Background: [FeFe]-Hydrogenases catalyze the reversible reduction of protons to H2.
Results: Substitution of strictly conserved amino acids in a putative proton transport pathway impairs hydrogenase activity.
Conclusion: The four strictly conserved residues studied in this work are critical for hydrogenase activity and are implicated in proton transfer.
Significance: Elucidating the method of intramolecular substrate transport in [FeFe]-hydrogenases is vital for understanding the enzymatic mechanism.
Keywords: Enzymatic Mechanisms, Iron-Sulfur Protein, Metalloenzymes, Metalloproteins, Proton Transport, Hydrogenase
Abstract
[FeFe]-Hydrogenases are complex metalloproteins that catalyze the reversible reduction of protons to molecular hydrogen utilizing a unique diiron subcluster bridged to a [4Fe4S] subcluster. Extensive studies have concentrated on the nature and catalytic activity of the active site, yet relatively little information is available concerning the mechanism of proton transport that is required for this activity. Previously, structural characterization of [FeFe]-hydrogenase from Clostridium pasteurianum indicated a potential proton transport pathway involving four residues (Cys-299, Glu-279, Ser-319, and Glu-282) that connect the active site to the enzyme surface. Here, we demonstrate that substitution of any of these residues resulted in a drastic reduction in hydrogenase activity relative to the native enzyme, supporting the importance of these residues in catalysis. Inhibition studies of native and amino acid-substituted enzymes revealed that Zn2+ specifically blocked proton transfer by binding to Glu-282, confirming the role of this residue in the identified pathway. In addition, all four of these residues are strictly conserved, suggesting that they may form a proton transport pathway that is common to all [FeFe]-hydrogenases.
Introduction
Hydrogenases are found in a diverse array of microorganisms, where they function mainly either to 1) reduce protons utilizing electrons accumulated during fermentation or 2) couple the oxidation of H2 to energy-yielding reactions (1). Hydrogenases are separated into three subclasses based on the composition of their active sites: [FeFe]-, [NiFe]-, and [Fe]-hydrogenases (2). [FeFe]-Hydrogenases demonstrate the highest in vitro catalytic turnover among hydrogen-producing enzymes, and there is great interest in elucidating the enzymatic mechanism of these hydrogenases (3, 4). Current research has focused primarily on examining the catalytic activity (5, 6), active site assembly (7–10), and irreversible oxygen inactivation of these enzymes (11–13), but relatively little data are available concerning the intramolecular transport of substrates between the active site and the enzyme surface (14–16). Proton transfer is an essential component of reversible hydrogen production by [FeFe]-hydrogenases, and biochemical investigation is required for a complete understanding of the enzymatic mechanism.
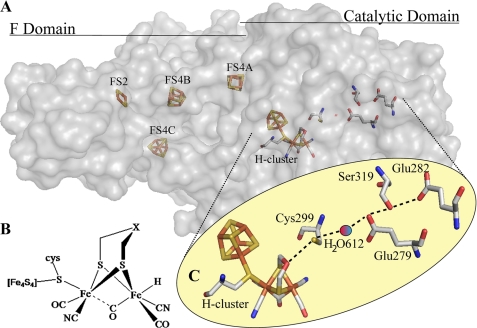
Two structures of [FeFe]-hydrogenases have been solved by crystallography (Clostridium pasteurianum (Fig. 1A) (17, 18) and Desulfovibrio desulfuricans (19)), and there is considerable structural homology near the active site. This active site (H-cluster) is best described as a [4Fe4S]-cubane cluster attached via a cysteinyl sulfur linkage to a diiron moiety. Each iron is coordinated by a terminal cyanide and a carbon monoxide ligand, and an additional CO bridges the two irons (17, 20). A five-atom dithiolate ligand bridges the diiron moiety (Fig. 1B). The exact identity of this ligand has not been determined through direct chemical or biochemical analysis, but this ligand has been proposed to be dithiomethylamine because of the close proximity of the amine group to the presumed H2 oxidation site and the ability of amines to cycle between protonation states at physiological pH (21). The assignment of an amine group at this position is consistent with the results of a recent ESEEM/HYSCORE spectroscopic study (22). Direct experimental results addressing whether an amine group within the dithiolate ligand could cycle between protonation states in the enzyme is still lacking, however.
FIGURE 1.
[FeFe]-hydrogenase structure and putative proton transfer pathway. A, crystal structure of the [FeFe]-hydrogenase from C. pasteurianum (Protein Data Bank code 3C8Y) resolved to 1.39 Å. The protein is a monomer separated into a catalytic domain containing the [6Fe6S] H-cluster and three N-terminal domains that in total contain three [4Fe4S] clusters and a [2Fe2S] cluster. B, chemical structure of the H-cluster emphasizing the diiron active site. X is generally postulated to be nitrogen, although oxygen and carbon remain possibilities. C, putative proton transfer relay inferred by multiple sequence alignment analysis and examination of the crystal structure of the [FeFe]-hydrogenase from C. pasteurianum. H-cluster → Cys-299 → H2O-612 → Glu-279 → Ser-319 → Glu-282. All distances are in angstroms and are measured between oxygen and sulfur atoms.
