Background: Obesity is associated with lipocalin 13 deficiency and fatty liver disease.
Results: Recombinant lipocalin 13 suppressed lipogenesis and promoted β-oxidation. It attenuated hepatic steatosis and insulin resistance in obese mice.
Conclusion: Lipocalin 13 is a new regulator of lipogenesis and β-oxidation; its deficiency contributes to fatty liver disease.
Significance: Lipocalin 13 or its related molecules have therapeutic potential in treating metabolic diseases.
Keywords: Fatty Acid Oxidation, Hepatocyte, Insulin Resistance, Lipid Oxidation, Lipogenesis, Liver Metabolism, Obesity, Hepatic Steatosis, Lipocalin, Nonalcoholic Fatty Liver Disease
Abstract
Obesity is associated with hepatic steatosis, partially due to increased lipogenesis and decreased fatty acid β-oxidation in the liver; however, the underlying mechanism of abnormal lipid metabolism is not fully understood. We reported previously that obesity is associated with LCN13 (lipocalin 13) deficiency. LCN13 is a lipocalin family member involved in glucose metabolism, and LCN13 deficiency appears to contribute to hyperglycemia in obese mice. Here, we show that LCN13 is also an important regulator of lipogenesis and β-oxidation in the liver. In primary hepatocytes, recombinant LCN13 directly suppressed lipogenesis and increased fatty acid β-oxidation, whereas neutralization of endogenous LCN13 had an opposite effect. Transgenic overexpression of LCN13 protected against hepatic steatosis in mice with either dietary or genetic (ob/ob) obesity. LCN13 transgenic overexpression also improved hyperglycemia, glucose intolerance, and insulin resistance in ob/ob mice. Short-term LCN13 overexpression via an adenovirus-mediated gene transfer similarly attenuated hepatic steatosis in db/db mice. LCN13 inhibited the expression of important lipogenic genes and stimulated the genes that promote β-oxidation. These results suggest that LCN13 decreases liver lipid levels by both inhibiting hepatic lipogenesis and stimulating β-oxidation. LCN13 deficiency is likely to contribute to fatty liver disease in obese mice.
Introduction
Hepatic lipid levels are determined by multiple physiological processes, including lipogenesis, fatty acid β-oxidation, lipid uptake, and VLDL secretion. These processes are tightly controlled by metabolites and metabolic hormones, and aberrant regulation results in hepatic steatosis, leading to nonalcoholic fatty liver disease (NAFLD),3 nonalcoholic steatohepatitis, cirrhosis, and liver failure (1–3). Obesity is an important risk factor for NAFLD (1, 4, 5); however, the underlying mechanism is poorly understood.
We recently identified LCN13 (lipocalin 13) as a new and important regulator of glucose metabolism, and LCN13 deficiency is associated with obesity (6). LCN13 is a member of the lipocalin family, which consists of a large number of small secretory proteins (6, 7). The family members share a highly conserved tertiary structure with a characteristic β-barrel at the center (8, 9). Lipocalins bind via their central cavities to small lipophilic molecules, including fatty acids, retinol, steroids, odorants, and pheromones (8, 9). Thus, lipocalins function as carriers to regulate the transportation, stability, release, and activity of these small bioactive molecules (9). Some lipocalin family members are also believed to stimulate cellular responses directly by binding to and activating their cognate receptors (10, 11).
Lipocalins are secreted by multiple cell types and are believed to regulate numerous biological processes, including chemical communication, cell proliferation, and cell differentiation (9, 12, 13). Several lipocalins have been reported to be involved in the regulation of glucose metabolism (9, 12, 14–18). For instance, abnormal secretion and action of RBP4 (retinol-binding protein 4) and lipocalin 2 contribute to insulin resistance in type 2 diabetes (12, 14–16, 19). MUP1 (major urinary protein 1), a lipocalin family member expressed primarily in hepatocytes, improves insulin sensitivity and glucose metabolism; MUP1 deficiency may contribute to insulin resistance and hyperglycemia in obese mice (17, 18). We reported recently that LCN13 is produced by multiple cell types and secreted into the bloodstream in mice (6). Circulating LCN13 levels decrease in the fasting state; importantly, LCN13 levels dramatically decrease in mice with either genetic (db/db) or diet-induced obesity (6). Moreover, LCN13 therapy improves insulin resistance, hyperglycemia, and glucose intolerance in these mice (6). Thus, LCN13 deficiency is likely to contribute to insulin resistance and hyperglycemia in obese mice. However, the role of LCN13 in lipid metabolism has not been examined.
The liver is a key organ for the maintenance of lipid homeostasis. In the fed state, glucose is taken up by hepatocytes and converted to fatty acids and triacylglycerols that are exported via VLDL to extrahepatic tissues. In the fasted state, fatty acids are oxidized to generate ATP and ketone bodies. Ketone bodies, a metabolic fuel, are exported to extrahepatic tissues. Hepatic lipogenesis and fatty acid β-oxidation are regulated by various metabolic hormones, including insulin and glucagon (20, 21). In this study, we show that, in primary hepatocytes, LCN13 directly inhibits lipogenesis and stimulates fatty acid β-oxidation. In mice with either genetic or diet-induced obesity, overexpression of LCN13 protects against hepatic steatosis. Our results indicate that LCN13 is a novel regulator of lipid metabolism in mouse liver.
EXPERIMENTAL PROCEDURES
Animals
LCN13 transgenic (Tg) animals have been generated previously and backcrossed for more than six generations in a C57BL/6 background (6). The expression of the LCN13 transgene is under the control of the chicken β-actin/rabbit β-globin hybrid promoter. This hybrid promoter is constitutively active in multiple tissues of the transgenic mice (22). Tg mice were crossed with ob+/− mice (The Jackson Laboratory, Bar Harbor, ME) to obtain ob/ob/Tg mice containing both the LCN13 transgene and a defective leptin gene. Mice were housed on a 12-h light/12-h dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan and fed either normal chow (9% fat; LabDiet) or a high fat diet (45% fat; Research Diets) ad libitum with free access to water.
