Background: γ-Secretase is composed of four subunits with 19 transmembrane domains.
Results: Transmembrane domain (TMD) 4 of presenilin 1 contains polar amino acids that are involved in ER retention, assembly, and stability.
Conclusion: TMD4 is a crucial interaction site in the γ-secretase complex.
Significance: Understanding TMD-TMD interactions in molecular detail is crucial for understanding of γ-secretase and other membrane protein complexes.
Keywords: Alzheimer Disease, Aspartic Protease, Endoplasmic Reticulum (ER), Membrane Proteins, Presenilin, Gamma-secretase
Abstract
γ-Secretase is composed of the four membrane proteins presenilin, nicastrin, Pen2, and Aph1. These four proteins assemble in a coordinated and regulated manner into a high molecular weight complex. The subunits constitute a total of 19 transmembrane domains (TMD), with many carrying important amino acids involved in catalytic activity, interaction with other subunits, or in ER retention/retrieval of unassembled subunits. We here focus on TMD4 of presenilin 1 (PS1) and show that a number of polar amino acids are critical for γ-secretase assembly and function. An asparagine, a threonine, and an aspartate form a polar interface important for endoplasmic reticulum retention/retrieval. A single asparagine in TMD4 of PS1 is involved in a protein-protein interaction by binding to another asparagine in Pen2. Intriguingly, a charged aspartate in TMD4 is critical for γ-secretase activity, most likely by stabilizing the newly formed complex.
Introduction
γ-Secretase is a protease cleaving its substrates in a process termed regulated intramembrane proteolysis. Among its many substrates are pivotal signal transduction components such as Notch, CD44, and cadherins (for review, see Refs. 1 and 2). Cleavage of substrates like APP and Notch critically contribute to pathologic conditions like Alzheimer disease and many tumors (3). γ-Secretase is composed of four different subunits (for review, see Ref. 1). The subunits nicastrin (Nct)2, Pen2, Aph1, and presenilin 1 or 2 (PS) contain one, two, seven, and nine transmembrane domains (TMDs), respectively, which sum up to a total of 19 TMDs in the γ-secretase complex. Not surprisingly, many important residues were identified in these TMDs, including the catalytically active aspartates in TMD6 and -7 (4), substrate binding sites in TMD2 and -6 (5, 6), protein-protein interaction sites in TMD4 (7–9), all in PS1. TMD1 of Pen2 contains a binding site for TMD4PS1 (9), and Aph1 carries residues in TMD3, -4, -5, and -6 that are important for complex formation/stability (10).
γ-Secretase assembles in the ER or the early secretory pathway by subsequent recruitment of PS to an initial assembly intermediate composed of Nct and Aph1, followed by the final recruitment of Pen2 (for review, see Refs. 11, 12, and 13). ER retention/retrieval signals in the subunits ensure that only fully assembled γ-secretase leaves the ER. Such signals have been identified in PS1 (14), Pen2 (15), and Nct (16). Interestingly, these motifs are located in TMDs and therefore do not resemble one of the well characterized ER retention/retrieval signals such as RXR found in cytoplasmic domains of many ion channels (17). Little is known about the machinery mediating TMD-based ER retention/retrieval of γ-secretase subunits. We recently identified Rer1 as a protein interacting with unassembled Pen2. Rer1 interacts with the ER retention/retrieval signal in TMD1 of Pen2 and localizes unassembled Pen2 to the ER. This interaction is dependent on an asparagine in TMD1 (15). In the assembled γ-secretase, Pen2 interacts with the TMD4 of PS1 (7, 8) via this asparagine (9). The TMD4PS1 also contains an ER retention/retrieval signal, and binding of TMD1Pen2 to TMD4PS1 masks the respective signals, allowing export of γ-secretase out of the ER (9). We here describe the characterization of a polar interface in TMD4PS1, which is involved in several important functions. A large part of the interface is involved in ER retention/retrieval, whereas a single asparagine is responsible for Pen2 binding. Interestingly, a critical aspartate in the interface was found to be essential for complex stability.
EXPERIMENTAL PROCEDURES
Antibodies
The following antibodies were used. For detection of Myc tags, monoclonal 9E10 (Santa Cruz Biotechnology) was used for immunofluorescence, immunoprecipitation, and Western blotting, and Alexa Fluor 488-conjugated 9E10 (AbD Serotec) was used for immunofluorescence. To detect human CD4, the following antibodies were used: monoclonal EDU-2 (Diatec Monoclonals) for immunofluorescence and polyclonal H-370 (Santa Cruz Biotechnology) for Western blotting. Monoclonal anti-calnexin MAB3126 (Chemicon) was used as an ER marker in immunofluorescence. Polyclonal anti-GFP A11122 (Invitrogen) was used in immunofluorescence, IP, and Western blot. Anti-β-actin polyclonal ab8227 (Abcam), anti-cleaved Notch 1 Val-1744 (Cell Signaling), polyclonal anti-Pen2 (Invitrogen), and polyclonal anti-Nct N1660 (Sigma) were used for Western blotting. Monoclonal antibody against PS1 N terminus, obtained from R. Nixon, and polyclonal anti-Aph1 antibody 433G have been described before (18, 19). Secondary HRP-conjugated antibodies for Western blot were obtained from Promega. Secondary antibodies conjugated to fluorophores (Alexa Fluor 488, Alexa Fluor 555) for immunofluorescence were obtained from Invitrogen.