Intramolecular transfer of substrates is necessary for efficient catalysis at the active site, which is sequestered ∼20 Å within the interior of the enzyme. Accessory iron-sulfur clusters provide pathways to facilitate the transport of electrons between the protein surface and the H-cluster (23, 24). Rationalizing pathways for bidirectional proton transfer is less intuitive, however. In previous work, it has been observed that proton exchange within proteins can be facilitated via negatively charged side chains and water molecules (25–27). Understanding this key mechanism in [FeFe]-hydrogenases is critical for targeted protein engineering, as perturbing proton transfer can cause unintended deleterious effects on the catalytic rate (28). To date, detailed experiments investigating proton transport within [FeFe]-hydrogenases have not been reported.
In this work, we provide biochemical evidence that four residues (Cys-299, Glu-279, Ser-319, and Glu-282) in the [FeFe]-hydrogenase from C. pasteurianum are important for hydrogenase activity and are likely to be involved in proton transfer between the active site and the enzyme surface. The strict conservation of these residues among this class of hydrogenases suggests a ubiquitous proton transport pathway. In addition, we have observed that Zn2+-based inhibition of [FeFe]-hydrogenase specifically targets this proton pathway.
EXPERIMENTAL PROCEDURES
Multiple Sequence Alignment
Using the C. pasteurianum [FeFe]-hydrogenase as the base sequence, we performed PSI-BLAST (29) to identify an additional 62 unique amino acid sequences. These 63 [FeFe]-hydrogenase sequences (acquired from the National Center for Biotechnology Information (NCBI)) were examined for side chain conservation using ClustalX (30).
Plasmid Construction
The coding sequence of the [FeFe]-hydrogenase from C. pasteurianum was amplified by PCR and ligated into the NcoI/SacI sites of pAC-BAD (a modified pBAD/D-TOPO vector from Invitrogen lacking the N-terminal thioredoxin tag) containing a kanamycin resistance cassette and an l-arabinose-inducible promoter. Constructs were transformed into DH5α competent Escherichia coli cells and selected for resistance to 50 μg/ml kanamycin.
Site-directed Mutagenesis
Specific amino acid codons in the [FeFe]-hydrogenase gene from C. pasteurianum were mutated by site-directed mutagenesis PCR (PfuTurbo, Stratagene) using appropriately designed primers (supplemental Table 1) (31). Segregation of mutated DNA from wild-type DNA was achieved by DpnI digestion of methylated DNA. After isolation of mutated DNA from E. coli, the sequences were confirmed, and the constructs were transformed into Shewanella oneidensis MR-1 ΔhydA/ΔhyaB electrocompetent cells as described by Ozawa et al. (32) and selected for resistance to 50 μg/ml kanamycin.
Cell Growth and Induction
A 0.5-ml inoculum of overnight transformant S. oneidensis MR-1 ΔhydA/ΔhyaB culture was transferred to 50 ml of 50 μg/ml kanamycin-supplemented LB medium in a 250-ml Erlenmeyer flask and shaken at 200 rpm at 30 °C until reaching an A600 of 0.4 (Eppendorf BioPhotometer). The entire culture was transferred into 500 ml of 50 μg/ml kanamycin-supplemented LB medium in a 2-liter Erlenmeyer flask and shaken at 30 °C until reaching an A600 of 0.4. The culture was then transferred to a 2-liter round-bottom flask, supplemented with ammonium iron citrate to a final concentration of 100 μm, and sparged with argon to remove ambient O2. After 30 min, 0.5 ml of 1.3 m l-arabinose (final concentration of 1.3 mm) was added, and the culture was sparged for an additional 30 min. The flask was then sealed with a rubber septum and shaken at 200 rpm at 30 °C for an additional 16 h.
Protein Purification
All steps for hydrogenase purification were performed anaerobically. Cells were collected by spinning the culture in airtight 250-ml Nalgene tubes at 4000 rpm for 20 min. Inside an anaerobic Coy chamber, the cell pellet was resuspended in 7.0 ml of nickel-nitrilotriacetic acid (Ni-NTA)2 wash buffer (100 mm Tris-HCl (pH 8.0), 200 mm NaCl, and 5.0% glycerol) supplemented with 10 mm sodium dithionite. The resuspended cell pellet was transferred to 1.7-ml Eppendorf tubes in 1.0-ml aliquots and sonicated eight times for 4 s each at a power setting of 4 (Fischer Scientific Model 100 Sonic Dismembrator). The sonicated cell suspension was cleared of cell debris by centrifugation at 13,000 rpm in airtight 40-ml tubes. A column containing 1.0 ml of Ni-NTA-agarose resin (Qiagen) was first equilibrated with 20 ml of wash buffer supplemented with 10 mm sodium dithionite to remove residual O2, and the supernatant was then passed over the column. The column was washed with 15 ml of wash buffer supplemented with 20 mm imidazole. The protein was eluted from the column in 1.0-ml fractions by increasing the concentration of imidazole to 100 mm.