Animal Experiments
db/db mice (9 weeks old) were infected with β-gal or LCN13 adenoviruses via tail veins as described previously (17). Blood samples were collected from mouse tail veins using heparin-pretreated capillary tubes. Blood glucose was measured using a glucometer (Bayer Corp., Tarrytown, NY). For glucose tolerance tests (GTTs), mice were fasted overnight or for 6 h and intraperitoneally injected with d-glucose. Blood glucose was measured 0, 15, 30, 60, and 120 min after glucose injection. For insulin tolerance tests (ITTs), mice were fasted for 6 h and intraperitoneally injected with human insulin. Blood glucose was monitored 0, 15, 30, and 60 min after insulin injection. Liver triacylglycerol (TAG) contents were measured as described previously (23). Recombinant LCN13 was chronically administrated to ob/ob mice via osmotic minipumps (33 pmol/h/mouse). Anti-LCN13 or preimmune serum (45 μl/kg of body weight) was injected daily into C57BL/6 males (7 weeks old) via tail veins. Animal experiments were conducted following the protocols approved by the University Committee on the Use and Care of Animals.
Conditioned Medium
HepG2 cells were infected with β-gal or LCN13 adenoviruses, and the culture medium was changed 24 h after infection. The conditioned medium was collected 24 h later.
Lipogenesis Assays
Primary hepatocytes were prepared by liver perfusion with type II collagenase (Worthington) and grown on collagen-coated plates as described previously (17). Primary hepatocytes were pretreated with recombinant LCN13 protein, conditioned medium, or anti-LCN13 antibody for 16 h. The cells were then incubated for an additional 4 h in Williams' Medium E (Sigma) supplemented with 0.5% BSA, 0.5 mm unlabeled acetate, and 4 μCi/ml [3H]acetate (Moravek Biochemicals Inc., Brea, CA). Cells were lysed in 0.1 m HCl, and lipids were extracted with chloroform/methanol (2:1). The organic phase was collected and dried by evaporation at 50 °C. The pellets were dissolved in 50 μl of hexane and 200 μl of H2SO4 (1.8% in methanol) and heated for 30 min at 100 °C. The mixtures were cooled down to room temperature, mixed with 125 μl of water, and extracted with 250 μl of petroleum twice. After centrifugation, the petroleum phase was collected and used to measure 3H radioactivity. Lipogenesis rates were normalized to total protein levels.
Fatty Acid β-Oxidation Assays
Primary hepatocytes were treated as described above. The treated cells were incubated for 1 h at 37 °C with 0.4 μCi/ml [9,10-3H]oleic acid (Moravek Biochemicals Inc.) and 100 μm unlabeled oleic acid (conjugated with BSA) in Krebs-Ringer buffer (119 mm NaCl, 5 mm KCl, 2 mm CaCl2, 2.6 mm MgSO4, 24.6 mm NaHCO3, 2.6 mm KH2PO4, and 10 mm HEPES, pH 7.4). Supernatants were collected, incubated with perchloric acid (1.3 m), and centrifuged at 16,000 × g for 10 min. Supernatants were neutralized with 2 m KOH and 0.6 m MOPS and loaded on an anion-exchange column. 3H activity in the effluent was measured and used to calculate β-oxidation rates.
Immunoblotting
Liver lysates, cell extracts, and conditioned medium were immunoblotted with anti-LCN13 antibody as described previously (6).
Quantitative Real-time RT-PCR (qRT-PCR)
Total RNAs were extracted from livers or primary hepatocytes and used to measure the mRNA abundance of lipogenic and oxidative genes using ABsoluteTM QPCR SYBR® Green kits (Thermo Scientific, Waltham, MA) and an Mx3000PTM real-time PCR system (Stratagene, La Jolla, CA) as described previously (6). The primer sequences were as follows: carbohydrate-responsive element-binding protein (ChREBP), 5′-CTGGGGACCTAAACAGGAGC-3′ (forward) and 5′-GAAGCCACCCTATAGCTCCC-3′ (reverse); carnitine palmitoyltransferase 1α (CPT1α), 5′-CTGATGACGGCTATGGTGTTT-3′ (forward) and 5′-GTGAGGCCAAACAAGGTGATA-3′ (reverse); fatty acid synthase (FAS), 5′-TTGACGGCTCACACACCTAC-3′ (forward) and 5′-CGATCTTCCAGGCTCTTCAG-3′ (reverse); long-chain acyl-CoA dehydrogenase, 5′-CACTCAGATATTGTCATGCCCT-3′ (forward) and 5′-TCCATTGAGAATCCAATCACTC-3′ (reverse); medium-chain acyl-CoA dehydrogenase, 5′-ACCCTGTGGAGAAGCTGATG-3′ (forward) and 5′-AGCAACAGTGCTTGGAGCTT-3′; peroxisome proliferator-activated receptor-γ (PPARγ), 5′-CCAGAGTCTGCTGATCTGCG-3′ (forward) and 5′-GCCACCTCTTTGCTCTGATC-3′ (reverse); SCD1 (stearoyl-CoA desaturase 1), 5′-AGGTGCCTCTTAGCCACTGA-3′ (forward) and 5′-CCAGGAGTTTCTTGGGTTGA-3′ (reverse); SREBP1c (sterol regulatory element-binding protein 1c), 5′-AACGTCACTTCCAGCTAGAC-3′ (forward) and 5′-CCACTAAGGTGCCTACAGAGC-3′ (reverse); and 36B4, 5′-AAGCGCGTCCTGGCATTGTCT-3′ (forward) and 5′-CCGCAGGGGCAGCAGTGGT-3′ (reverse).
Luciferase Assays
Primary hepatocytes were seeded in 24-well plates (1.5 × 105 cells/well) and transfected with luciferase reporter plasmids using polyethylenimine (1 mg/ml). Briefly, cells were incubated for 4 h in 200 μl of serum-free Williams' Medium E containing luciferase reporter plasmids (600 ng), Renilla expression vectors (50 ng), and polyethylenimine (3 μl) and then grown in Williams' Medium E supplemented with 2% FBS for ∼24 h. The cells were subsequently incubated for 16 h in serum-free Williams' Medium E supplemented with 0.5% BSA and recombinant LCN13. Luciferase activity was measured using the Dual-Luciferase® reporter assay System (Promega, Madison, WI) and normalized to Renilla activity.
