm sodium vanadate, and 1 μg/ml leupeptin/aprotinin). Attached cells were washed with ice-cold PBS and lysed in 400 μl of 0.5× Nonidet P-40 lysis buffer on ice. All of the lysates were clarified by centrifugation at 12,000 × G for 20′ at 4 °C, assayed for protein concentration (Pierce), and equalized for protein content. FAK was immunoprecipitated from 0.5 mg of cell lysate with 1 μg of purified antibody and goat anti-mouse magnetic beads (Pierce) at 4 °C for 1 h, then washed, eluted in sample buffer separated by SDS-PAGE, and transferred to Immobilon P (Millipore) by Western blot. Where shown, quantitation was performed from ECL chemiluminescent images using a FluorChem HD2 CCD image capture camera and software (Alpha Innotech).
RESULTS
Paxillin-null and HIC5 Knockdown Cell Lines
Fibroblasts that are derived from ES cells (termed T17 cells) or fibroblasts derived from paxillin-null mouse embryos both express HIC5 (27, 28). To derive cells that express neither paxillin nor HIC5, paxillin-null T17 cells were transduced with shRNA directed against HIC5, and drug-resistant pools of cells were selected. shRNA KD1 yielded a 90% knockdown of HIC5 protein, whereas KD2 knocked down HIC5 by greater than 95% (Fig. 1A). The resulting T17-KD2 cells were then retrovirally transduced either with wild-type paxillin, paxillin mutants, or human HIC5 cDNAs and used for subsequent experiments to examine the role of HIC5 or paxillin in FAK tyrosine phosphorylation.
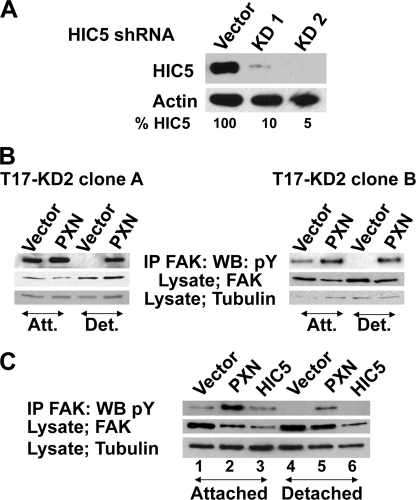
FIGURE 1.
Paxillin supports attachment-independent FAK tyrosine phosphorylation. A, generation of T17 KD cell lines. Cloned, paxillin-null T17 cells were stably transduced with shRNA retrovirus against HIC5. Pooled drug-resistant cells were analyzed for HIC5 protein expression. KD1 shRNA yielded a 90% knockdown of HIC5, whereas KD2 knocked down HIC5 levels by 95%; protein levels were normalized to actin levels (bottom panel) and quantitated using a Kodak imaging station. B, FAK was immune precipitated (IP) from cells that were either attached (Att.) or detached (Det.) for 1 h, and immunoblots were probed with an anti-phosphotyrosine antibody (top panel). Cell lysates were Western blotted (WB) and probed with antibodies against FAK or tubulin. Independently isolated and cloned cell lines were tested for the ability to retain FAK tyrosine phosphorylation in detached cells. Two separate T17 fibroblast clones were transduced with HIC5 shRNA KD2 and then reconstituted with either empty vector or paxillin. Both T17 clone A and clone B cells require paxillin for attachment-independent FAK tyrosine phosphorylation. Clone A T17-KD2 cells were used for the rest of this study. C, T17-KD2 cells re-expressing human HIC5 or paxillin were tested for attachment-independent FAK tyrosine phosphorylation as in A by detachment for 1 h. PXN supported FAK tyrosine phosphorylation in detached cells, whereas HIC5 did not.
Paxillin Is Required for the Attachment-independent Tyrosine Phosphorylation of FAK
In our prior studies, we observed that in normal ES cells expressing paxillin, FAK is tyrosine-phosphorylated in both attached and detached cells. In contrast, in ES cells null for paxillin there is little FAK tyrosine phosphorylation in attached or detached cells; undifferentiated ES cells express only paxillin and not the closely related family member HIC5 (27). In contrast, FAK was tyrosine-phosphorylated when T17-KD2 fibroblasts were attached overnight with or without paxillin, although reproducibly more so when paxillin was expressed (Fig. 1B). When we compared FAK phosphorylation in attached and detached fibroblasts, we observed something similar to our prior observations in ES cells. When detached for 1 h, only those cells with paxillin retained appreciable FAK tyrosine phosphorylation (Fig. 1B). This result was not due to a unique clonal selection of this cell line because we observed the same paxillin-dependent but attachment-independent FAK tyrosine phosphorylation in another separately derived and cloned T17-KD2 cell line (clone B; Fig. 1B). In contrast to paxillin, HIC5 re-expression was not able to support FAK phosphorylation in detached T17-KD2 cells (Fig. 1C).
Structural Features of Paxillin That Are Required for Attachment-independent FAK Tyrosine Phosphorylation
To determine the domains of paxillin that are necessary for attachment-independent FAK tyrosine phosphorylation in fibroblasts, we stably expressed FLAG epitope-tagged paxillin and paxillin mutants in T17-KD2 paxillin-null cells (Fig. 2) and assayed FAK tyrosine phosphorylation in cells attached overnight or detached for 1 h prior to lysis (Fig. 3, A–E). As noted above in Fig. 1, FAK was tyrosine-phosphorylated in paxillin-null fibroblasts attached to tissue culture plates overnight, but attachment-independent FAK tyrosine phosphorylation was dependent upon re-expression of paxillin. Mutation of paxillin tyrosine phosphorylation sites 31, 33, 40, or 118 did not effect FAK tyrosine phosphorylation in detached cells (Fig. 3A), indicating that this phenotype was not maintained through signaling complexes that are recruited to these tyrosine phosphorylation sites.
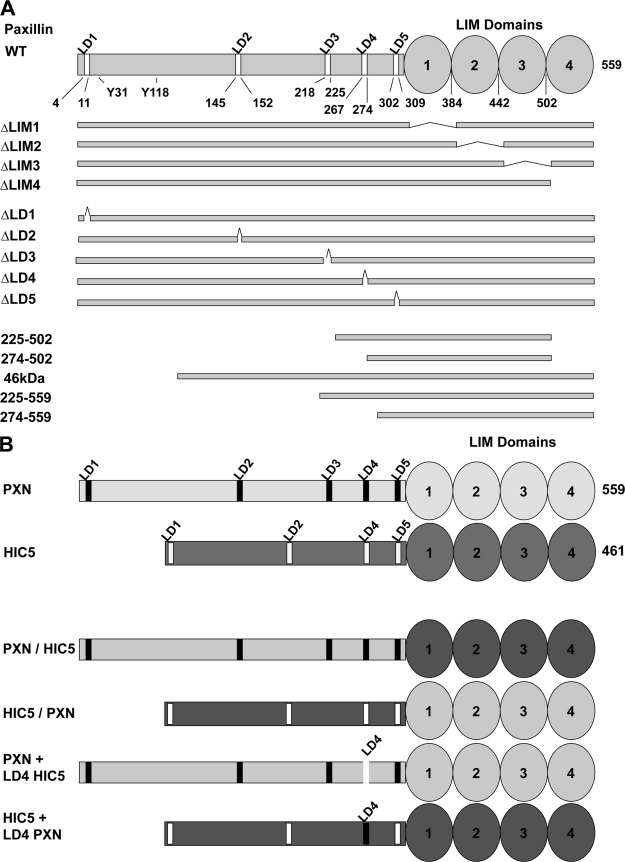
FIGURE 2.
