Background: Cell surface-expressed glycans play a role in leukocyte trafficking and recruitment.
Results: Deficiency of MGAT5 causes attenuation of allergen-induced eosinophilia and Th2 cytokines but increases neutrophilic inflammation and airway hyperreactivity.
Conclusion: Recruitment of eosinophils and neutrophils is differentially regulated by MGAT5-modified N-glycans during airway inflammation.
Significance: This study demonstrates a significant role for N-glycans in the development of allergic airway inflammation and asthma.
Keywords: Allergy, Carbohydrate Processing, Eosinophils, Neutrophil, Trafficking, Allergic Airway Inflammation
Abstract
Allergic airway inflammation, including asthma, is usually characterized by the predominant recruitment of eosinophils. However, neutrophilia is also prominent during severe exacerbations. Cell surface-expressed glycans play a role in leukocyte trafficking and recruitment during inflammation. Here, the involvement of UDP-N-acetylglucosamine:α-6-d-mannoside β1,6-N-acetylglucosaminyltransferase V (MGAT5)-modified N-glycans in eosinophil and neutrophil recruitment during allergic airway inflammation was investigated. Allergen-challenged Mgat5-deficient (Mgat5−/−) mice exhibited significantly attenuated airway eosinophilia and inflammation (decreased Th2 cytokines, mucus production) compared with WT counterparts, attributable to decreased rolling, adhesion, and survival of Mgat5−/− eosinophils. Interestingly, allergen-challenged Mgat5−/− mice developed airway neutrophilia and increased airway reactivity with persistent elevated levels of proinflammatory cytokines (IL-17A, TNFα, IFNγ)). This increased neutrophil recruitment was also observed in LPS- and thioglycollate (TG)-induced inflammation in Mgat5−/− mice. Furthermore, there was significantly increased recruitment of infused Mgat5−/− neutrophils compared with WT neutrophils in the peritoneal cavity of TG-exposed WT mice. Mgat5−/− neutrophils demonstrated enhanced adhesion to P-selectin as well as increased migration toward keratinocyte-derived chemokine compared with WT neutrophils in vitro along with increased calcium mobilization upon activation and expression of elevated levels of CXCR2, which may contribute to the increased neutrophil recruitment. These data indicate an important role for MGAT5-modified N-glycans in differential regulation of eosinophil and neutrophil recruitment during allergic airway inflammation.
Introduction
Allergic airway inflammation, including asthma, is characterized by the influx of inflammatory leukocytes to the airways (1). Although eosinophils are the predominant cells recruited, there is increasing evidence for the significant involvement of neutrophils in asthma (2, 3). Several studies have indicated that cell surface-expressed glycans play an important role in trafficking, migration and recruitment of leukocytes during inflammation by virtue of their ability to bind to selectins (4–6). A classic example is the role played by N-glycans in L-selectin-mediated lymphocyte homing and recruitment (7). However, the role played by N-glycans in eosinophil and neutrophil recruitment, particularly in the context of allergic airway inflammation, is not known.
In addition to L-selectin and α4 integrins (8), a significant role has been demonstrated for galectin-3 in mediating eosinophil rolling and adhesion by binding to α4 integrins (9) and in promoting allergic airway inflammation, including airway remodeling, in vivo (10). Galectins bind to N-glycan residues of cell surface glycoproteins in a manner proportional to the number of N-glycan residues and the degree of N-acetylglucosamine branching. This results in a molecular lattice that controls the concentration of surface glycoproteins to effect signaling, cell growth, differentiation, and disease states (11, 12). The direct relevance of dysregulated N-glycosylation to human disease was recently confirmed in the inflammatory demyelinating disease multiple sclerosis (13). UDP-N-acetylglucosamine:α-6-d-mannoside β1,6 N-acetylglucosaminyltransferase V (MGAT5)2 modifies N-glycans by transferring N-acetyllactosamine to the OH on carbon 6 of the α1,6-linked mannose, which are then preferentially extended with poly-N-acetyllactosamine units, resulting in high affinity ligands for galectins, particularly galectin-3 (14).
Previous studies have demonstrated that MGAT5-modified N-glycans regulate T cell inflammatory responses (15, 16) and promote Th2 over Th1 differentiation (17). Mice deficient for Mgat5 (Mgat5−/−) display more severe delayed type hypersensitivity reactions, a greater tendency for autoimmune kidney disease, and susceptibility to experimental autoimmune encephalomyelitis (16–18). Because MGAT5-modified N-glycans have the capacity to regulate Th1/Th2 polarization as well as function as ligands for galectin-3 (14), which promotes eosinophil trafficking and recruitment (9, 10), MGAT5 activity may well play a role in the regulation of allergic inflammation including asthma. Using Mgat5−/− mice, we examined in detail the contribution of MGAT5-modified N-glycans to trafficking and recruitment of eosinophils and neutrophils during the development of allergic airway inflammation.