Hydrogenase Activity Assays
Hydrogen evolution and uptake assays were modified from those of King et al. (33). Briefly, H2 evolution was measured by incubating 0.1 ml of protein sample in 1.9 ml of H2 evolution assay buffer (50 mm HEPES (pH 7.0), 500 mm NaCl, 100 mm sodium dithionite, and 10 mm methyl viologen) in a 13-ml serum vial at 25 °C with continuous shaking. A 100-μl syringe was used to inject 50 μl of headspace gas into a TRACE GC Ultra gas chromatograph (Thermo Scientific), and H2 accumulation was measured over time by plotting the peak area against a standard curve. To measure hydrogen consumption, 0.1 ml of protein sample was incubated in 1.9 ml of H2 uptake assay buffer (50 mm Tris-HCl (pH 8.0), 10 mm benzyl viologen, and 0.2% Triton X-100) in a 13-ml serum vial at 25 °C with 2.5% hydrogen in the headspace and shaken continuously. Uptake of H2 was measured over time by injecting 50 μl of the headspace into the gas chromatograph and plotting the peak area against a standard curve.
Azide Rescue Assay
To determine the effect of sodium azide on hydrogenase activity, the H2 evolution activity of native and variant enzymes was measured (see Hydrogenase Activity Assays) in the presence of different amounts of sodium azide (0, 50, 100, and 250 mm).
Zn2+ Inhibition Assay
Zn2+ inhibition assays were performed by measuring H2 evolution activity (see Hydrogenase Activity Assays) in a 2.0-ml total volume in the presence of ZnCl2. Because ZnCl2 dissolves in the H2 evolution assay buffer only below pH 6.3, 0.02 ml of the buffer (pH 6.3), with or without dissolved ZnCl2, was added to each assay. The effect of Zn2+ on the native enzyme was assayed by measuring H2 evolution activity over several ZnCl2 concentrations (0, 0.05, 0.125, 0.25. and 0.50 mm) as the concentration of methyl viologen was varied (3.0, 5.0, 8.0, and 10.0 mm), and the data were graphed on both Dixon and Cornish-Bowden plots. The effect of ZnCl2 on the activity of amino acid-substituted [FeFe]-hydrogenases was assayed at either 0 or 0.50 mm ZnCl2.
Activation Energy Assays
H2 evolution and uptake assays were performed as noted previously over a range of temperatures (0, 8, 25, 30, 35, and 40 °C). The natural log of kobs was plotted against the reciprocal of the temperature. The resulting slope was multiplied by the negative value of the gas constant R (8.314 J K−1 mol−1) to calculate the activation energy (Ea).
RESULTS
Proton Pathway Identification
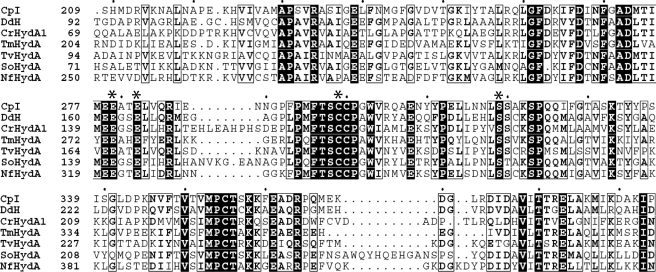
Based on their crystallographic analysis, a putative proton transfer relay composed of Cys-299, a modeled water, Glu-279, Ser-319, and Glu-282 that connect the enzyme surface to the H-cluster of the C. pasteurianum [FeFe]-hydrogenase was initially proposed by Peters et al. (17). Reasoning that residues involved in proton transport should be highly conserved, we performed multiple sequence alignment on 63 unique [FeFe]-hydrogenases (Fig. 2 and supplemental Fig. 1). Our results revealed that these four residues are strictly conserved among both algal and bacterial [FeFe]-hydrogenase genes. In comparison, these residues are not conserved among Nar1 proteins, a class of proteins that share a number of sequence motifs with [FeFe]-hydrogenases (8, 34). Together, these data strongly imply the importance of these four residues to hydrogenase function.
FIGURE 2.
Multiple sequence alignment. Shown is a partial multiple sequence alignment (ClustalX) of [FeFe]-hydrogenases from C. pasteurianum (Cp), D. desulfuricans (Dd), Chlamydomonas reinhardtii (Cr), Thermotoga maritima (Tm), Trichomonas vaginalis (Tv), S. oneidensis (So), and Neocallimastix frontalis (Nf). Amino acids predicted to participate in proton transfer are denoted by asterisks. Strictly conserved residues are indicated by black boxes, whereas highly conserved residues are in boldface. Residues with low conservation but chemically similar side chains are boxed. Every tenth amino acid of the C. pasteurianum sequence is denoted by a black dot. A multiple sequence alignment with 63 amino acid sequences can be found in supplemental Fig. 1.
Amino Acid-substituted Enzyme Studies
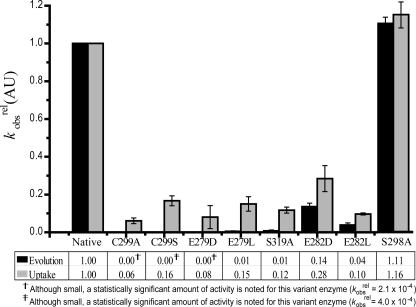
The coding sequence of the putative proton transport residues was mutated by site-directed mutagenesis PCR in the [FeFe]-hydrogenase gene from C. pasteurianum. N-terminally His-tagged wild-type and mutated genes were overexpressed anaerobically in a ΔhydA/ΔhyaB strain of S. oneidensis MR-1, and the resulting enzymes were purified by Ni-NTA affinity chromatography. The kcat of native C. pasteurianum hydrogenase H2 evolution activity was calculated to be ∼1000 s−1. Compared with the native enzyme, all amino acid-substituted (variant) enzymes demonstrated sharp decreases in hydrogenase activity (Fig. 3). Interestingly, in all variant enzymes, the hydrogen evolution activity was depressed to a greater extent than the hydrogen uptake activity.