Diagram of the paxillin mutants used in this study. A, The location of five LD motifs (peptide motifs with the consensus sequence LXXLLXXL) and four zinc finger LIM domains are shown. Tyr-31 and Tyr-118 are tyrosine phosphorylation sites. B, chimeric molecules between paxillin and HIC5 are illustrated.
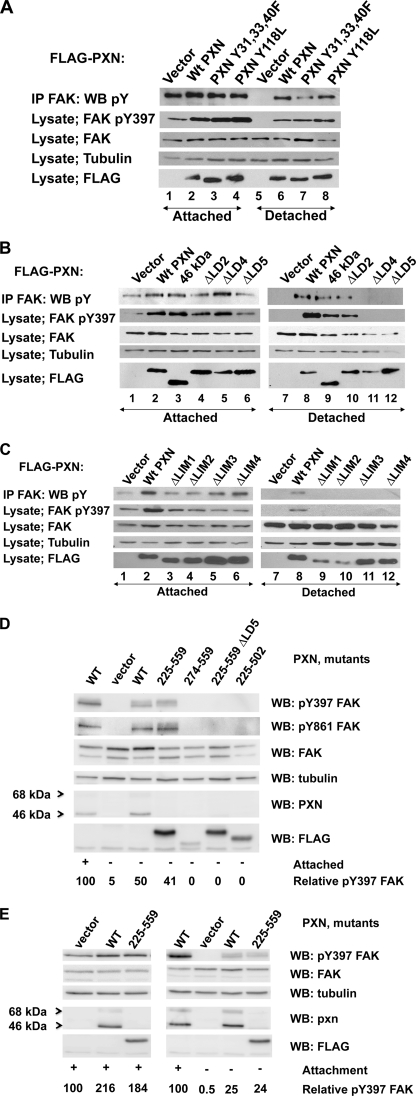
FIGURE 3.
Regions of paxillin required to support attachment-independent tyrosine phosphorylation of FAK. A, major tyrosine phosphorylation sites of paxillin do not regulate attachment-independent FAK tyrosine phosphorylation. T17-KD2 cells expressing FLAG-tagged paxillin or paxillin tyrosine mutants were either attached (lanes 1–4) or detached (lanes 5–8) for 1 h and assayed for FAK tyrosine phosphorylation, FAK phosphorylation at tyrosine 397 (pY397), total FAK, tubulin, and FLAG (FLAG-tagged paxillin mutants). Paxillin tyrosines 31, 33, 40, and 118 are dispensable for FAK tyrosine phosphorylation in detached cells. B, LD motifs 4 and 5 are required for attachment-independent FAK tyrosine phosphorylation. In the upper set of panels, T17-KD2 cells stably transduced with FLAG-tagged full-length paxillin, 46-kDa paxillin, and paxillin mutants containing in-frame LD deletions were tested as in A, and FAK tyrosine phosphorylation was measured in attached (lanes 1–6) or detached cells (lanes 7–12). Attachment-independent FAK tyrosine phosphorylation required both LD4 and LD5 in T17-KD2 cells, but not the FAK-binding LD2 motif or LD1. C, the LIM domains of paxillin are all required for attachment-independent FAK tyrosine phosphorylation. In-frame LIM domain deletion mutants of FLAG-tagged paxillin stably transduced into T17-KD2 cells were analyzed as in B in attached (lanes 1–6) and detached (lanes 7–12) conditions and also by using phosphospecific antibody to FAK tyrosine 397. All four paxillin LIM domains were required to support attachment-independent FAK tyrosine phosphorylation. D, the minimal fragment of paxillin that supports attachment-independent FAK tyrosine phosphorylation. Deletion mutants of paxillin were expressed in T17-KD2 cells, and SDS cell lysates were tested for FAK tyrosine phosphorylation by using phosphospecific antibody to FAK tyrosine 397 and then probed with the other indicated antibodies. Lastly, the membrane was then stripped and probed for FAK phosphospecific antibody to Tyr-861. The minimal fragment of paxillin that supported attachment-independent FAK tyrosine phosphorylation in T17-KD2 cells was amino acids 225–559 containing LD4, LD5, and all four LIM domains. Quantitation of relative FAK Tyr(P)-397 phosphorylation is shown at the bottom normalized to that obtained with wild-type paxillin in attached cells. E, mouse embryo fibroblasts require paxillin to support attachment-independent FAK tyrosine phosphorylation. Wild-type paxillin or the paxillin deletion mutant 225–559 was expressed in paxillin-null MEFs and assayed for FAK tyrosine phosphorylation as in D. IP, immunoprecipitation; WB, Western blot.
We next examined the role of LD motifs by sequential in-frame deletion of the motifs. Paxillin mutants deleted of either LD4 or LD5 were unable to support FAK tyrosine phosphorylation in detached T17-KD2 cells, whereas deletion of either the first 134 amino acids including LD1 (46-kDa PXN in Fig. 3B, lane 3) or an in-frame deletion of LD2 had a modest effect. Thus, attachment-independent tyrosine phosphorylation of FAK was not mediated through associations with either amino-terminal tyrosine phosphorylation sites of paxillin (Src family interactions at Tyr(P)-31 or CRK family interactions at Tyr(P)-118) or FAK interaction with the LD2 motif of paxillin.
Deletion of each of the four LIM domains of paxillin ablated attachment-independent FAK tyrosine phosphorylation in T17-KD2 cells, including LIM4 (Fig. 3C), which together with LIM3 interacts with PTP-PEST. A paxillin fragment consisting of LD4-LIM4 (225–559) was the minimal paxillin fragment able to support FAK tyrosine phosphorylation in detached cells (Fig. 3D). Within this minimal 225–559 fragment, there was a requirement for both LD4 and LD5, as well as LIM4, because mutants of paxillin that consisted of 274–559 or the 225–559 fragment with an in-frame deletion of LD5, or deletion of LIM4 were not able to support FAK tyrosine phosphorylation in detached cells (Fig. 3D, compare fourth lane with fifth through seventh lanes). Thus, we infer that the phenotype of FAK tyrosine phosphorylation in detached T17-KD2 fibroblasts requires at the minimum, paxillin LD4, LD5, and all four LIM domains.