Cell Lines
HEK293 cells stably expressing Swedish mutant APP (Swe) were described previously (20). Mouse embryonic fibroblast (MEF) cells from PS1−/−/PS2−/− mice (dKO cells) are described in Ref. 21 and were kindly provided by B. De Strooper (Leuven, Belgium). HeLa Kyoto cells were kindly provided by Rainer Pepperkok (EMBL). All cells were grown under standard culture conditions with DMEM and 10% FCS.
cDNA Constructs and Transfections
CD4-RXR was obtained from Lily E. Jan (Howard Hughes Medical Institute, San Francisco, CA)(22). CD4-TMD4PS1 with a C-terminal Myc tag has been described previously (15) and a C-terminally Myc-tagged CD4-RXR was generated in a similar manner. PS1-EGFP (PE), C99-EGFP, and GFP-Pen2 have been described previously (15, 23, 24). NotchΔE with six C-terminal Myc tags was obtained from Raphael Kopan (Washington University, St. Louis, MO, (25)). Mutations in CD4-TMD4PS1 and the TMD4 of PE were introduced by standard site-directed mutagenesis. Primer sequences and cloning details are available upon request. dKO cells were transfected using Turbofect (Fermentas). All other cell lines were transfected using Lipofectamine 2000 (Invitrogen).
Immunoprecipitation, Co-immunoprecipitation, and Western Blot
For Western blot, cells were lysed in STEN lysis buffer (50 mm Tris, pH 7.6, 150 mm NaCl, 2 mm EDTA, 1% Nonidet P-40, and protease inhibitor mix). Subsequently, proteins were separated on 10% SDS-PAGE gels or 10–20% Tris-Tricine gels (Invitrogen) and transferred to PVDF membranes (Millipore). Membranes were cut at appropriate positions and blotted with antibodies as indicated. For immunoprecipitation STEN lysates were incubated with antibody and paramagnetic protein A or protein G beads (Invitrogen) overnight. Subsequently, beads were washed successively with STEN-NaCl (50 mm Tris-HCl, pH 7.6, 325 mm NaCl, 2 mm EDTA, 0.2% Nonidet P-40), STEN-SDS (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 2 mm EDTA, 0.2% Nonidet P-40, 0.1% SDS), and STEN (50 mm Tris-HCl, pH 7.6, 150 mm NaCl, 2 mm EDTA, 0.2% Nonidet P-40). For protein elution, beads were boiled with Laemmli sample buffer. Co-immunoprecipitation was performed as described in Ref. 26, with the exception that paramagnetic protein A or protein G beads (Invitrogen) were used.
Deglycosylation of CD4-Myc Variants
For deglycosylation, the different CD4-Myc species were immunoprecipitated using monoclonal 9E10 anti-Myc antibody. Subsequently, proteins were eluted from paramagnetic beads with 30 μl of elution buffer (100 mm Tris-HCl, pH 7.6, 10 mm DTT, 1% SDS) and the sample was split into three equal parts. Part 1 was directly boiled with Laemmli sample buffer. Part 2 was incubated together with 88 μl of NGF buffer (100 mm phosphate buffer, pH8.0, 25 mm EDTA, 0.1% Triton X-100, 0.2% SDS, 0.1% β-mercaptoethanol), and 2 μl of N-Glycosidase F (New England Biolabs) overnight. Part 3 was incubated in a total volume of 100 μl with 1 μl of Endo-H (NEB) according to the manufacturer's protocols overnight. After incubation, proteins were TCA-precipitated and dissolved in Laemmli sample buffer. All three samples were subsequently subjected to standard SDS-PAGE and Western blot protocols.
Microscopy
Immunofluorescence was performed using standard protocols (27). Fixed cells were analyzed on a Zeiss Axio Imager microscope (Carl Zeiss, Jena, Germany) equipped with an ×63/1.4 objective and standard FITC, TRITC, and Alexa Fluor 660 fluorescence filter sets, using an Axiocam Mrm Camera and AxioVision software. For some images, a Zeiss Apotome was used (Carl Zeiss, Jena, Germany). Images were assembled and processed using Adobe Photoshop.
Statistics
Western blots were quantified by direct digital acquisition with a Luminescence Image Analyzer (Fuji). Signal detection using this machine is linear over four orders of magnitude and allows appropriate quantitation. For determination of Myc-Pen2 half-lives, curve fitting with quantified time points was performed using SciDAVis freeware. t½ was then calculated from the resulting equations. For statistical analysis, a paired Student's t test was applied.
RESULTS
Polar Interface in TMD4 of PS1 Is Involved in ER Retention/Retrieval
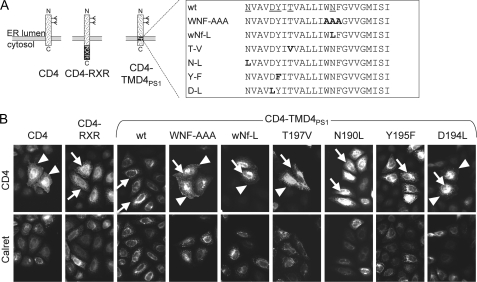
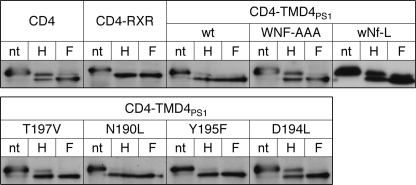
We previously showed that the TMD4PS1 contains an ER retention/retrieval signal. Mutations of the three amino acids WNF in TMD4PS1 partially abolished the ER localization of a CD4-based reporter (9), and we now wanted to identify additional important residues in this TMD. Close inspection of TMD4PS1 revealed a number of polar amino acids in the TMD (Fig. 1A). They are highly conserved across species and between PS1 and -2 (for an alignment, see Fig. 10A in Ref. 7). To define the role of these amino acids, we individually mutated the polar amino acids Asn-190, Asp-194, Tyr-195, Thr-197, and Asn-204 to similar-sized hydrophobic amino acids like Leu, Val, and Phe in our CD4-TMD4PS1 reporter (for a scheme, see Fig. 1A). After transient transfection in HeLa cells, the localization was determined by immunofluorescence (Fig. 1B). As shown previously (9), CD4-TMD4PS1 localized to the ER, whereas mutation of WNF(203–205) to AAA led to a mixed localization in ER and plasma membrane. Mutation of only Asn-204 to Leu in the WNF motif (wNf-L) led to a similar mixed distribution, as did mutations T197V and D194L, respectively. In contrast, mutations N190L and Y195F had no effect on the ER localization of the CD4 reporter (Fig. 1B). Analysis of subcellular localization of CD4 constructs by deglycosylation confirmed these observations (Fig. 2). Similar to TMD4PS1 WT, N190L and Y195F mutations localized to the early secretory pathway, as indicated by the same mobility of the Endo-H and the N-glycosidase F-digested samples. In contrast, WNF-AAA, wNf-L, D194L, and T197V in addition displayed an Endo-H resistant band similar to CD4, indicating that parts of the reporter protein traveled beyond the Golgi. Taken together, these data suggest that the three polar amino acids Asp-194, Thr-197, and Asn-204 form a polar interface that is involved in the ER localization of the CD4 reporter.