FIGURE 3.
Relative reduction in hydrogenase activity of proton pathway variants. kobsrel (rate of the variant enzyme relative to that of the native enzyme) values for H2 evolution and uptake activities are shown. To determine the effect of substitution, the specific activity of the native enzyme was normalized to 1.0. Variant enzyme kobsrel values were calculated from individual experiments and averaged. Error bars denote S.D. (n = 3–8). AU, arbitrary units.
In close proximity to the H-cluster, Cys-299 is an ideal candidate for donating protons to the dithiolate ligand at the active site. Substitution of this residue with alanine or serine strongly decreased the kobsrel (specific activity of the variant enzyme relative to that of the native enzyme) for both evolution and uptake activities. Substitution with serine retained slightly more hydrogenase activity, which may be due to the fact that the serine side chain preserves a hydrogen-bonding hydroxyl group, whereas the alanine side chain is aliphatic and unable to participate in proton transfer.
The residues adjacent to Cys-299 in the putative pathway, Glu-279 and Ser-319, are also important for enzyme activity. When these residues were substituted, the H2 uptake activity of the resulting enzymes was reduced to ∼85% of the kobsrel, whereas the evolution activity was decreased by >99%.
At the enzyme surface, Glu-282 is solvent-exposed and ideally positioned to exchange protons between Ser-319 and bulk solvent (supplemental Fig. 2). The residual activity of Glu-282 variant enzymes was dependent on the specific substitution. A greater reduction in activity was observed in the E282L variant compared with the E282D variant, especially with regard to H2 uptake. These results are consistent with the structural differences of the side chains. Whereas aspartic acid and glutamic acid differ only in side chain length, leucine lacks a protonatable side chain.
To probe the apparent discrepancy noted between the percent reduction in H2 uptake versus evolution activities in the amino acid-substituted hydrogenases, we calculated the Ea for each direction for the native enzyme. Our results revealed activation energies of 24 ± 3 kJ/mol (n = 5) and 45 ± 2 kJ/mol (n = 3) for H2 evolution and uptake, respectively. The difference in the activation energies could partially explain the apparent discrepancy in the activities.
A second putative proton transfer pathway can be imagined from the C. pasteurianum hydrogenase crystal structure, starting at Cys-299, passing through several modeled water molecules and Ser-298, and ending at the non-conserved Lys-571 residue at the enzyme surface (supplemental Fig. 3). However, when we substituted Ser-298 with alanine, the resulting enzyme had activity similar to the native enzyme (Fig. 3), indicating that this residue is not critical for activity.
Chemical Rescue of Variant Enzyme Activity by Sodium Azide
To examine further the role of the residues identified as potentially involved in proton transfer, hydrogen evolution activity was stimulated by the addition of a chemical recovery agent. Sodium azide has been previously demonstrated to enhance specifically the in vitro proton transfer activity of variant enzymes deficient in proton transport (35–37). Importantly, the hydrogen evolution activity of the native enzyme was unaffected by the addition of sodium azide; the chemical neither significantly promoted nor repressed the hydrogenase activity. The activities of Cys-299 and Glu-279 variants were not stimulated by the addition of sodium azide, possibly as a result of the inability of azide to penetrate into the interior of the protein. In contrast, the hydrogen evolution activities of the S319A, E282D, and E282L variants were all stimulated as the concentration of sodium azide was increased (Table 1), providing additional evidence that Ser-319 and Glu-282 participate in proton transfer.
TABLE 1.
Measured properties of native and variant enzymes
The kobsrel values of evolution activities were calculated as described for Fig. 3. Zn2+-binding constants (Ki) were determined for native and variant enzymes at 0.50 mm ZnCl2. -Fold stimulation values are the ratio of enzyme activity with and without the addition of 250 mm sodium azide (n = 3–8).
| kobsrel (evolution) | Ki | Stimulation | |
|---|---|---|---|
| mm | -fold | ||
| Native | 1.00 | 171 ± 25 | 0.89 |
| C299S | 0.00a | 477 ± 36 | 0.28b |
| E279D | 0.00a | 236 ± 68 | 0.73b |
| E279L | 0.01 | 283 ± 99 | 0.30b |
| S319A | 0.01 | 107 ± 8 | 1.30b |
| E282D | 0.14 | 383 ± 10 | 2.37 |
| E282L | 0.04 | 3550 ± 98 | 3.57b |
a Although small, a statistically significant amount of activity was noted for this variant enzyme (kobsrel = 4.0 × 10−4).
b p < 0.1.