The ability of paxillin to support attachment-independent FAK tyrosine phosphorylation was also observed in paxillin-null MEFs when paxillin was re-expressed (Fig. 3E). These MEFs were independently created from a separate targeting event in a different genetic background than the T17-KD2 fibroblasts, which are derived from differentiated paxillin-null ES cells (28). In MEFs as in the T17KD2 cells, the 225–559 fragment of paxillin supported attachment-independent FAK tyrosine phosphorylation. Antibodies to phosphotyrosine against immune precipitated FAK and phosphospecific antibodies to the major autophosphorylation site of FAK (Tyr(P)-397) in total cell lysates gave similar results (Fig. 3) as did phosphospecific antibody to tyrosine 861 (Fig. 3D). Additional available phosphospecific antibodies to other characterized FAK tyrosine phosphorylation sites (tyrosines 525, 577, and 925) were of insufficient sensitivity to reproducibly distinguish differences in phosphorylation at normal expression levels in these cells (data not shown).
The Essential Difference between HIC5 and Paxillin in Supporting Attachment-independent FAK Tyrosine Phosphorylation Resides within the LIM Domains
Paxillin and HIC5 are highly conserved within the LD motifs and the LIM domains, and both proteins modulate migration, yet they differ in their ability to support FAK tyrosine phosphorylation in detached cells. To determine whether HIC5 and paxillin might have qualitatively different protein associations that might explain their different phenotypes, both molecules were FLAG epitope-tagged, stably transduced into paxillin-null MEFs, and immunopurified, and associated cellular proteins were identified by mass spectrometry. Complexes unique to paxillin and not HIC5 were not identified by this technique (data not shown). Therefore, we pursued further genetic analysis of the differences between HIC5 and paxillin. Because our genetic experiments demonstrated that LD4 was essential for attachment-independent FAK tyrosine phosphorylation, chimeras between HIC5 and paxillin were constructed, making paxillin with the LD4 motif of HIC5 (PXN-LD4 HIC5), HIC5 with the LD4 of paxillin (HIC5-LD4 PXN), and additional chimeras that swap the entire amino terminus and LIM domains (PXN/HIC5 and HIC5/PXN) as illustrated in Fig. 2B. All of the chimeric molecules, as well as native HIC5 and paxillin, were stably expressed, localized to focal adhesions (Fig. 4A), and augmented cell spreading when re-expressed in T17-KD2 cells (Fig. 4B). This indicated that each chimera retained biological activities. As previously shown in Fig. 1C, only re-expressed paxillin and not HIC5 could support the attachment-independent tyrosine phosphorylation of FAK (Fig. 4C). Only molecules containing the paxillin LIM domains and not the HIC5 LIM domains could support attachment-independent FAK Tyr(P)-397, whereas either the paxillin or HIC5 LD4 motifs supported attachment-independent FAK Tyr(P)-397 (Fig. 4C). Thus, a critical difference between HIC5 and paxillin for this activity resided within the LIM domains. Parallel experiments that expressed the same chimeric paxillin and HIC5 molecules in paxillin-null MEFs gave similar results as shown in Fig. 4C (data not shown).
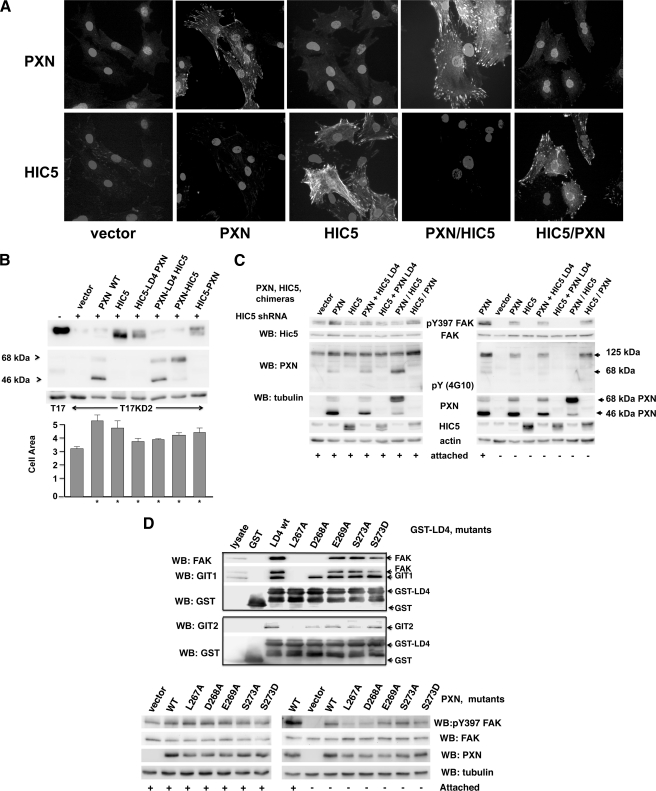
FIGURE 4.
Paxillin LIM domains support attachment-independent FAK tyrosine phosphorylation, whereas the LIM domains of HIC5 do not. Chimeric molecules composed of paxillin and HIC5, illustrated in Fig. 2B, were introduced into T17-KD2 cells by retroviral transduction. A, localization of HIC5, paxillin, and chimeric molecules to focal adhesions. Transduced T17-KD2 cells (indicated below the bottom row) were grown attached to glass coverslips overnight, fixed with formalin, permeablized with 0.1% Tween, and stained using polyclonal rabbit antibody to the amino terminus of paxillin and mouse monoclonal antibody to the amino terminus of HIC5 followed by fluorescently labeled secondary antibodies and DAPI nuclear counterstain. Note that the HIC5 antibody faintly stains HIC5 in vector-transduced cells that is residual HIC5 (i.e. incompletely knocked-down by the KD2 shRNA). B, paxillin and HIC5 chimeric molecules augment cell spreading in vivo. In the top Western blot (WB) panels, T17-KD2 cells re-expressing HIC5, paxillin, or the indicated HIC5/PXN chimeric molecules were lysed in SDS and analyzed by immunoblot as indicated in the figure. The expression of endogenous murine HIC5 in T17 cells (lane 1) is compared with T17-KD2 cells (lane 3) and re-expressed human HIC5 in T17-KD2 cells (lane 4) and the indicated paxillin and chimeric paxillin-HIC5 molecules in the remaining lanes. Below, the bar graph illustrates the median spread cell area (arbitrary units) of each T17-KD2 derived cell line when attached to tissue culture plastic in complete media overnight as described in the methods. Error bars show the standard deviation, and an asterisk denotes P significance values of less than 0.01 compared with T17-KD2 cells by Student t test. Paxillin, HIC5, and all the chimeras augment cell spreading compared with vector transduced T17-KD2 cells. C, LIM domains of paxillin but not HIC5 are required to support attachment-independent tyrosine phosphorylation of FAK. Western blot analysis of T17-KD2 cells transduced with the indicated genes were grown attached or detached for 1 h as indicated, lysed in SDS, and analyzed by Western blot for Tyr(P)-397 (pY397) FAK. Only genes encoding the paxillin LIM domains support FAK Tyr-397 phosphorylation. D, association of FAK with LD4 of paxillin is associated with attachment-independent Tyr(P)-397 FAK phosphorylation in T17KD2 cells. Nonidet P-40-clarified cell lysates were prepared from T17KD2 cells transduced with wild-type paxillin and tested for in vitro binding to GST, GST-LD4 fusion, or GST fused to LD4 motifs with the indicated amino acid changes (numbered by the full-length paxillin sequence). The beads were collected by centrifugation and washed three times, and bound proteins were eluted in SDS. Resolved proteins are shown in the upper set of boxed Western blots that were sequentially probed with antibodies to FAK, GIT1, or GIT2, and finally GST. In the lower set of Western blots, SDS lysates were prepared from T17KD2 cells transduced with vector, paxillin, or paxillin molecules with the indicated mutations in LD4. Cells were either attached or detached as described in Fig. 3 (shown by plus or minus signs), and blots were probed with antibodies to paxillin, FAK, tubulin, and Tyr(P)-397 FAK.