FIGURE 1.
Polar amino acids in TMD4 of PS1 are involved in ER retention/retrieval. A, scheme of CD4, CD4-RXR with an unrelated ER localization signal, and CD4-TMD4PS1. The box shows the sequence of TMD4 wild type with polar amino acids underlined. Variants with mutations (mutated amino acids in boldface) are listed below. B, HeLa cells were transiently transfected with CD4 variants, processed for immunofluorescence with antibodies against CD4 and calreticulin, and analyzed by fluorescence microscopy. Arrowheads depict plasma membrane staining, and arrows depict the ER network and the nuclear envelope. For each variant, at least 100 cells from three independent experiments were analyzed, and representative images were chosen.
FIGURE 2.
Deglycosylation identifies polar amino acids in TMD4 of PS1 involved in ER retention/retrieval. HeLa cells were transiently transfected with CD4 variants, lysed after 16 h, immunoprecipitated with CD4 antibodies, and processed for deglycosylation. After TCA precipitation, digests were loaded on SDS gels, blotted, and probed with anti-CD4 antibodies. nt, non-treated; H, endoglycosidase H-treated; F, N-glycosidase F-treated. Representative blots of at least three independent experiments are shown.
Single Asparagine Mediates Binding of TMD4PS1 to TMD1Pen2
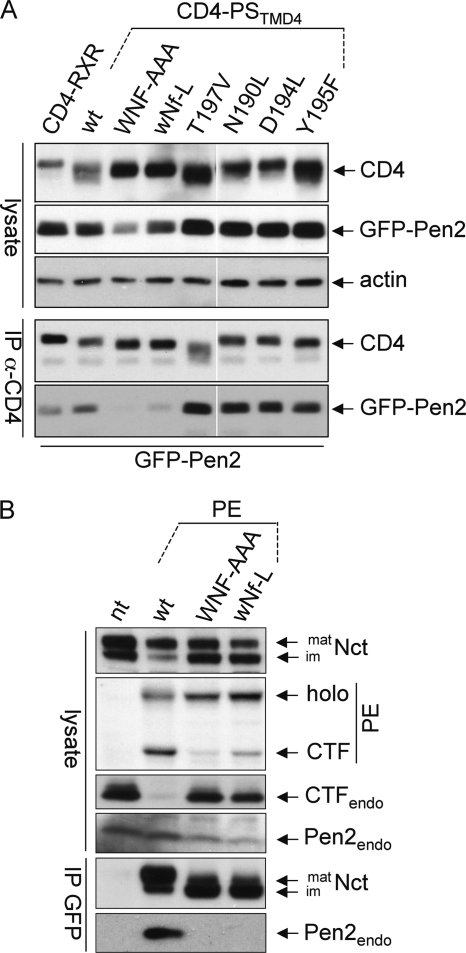
The TMD4PS1 binds to TMD1Pen2 (9). To test whether one or more of the polar amino acids in TMD4PS1 contribute to Pen2 binding, co-immunoprecipitation experiments were performed using transiently transfected CD4-TMD4PS1 with or without mutations and GFP-tagged Pen2. Cell lysates were immunoprecipitated with CD4 antibodies and probed for CD4 and GFP (Fig. 3). As positive and negative controls, CD4-TMD4PS1 and CD4-RXR were used, respectively. Only the mutation of the Asn-204 in the WNF motif abolished Pen2 binding; mutation of all other polar amino acids had no effect (Fig. 3A). To confirm the importance of Asn-204 in the context of presenilin, we made use of Swe cells (HEK293 cells expressing APPswe) stably expressing fully functional PS1-EGFP (PE (23)) and the mutations WNF-AAA and wNf-L. After co-immunoprecipitation with GFP antibodies, only PE WT precipitated endogenous Pen2, but not the WNF-AAA or the wNf-L mutation (Fig. 3B). The lack of Pen2 binding and consequently defective γ-secretase complex formation in the two mutants is also indicated by reduced PE endoproteolysis to N- and C-terminal fragments (NTF and CTF), and by the relatively low levels of co-precipitated mature Nct (Fig. 3B, for additional data, see Fig. 6). In addition, PE WT but neither of the mutants replaced the endogenous PS (indicated by the absence/presence of PS1CTF in Fig. 3B), a phenomenon observed for stably expressed exogenous functional PS (28). These data suggest that a single asparagine (Asn-204) in TMD4PS1 is responsible for binding to TMD1Pen2.