Zn2+-based Inhibition
Previous studies have revealed that certain divalent metal cations inhibit [FeFe]-hydrogenase, but the specific mechanism for this inhibition was not elucidated (38, 39). Importantly, divalent cations such as Zn2+ have been previously shown to inhibit intramolecular proton transfer in a variety of biomolecular reactions (27, 40). To determine the inhibitory effect of Zn2+ on C. pasteurianum [FeFe]-hydrogenase activity, we measured the H2 evolution activity over a range of ZnCl2 and methyl viologen concentrations. H2 evolution activity decreased as the Zn2+ concentration increased. Kinetic analyses from Dixon and Cornish-Bowden plots indicated non-competitive mixed inhibition (supplemental Fig. 4), with nearly equal values of Ki and Ki′ (0.171 and 0.170 mm, respectively). These results indicate that Zn2+ likely binds with similar affinity to the enzyme and substrate-bound enzyme and is not competing for binding with methyl viologen. The addition of 0.50 mm CaCl2 or NaCl had only a negligible effect on hydrogenase activity (data not shown), indicating that the inhibition is not due to chloride ions or a change in ionic strength. When the Zn2+ chelator EDTA was titrated into a reaction containing Zn2+, the enzyme activity was restored in proportion to the amount of chelator added. Together, these results clarify that Zn2+ does not occlude methyl viologen binding to the C. pasteurianum [FeFe]-hydrogenase and that Zn2+ may depress enzyme activity by inhibiting proton transport.
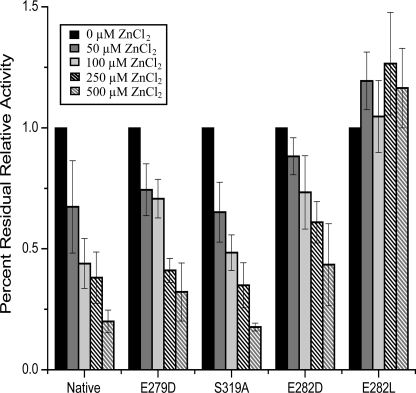
Recognizing that Zn2+ has high affinity for negatively charged residues, we hypothesized that Zn2+ might inhibit [FeFe]-hydrogenase activity through coordination to the surface-exposed Glu-282 residue, thereby blocking proton transfer. To examine more fully the effect of ZnCl2, native and variant enzymes were assayed for H2 evolution activity in both the presence and absence of 0.50 mm ZnCl2. The addition of 0.50 mm ZnCl2 to the assay mixture resulted in a 70–80% reduction in relative H2 evolution activity for native and most variant enzymes, whereas the activity of the E282D variant was depressed by only ∼40% (Fig. 4). The binding constants (Ki) of Zn2+ for the native and most variant enzymes ranged between 0.1 and 0.4 mm (Table 1), with the exception of E282L. This variant enzyme maintained the same level of activity independent of Zn2+ concentration, with a binding constant an order of magnitude greater than that observed in the native enzyme. These data indicate that the E282L substitution renders the enzyme insensitive to Zn2+, providing experimental evidence that Zn2+ inhibits [FeFe]-hydrogenases by binding to Glu-282.
FIGURE 4.
Inhibitory effects of ZnCl2 on activity of native and variant enzymes. Shown is the relative hydrogen evolution activity after the addition of 0.05, 0.10, 0.25, or 0.50 mm ZnCl2. Residual activity is relative to enzyme activity with 0 mm ZnCl2 (1.0). Error bars denote S.D. (n = 3–8).
DISCUSSION
The activity of [FeFe]-hydrogenase is dependent on the transfer of protons between the enzyme surface and the H-cluster. Active intramolecular proton transport is an essential function for a variety of enzymes, and this process occurs via a proton-hopping method (Grotthuss mechanism) between water molecules and the side chains of amino acid residues (26, 41, 42). Although hypothesized, this essential transfer activity has not been characterized experimentally for [FeFe]-hydrogenases.
The C. pasteurianum [FeFe]-hydrogenase is a soluble 63-kDa periplasmic protein. Four iron-sulfur clusters, termed F-clusters, are sequestered in the three N-terminal domains, whereas the H-cluster is buried within the catalytic domain (Fig. 1A). From analysis of the crystal structure, the cysteine residue at position 299 has been proposed to act as a proton donor/acceptor for the H-cluster (17, 43).
When the structure of the C. pasteurianum hydrogenase was first determined, a putative proton transfer pathway was envisioned involving residues within hydrogen-bonding distance that link the H-cluster to the enzyme surface (17). This pathway consists of Cys-299, a water molecule, Glu-279, Ser-319, and Glu-282 (Fig. 1C) (24). Importantly, we determined that these residues are strictly conserved, supporting the viability of this pathway (Fig. 2 and supplemental Fig. 1).
Recent theoretical work has examined this proton pathway via quantum mechanical and molecular mechanics calculations (44). As part of this study, direct proton transport between the active site dithiolate ligand and a nearby cysteine residue (analogous to Cys-299) in the D. desulfuricans [FeFe]-hydrogenase was calculated to be energetically favorable. Parallel simulations on the C. pasteurianum hydrogenase demonstrated low energy barriers for proton transfer from Glu-282 to Glu-279 and from Glu-279 to Cys-299 (44). This work therefore provides computational evidence for a putative proton transfer relay in C. pasteurianum [FeFe]-hydrogenase, and it establishes a basis for direct biochemical experimentation.