Interaction between FAK and Paxillin at LD4 Are Required for Attachment-independent Tyrosine Phosphorylation of FAK
Both FAK and GIT1 compete for interaction at LD4 of paxillin. We wished to determine whether GIT1 interaction or FAK interaction at LD4 was required for attachment-independent tyrosine phosphorylation of FAK. Attempts to make stable knockdowns of GIT1 were unsuccessful, so point mutations in the paxillin LD4 domain were constructed in full-length paxillin and within GST fusions to LD4. The LD4 point mutants were screened for in vitro interaction with FAK and GIT1 in an attempt to find a mutant that dissociated FAK and GIT1 interactions at LD4 (Fig. 4D, boxed gels). The upper boxed gels of Fig. 4D show that although mutations of the leucines ablated interaction of both proteins, a single mutation, D268A in LD4, retained interaction with FAK but lost interaction with GIT1 in vitro. These LD4 point mutations in the context of full-length paxillin were introduced into T17KD2 cells and tested for attachment-independent FAK Tyr-397 phosphorylation in the lower set of Fig. 4D gels. Paxillin with point mutations in LD4 that retained interaction with FAK were able to support attachment-independent tyrosine phosphorylation of FAK, whereas mutants that lost association with FAK did not support attachment-independent tyrosine phosphorylation of FAK. Paxillin D268A, which retains FAK association but loses GIT1 association, was able to retain attachment-independent tyrosine phosphorylation of FAK.
Paxillin-null Fibroblasts Are Impaired in Anchorage-independent Cell Proliferation Induced by Activated RAS
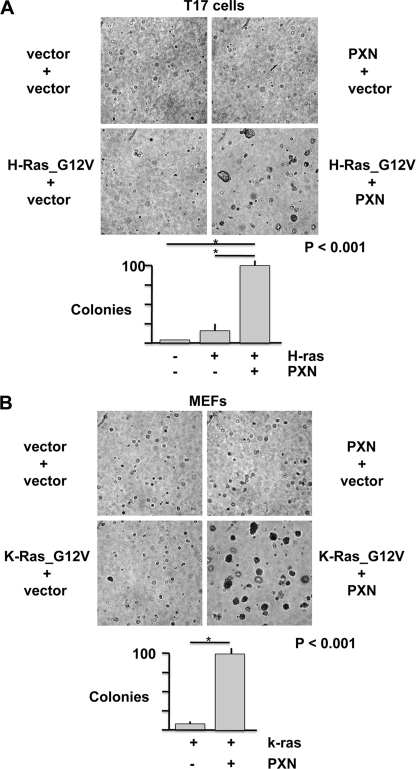
The requirement of paxillin for the tyrosine phosphorylation of FAK in detached cells suggested that paxillin might support attachment-independent signaling that is correlated with cell survival and tumorigenicity. To test the role of paxillin in anchorage-independent cell proliferation, paxillin-null MEFs, and T17-KD2 cells were transduced with paxillin or empty vector conferring puromycin resistance, and then further transduced with either empty vector or activated alleles of RAS with a blasticidin resistance marker. The oncogene-expressing retroviruses express a single bicistronic transcript encoding both the oncogene and blasticidin resistance to ensure oncogene expression in blasticidin-resistant cells; transduced cells were selected and expanded at subconfluence and seeded into agar at passage 2. Fig. 5 demonstrates that paxillin expression augmented RAS_G12V-induced colony formation in both T17 (Fig. 5A) fibroblasts and paxillin-null MEFs (Fig. 5B). Because the MEFs were more easily transduced and selected with K-RAS_G12V, subsequent analysis was performed in these cells.
FIGURE 5.
Paxillin augments anchorage-independent transformation by activated G12V RAS. A, paxillin-null T17 cells were retrovirally transduced with empty vector or paxillin, puromycin-selected, and then retrovirally transduced with mutation-activated and Myc-tagged H-RAS_G12V and selected in blasticidin. Drug-resistant cells were selected and expanded at subconfluence for one passage and seeded into agarose for 10 days. B, paxillin-null MEFs were transduced with paxillin and or FLAG-tagged and mutation-activated K-RAS_G12V and assayed for anchorage-independent colony formation as in A. The error bars show S.E., and an asterisk denotes p < 0.01 difference by Student's t test.