FIGURE 3.
A polar asparagine is the sole determinant for binding of TMD4 of PS1 to Pen2. A, HEK293 cells were transiently transfected with CD4 variants and GFP-tagged Pen2. Immunoprecipitation was performed with anti-CD4 antibodies, and after processing for Western blotting, membranes were probed with antibodies against CD4, GFP, and actin as loading control. B, Swe cells stably expressing PE variants as indicated were lysed or subjected to immunoprecipitation with anti-GFP antibodies (IP GFP), loaded on SDS gels, blotted, and probed with antibodies against Nct, PS1CTF, and Pen2. Note that due to GFP moiety, the endogenous PS1CTF is much smaller (17 kDa) than the PECTF; therefore, they are on different parts of the membrane. nt, non-transfected Swe cells; mat/im, mature/immature; endo, endogenous. Representative blots of at least three independent experiments are shown.
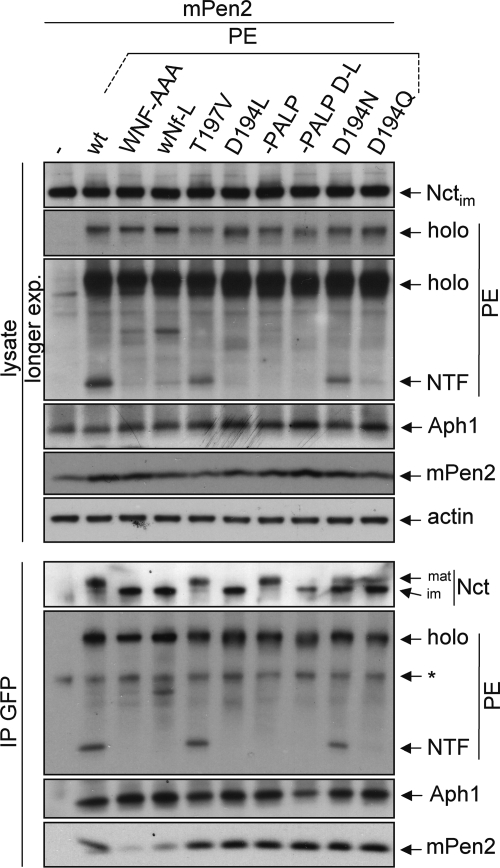
FIGURE 6.
Mutation of Asn-204 but not Asp-194 inhibits complex formation. PS1−/−/PS2−/− MEF (dKO) cells were transiently transfected with PE variants and Myc-tagged Pen2 (mPen2) as indicated. After 24 h of expression, cells were lysed, and lysates were either directly loaded on an SDS gel (top) or subjected to immunoprecipitation using anti-GFP antibodies followed by Western blotting. Membranes were probed as indicated. *, unspecific band. Representative blots of at least three independent experiments are shown. nt, non-transfected Swe cells; mat/im, mature/immature; endo, endogenous.
Mutations of Asn-204 and Asp-194 Completely Abolish γ-Secretase Activity
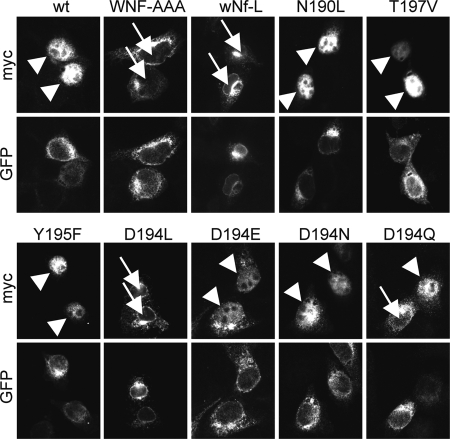
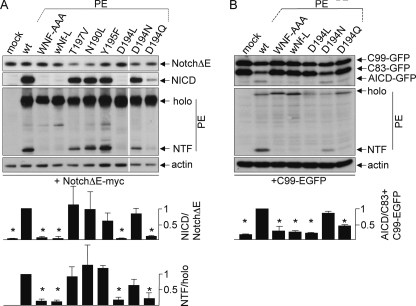
Next, it was tested whether the mutations of the polar amino acids in TMD4PS1 would have an impact on γ-secretase activity. To this end, mutations in TMD4PS1 were generated in PE, and γ-secretase activity was tested in mouse embryonic fibroblasts derived from PS1/2 double KO mice (dKO cells (29)). PE variants were transiently transfected together with NotchΔE, a reporter for γ-secretase activity (25). In untransfected dKO cells, NotchΔE accumulates in the secretory pathway and at the plasma membrane, whereas in cells that express a functional PE, γ-secretase is reconstituted and cleaves NotchΔE, and the resulting Notch intracellular domain (NICD) accumulates in the nucleus (14). As described previously, transfection of PE resulted in nuclear staining of NICD, whereas PEWNF/AAA was not active, because no γ-secretase complex was assembled (Fig. 4) (9). Likewise, mutation of the asparagine alone (wNf-L) did not rescue γ-secretase activity, confirming that it is Asn-204 in the WNF motif that is the critical amino acid. Mutations T197V, N190L, and Y195F did not affect reconstitution of γ-secretase activity in this assay (Fig. 4). Surprisingly, mutation of Asp-194 to leucine completely abolished nuclear accumulation of NICD (Fig. 4). Introductions of more conserved mutations ranged from complete reconstitution (D194E, D194N) to partial reconstitution of γ-secretase activity (D194Q, Fig. 4). The functional D194E and D194N mutations suggested that Asp-194 is not catalytically active. To further test catalytic activity, lysates of dKO cells transfected with PE variants and NotchΔE or another direct substrate of γ-secretase, C99-EGFP (24) were subjected to Western blotting and probed for NICD and APP intracellular domain, respectively (Fig. 5). The results confirmed the data of Fig. 4 on a biochemical level and demonstrated that total γ-secretase activity is affected and not activity toward a specific substrate. In addition, PE endoproteolysis correlated with activity and was high in the WT and fully functional mutants, absent in the non-functional mutants, and reduced in the case of D194N and strongly reduced in D194Q (Fig. 5).