Statistical Analysis
Data are presented as means ± S.E. Differences between groups were determined by two-tailed Student's t tests. p < 0.05 was considered statistically significant.
RESULTS
LCN13 Suppresses Lipogenesis in Primary Hepatocytes
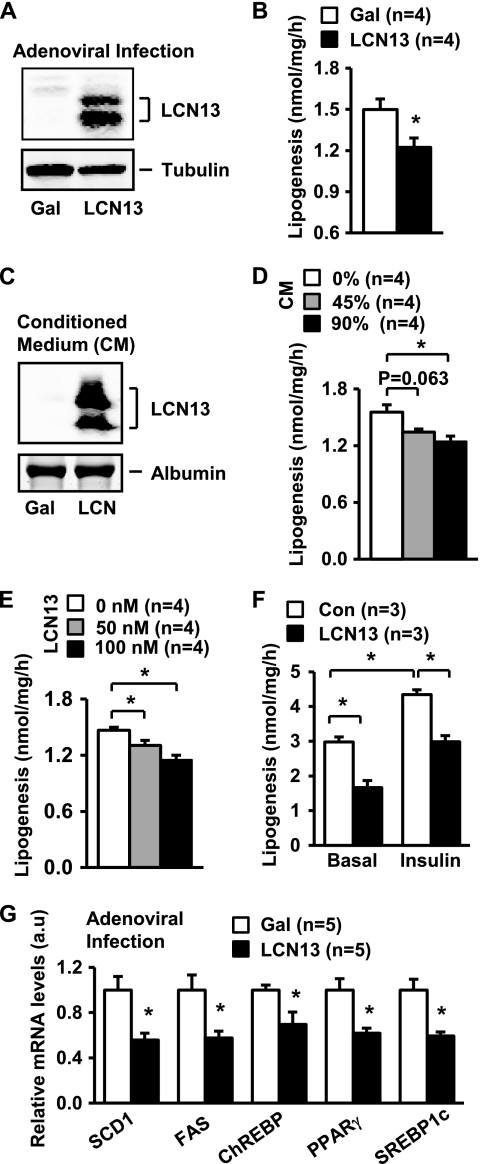
To overexpress LCN13, primary hepatocytes were prepared from C57BL/6 male mice (8–9 weeks old) and infected with LCN13 or β-gal adenoviruses as described previously (6). LCN13 was detected in LCN13 but not β-gal adenovirus-infected cells (Fig. 1A). In agreement with our previous report (6), we detected two forms of recombinant LCN13. Hepatocytes were subjected to lipogenesis assays 16 h after infection. LCN13 overexpression reduced lipogenesis rates by 18% (Fig. 1B).
FIGURE 1.
LCN13 suppresses lipogenesis in primary hepatocytes. A, primary hepatocytes were infected with β-gal or LCN13 adenoviruses. Cell extracts were prepared 16 h after viral infection and immunoblotted with anti-LCN13 or anti-tubulin antibody. B, primary hepatocytes were infected with β-gal or LCN13 adenoviruses and subjected to lipogenesis assays 16 h after infection. C, HepG2 cells were infected with β-gal or LCN13 adenoviruses, and the conditioned medium was collected 24 h after viral infection. The conditioned medium (10 μl) was immunoblotted with anti-LCN13 antibody (upper panel) or subjected to Coomassie Blue staining to visualize BSA (lower panel). D, primary hepatocytes were treated with conditioned medium for 16 h and subjected to lipogenesis assays. E, primary hepatocytes were treated with recombinant LCN13 protein for 16 h and subjected to lipogenesis assays. F, primary hepatocytes were treated with or without LCN13 (100 nm) in the presence or absence of insulin (100 nm). Lipogenesis was measured 16 h after the treatments. G, primary hepatocytes were infected with β-gal or LCN13 adenoviruses. Total RNAs were extracted 16 h after infection and used to measure the mRNA abundance by qRT-PCR. The expression of individual genes was normalized to 36B4 expression. Error bars represent S.E. *, p < 0.05. Con, control; a.u., arbitrary units.
To verify LCN13 suppression of lipogenesis, we prepared LCN13 conditioned medium from HepG2 cells (human hepatoblastoma cells). HepG2 cells were infected with LCN13 or β-gal adenoviruses and used to prepare LCN13 or β-gal conditioned medium. Recombinant LCN13 proteins were detected in LCN13 but not β-gal conditioned medium (Fig. 1C). Primary hepatocytes were treated with LCN13 or β-gal conditioned medium for 16 h and then subjected to lipogenesis assays. LCN13 conditioned medium inhibited lipogenesis in a dose-dependent manner (Fig. 1D). To further analyze LCN13 action, recombinant LCN13 was produced and purified from bacteria as described previously (6). Primary hepatocytes were treated with recombinant LCN13 for 16 h and subjected to lipogenesis assays. Recombinant LCN13 also dose-dependently inhibited lipogenesis (Fig. 1E). Insulin has been shown to promote lipogenesis in the liver (20, 24). We observed that insulin increased lipogenesis rates in primary hepatocytes; LCN13 inhibited both basal and insulin-stimulated lipogenesis (Fig. 1F). Together, these results demonstrate that LCN13 is a novel suppressor of hepatic lipogenesis.
Hepatic lipogenesis rates are controlled by key transcription factors and metabolic enzymes, including PPARγ, SREBP1c, ChREBP, SCD1, and FAS (25–30). To determine whether LCN13 inhibits the expression of these lipogenic genes, we measured the mRNA abundance of these genes. Primary hepatocytes were infected with LCN13 or β-gal adenoviruses, and total RNAs were extracted 16 h after infection and used to measure the mRNA abundance of these lipogenic regulators by qRT-PCR. LCN13 overexpression significantly inhibited the expression of SCD1, FAS, ChREBP, PPARγ, and SREBP1c (Fig. 1G). LCN13 may also decrease the mRNA stability of these molecules. These data suggest that LCN13 directly inhibits the hepatic lipogenic program.