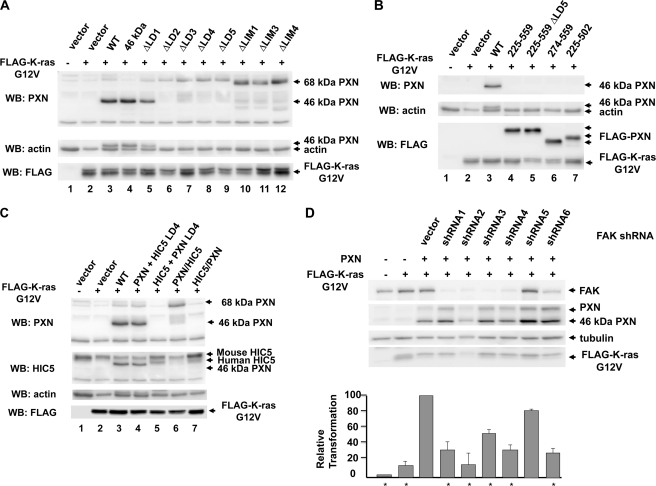
To determine which domains of paxillin are required to support colony formation by K-RAS_G12V, untagged paxillin or paxillin mutants were transduced, selected, and then retransduced with activated K-RAS_G12V. Amino-terminal paxillin deletion mutants were epitope-tagged with FLAG. Western blot analysis showed expression of paxillin and K-RAS_G12V in the transduced cells (Fig. 6, A–C). Re-expressed native paxillin is present in the full-length 68-kDa form but is mostly present in the 46-kDa form (Figs. 3, D and E, 4B, and 6; note that deletion mutants are altered in their migration). We determined by immune precipitation and mass spectrometry that the 46-kDa form produced in MEFs has an acetylated amino terminus beginning at amino acid 134 (threonine), consistent with translation at an internal methionine at amino acid 133 as has been previously proposed (30), followed by methionine cleavage and acetylation.4 Interestingly, the 68-kDa form is predominantly expressed when paxillin is internally deleted (Fig. 6A) or amino-terminally epitope-tagged (in Figs. 3, B–D, paxillin was FLAG epitope-tagged). The 46-kDa form of paxillin was active for both attachment-independent tyrosine phosphorylation of FAK (Fig. 3B) and anchorage-independent colony formation (Table 1).
FIGURE 6.
Expression of K-RAS_G12V, PXN, PXN mutants, HIC5, and chimeric molecules in paxillin-null MEFs. A, analysis of FLAG-K-RAS_G12V expression in paxillin-null MEFs reconstituted with paxillin and paxillin mutants in LD and LIM domains. Western blots (WB) from Nonidet P-40 cell lysates were analyzed by the indicated antibodies and probed in order with polyclonal anti-paxillin, anti-actin, and anti-FLAG with sequentially captured images. B, analysis of FLAG-K-RAS_G12V protein expression in paxillin-null MEFs reconstituted with paxillin and paxillin deletion mutants as described in A. C, analysis of FLAG-K-RasG12V expression in paxillin-null MEFs reconstituted with paxillin and paxillin-HIC5 chimeras as described in A. The blot was probed first with rabbit polyclonal antibody to the amino terminus of paxillin and then mouse monoclonal antibody to HIC5, anti-actin, and anti-FLAG with sequentially captured images. D, shRNA modulation of FAK expression and its effect upon colony formation by ras. Paxillin-null MEFs that had been previously reconstituted with wild-type paxillin were infected with lentiviruses expressing the indicated shRNA directed against FAK and then transduced with retrovirus expressing FLAG-tagged K-RasG12V. Pooled drug-resistant cells were passed twice; analyzed by Western blot for paxillin, FAK, FLAG-K-RasG12V, and tubulin expression; suspended in 0.3% agarose for 10 days; and photographed; and then the photographs were analyzed for colony number (bar graph). Transformation results are the mean results of three experiments normalized to cells expressing ras, paxillin, and lentiviral control shRNA.
TABLE 1.
Paxillin domains that augment transformation by K-RAS_G12V
| Transduced genesa | Colony numbersb | p value vs. WT PXNc | p value vs. vectord |
|---|---|---|---|
| Vectors, no K-RAS | 0 | p < 0.001 | |
| Vector | 7.8 ± 2.0 | NA | |
| PXN WT | 100 | NA | p < 0.001 |
| PXN ΔLD1 | 112 ± 12.8 | p < 0.014 | |
| PXN ΔLD2 | 53 ± 6.2 | p < 0.014 | p < 0.001 |
| PXN ΔLD3 | 68 ± 12.1 | p < 0.04 | p < 0.001 |
| PXN ΔLD4 | 15 ± 7.0 | p < 0.006 | p < 0.36 |
| PXN ΔLD5 | 18 ± 7.9 | p < 0.008 | p < 0.26 |
| PXN ΔLIM1 | 28 ± 11 | p < 0.002 | p < 0.12 |
| PXN ΔLIM3 | 12.1 ± 5.1 | p < 0.002 | p < 0.46 |
| PXN ΔLIM4 | 9.0 ± 2.5 | p < 0.002 | p < 0.7 |
| PXN 46 kDa | 101 ± 13 | p < 0.56 | p < 0.001 |
| PXN 225–559 | 42 ± 11 | p < 0.004 | p < 0.01 |
| PXN 274–559 | 7.6 ± 4.0 | p < 0.001 | p < 0.9 |
| PXN, LD4-HIC5 | 125 ±49 | p < 0.8 | p < 0.03 |
| HIC5, LD4-PXN | 7.4 ± 2.1 | p < 0.001 | p < 0.89 |
| PXN/HIC5 | 25 ± 7.0 | p < 0.001 | p < 0.03 |
| HIC5/PXN | 59 ± 14 | p < 0.03 | p < 0.01 |
a Paxillin-null MEFs were transduced with puromycin-selected retroviruses expressing either empty vector or paxillin, paxillin mutants, or chimeras between paxillin or HIC5. FLAG-tagged K-RAS with an activating G12V mutation was retrovirally transduced and selected by blasticidin resistance except in the first row, as noted.
b Colony growth in 0.3% agarose after 10 days normalized to colony numbers produced by cells transduced by K-RAS_G12V and wild-type paxillin.
c Student's t test of normalized colony numbers produced by K-RAS_G12V plus sample compared to K-RAS_G12V plus wild-type paxillin. The data represent the combined results of between four and six independent experiments for each sample.
d Student t test of normalized colony numbers produced by K-RAS_G12V plus sample compared with K-RAS_G12V plus vector. The data represent the combined results of between four and six independent experiments for each sample.
As shown in Table 1, colony formation by K-RAS_G12V alone in paxillin-null cells was augmented by re-expression of paxillin. Deletion of LD4, LD5, or deletion of LIM domains greatly diminished colony formation. The minimal paxillin fragment of amino acids 225–559 that supported FAK tyrosine phosphorylation in detached cells (Fig. 3, D and E) significantly supported anchorage-independent colony formation by K-RAS_G12V, although it was significantly less active than WT paxillin. The 274–559 fragment (which does not support FAK tyrosine phosphorylation in detached T17-KD2 cells) was inactive in colony formation, indicating a critical role for the LD4 motifs and the LIM domains of paxillin in supporting anchorage-independent cell proliferation (Table 1).
Fig. 3 and Table 1 show a correlation between the activity of paxillin in supporting attachment-independent FAK tyrosine phosphorylation and colony formation in agar. The LD4 motif is the sole FAK-binding LD motif in paxillin 225–559, and deletion of LD4 both ablated anchorage-independent FAK tyrosine phosphorylation and greatly reduced colony formation. Although FAK-null fibroblasts are decreased for RAS transformation (31), the requirement for LD4 in this fragment may be more complex than simply interacting with FAK, because LD4 interacts directly and competitively with both FAK and GIT1 (10, 32).