To investigate experimentally the role of Cys-299, Glu-279, Ser-319, and Glu-282 in the C. pasteurianum hydrogenase, we generated amino acid substitutions of these residues to limit hydrogen bonding and thus proton transfer. Substitution of these residues caused a dramatic reduction in both H2 uptake and evolution, indicating their importance for hydrogenase activity. Based on the crystal structure, these residues are unlikely to participate in electron transfer or significantly alter the redox potential of any of the N-terminal [FeS] clusters (Fig. 1A) (45). Substitution of a residue responsible for coordinating either the FS2 or FS4C [FeS] cluster (C34S and C98S, respectively) had only a moderate effect on hydrogenase activity when methyl viologen was the electron donor (data not shown). Additionally, the similarity between Ki values of the native and proton pathway variant enzymes indicates that the reduction in kobsrel was not due to structural changes. Finally, to examine the proton transfer activity of these residues, sodium azide was used as a chemical rescue agent. The addition of sodium azide stimulated the H2 evolution activity of the Glu-282 and Ser-319 variants, whereas the native enzyme was not enhanced by azide (Table 1). Based on this stimulation of variant enzyme activity by azide, the noted Zn2+ insensitivity of the E282L variant, and computational calculations (44), the most parsimonious explanation for the observed reduction in activity of the variants is that proton transfer was impaired.
Within hydrogen-bonding distance of the H-cluster dithiolate ligand, Cys-299 is an ideal candidate as the direct proton donor to the active site. Substitution of this residue demonstrated a dramatic reduction in hydrogenase activity and matched quantum mechanical and molecular mechanics predictions described previously (44). The C299S variant retained low level activity and could be further inhibited by the addition of Zn2+, with a Ki similar in value to that of the native enzyme. Together with its close proximity to the dithiolate ligand, these results indicate that Cys-299 could participate in proton transfer to the H-cluster.
In recent work examining H2 diffusion channels, Lautier et al. (16) measured the effect of substitution of the residue analogous to Cys-299 in the [FeFe]-hydrogenase from Clostridium acetobutylicum (C298A). The reported H2 oxidation kcat value of the C298A variant was 6-fold less than that of the native enzyme, indicating that, under their assay conditions, a significant decrease in activity could be noted, albeit to a lesser extent than noted in our experiments (∼20-fold decrease for H2 uptake). This difference in the magnitude of reduced activity for the Cys-299/Cys-298 variant enzyme might be explained by the very different reaction conditions used by Lautier et al. (16). Nevertheless, both studies clearly indicate the importance of this residue for hydrogenase activity.
The solvent-exposed Glu-282 residue is expected to act as a proton acceptor at the protein surface. Substitution of this residue with either leucine or aspartate caused a decrease in kobsrel. Interestingly, whereas the E282D variant was susceptible to Zn2+-based inhibition, the E282L variant was unaffected even at 0.50 mm ZnCl2. These results are consistent with previous work that established that divalent metal cations can specifically inhibit proton transfer via interactions with negatively charged residue side chains (27, 40). Our work suggests a mechanism for preferential binding of Zn2+ to the surface-exposed Glu-282 residue, presumably occluding proton binding and transfer (supplemental Fig. 2).
An apparent discrepancy was noted between the kobsrel(uptake) and kobsrel(evolution) of the variant enzymes, with H2 evolution activity more strongly impaired by substitution relative to uptake activity (Fig. 3). To investigate this apparent discrepancy, we measured the activities of native and variant enzymes (C299S, E279D, and E282D) with a variety of buffers, salt concentrations, and varying pH values. No set of tested conditions resulted in kobsrel(uptake) being equal to kobsrel(evolution). Next, we determined the Ea of the native enzyme for both evolution and uptake (24 ± 3 and 45 ± 2 kJ/mol, respectively). The fact that the two values are different is not surprising because the forward and reverse directions of a reaction will always have different Ea values unless the free energies of the products and reactants are identical. In this particular case, however, this difference is also impacted by the fact that the H2 uptake and evolution reactions were performed under distinct assay conditions (pH and redox partner).
The noted discrepancy (kobsrel of evolution versus uptake) is likely a result of this disparity between the calculated Ea values. The effect of mutating a residue important for hydrogenase activity is expected to increase the energy of the transition state by the same magnitude for both directions of the reaction. Because H2 uptake has a higher activation energy under our reaction conditions, however, the percent change in the Ea between native and variant enzymes will be less for H2 uptake. Thus, one would predict that mutations would have a smaller observed effect on the rate constant for H2 uptake, which is exactly what we observed experimentally. Ultimately, because of the different reaction conditions, these systems are not directly comparable, and the apparent discrepancies therefore do not imply different proton pathways for H2 evolution and uptake.
Hong et al. (44) identified a second potential proton transfer pathway, composed of Cys-299, Ser-298, five modeled water molecules, and Lys-571 (supplemental Fig. 3). However, the surface-exposed lysine is not well conserved among [FeFe]-hydrogenase genes. In addition, our results indicated that the activity of the S298A variant enzyme was not impaired under our reaction conditions relative to the native enzyme. Thus, this potential pathway does not appear to be essential for proton transfer activity, although its potential role as a substitute pathway under certain conditions cannot be dismissed.