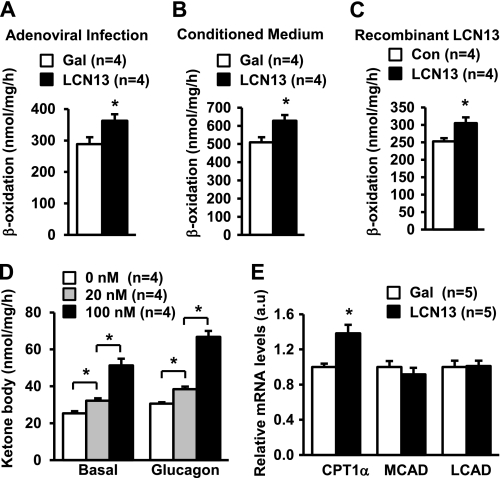
LCN13 Stimulates Fatty Acid β-Oxidation in Primary Hepatocytes
Primary hepatocytes were infected with LCN13 or β-gal adenoviruses as described in the legend to Fig. 1A and subjected to β-oxidation assays 16 h after infection. LCN13 overexpression increased fatty acid β-oxidation rates by 26% (Fig. 2A). To confirm these findings, primary hepatocytes were treated with LCN13 or β-gal conditioned medium for 16 h and subjected to β-oxidation assays. LCN13 conditioned medium treatments increased β-oxidation rates by 23% (Fig. 2B). To further examine LCN13 action, primary hepatocyte cultures were treated with purified bacterially derived recombinant LCN13. Recombinant LCN13 also stimulated β-oxidation rates (Fig. 2C). Additionally, LCN13 dose-dependently stimulated the secretion of ketone bodies from primary hepatocytes (Fig. 2D). Glucagon has been reported to stimulate β-oxidation and ketone body secretion from the liver during fasting (21). We showed that glucagon stimulated ketone body secretion in primary hepatocytes; LCN13 further increased ketone body secretion in glucagon-stimulated cells (Fig. 2D). We also examined the effect of LCN13 on the expression of CPT1α (liver form) and long- and medium-chain acyl-CoA dehydrogenases, which mediate β-oxidation. Primary hepatocytes were infected with LCN13 or β-gal adenoviruses, and total RNAs were extracted 16 h after infection and used to measure the mRNA abundance of these genes by qRT-PCR. LCN13 overexpression significantly increased the expression of CPT1α but not medium- and long-chain acyl-CoA dehydrogenases (Fig. 2E). Therefore, LCN13 directly stimulates fatty acid β-oxidation in hepatocytes at least in part by stimulating CPT1α expression.
FIGURE 2.
LCN13 promotes fatty acid β-oxidation in primary hepatocytes. A, primary hepatocytes were infected with β-gal or LCN13 adenoviruses, and fatty acid β-oxidation rates were measured 16 h after viral infection. B, primary hepatocytes were treated with the conditioned medium for 16 h and subjected to β-oxidation assays. C, primary hepatocytes were treated with recombinant LCN13 protein for 16 h and subjected to β-oxidation assays. D, primary hepatocytes were stimulated with LCN13 in the absence (Basal) and presence of glucagon (50 nm). Ketone body secretion was measured 8 h after stimulation. E, primary hepatocytes were infected with β-gal or LCN13 adenoviruses. Total RNAs were extracted 16 h after infection and used to measure mRNA abundance by qRT-PCR. The expression of individual genes was normalized to 36B4 expression. Error bars represent S.E. *, p < 0.05. a.u., arbitrary units; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase.
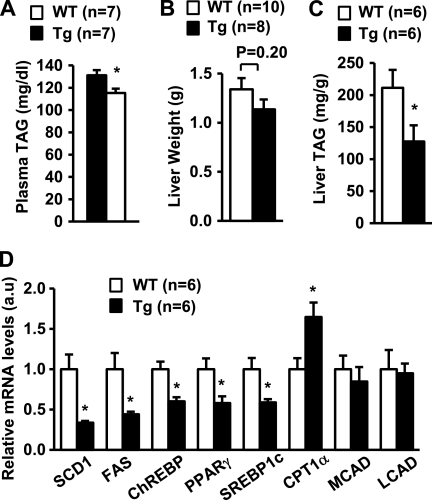
Chronic Overexpression of LCN13 Protects against Hepatic Steatosis in Mice with Dietary Obesity
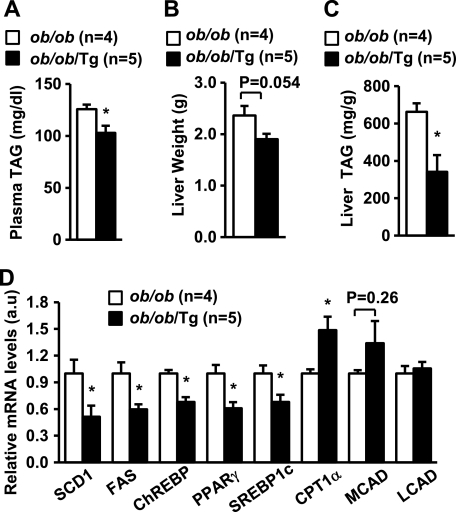
To determine whether LCN13 regulates hepatic lipid metabolism in animals, we performed studies in LCN13 Tg mice that have been generated previously (6). In Tg mice, the expression of the LCN13 transgene is under the control of constitutively active chicken β-actin/rabbit β-globin hybrid promoter (6). We verified recently that recombinant LCN13 is detected in the blood of Tg mice but not control littermates and that Tg mice resist diet-induced insulin resistance and hyperglycemia (6). To determine whether chronic LCN13 overexpression protects against diet-induced hepatic steatosis, Tg and control male littermates (7 weeks old) were fed a high fat diet. Body weights were similar between Tg and WT mice at 18 weeks of age: 39.5 ± 1.4 g (n = 9) and 41.6 ± 1.5 g (n = 10), respectively (p = 0.38). Plasma TAG levels decreased by 12% in Tg mice at 18 weeks of age (Fig. 3A). Liver weights were similar between WT and Tg mice (Fig. 3B); however, liver TAG contents decreased by 40% in Tg mice at 20 weeks of age (Fig. 3C). Chronic LCN13 overexpression significantly inhibited the expression of key lipogenic genes (e.g. SCD1, FAS, ChREBP, PPARγ, and SREBP1c) but stimulated CPT1α expression in the liver (Fig. 3D). These results suggest that LCN13 also suppresses lipogenesis and enhances fatty acid β-oxidation in the liver.
FIGURE 3.