To determine whether FAK expression contributes to transformation by K-RAS_G12V in paxillin-null MEFs, shRNAs to FAK were transduced into paxillin-null MEFs that re-expressed paxillin. Seven shRNA transduced cell lines were selected for further analysis: four cell lines with effective knockdowns, one cell line where the shRNA did not knockdown FAK, and an irrelevant shRNA control. The seven selected cell lines were then infected with retroviruses expressing activated K-RAS_G12V, and drug-resistant cells were tested for anchorage-independent colony formation. shRNAs that effectively knocked down FAK expression reduced the frequency of colony formation by ras (Fig. 6D). Taken together, these results indicate that paxillin augments anchorage-independent colony formation through essential interactions at LD4, LD5, and the LIM domains that result in the tyrosine phosphorylation of FAK. The regions of paxillin required for colony formation and attachment-independent tyrosine phosphorylation of FAK in T17-KD2 cells and ES cells are illustrated in Fig. 7.
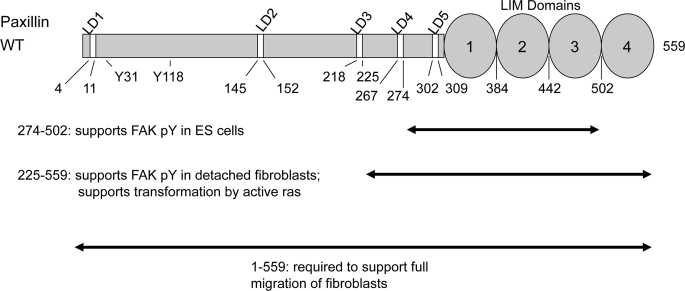
FIGURE 7.
Paxillin domains required for attachment-independent cell proliferation and tyrosine phosphorylation of FAK. The domains of paxillin required to support FAK tyrosine phosphorylation in ES cells (11) are compared with those domains required to support attachment-independent FAK tyrosine phosphorylation in fibroblasts (from Fig. 3) and oncogene-induced colony formation in fibroblasts (Table 1).
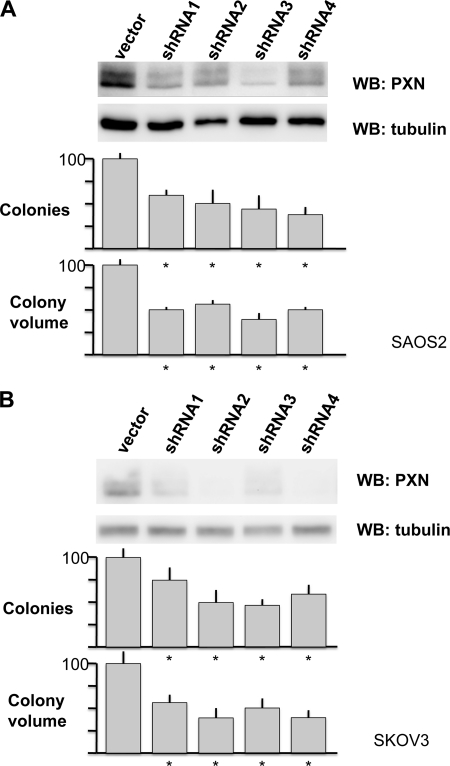
To determine whether paxillin expression supports anchorage-independent colony formation by human tumor cell lines, shRNA to paxillin were introduced into human SAOS2 (osteosarcoma) C33A (HPV-negative cervical carcinoma) and SKOV3 (ovarian carcinoma) cells, and the resultant cell lines were tested for anchorage-independent colony formation. shRNA against paxillin significantly reduced both the number and the size of SAOS2 and SKOV3 soft agar colonies (Fig. 8) but did not affect colony formation by C33A cells (data not shown), indicating that paxillin is not universally important for attachment-independent cell proliferation in human cancer cell lines.
FIGURE 8.
Paxillin augments anchorage-independent cell proliferation in human cancer cell lines. A, SAOS2 osteosarcoma cells were transduced by lentiviral shRNA control or the indicated lentiviruses expressing shRNA to human paxillin. Pooled drug-resistant cells were analyzed by Western blot for paxillin and tubulin expression, then suspended in 0.3% agarose for 10 days, and photographed, and the photographs were analyzed for colony number (top bar graph) and size (bottom bar graph) using NIH Image J software. Median normalized sizes from three separate experiments are shown relative to control-transduced cells. The error bars are S.E., and an asterisk indicates p values < 0.01 by Student's t test. B, same experiment as in A performed in SKOV3 ovarian cancer cell line.
DISCUSSION
The association of viral oncoproteins with their cellular targets has provided critical clues for the identification of cellular proteins that regulate signal transduction and the cell cycle. The E6 oncoprotein of bovine papillomavirus type 1 associates with the LD motifs of paxillin (12, 13), and we have recently determined that paxillin is required for transformation induced by bovine papillomavirus type 1 E6 (14). The provocative association of E6 with paxillin prompted us to initiate studies of paxillin and its possible role in attachment-independent signaling. Because paxillin associates with FAK, localizes to focal adhesions, and modulates cell migration, the role of paxillin in signal transduction has naturally focused upon attachment-induced signaling events. Because paxillin is tyrosine-phosphorylated by FAK upon cell attachment, paxillin has naturally been considered an attachment-dependent substrate of FAK rather than a factor that contributes to the tyrosine phosphorylation of FAK. Our results demonstrate that paxillin is a key regulator of FAK activation and attachment-independent signaling in both ES cells and fibroblasts.
Both paxillin-null MEFs and T17 cells express HIC5, which shares functionality with paxillin and associates with a common set of cellular proteins. To learn more about the role of paxillin in differentiated fibroblast cells, we generated paxillin-null differentiated T17-KD2 fibroblasts in which we stably knocked down HIC5 by shRNA to prevent possible trans-complementation by HIC5 of one or more paxillin functions.
We found two circumstances that result in FAK tyrosine phosphorylation in fibroblasts. First is an attachment-dependent mechanism that is independent of either paxillin or HIC5 expression that permits FAK tyrosine phosphorylation in cells attached to plates overnight (Figs. 1B and 3). Second, in cells that express paxillin, FAK can remain partially tyrosine-phosphorylated in detached cells; this is a function of paxillin and not HIC5 (Figs. 1C and 4). Attachment-independent FAK tyrosine phosphorylation in fibroblasts resembles ES cells where FAK tyrosine phosphorylation is attachment-independent (11). Together, these results indicate that during differentiation of ES cells an additional mechanism becomes active that facilitates the adhesion-dependent tyrosine phosphorylation of FAK independently of paxillin or HIC5 expression. That this is due to differentiation is inferred because the T17 fibroblasts used in these studies are derived from differentiation of the same paxillin-null clone 17 ES cells used in our prior studies (11, 27). FAK has been shown to directly interact with certain integrins and a number of other regulatory adapter molecules and kinases such as Src family kinases; further study is needed to identify those gene products that support attachment-dependent FAK tyrosine phosphorylation in the absence of either paxillin or HIC5.