FIGURE 4.
Mutation of Asn-204 and Asp-194 abolish γ-secretase-mediated Notch processing. PS1−/−/PS2−/− MEF (dKO) cells were transiently transfected with Myc-tagged NotchΔE together with PE or indicated PE variants. After 24 h of expression, cells were fixed and processed for immunofluorescence using anti-Myc to visualize NotchΔE/NICD and anti-GFP antibodies to visualize PE. Arrowheads point toward nuclear NICD staining, indicative of γ-secretase activity, arrows point toward to unstained nuclei, indicating that γ-secretase was not restored. For each variant, at least 100 cells from three independent experiments were analyzed, and representative images were chosen.
FIGURE 5.
Mutation of Asn-204 and Asp-194 abolish γ-secretase-activity. PS1−/−/PS2−/− MEF (dKO) cells were transiently transfected with Myc-tagged NotchΔE (A) or C99-EGFP (B) together with PE or indicated PE variants. After 24 h of expression, cells were lysed, subjected to Western blotting, and probed with Myc, anti-NICD, and GFP antibodies to detect NotchΔE, NICD, and PE variants, respectively. The C83-EGFP originates from C99-EGFP processed by α-secretase (24), and both can produce the APP intracellular domain (AICD). In A, two unrelated lanes were cut out, indicated by the white line. Actin serves as a loading control. Representative blots of at least three independent experiments are shown. For quantitation of activity, the ratio product/substrate was determined, set to 1 in the case of PE WT and the other ratios related to that. PS endoproteolysis was quantified accordingly by determining the ratios NTF/holoprotein. Error bars, S.D.; (n = 3); *, p < 0.01.
Asp-194 Is Not Involved in Assembly of γ-Secretase
The experiments in Figs. 4 and 5 using dKO cells and NotchΔE do not allow to discriminate whether the lack of NICD or APP intracellular domain production is due to defects in complex assembly, stability, substrate binding, or due to catalytic inactivity. To discriminate between these possibilities, co-immunoprecipitation experiments were performed in dKO cells transfected with Myc-tagged Pen2 and PE variants (Fig. 6). 24 h post-transfection, cells were lysed and subjected to immunoprecipitation using GFP antibodies followed by Western blotting with antibodies against γ-secretase subunits. As a control for a non-complex forming variant PEWNF/AAA was used (7, 9), for a mutant forming a complex but being defective in autoproteolysis PE-PALP was used, where the critical PALP motif was deleted (14). PE and PET197V reconstituted a γ-secretase complex, indicated by co-precipitation of mature Nct, Aph1, and Pen2 and endoproteolysis to PS1NTF. The other functional mutations N190L and Y195F behaved identically (data not shown). PE-PALP formed a complex as a holoprotein; therefore, no PS1NTF was detected, but mature Nct, Aph1, and Pen2 co-precipitated. PEwNf-L, similar to PEWNF/AAA, bound immature Nct and Aph1 but did not co-precipitate Pen2, and no endoproteolysis occurred. This was indicative of a trimeric complex intermediate PE+Nct+Aph1 and confirmed the data from Fig. 3 that solely Asn-204 is responsible for Pen2 binding. In PED194L, a complex of all four subunits was formed, indicated by the co-precipitation of Aph1, Nct, and Pen2. However, only immature Nct co-precipitated, and no PE endoproteolysis occurred, suggesting that the complex was non-functional and not exported out of the ER. Likewise, when the D194L mutation was introduced in PE-PALP, also only immature Nct co-precipitated. This indicated that Asp-194 is not involved in endoproteolysis as a prerequisite for Nct maturation. Interestingly, D194N and D194Q partially rescued γ-secretase formation indicated by partial Nct maturation, Aph1 and Pen2 binding, and generation of PS1NTF. The D194Q mutation seemed to have a greater effect because less PE endoproteolysis occurred (Figs. 5 and 6). These results confirm that mutation of Asp-194 does not interfere with Pen2 binding to the nascent complex and suggest that the aspartate is involved in other important functions.