The five crystallographically modeled water molecules described above are in close proximity to the putative proton transfer pathway examined in this study. Thus, it could be argued that the residues we have examined play only an indirect role in proton transfer via interactions with this water channel. Although theoretically possible, this scenario seems unlikely given the insensitivity of hydrogenase activity to amino acid substitutions of Ser-298 and the importance of Glu-282 to proton transport as determined by Zn2+ inhibition. Importantly, there does not appear to be any other chain of crystallographically observed water molecules in the vicinity of our putative proton channel.
In conclusion, we have demonstrated the importance of Cys-299, Glu-279, Ser-319, and Glu-282 for activity in the [FeFe]-hydrogenase from C. pasteurianum. Together with a crystallographically characterized water molecule, these residues form a potential hydrogen-bonding chain from the active site to the enzyme surface. Amino acid substitution of any of these residues caused a severe reduction in hydrogenase activity. In many cases, this activity could be partially restored by incubation with sodium azide, providing additional evidence that these residues (Ser-319 and Glu-282) are important for proton transfer. Substitution of the solvent-exposed Glu-282 with leucine rendered the enzyme insensitive to ZnCl2, indicating that Zn2+ inhibits enzyme activity by binding to this residue and occluding the proton transfer pathway. Because Cys-299, Glu-279, Ser-319, and Glu-282 are strictly conserved, these residues may constitute a key proton transport relay in all [FeFe]-hydrogenases.
Supplementary Material
Acknowledgments
We greatly appreciate the donation of the ΔhydA/ΔhyaB strain of S. oneidensis MR-1 by Drs. Jim Fredrickson and Matthew Marshall (Pacific Northwest National Laboratory) (46). We also thank Dr. Ruth Pachter (Air Force Research Laboratory) for insight and Dr. Charles Hoogstraten (Michigan State University) for helpful discussions on enzyme kinetics.
This work was supported by Air Force Office of Scientific Research Grant FA9550-05-1-0365 and by the United States Department of Energy Great Lakes Bioenergy Research Center (Biological and Environmental Research Office of Science Grant DE-FC02-07ER64494).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4 and Table 1.
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1. Ghirardi M. L., Posewitz M. C., Maness P. C., Dubini A., Yu J., Seibert M. (2007) Annu. Rev. Plant Biol. 58, 71–91 [DOI] [PubMed] [Google Scholar]
- 2. Vignais P. M., Billoud B., Meyer J. (2001) FEMS Microbiol. Rev. 25, 455–501 [DOI] [PubMed] [Google Scholar]
- 3. Frey M. (2002) ChemBioChem 3, 153–160 [DOI] [PubMed] [Google Scholar]
- 4. Adams M. W. (1990) Biochim. Biophys. Acta 1020, 115–145 [DOI] [PubMed] [Google Scholar]
- 5. Wright J. A., Pickett C. J. (2009) Chem. Commun. 5719–5721 [DOI] [PubMed] [Google Scholar]
- 6. Vannucci A. K., Wang S., Nichol G. S., Lichtenberger D. L., Evans D. H., Glass R. S. (2010) Dalton Trans. 39, 3050–3056 [DOI] [PubMed] [Google Scholar]
- 7. Shepard E. M., Duffus B. R., George S. J., McGlynn S. E., Challand M. R., Swanson K. D., Roach P. L., Cramer S. P., Peters J. W., Broderick J. B. (2010) J. Am. Chem. Soc. 132, 9247–9249 [DOI] [PubMed] [Google Scholar]
- 8. Mulder D. W., Boyd E. S., Sarma R., Lange R. K., Endrizzi J. A., Broderick J. B., Peters J. W. (2010) Nature 465, 248–251 [DOI] [PubMed] [Google Scholar]
- 9. Shepard E. M., McGlynn S. E., Bueling A. L., Grady-Smith C. S., George S. J., Winslow M. A., Cramer S. P., Peters J. W., Broderick J. B. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 10448–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Czech I., Stripp S., Sanganas O., Leidel N., Happe T., Haumann M. (2011) FEBS Lett. 585, 225–230 [DOI] [PubMed] [Google Scholar]
- 11. Stripp S. T., Goldet G., Brandmayr C., Sanganas O., Vincent K. A., Haumann M., Armstrong F. A., Happe T. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 17331–17336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liebgott P. P., Leroux F., Burlat B., Dementin S., Baffert C., Lautier T., Fourmond V., Ceccaldi P., Cavazza C., Meynial-Salles I., Soucaille P., Fontecilla-Camps J. C., Guigliarelli B., Bertrand P., Rousset M., Léger C. (2010) Nat. Chem. Biol. 6, 63–70 [DOI] [PubMed] [Google Scholar]