LCN13 Tg mice resist diet-induced hepatic steatosis. Tg and WT male littermates (7 weeks old) were fed a high fat diet. Mice were fasted for 16 h and killed. A, plasma TAG levels at 18 weeks of age. B, liver weights at 20 weeks of age. C, liver TAG levels at 20 weeks of age (normalized to liver weights). D, gene expression measured by qRT-PCR and normalized to 36B4 expression. Error bars represent S.E. *, p < 0.05. a.u., arbitrary units; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase.
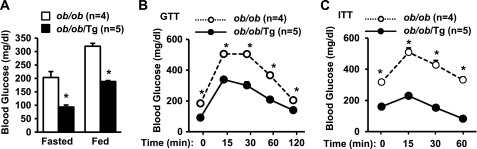
LCN13 Protects against Hyperglycemia, Glucose Intolerance, and Hepatic Steatosis in Mice with Genetic Obesity
To determine whether overexpression of LCN13 protects against hyperglycemia, insulin resistance, and glucose intolerance in mice with genetic obesity, Tg mice were crossed with ob+/− mice to generate LCN13 Tg and leptin-deficient double mutant mice (ob/ob/Tg). ob/ob mice are commonly used as a genetic model of obesity. LCN13 overexpression did not alter the body weights of ob/ob/Tg mice (data not shown); however, transgenic overexpression of LCN13 markedly reduced both fasted (by 54%) and randomly fed (by 41%) blood glucose in ob/ob/Tg mice (Fig. 4A). To further analyze glucose metabolism, we performed GTTs and ITTs. In GTTs, blood glucose levels were significantly lower in ob/ob/Tg mice compared with ob/ob mice at each time point after glucose injection (Fig. 4B). In ITTs, insulin was unable to reduce blood glucose in ob/ob mice due to insulin resistance; however, chronic LCN13 overexpression increased the ability of insulin to reduce blood glucose in ob/ob/Tg mice (Fig. 4C). These results are consistent with our previous observation that LCN13 improves glucose metabolism at least in part by sensitizing insulin action (6).
FIGURE 4.
Transgenic overexpression of LCN13 improves insulin resistance, hyperglycemia and glucose intolerance in ob/ob mice. ob/ob and ob/ob/Tg males were fed normal chow. A, fasting (16 h) and randomly fed blood glucose levels at 10 weeks of age. B, GTT. Mice (10 weeks old) were fasted overnight and intraperitoneally injected with d-glucose (0.5 g/kg of body weight). C, ITT. Mice (10 weeks old) were fasted for 6 h and intraperitoneally injected with human insulin (2.5 units/kg of body weight). Error bars represent S.E. *, p < 0.05.
To determine whether overexpression of LCN13 protects against hepatic steatosis in ob/ob mice, we analyzed lipid metabolism in ob/ob/Tg mice. Plasma TAG levels decreased by 18% in ob/ob/Tg mice compared with ob/ob mice (Fig. 5A). Liver weights also decreased in ob/ob/Tg mice (13 weeks old) (Fig. 5B). Importantly, liver TAG contents were significantly lower in ob/ob/Tg than in ob/ob mice (Fig. 5C). Hematoxylin and eosin staining of liver sections revealed that both the sizes of lipid droplets and the number of large lipid droplets were decreased in ob/ob/Tg mice (data not shown). Furthermore, the expression of lipogenic genes (e.g. SCD1, FAS, ChREBP, PPARγ and SREBP1c) was significantly reduced in ob/ob/Tg mice, whereas CPT1α expression increased in LCN13-overexpressing mice with genetic obesity (Fig. 5D). These data indicate that LCN13 is able to suppress hepatic lipogenesis, promote β-oxidation, and ameliorate hepatic steatosis in obesity.
FIGURE 5.
Transgenic overexpression of LCN13 ameliorates hepatic steatosis in ob/ob mice. A, plasma TAG levels at 11 weeks of age. B, liver weights at 13 weeks of age. C, liver TAG levels measured at 13 weeks of age and normalized to liver weights. D, gene expression measured by qRT-PCR and normalized to 36B4 expression. Error bars represent S.E. *, p < 0.05. a.u., arbitrary units; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase.
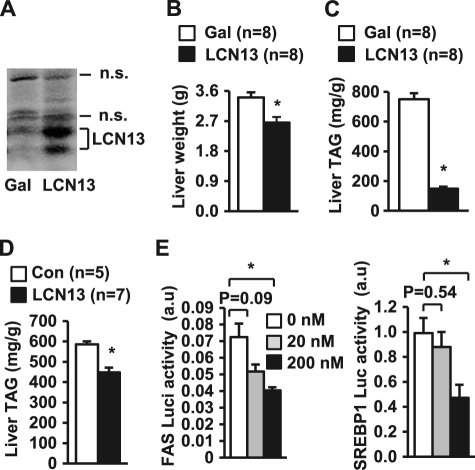
To determine whether short-term LCN13 treatment improves hepatic steatosis in obesity, db/db mice (9 weeks old) were infected with LCN13 or β-gal adenoviruses via tail vein injection, and livers were harvested 16 days after infection. db/db mice are deficient in functional leptin receptors and are widely used as a genetic model of obesity. Recombinant LCN13 was detected as two forms in LCN13 adenovirus-infected livers (Fig. 6A). Liver weights decreased in LCN13-overexpressing mice (Fig. 6B). Liver TAG contents decreased by 80% in LCN13 adenovirus-infected mice compared with β-gal adenovirus-infected mice (Fig. 6C). To further verify LCN13 action in vivo, purified LCN13 was chronically administrated to ob/ob mice via osmotic minipumps as described previously (6). Recombinant LCN13 also significantly decreased liver TAG levels under these conditions (Fig. 6D). These data suggest that LCN13 therapy may be useful in treating NAFLD.
FIGURE 6.