Asp-194 Stabilizes Pen2 and PS1NTF in Nascent γ-Secretase Complex
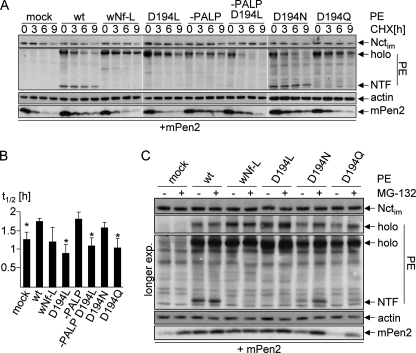
If Asp-194 in TMD4PS1 is not catalytically active and not involved in binding to Pen2, could it be involved in the stabilization of the complex? The stability of Pen2 is indicative of its incorporation into a γ-secretase complex as well as the stability of this complex (19). We performed a cycloheximide (CHX) chase and analyzed the stability of transiently transfected Myc-Pen2 in dKO cells co-transfected with PE variants (Fig. 7A, determination of t½ in B). As expected, Pen2 is rapidly degraded in the absence of PE but stabilized in PE and in PE-PALP transfected cells, where a γ-secretase complex is formed. Because Pen2 binding is abolished in PEwNf-L, it is not stabilized under these conditions. In PED194L-transfected cells, Pen2 binds (Figs. 3 and 6) but is not stabilized (Fig. 7, A and B), suggesting that initially a complex is formed but that this complex is not stable. The D194N and D194Q mutations rescued γ-secretase activity and PS1 endoproteolysis but not completely, and D194N was better than D194Q (Figs. 5 and 6). This is also reflected in the CHX chase because Pen2 levels in PED194N are stabilized similar to PE WT levels, whereas in PED194Q, no or very little Pen2 stabilization was observed (Fig. 7, A and B). To further assess whether the complex is destabilized if Asp-194 is mutated, we inhibited proteasomal degradation by MG132 and analyzed PS1NTF and Pen2 levels (Fig. 7C). In cells expressing PE and Myc-Pen2 and treated with MG132, neither Pen2 nor PS1NTF levels were increased because the complex is very stable, and there is no free Pen2 or PS1NTF whose degradation could be inhibited. In PED194L- and in PEwNf-L-transfected cells, no PS1NTF is produced at all, therefore no accumulation is visible after proteosomal blockage. In contrast, in PED194Q- and PED194N-transfected cells, PS1NTF accumulates after MG132 treatment. Because PS1NTF most likely is only produced in a γ-secretase complex, this indicated that a complex must have been formed, endoproteolysis must have occurred, but then the PS1NTF was not stabilized in the complex and degraded, unless proteasomal degradation was inhibited. Taken together, these data suggest that Asp-194 is involved in stabilization of the γ-secretase complex.
FIGURE 7.
Pen2 binds to the nascent complex but is not stabilized when Asp-194 is mutated. A, PS1−/−/PS2−/− MEF (dKO) cells were transiently transfected with PE variants and Myc-tagged Pen2 (mPen2) as indicated. After 24 h of expression, a CHX chase was performed for the indicated time periods. Subsequently, cell lysates were subjected to Western blotting. Membranes were probed as indicated. B, half-lives (t½) of Myc-tagged Pen2 were determined from experiments performed as in A. Asterisks denote significant shorter half-lives compared with cells transfected with PE WT. p < 0.02 (n = 3). C, dKO cells were transiently transfected with PE variants and Myc-tagged Pen2 as indicated. After 24 h of expression, cells were incubated with or without MG132 for 4 h. Subsequently, cell lysates were subjected to Western blotting. Membranes were probed as indicated. Representative blots of at least three independent experiments are shown. nt, non-transfected Swe cells; mat/im, mature/immature; endo, endogenous; exp., exposure.
DISCUSSION
Protein-protein interactions via TMDs are important for an increasing number of polytopic membrane proteins such as ion channels and cell surface receptors. A prominent example is the hetero-oligomeric complex γ-secretase, where the TMDs of the four subunits sum up to a total of 19, and some of the subunits such as Pen2 and Aph1 basically consist of TMDs connected by short loops (depicted in Ref. 30). It is therefore not surprising that TMDs within γ-secretase are very important. The TMD of Nct was shown to bind to the C terminus of PS1 (14), which is probably membrane-spanning or membrane-embedded (31, 32). In PS1, TMD1 could be part of the catalytic pore (33), TMD2 and TMD6 are involved in substrate binding (5, 6). TMDs in Aph1 were shown to be involved in binding to Nct and PS (34). Polar amino acids in TMDs have been shown to be part of TMD-TMD interaction motifs in Pen2 (9), Aph1 (10), and PS1 (7, 9, 35).
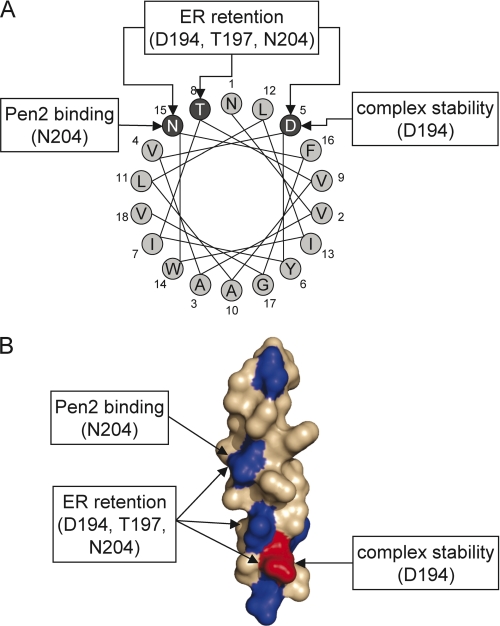
We here show that a number of polar amino acids in TMD4PS1 play important, but different roles in ER retention/retrieval, complex assembly, and complex stability (Fig. 8). Three polar amino acids, Asp-194–Thr-197–Asn-204, form a polar interface that is involved in ER retention/retrieval of unassembled PS1. Mutation of each of them influenced the localization of a CD4 reporter protein. Studying the impact of these amino acids on the localization of PS1 is difficult because PS1 carries at least one more ER retention/retrieval signal (14) and because PS1 is present mostly as tetramer or as trimer without Pen2 if the Pen2 binding site is mutated. The other complex components presumably have their own set of ER retention/retrieval signals, aggravating localization analysis. Little is known about TMD-based ER retention/retrieval signals. We recently showed that TMD1Pen2 is recognized via an asparagine by the