The role that attachment-independent FAK tyrosine phosphorylation will play in normal cell biology is as yet unclear, but residual attachment-independent FAK phosphorylation could promote cell survival in adverse circumstances or during differentiation induced by detachment from matrix. In cancer cell biology, numerous studies have associated FAK overexpression and activation with malignancies and metastasis (reviewed in Refs. 33 and 34).
In this report we show that a fragment of paxillin with two LD motifs (amino acids 225–559; LD4 to LIM4) is the minimal fragment that supports FAK tyrosine phosphorylation in detached fibroblast cells (Fig. 3D and illustrated in Fig. 7). In our previous work using ES cells, we identified a slightly smaller paxillin fragment consisting of LD5 to LIM3 (amino acids 302–502) as sufficient for supporting FAK tyrosine phosphorylation. This paxillin fragment does not associate with FAK because it is deleted of both the LD2 and LD4 sites that interact directly with FAK, indicating that in ES cells, paxillin modulates FAK tyrosine phosphorylation independently of a detectable direct association with FAK (11). Thus, fibroblasts require additional domains of paxillin compared with their progenitor ES cells. It is possible that FAK tyrosine phosphorylation in ES cells and fibroblasts results from distinctly different mechanisms, but more likely the requirement for additional domains of paxillin in fibroblasts may reflect the need for additional components in a similar multimeric signaling complex. The 225–559 fragment contains at least six protein interaction domains thought to have multiple discrete interactions: LD4 (associates with FAK and the GIT-PAK-PIX complex), LD5 (unknown associations), LIM1 (unknown associations), LIM2 and LIM3 (localize paxillin to focal adhesions through unknown associations), and LIM3 and LIM4 (associate with PTP-PEST) (17). Our mutational analysis demonstrates that each of these domains is required for paxillin to support attachment-independent FAK tyrosine phosphorylation in fibroblasts. The requirement for LIM domains 2 and 3 is especially intriguing because these domains are required for focal adhesion localization of paxillin and thereby also influence the localization of FAK to focal adhesions (28). However, in our assays in detached cells, LIM domains 2 and 3 clearly are required for FAK tyrosine phosphorylation independently of focal adhesion formation in these detached cells. Given the number of required binding sites for multiple factors contained within the paxillin 225–559 fragment, elucidating the complete mechanism by which paxillin augments FAK tyrosine phosphorylation is quite complex and beyond the scope of this study.
Attachment-independent signaling and cell cycle progression are a hallmark of malignant cells. It was first observed that primary cell cultures require attachment for cell proliferation, whereas virally transformed cells do not and that attachment-dependent cells arrest in G1 upon suspension (35, 36). There is a close correlation between attachment-independent colony formation and tumorigenicity in nude mice (37, 38). In our experiments, paxillin strongly augmented the size and frequency of anchorage-independent colonies induced by an activated RAS (Figs. 5 and 7). Because the paxillin-null MEFs used in this experiment expressed HIC5, this implicates structural features found in paxillin 225–559 and not in the closely related HIC5 protein. Activated RAS alleles are common in multiple human malignancies (39), so the means by which paxillin influences RAS transformation could be clinically significant.
Overexpression of a FAK molecule with elevated kinase activity enhances the size of colonies induced by an activated RAS (31), so it is possible that the role of paxillin in transformation by RAS is simply to enable FAK tyrosine phosphorylation in detached cells. This is a correlation, but it as yet remains unproven. Supporting this interpretation is the reduction of RAS-induced anchorage-independent colonies in cells expressing shRNA to FAK (Fig. 6D). Deletion of LD4 abolished the effect of paxillin upon RAS transformation as expected because this ablates interaction of LD4 with FAK and GIT1. However, our results cannot be ascribed entirely to the interaction of LD4 with FAK. The 225–559 fragment of paxillin has additional and complex overlapping associations. The most consistent interpretation of these data is that dynamic overlapping associations between multiple protein complexes and paxillin support RAS transformation. It is notable that both anchorage-independent FAK tyrosine phosphorylation and anchorage-independent cell proliferation are independent of the amino-terminal tyrosine phosphorylation sites of paxillin that are phosphorylated upon cell attachment (amino acids 31, 33, and 118) and are therefore independent of SH2 dependent interactions with CRK and Src family associations that have been mapped to these sites. Because mutation of these tyrosine phosphorylation sites ablates the influence of paxillin upon cell migration (5), our experimental system dissociates paxillin-mediated signaling that regulates migration from signaling that supports anchorage-independent cell proliferation.
It is striking that although paxillin supported anchorage-independent colony formation, the closely related HIC5 protein did not. Both paxillin and HIC5 regulate the actin cytoskeleton and cell migration (20, 40, 41). More recently, a number of studies have implicated HIC5 as a transcriptional co-activator (42) (22, 43, 44) and both an effector and regulator of TGF-β signaling (20, 45, 46), and recent work has shown paxillin nuclear to cytoplasmic shuttling and paxillin modulation of transcription (21). A careful evaluation of the differences between paxillin and HIC5 in the 225–559 region should elucidate some of the feature(s) of paxillin responsible for anchorage-independent colony formation. We are currently analyzing the activation of signaling pathways in paxillin-null MEFs reconstituted with paxillin mutants and RAS to elucidate which known RAS signaling effector pathways are sensitive to paxillin expression in detached cells.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA-69292 and CA-120352 (to S. V.). This work was also supported by the University of Virginia.

The on-line version of this article (available at http://www.jbc.org) contains supplemental text.