LCN13 therapy attenuates hepatic steatosis in genetically obese mice. A–C, db/db males (9 weeks old) were infected with β-gal or LCN13 adenoviruses via tail vein injection, and the mice were killed 16 days after viral infection. A, liver extracts were immunoblotted with anti-LCN13 antibody. n.s., nonspecific bands. B, liver weights. C, liver TAG levels (normalized to liver weights). D, ob/ob mice (10 weeks old) were treated with either 0.9% NaCl vehicle (control (Con)) or recombinant LCN13 via osmotic minipumps. Liver TAG levels were measured 28 days after treatments. E, primary hepatocytes were cotransfected with Renilla expression vectors and FAS- or SREBP1-luciferase reporter plasmids and treated with LCN13. Luciferase (Luci) activity was measured 16 h after LCN13 treatments and normalized to Renilla expression. Error bars represent S.E. *, p < 0.05. a.u., arbitrary units.
To determine whether LCN13 directly inhibits the transcription of lipogenic genes, we analyzed the activity of the FAS and SREBP1 promoters using luciferase reporter assays. LCN13 inhibited both FAS and SREBP1 promoter activity (Fig. 6E). These results further suggest that LCN13 directly suppresses the hepatic lipogenic program.
Endogenous LCN13 Regulates Hepatic Lipid Metabolism
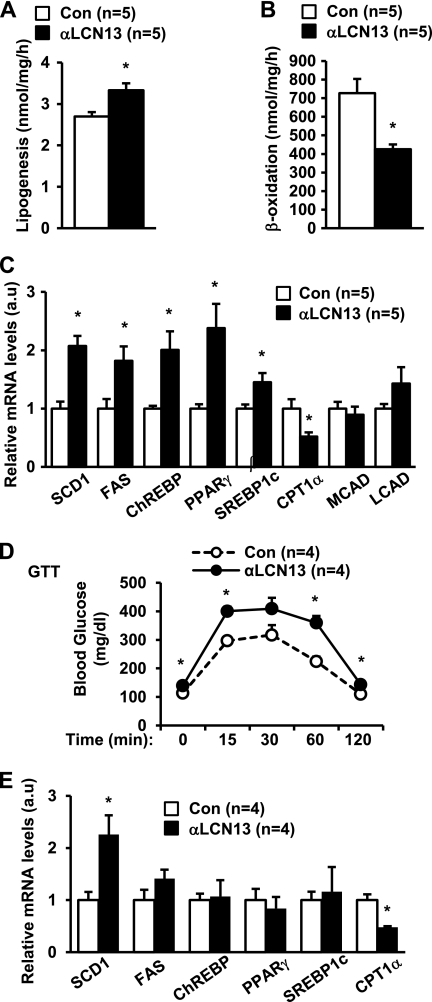
The liver is able to secrete LCN13 (6). To determine whether endogenous LCN13 regulates lipogenesis and β-oxidation, primary hepatocytes were treated with anti-LCN13 serum to neutralize hepatocyte-secreted LCN13 in the culture medium. Preimmune serum was used as a control. The treated cells were then subjected to lipogenesis or β-oxidation assays. Neutralization of endogenous LCN13 increased lipogenesis by 24% (Fig. 7A) and decreased fatty acid β-oxidation by 41% (Fig. 7B). We also examined the expression of genes that control lipogenesis and β-oxidation by qRT-PCR. In contrast to LCN13 treatments, neutralization of LCN13 significantly increased the expression of lipogenic genes (e.g. SCD1, FAS, ChREBP, PPARγ, and SREBP1c) and decreased CPT1α expression (Fig. 7C). These data suggest that endogenous LCN13 in the liver regulates hepatic lipogenesis and β-oxidation in an autocrine/paracrine manner.
FIGURE 7.
Endogenous LCN13 regulates hepatic lipogenesis and β-oxidation. A–C, primary hepatocytes were treated with anti-LCN13 antibody or preimmune serum (control (Con)). Lipogenesis (A) and fatty acid β-oxidation (B) rates were measured 16 h after treatments. Total RNAs were extracted 16 h after treatments and used to measure gene expression (normalized to 36B4 expression) by qRT-PCR (C). D and E, C57BL/6 males (7 weeks old) were treated with anti-LCN13 antibody or preimmune serum. A GTT (2 g of d-glucose/kg of body weight; 6-h fasting) was performed 5 days after treatments (D). Gene expression was measured 8 days after treatments and normalized to 36B4 expression (E). Error bars represent S.E. *, p < 0.05. a.u., arbitrary units; MCAD, medium-chain acyl-CoA dehydrogenase; LCAD, long-chain acyl-CoA dehydrogenase.
To determine whether endogenous LCN13 regulates glucose and lipid metabolism in vivo, anti-LCN13 serum was injected into C57BL/6 mice (with preimmune serum used as a control), and a GTT was performed 5 days after injection. Anti-LCN13 serum treatments increased fasting blood glucose (at the 0 point in the GTT) and promoted glucose intolerance (Fig. 7D). These observations suggest that endogenous LCN13 is required for the maintenance of normal glucose homeostasis in mice. Neutralization of endogenous LCN13 also increased SCD1 expression and decreased CPT1α expression in the liver (Fig. 7E), furthering supporting the important role of LCN13 in regulating lipogenesis and β-oxidation. However, the expression of FAS, ChREBP, PPARγ, and SREBP1c was not significantly changed, suggesting that, in vivo, the expression of these genes is controlled by other factors in addition to LCN13. Surprisingly, liver TAG levels were similar in anti-LCN13 and preimmune serum-treated mice: 201 ± 5 (n = 4) and 193 ± 9 (n = 4) mg/g of liver weight, respectively (p = 0.4338). One possibility is that anti-LCN13 serum treatments may not be able to reduce LCN13 in the liver below a threshold level. Residual hepatic LCN13 may be sufficient to maintain relatively normal TAG contents in the anti-LCN13 serum-treated mice. Additionally, anti-LCN13 serum treatments may trigger a compensatory response that maintains relatively normal liver TAG levels by overcoming relative LCN13 deficiency under these conditions.
DISCUSSION
Hepatic lipid metabolism is tightly controlled by nutrients and metabolic hormones, and dysregulation of hepatic lipid metabolism plays an important role in metabolic diseases (1, 2, 5). Abnormal accumulation of lipids in the liver impairs hepatocyte function, leading to liver injury (1, 31). Hepatic steatosis is believed to contribute to liver inflammation and nonalcoholic steatohepatitis progression (1, 3, 4, 32). Therefore, it is important to identify factors that regulate hepatic lipid synthesis and β-oxidation.