putative Golgi-ER retrieval receptor Rer1 (15), but TMD4PS1 is not, despite the fact that is also has an asparagine as part of an ER retention/retrieval signal (9). Therefore, an Rer1-independent yet elusive machinery must operate on TMD4PS1. The amino acids WNF (7) or only NF (8) in TMD4PS1 were shown previously to be critical for the interaction of PS1 with Pen2, more precisely with TMD1 of Pen2 (9). By a single point mutation in a CD4 reporter and in PS1, we show here that it is the asparagine only, which mediates the binding to TMD1Pen2. That the flanking tryptophane and phenylalanine do not contribute to the Pen2 binding is also supported by the fact that in a helical wheel projection they protrude into different directions (Fig. 8A). As it is known that polar amino acids can pair to connect two TMDs (36–38) and because it was shown by us that a corresponding asparagine in TMD1Pen2 is important for binding (15), we speculate that an intramembraneous NN pairing is responsible for the interaction of PS1 and Pen2. An aspartate (Asp-194) in TMD4 of PS1 turned out to be very interesting. It is part of the ER retention/retrieval signal, but it is not involved in Pen2 binding. Nevertheless, mutation of this residue to leucine completely abolished γ-secretase activity. An aspartate in a TMD might contribute to the catalytic center of the γ-secretase, an intramembraneous aspartyl protease after all (4). However, more conserved mutations of the Asp to Glu, Asn, and Gln, all rescued γ-secretase activity, albeit to different degrees. This demonstrated that Asp is rather playing a structural role. Indeed, our studies revealed that Asp-194 is a critical amino acid for the stabilization of the γ-secretase complex. When Asp-194 is mutated to Leu, Pen2 could be co-immunoprecipitated with the other complex components, but no endoproteolysis and no Nct maturation were observed. In addition, Pen2 is less stabilized after treatment with CHX. In the partially rescuing D194N and D194Q mutants endoproteolysis did occur to some extent, but the fragments are not stabilized in a γ-secretase complex and can only be visualized after inhibition of protein degradation. This suggests that without Asp-194, the nascent γ-secretase complex falls apart, and the subunits are degraded before endoproteolysis and Nct maturation occur. Alternatively, the aspartate is, in addition to stabilizing the complex, directly involved in endoproteolysis and maturation. However, arguing against a direct involvement in endoproteolysis is the fact that in PE-PALP, which is incorporated into a maturing γ-secretase complex without endoproteolysis (14), mutating the Asp-194 also prevents Pen2 stabilization and Nct maturation. Why has the relevance of Asp-194 not been appreciated previously? Watanabe et al. (7) noted that exchanging the first third of TMD4PS1, where the aspartate is located, by corresponding CD4 TMD sequences, led to defective Nct maturation, endoproteolysis, and Pen2 binding. This finding was not analyzed in detail but is in line with our data, except that we find Pen2 binding in the absence of Asp-194. The discrepancy can be explained because they exchanged eight amino acids, which might have had a greater impact on Pen2 binding than our point mutation. Previously, Sisodia and colleagues did not find an involvement of the first third of TMD4PS1, but the TMD they used for swapping from SCAP introduces a glutamate close to the position of Asp-194, probably replacing it functionally (8). This might explain why their approach did not identify the importance of Asp-194 and is in line with our finding that an Asp→Glu mutation is fully functional. The molecular role of the aspartate could be a pairing via hydrogen bonding with another polar or charged amino acid in a TMD of one of the four subunits. This pairing could tightly bind the subunits together and trigger endoproteolysis. More structural information about the alignment of the TMDs within the complex will be needed to address this point. Taken together, the TMD4PS1 is a very important TMD in the γ-secretase complex. It helps to keep unassembled PS1 in the ER, binds Pen2 to the nascent complex, and is very important for the stability of the fully assembled complex.
FIGURE 8.
Model of the polar interface in TMD4PS1 and assigned function(s) of polar amino acids. A, helical wheel projection. The three amino acids forming the polar interface are highlighted in dark gray. B, surface representation of a model of TMDPS1 with polar amino acids in blue and the charged aspartate in red.
Acknowledgments
We thank R. Kopan for providing NotchΔE cDNA, R. Nixon for anti PS1-NTF antibody, and Christian Haass and Harald Steiner for anti-APH1 antibody. We are grateful to Karl Bauer for critically reading the manuscript.
This work was supported by Grant KA 1751/4-1 from the Deutsche Forschungsgemeinschaft (to C. K.) and the Hans-and-Ilse-Breuer Stiftung (to M. F.).
- Nct
- nicastrin
- CHX
- cycloheximide
- CTF
- C-terminal fragment
- dKO
- PS1−/−/PS2−/− double knock-out
- ER
- endoplasmic reticulum
- MEF
- mouse embryonic fibroblast
- NICD
- Notch intracellular domain
- NTF
- N-terminal fragment
- EGFP
- enhanced GFP
- PE
- PS1-EGFP
- PS
- presenilin
- TMD
- transmembrane domain.