- 13. Goldet G., Brandmayr C., Stripp S. T., Happe T., Cavazza C., Fontecilla-Camps J. C., Armstrong F. A. (2009) J. Am. Chem. Soc. 131, 14979–14989 [DOI] [PubMed] [Google Scholar]
- 14. Fontecilla-Camps J. C., Amara P., Cavazza C., Nicolet Y., Volbeda A. (2009) Nature 460, 814–822 [DOI] [PubMed] [Google Scholar]
- 15. English C. M., Eckert C., Brown K., Seibert M., King P. W. (2009) Dalton Trans. 45, 9970–9978 [DOI] [PubMed] [Google Scholar]
- 16. Lautier T., Ezanno P., Baffert C., Fourmond V., Cournac L., Fontecilla-Camps J. C., Soucaille P., Bertrand P., Meynial-Salles I., Léger C. (2011) Faraday Discuss. 148, 385–407 [DOI] [PubMed] [Google Scholar]
- 17. Peters J. W., Lanzilotta W. N., Lemon B. J., Seefeldt L. C. (1998) Science 282, 1853–1858 [DOI] [PubMed] [Google Scholar]
- 18. Pandey A. S., Harris T. V., Giles L. J., Peters J. W., Szilagyi R. K. (2008) J. Am. Chem. Soc. 130, 4533–4540 [DOI] [PubMed] [Google Scholar]
- 19. Nicolet Y., Piras C., Legrand P., Hatchikian C. E., Fontecilla-Camps J. C. (1999) Structure 7, 13–23 [DOI] [PubMed] [Google Scholar]
- 20. Bethel R. D., Singleton M. L., Darensbourg M. Y. (2010) Angew. Chem. Int. Ed. Engl. 49, 8567–8569 [DOI] [PubMed] [Google Scholar]
- 21. Nicolet Y., de Lacey A. L., Vernède X., Fernandez V. M., Hatchikian E. C., Fontecilla-Camps J. C. (2001) J. Am. Chem. Soc. 123, 1596–1601 [DOI] [PubMed] [Google Scholar]
- 22. Silakov A., Wenk B., Reijerse E., Lubitz W. (2009) Phys. Chem. Chem. Phys. 11, 6592–6599 [DOI] [PubMed] [Google Scholar]
- 23. Florin L., Tsokoglou A., Happe T. (2001) J. Biol. Chem. 276, 6125–6132 [DOI] [PubMed] [Google Scholar]
- 24. Peters J. W. (1999) Curr. Opin. Struct. Biol. 9, 670–676 [DOI] [PubMed] [Google Scholar]
- 25. Schultz B. E., Chan S. I. (2001) Annu. Rev. Biophys. Biomol. Struct. 30, 23–65 [DOI] [PubMed] [Google Scholar]
- 26. Cukierman S. (2006) Biochim. Biophys. Acta 1757, 876–885 [DOI] [PubMed] [Google Scholar]
- 27. Whitehead S. J., Iwaki M., Cotton N. P., Rich P. R., Jackson J. B. (2009) Biochim. Biophys. Acta 1787, 1276–1288 [DOI] [PubMed] [Google Scholar]
- 28. Dementin S., Burlat B., De Lacey A. L., Pardo A., Adryanczyk-Perrier G., Guigliarelli B., Fernandez V. M., Rousset M. (2004) J. Biol. Chem. 279, 10508–10513 [DOI] [PubMed] [Google Scholar]
- 29. Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]
- 31. Zheng L., Baumann U., Reymond J. L. (2004) Nucleic Acids Res. 32, e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozawa K., Yasukawa F., Fujiwara Y., Akutsu H. (2001) Biosci. Biotechnol. Biochem. 65, 185–189 [DOI] [PubMed] [Google Scholar]
- 33. King P. W., Posewitz M. C., Ghirardi M. L., Seibert M. (2006) J. Bacteriol. 188, 2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fujii M., Adachi N., Shikatani K., Ayusawa D. (2009) Genes Cells 14, 457–468 [DOI] [PubMed] [Google Scholar]
- 35. Takahashi E., Wraight C. A. (2006) J. Biol. Chem. 281, 4413–4422 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi E., Wraight C. A. (1991) FEBS Lett. 283, 140–144 [DOI] [PubMed] [Google Scholar]
- 37. Chang Y. H., Chuang L. Y., Hwang C. C. (2007) J. Biol. Chem. 282, 34306–34314 [DOI] [PubMed] [Google Scholar]
- 38. Fernandez V. M., Rua M. L., Reyes P., Cammack R., Hatchikian E. C. (1989) Eur. J. Biochem. 185, 449–454 [DOI] [PubMed] [Google Scholar]
- 39. Kamachi T., Uno S., Hiraishi T., Okura I. (1995) J. Mol. Catal. A Chemo. 96, 329–333 [Google Scholar]
- 40. Faxén K., Salomonsson L., Adelroth P., Brzezinski P. (2006) Biochim. Biophys. Acta 1757, 388–394 [DOI] [PubMed] [Google Scholar]
- 41. Nagle J. F., Morowitz H. J. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 298–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu J., Voth G. A. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 6795–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nicolet Y., Lemon B. J., Fontecilla-Camps J. C., Peters J. W. (2000) Trends Biochem. Sci. 25, 138–143 [DOI] [PubMed] [Google Scholar]
- 44. Hong G., Cornish A. J., Hegg E. L., Pachter R. (2011) Biochim. Biophys. Acta 1807, 510–517 [DOI] [PubMed] [Google Scholar]
- 45. Moulis J. M., Davasse V. (1995) Biochemistry 34, 16781–16788 [DOI] [PubMed] [Google Scholar]
- 46. Marshall M. J., Plymale A. E., Kennedy D. W., Shi L., Wang Z., Reed S. B., Dohnalkova A. C., Simonson C. J., Liu C., Saffarini D. A., Romine M. F., Zachara J. M., Beliaev A. S., Fredrickson J. K. (2008) Environ. Microbiol. 10, 125–136 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.