LCN13 is a recently identified lipocalin family member that is secreted into the bloodstream by multiple cell types, including hepatocytes (6). Circulating LCN13 levels are markedly reduced in mice with either dietary or genetic obesity (6). In this study, we showed that chronic overexpression of LCN13 significantly attenuated hepatic steatosis in LCN13 transgenic mice with either diet-induced or genetic (ob/ob) obesity. Additionally, short-term overexpression of LCN13 via adenovirus-mediated gene transfer similarly ameliorated hepatic steatosis in db/db mice. Administration of purified recombinant LCN13 proteins also decreased liver TAG levels in ob/ob mice. Collectively, these data suggest that LCN13 is a previously unknown regulator of liver lipid metabolism. LCN13 deficiency may contribute to NAFLD in obesity.
LCN13 appears to directly regulate the genetic programs that govern lipogenesis and fatty acid β-oxidation in hepatocytes. We first showed that, in primary hepatocytes, adenovirus-mediated overexpression of LCN13, which was secreted into the culture medium and acted on hepatocytes themselves, suppressed lipogenesis. We then demonstrated that LCN13 conditioned medium similarly inhibited lipogenesis in primary hepatocytes. Finally, we showed that purified bacterially derived recombinant LCN13 proteins suppressed lipogenesis in primary hepatocytes. In agreement, LCN13 inhibited the expression of key lipogenic genes (e.g. ChREBP, SREBP1c, PPARγ, FAS, and SCD1) in both primary hepatocytes and livers. In contrast to lipogenesis, fatty acid β-oxidation in primary hepatocytes was robustly stimulated by adenovirus-mediated overexpression of LCN13, LCN13 conditioned medium, and purified recombinant LCN13 proteins. Furthermore, in both primary hepatocytes and livers, LCN13 stimulated the expression of CPT1α, which promotes β-oxidation. These results suggest that LCN13 directly suppresses the hepatic lipogenic program and enhances β-oxidation, thus reducing hepatic lipid levels. Interestingly, amino acid sequences of LCN13 are highly homologous to those of several other lipocalin family members (e.g. LCN1, LCN3, LCN4, LCN14, OBP2B, and OBP2A), raising the possibility that these LCN13-like molecules may also regulate lipogenesis and β-oxidation in a similar fashion.
Interestingly, neutralization of LCN13 using anti-LCN13 antibody increased lipogenesis and decreased β-oxidation in primary hepatocytes. Neutralization of LCN13 also increased the expression of lipogenic genes and decreased CPT1α expression. These observations raise the possibility that, in hepatocytes, endogenous LCN13 may regulate lipogenesis and fatty acid β-oxidation in an autocrine/paracrine fashion. In mice, anti-LCN13 serum treatments also increased SCD1 expression and suppressed CPT1α expression in the liver, suggesting that endogenous LCN13 may similarly regulate lipogenesis and β-oxidation in vivo.
We reported previously that transgenic overexpression of LCN13 improves insulin resistance and glucose intolerance in diet-induced obesity (6). Here, we showed that transgenic overexpression of LCN13 markedly reduced hyperglycemia, glucose intolerance, and insulin resistance in ob/ob mice, whereas anti-LCN13 serum treatments induced glucose intolerance in mice fed normal chow. These data further support our previous observation that endogenous LCN13 is an important regulatory of insulin sensitivity and glucose metabolism in mice (6). We reported that LCN13 is able to directly enhance insulin signaling (6). Importantly, abnormal lipid accumulation has been reported to impair insulin action (lipotoxicity) (33–37), and increased β-oxidation improves insulin sensitivity (38). Our current data raise the possibility that LCN13 may also improve insulin sensitivity indirectly by decreasing lipogenesis and/or increasing β-oxidation in the liver.
The molecular mechanisms of LCN13 action are currently unclear. We speculate that LCN13 may bind via its central cavity to bioactive small molecules, thus serving as a carrier to regulate the transportation, stability, release, and/or activity of these bioactive molecules. These hydrophobic small molecules in turn regulate lipogenesis and/or β-oxidation. Alternatively, LCN13 may bind to and activate its receptors, which in turn mediate LCN13 action. Additional studies are needed to identify putative LCN13-bound bioactive small molecules and/or LCN13 cognate receptors.
In conclusion, we have shown that LCN13 protects against hepatic steatosis in obesity. LCN13 protects mice from fatty liver disease at least in part by suppressing lipogenesis and promoting β-oxidation in hepatocytes. LCN13 may also improve insulin resistance and glucose intolerance in obesity indirectly by ameliorating hepatic steatosis in addition to enhancing insulin signaling directly. Therefore, LCN13 or its related molecules may be useful in treating NAFLD and insulin resistance.
Acknowledgments
We thank Drs. Lin Jiang and Zheng Chen for technique support and helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK065122 and R01 DK073601. This work was also supported by American Diabetes Association Research Award 1-09-RA-156. This work utilized the cores supported by the Michigan Diabetes Research and Training Center (funded by National Institutes of Health Grant 5P60 DK20572), the University of Michigan Cancer Center (funded by National Institutes of Health Grant 5P30 CA46592), the University of Michigan Nathan Shock Center (funded by National Institutes of Health Grant P30 AG013283), and the University of Michigan Gut Peptide Research Center (funded by National Institutes of Health Grant DK34933).
- NAFLD
- nonalcoholic fatty liver disease
- Tg
- transgenic
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- TAG
- triacylglycerol
- qRT-PCR
- quantitative real-time RT-PCR
- ChREBP
- carbohydrate-responsive element-binding protein
- CPT1α
- carnitine palmitoyltransferase 1α
- FAS
- fatty acid synthase
- PPARγ
- peroxisome proliferator-activated receptor-γ.