REFERENCES
- 1. Haass C. (2004) EMBO J. 23, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarthy J. V., Twomey C., Wujek P. (2009) Cell Mol. Life Sci. 66, 1534–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shih IeM., Wang T. L. (2007) Cancer Res. 67, 1879–1882 [DOI] [PubMed] [Google Scholar]
- 4. Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- 5. Watanabe N., Takagi S., Tominaga A., Tomita T., Iwatsubo T., (2010) J. Biol. Chem. 285, 19738–19746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gong P., Vetrivel K. S., Nguyen P. D., Meckler X., Cheng H., Kounnas M. Z., Wagner S. L., Parent A. T., Thinakaran G. (2010) J. Biol. Chem. 285, 38042–38052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watanabe N., Tomita T., Sato C., Kitamura T., Morohashi Y., Iwatsubo T. (2005) J. Biol. Chem. 280, 41967–41975 [DOI] [PubMed] [Google Scholar]
- 8. Kim S. H., Sisodia S. S. (2005) J. Biol. Chem. 280, 41953–41966 [DOI] [PubMed] [Google Scholar]
- 9. Fassler M., Zocher M., Klare S., de la Fuente A. G., Scheuermann J., Capell A., Haass C., Valkova C., Veerappan A., Schneider D., Kaether C. (2010) Traffic 11, 250–258 [DOI] [PubMed] [Google Scholar]
- 10. Pardossi-Piquard R., Yang S. P., Kanemoto S., Gu Y., Chen F., Böhm C., Sevalle J., Li T., Wong P. C., Checler F., Schmitt-Ulms G., St George-Hyslop P., Fraser P. E. (2009) J. Biol. Chem. 284, 16298–16307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaether C., Haass C., Steiner H. (2006) Neurodegener. Dis. 3, 275–283 [DOI] [PubMed] [Google Scholar]
- 12. Spasic D., Annaert W. (2008) J. Cell Sci. 121, 413–420 [DOI] [PubMed] [Google Scholar]
- 13. Dries D. R., Yu G. (2008) Curr. Alzheimer Res. 5, 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaether C., Capell A., Edbauer D., Winkler E., Novak B., Steiner H., Haass C. (2004) EMBO J. 23, 4738–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaether C., Scheuermann J., Fassler M., Zilow S., Shirotani K., Valkova C., Novak B., Kacmar S., Steiner H., Haass C. (2007) EMBO Rep. 8, 743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spasic D., Raemaekers T., Dillen K., Declerck I., Baert V., Serneels L., Füllekrug J., Annaert W. (2007) J. Cell Biol. 176, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwappach B. (2008) Mol. Membr. Biol. 25, 270–278 [DOI] [PubMed] [Google Scholar]
- 18. Capell A., Saffrich R., Olivo J. C., Meyn L., Walter J., Grünberg J., Mathews P., Nixon R., Dotti C., Haass C. (1997) J. Neurochem. 69, 2432–2440 [DOI] [PubMed] [Google Scholar]
- 19. Prokop S., Shirotani K., Edbauer D., Haass C., Steiner H. (2004) J. Biol. Chem. 279, 23255–23261 [DOI] [PubMed] [Google Scholar]
- 20. Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. (1992) Nature 360, 672–674 [DOI] [PubMed] [Google Scholar]
- 21. Herreman A., Van Gassen G., Bentahir M., Nyabi O., Craessaerts K., Mueller U., Annaert W., De Strooper B. (2003) J. Cell Sci. 116, 1127–1136 [DOI] [PubMed] [Google Scholar]
- 22. Zerangue N., Schwappach B., Jan Y. N., Jan L. Y. (1999) Neuron 22, 537–548 [DOI] [PubMed] [Google Scholar]
- 23. Kaether C., Lammich S., Edbauer D., Ertl M., Rietdorf J., Capell A., Steiner H., Haass C. (2002) J. Cell Biol. 158, 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaether C., Schmitt S., Willem M., Haass C. (2006) Traffic 7, 408–415 [DOI] [PubMed] [Google Scholar]
- 25. Schroeter E. H., Kisslinger J. A., Kopan R. (1998) Nature 393, 382–386 [DOI] [PubMed] [Google Scholar]
- 26. Morohashi Y., Hatano N., Ohya S., Takikawa R., Watabiki T., Takasugi N., Imaizumi Y., Tomita T., Iwatsubo T. (2002) J. Biol. Chem. 277, 14965–14975 [DOI] [PubMed] [Google Scholar]
- 27. Wacker I., Kaether C., Krömer A., Migala A., Almers W., Gerdes H. H. (1997) J. Cell Sci. 110, 1453–1463 [DOI] [PubMed] [Google Scholar]
- 28. Thinakaran G., Harris C. L., Ratovitski T., Davenport F., Slunt H. H., Price D. L., Borchelt D. R., Sisodia S. S. (1997) J. Biol. Chem. 272, 28415–28422 [DOI] [PubMed] [Google Scholar]
- 29. Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., De Strooper B. (2000) Nat. Cell Biol. 2, 461–462 [DOI] [PubMed] [Google Scholar]
- 30. De Strooper B., Annaert W. (2010) Annu. Rev. Cell Dev. Biol. 26, 235–260 [DOI] [PubMed] [Google Scholar]
- 31. Laudon H., Hansson E. M., Melén K., Bergman A., Farmery M. R., Winblad B., Lendahl U., von Heijne G., Näslund J. (2005) J. Biol. Chem. 280, 35352–35360 [DOI] [PubMed] [Google Scholar]
- 32. Spasic D., Tolia A., Dillen K., Baert V., De Strooper B., Vrijens S., Annaert W. (2006) J. Biol. Chem. 281, 26569–26577 [DOI] [PubMed] [Google Scholar]
- 33. Takagi S., Tominaga A., Sato C., Tomita T., Iwatsubo T. (2010) J. Neurosci. 30, 15943–15950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiang P. M., Fortna R. R., Price D. L., Li T., Wong P. C. (2010) Neurobiol. Aging, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S. H., Sisodia S. S. (2005) J. Biol. Chem. 280, 1992–2001 [DOI] [PubMed] [Google Scholar]
- 36. Zhou F. X., Cocco M. J., Russ W. P., Brunger A. T., Engelman D. M. (2000) Nat. Struct. Biol. 7, 154–160 [DOI] [PubMed] [Google Scholar]
- 37. Gratkowski H., Lear J. D., DeGrado W. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou F. X., Merianos H. J., Brunger A. T., Engelman D. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]