N. Brimer, C. Lyons, and S. Vande Pol, unpublished observations.
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- ES
- embryonic stem
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1. Zimerman B., Volberg T., Geiger B. (2004) Cell Motil. Cytoskeleton 58, 143–159 [DOI] [PubMed] [Google Scholar]
- 2. Hehlgans S., Haase M., Cordes N. (2007) Biochim. Biophys. Acta 1775, 163–180 [DOI] [PubMed] [Google Scholar]
- 3. Deakin N. O., Turner C. E. (2008) J. Cell Sci. 121, 2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webb D. J., Brown C. M., Horwitz A. F. (2003) Curr. Opin. Cell Biol. 15, 614–620 [DOI] [PubMed] [Google Scholar]
- 5. Webb D. J., Donais K., Whitmore L. A., Thomas S. M., Turner C. E., Parsons J. T., Horwitz A. F. (2004) Nat. Cell Biol. 6, 154–161 [DOI] [PubMed] [Google Scholar]
- 6. Brown M. C., Turner C. E. (2004) Physiol. Rev. 84, 1315–1339 [DOI] [PubMed] [Google Scholar]
- 7. Vallés A. M., Beuvin M., Boyer B. (2004) J. Biol. Chem. 279, 44490–44496 [DOI] [PubMed] [Google Scholar]
- 8. Nikolopoulos S. N., Turner C. E. (2000) J. Cell Biol. 151, 1435–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikolopoulos S. N., Turner C. E. (2001) J. Biol. Chem. 276, 23499–23505 [DOI] [PubMed] [Google Scholar]
- 10. Turner C. E., Brown M. C., Perrotta J. A., Riedy M. C., Nikolopoulos S. N., McDonald A. R., Bagrodia S., Thomas S., Leventhal P. S. (1999) J. Cell Biol. 145, 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wade R., Vande Pol S. (2006) Biochem. J. 393, 565–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tong X., Howley P. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 4412–4417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vande Pol S. B., Brown M. C., Turner C. E. (1998) Oncogene 16, 43–52 [DOI] [PubMed] [Google Scholar]
- 14. Wade R., Brimer N., Vande Pol S. (2008) J. Virol. 82, 5962–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown M. C., Perrotta J. A., Turner C. E. (1996) J. Cell. Biol. 135, 1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen Y., Schneider G., Cloutier J. F., Veillette A., Schaller M. D. (1998) J. Biol. Chem. 273, 6474–6481 [DOI] [PubMed] [Google Scholar]
- 17. Côté J. F., Turner C. E., Tremblay M. L. (1999) J. Biol. Chem. 274, 20550–20560 [DOI] [PubMed] [Google Scholar]
- 18. Jamieson J. S., Tumbarello D. A., Hallé M., Brown M. C., Tremblay M. L., Turner C. E. (2005) J. Cell Sci. 118, 5835–5847 [DOI] [PubMed] [Google Scholar]
- 19. Shibanuma M., Mashimo J., Kuroki T., Nose K. (1994) J. Biol. Chem. 269, 26767–26774 [PubMed] [Google Scholar]
- 20. Tumbarello D. A., Turner C. E. (2007) J. Cell. Physiol. 211, 736–747 [DOI] [PubMed] [Google Scholar]
- 21. Dong J. M., Lau L. S., Ng Y. W., Lim L., Manser E. (2009) Biochem. J. 418, 173–184 [DOI] [PubMed] [Google Scholar]
- 22. Heitzer M. D., DeFranco D. B. (2006) Cancer Res. 66, 7326–7333 [DOI] [PubMed] [Google Scholar]
- 23. Ili D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. (1995) Nature 377, 539–544 [DOI] [PubMed] [Google Scholar]
- 24. Parsons J. T. (2003) J. Cell Sci. 116, 1409–1416 [DOI] [PubMed] [Google Scholar]
- 25. Mitra S. K., Hanson D. A., Schlaepfer D. D. (2005) Nat. Rev. Mol. Cell Biol. 6, 56–68 [DOI] [PubMed] [Google Scholar]
- 26. Hanks S. K., Ryzhova L., Shin N. Y., Brábek J. (2003) Front. Biosci. 8, d982–d996 [DOI] [PubMed] [Google Scholar]
- 27. Wade R., Bohl J., Vande Pol S. (2002) Oncogene 21, 96–107 [DOI] [PubMed] [Google Scholar]
- 28. Hagel M., George E. L., Kim A., Tamimi R., Opitz S. L., Turner C. E., Imamoto A., Thomas S. M. (2002) Mol. Cell. Biol. 22, 901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bohl J., Das K., Dasgupta B., Vande Pol S. B. (2000) Virology 271, 163–170 [DOI] [PubMed] [Google Scholar]
- 30. Tumbarello D. A., Brown M. C., Hetey S. E., Turner C. E. (2005) J. Cell Sci. 118, 4849–4863 [DOI] [PubMed] [Google Scholar]
- 31. Renshaw M. W., Price L. S., Schwartz M. A. (1999) J. Cell Biol. 147, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Z. M., Simmerman J. A., Guibao C. D., Zheng J. J. (2008) J. Biol. Chem. 283, 18685–18693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLean G. W., Carragher N. O., Avizienyte E., Evans J., Brunton V. G., Frame M. C. (2005) Nat. Rev Cancer 5, 505–515 [DOI] [PubMed] [Google Scholar]
- 34. Siesser P. M., Hanks S. K. (2006) Clin. Cancer Res. 12, 3233–3237 [DOI] [PubMed] [Google Scholar]
- 35. MacPherson I., Montagnier L. (1964) Virology 23, 291–294 [DOI] [PubMed] [Google Scholar]
- 36. Stoker M., O'Neill C., Berryman S., Waxman V. (1968) Int. J. Cancer 3, 683–693 [DOI] [PubMed] [Google Scholar]
- 37. Shin S. I., Freedman V. H., Risser R., Pollack R. (1975) Proc. Natl. Acad. Sci. 72, 4435–4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Freedman V. H., Shin S. I. (1974) Cell 3, 355–359 [DOI] [PubMed] [Google Scholar]
- 39. Malumbres M., Barbacid M. (2003) Nat. Rev. Cancer 3, 459–465 [DOI] [PubMed] [Google Scholar]
- 40. Avraamides C., Bromberg M. E., Gaughan J. P., Thomas S. M., Tsygankov A. Y., Panetti T. S. (2007) Am. J. Physiol. Heart Circ. Physiol. 293, H193–H203 [DOI] [PubMed] [Google Scholar]
- 41. Nishiya N., Tachibana K., Shibanuma M., Mashimo J. I., Nose K. (2001) Mol. Cell. Biol. 21, 5332–5345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujimoto N., Yeh S., Kang H. Y., Inui S., Chang H. C., Mizokami A., Chang C. (1999) J. Biol. Chem. 274, 8316–8321 [DOI] [PubMed] [Google Scholar]
- 43. Shibanuma M., Kim-Kaneyama J. R., Sato S., Nose K. (2004) J. Cell. Biochem. 91, 633–645 [DOI] [PubMed] [Google Scholar]
- 44. Drori S., Girnun G. D., Tou L., Szwaya J. D., Mueller E., Xia K., Shivdasani R. A., Spiegelman B. M. (2005) Genes Dev. 19, 362–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang H., Song K., Sponseller T. L., Danielpour D. (2005) J. Biol. Chem. 280, 5154–5162 [DOI] [PubMed] [Google Scholar]
- 46. Wang H., Song K., Krebs T. L., Yang J., Danielpour D. (2008) Oncogene 27, 6791–6805 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.