REFERENCES
- 1. Lewis J. R., Mohanty S. R. (2010) Dig. Dis. Sci. 55, 560–578 [DOI] [PubMed] [Google Scholar]
- 2. Méndez-Sánchez N., Arrese M., Zamora-Valdés D., Uribe M. (2007) Liver Int. 27, 423–433 [DOI] [PubMed] [Google Scholar]
- 3. Caldwell S., Ikura Y., Dias D., Isomoto K., Yabu A., Moskaluk C., Pramoonjago P., Simmons W., Scruggs H., Rosenbaum N., Wilkinson T., Toms P., Argo C. K., Al-Osaimi A. M., Redick J. A. (2010) J. Hepatol. 53, 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larter C. Z., Yeh M. M. (2008) J. Gastroenterol. Hepatol. 23, 1635–1648 [DOI] [PubMed] [Google Scholar]
- 5. Reddy J. K., Rao M. S. (2006) Am. J. Physiol. Gastrointest. Liver Physiol. 290, G852–G858 [DOI] [PubMed] [Google Scholar]
- 6. Cho K. W., Zhou Y., Sheng L., Rui L. (2011) Mol. Cell. Biol. 31, 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suzuki K., Lareyre J. J., Sánchez D., Gutierrez G., Araki Y., Matusik R. J., Orgebin-Crist M. C. (2004) Gene 339, 49–59 [DOI] [PubMed] [Google Scholar]
- 8. Schlehuber S., Skerra A. (2005) Drug Discov. Today 10, 23–33 [DOI] [PubMed] [Google Scholar]
- 9. Zhou Y., Rui L. (2010) Vitam. Horm. 83, 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morè L. (2006) Chem. Senses 31, 393–401 [DOI] [PubMed] [Google Scholar]
- 11. Chamero P., Marton T. F., Logan D. W., Flanagan K., Cruz J. R., Saghatelian A., Cravatt B. F., Stowers L. (2007) Nature 450, 899–902 [DOI] [PubMed] [Google Scholar]
- 12. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., Kahn B. B. (2005) Nature 436, 356–362 [DOI] [PubMed] [Google Scholar]
- 13. Brennan P. A., Kendrick K. M. (2006) Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 2061–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Graham T. E., Yang Q., Blüher M., Hammarstedt A., Ciaraldi T. P., Henry R. R., Wason C. J., Oberbach A., Jansson P. A., Smith U., Kahn B. B. (2006) N. Engl. J. Med. 354, 2552–2563 [DOI] [PubMed] [Google Scholar]
- 15. Klöting N., Graham T. E., Berndt J., Kralisch S., Kovacs P., Wason C. J., Fasshauer M., Schön M. R., Stumvoll M., Blüher M., Kahn B. B. (2007) Cell Metab. 6, 79–87 [DOI] [PubMed] [Google Scholar]
- 16. Yan Q. W., Yang Q., Mody N., Graham T. E., Hsu C. H., Xu Z., Houstis N. E., Kahn B. B., Rosen E. D. (2007) Diabetes 56, 2533–2540 [DOI] [PubMed] [Google Scholar]
- 17. Zhou Y., Jiang L., Rui L. (2009) J. Biol. Chem. 284, 11152–11159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hui X., Zhu W., Wang Y., Lam K. S., Zhang J., Wu D., Kraegen E. W., Li Y., Xu A. (2009) J. Biol. Chem. 284, 14050–14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Law I. K., Xu A., Lam K. S., Berger T., Mak T. W., Vanhoutte P. M., Liu J. T., Sweeney G., Zhou M., Yang B., Wang Y. (2010) Diabetes 59, 872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foretz M., Guichard C., Ferré P., Foufelle F. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Longuet C., Sinclair E. M., Maida A., Baggio L. L., Maziarz M., Charron M. J., Drucker D. J. (2008) Cell Metab. 8, 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Niwa H., Yamamura K., Miyazaki J. (1991) Gene 108, 193–199 [DOI] [PubMed] [Google Scholar]
- 23. Ren D., Li M., Duan C., Rui L. (2005) Cell Metab. 2, 95–104 [DOI] [PubMed] [Google Scholar]
- 24. Shimomura I., Matsuda M., Hammer R. E., Bashmakov Y., Brown M. S., Goldstein J. L. (2000) Mol. Cell 6, 77–86 [PubMed] [Google Scholar]
- 25. Iizuka K., Bruick R. K., Liang G., Horton J. D., Uyeda K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. (2007) Cell Metab. 6, 484–496 [DOI] [PubMed] [Google Scholar]
- 27. Uyeda K., Repa J. J. (2006) Cell Metab. 4, 107–110 [DOI] [PubMed] [Google Scholar]
- 28. Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimomura I., Bashmakov Y., Horton J. D. (1999) J. Biol. Chem. 274, 30028–30032 [DOI] [PubMed] [Google Scholar]
- 30. Matsusue K., Haluzik M., Lambert G., Yim S. H., Gavrilova O., Ward J. M., Brewer B., Jr., Reitman M. L., Gonzalez F. J. (2003) J. Clin. Invest. 111, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feldstein A. E., Canbay A., Angulo P., Taniai M., Burgart L. J., Lindor K. D., Gores G. J. (2003) Gastroenterology 125, 437–443 [DOI] [PubMed] [Google Scholar]
- 32. Richardson M. M., Jonsson J. R., Powell E. E., Brunt E. M., Neuschwander-Tetri B. A., Bhathal P. S., Dixon J. B., Weltman M. D., Tilg H., Moschen A. R., Purdie D. M., Demetris A. J., Clouston A. D. (2007) Gastroenterology 133, 80–90 [DOI] [PubMed] [Google Scholar]
- 33. Poitout V., Robertson R. P. (2008) Endocr. Rev. 29, 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim J. K., Fillmore J. J., Sunshine M. J., Albrecht B., Higashimori T., Kim D. W., Liu Z. X., Soos T. J., Cline G. W., O'Brien W. R., Littman D. R., Shulman G. I. (2004) J. Clin. Invest. 114, 823–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim Y. B., Shulman G. I., Kahn B. B. (2002) J. Biol. Chem. 277, 32915–32922 [DOI] [PubMed] [Google Scholar]
- 36. Taube A., Eckardt K., Eckel J. (2009) Am. J. Physiol. Endocrinol. Metab. 297, E1004–E1012 [DOI] [PubMed] [Google Scholar]
- 37. Liu L., Zhang Y., Chen N., Shi X., Tsang B., Yu Y. H. (2007) J. Clin. Invest. 117, 1679–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bruce C. R., Hoy A. J., Turner N., Watt M. J., Allen T. L., Carpenter K., Cooney G. J., Febbraio M. A., Kraegen E. W. (2009) Diabetes 58, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]