EXPERIMENTAL PROCEDURES
Mouse Model of Allergic Airway Inflammation
Mgat5−/− mice on a C57BL/6 background were obtained from the Consortium for Functional Glycomics and bred in-house. Offspring were genotyped by PCR using genomic DNA isolated from tail clippings. Age- and gender-matched Mgat5−/− and WT C57BL/6 mice were sensitized with 20 μg of ovalbumin (OVA, Grade V, Sigma) in 2 mg of aluminum hydroxide gel intraperitoneally. Control mice received aluminum hydroxide gel alone. Sixteen days later, control and OVA-sensitized mice were exposed to aerosolized saline alone or OVA in saline (20 mg/ml), respectively, for 5 days via a nebulizer. Mice were sacrificed 18–24 h after the last challenge. All animal studies were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Measurement of Airway Hyperresponsiveness (AHR)
Eighteen to twenty-four hours after the last aerosol challenge, AHR was measured in allergen-challenged and control mice using a single chamber, whole-body plethysmograph (Buxco, Wilmington, NC) as described previously (19). The enhanced pause in breathing after each nebulization of methacholine (MCh, from 3–25 mg/ml in saline, Sigma) was monitored, and results were expressed as a percentage of baseline enhanced pause values following exposure to saline.
Bronchoalveolar Fluid (BALF) Analysis
Total cells and differential cell counts based on morphologic and histologic criteria after staining with Hema 3 System (Thermo Fisher Scientific Co., Pittsburgh, PA) were determined. Phaseolus vulgaris leucoagglutinin (PHA)-L binding by gated eosinophils in the BALF after 30 min was analyzed using FITC-PHA-L (1 μg/ml, Vector Laboratories, Burlingame, CA) or FITC-BSA (as control) with a FACScan flow cytometer (BD Biosciences) and FlowJo flow cytometry analysis software (Tree Star, Ashland, OR) for data acquisition.
Lung Immunohistology
Paraformaldehyde-fixed, paraffin-embedded lung tissue sections (4 μm) were used for most analyses. VECTASTAIN ABC kits containing appropriate biotinylated secondary antibodies and avidin-biotin HRP complex (Vector Laboratories) and the Peroxidase AEC substrate kit (Vector Laboratories) were used for detection. Stained slides were evaluated using a Nikon Microphot EPI-FL microscope (Nikon Instruments, Inc., Melville, NY). Images were captured with an Olympus DP71 camera and quantitated by image analysis with ImageJ software (20). Sections were stained with H&E (Thermo Fisher Scientific) to determine cellular infiltration. Infiltrated eosinophils were assessed after staining with rat mAb against murine major basic protein (MBP) (21). Expression and quantitation of FIZZ1 (found in inflammatory zone 1) and α-smooth muscle actin was performed as described previously (10). Intelectin-1 expression was evaluated using sheep anti mouse intelectin-1 (0.8 μg/ml, R&D Systems, Minneapolis, MN), and results were expressed as μm2 intelectin-1 positive area/100 μm basement membrane length. Airway mucus secretion was assessed after periodic acid-Schiff's (PAS) staining as described (10). Neutrophils within blood vessels were assessed in cryopreserved lung sections using NIMP-R14 rat mAbs specific for murine neutrophils (Abcam, Cambridge, MA), whereas peribronchial neutrophils were detected in paraffin-embedded lung sections by immunofluorescence staining after antigen retrieval with proteinase K using FITC-conjugated goat anti-rat IgG (7.5 μg/ml, Jackson ImmunoResearch Laboratories, West Grove, PA) as the secondary antibody. DAPI was used to visualize the cells. Slides were observed at ambient temperature using a FluoView FV1000/BX61 confocal laser scanning biological microscope equipped with an UPlanSApo lens (20×/numerical aperture 0.85 (oil)) and a PlanApo N lens (60×/numerical aperture 1.42 (oil)). FV10-ASW 2.0 software was used for image acquisition (Olympus, Melville, NY).
Gr-1 Levels in Lung Tissue by Western Blot
Lung tissue lysates (50 μg protein) were evaluated by Western blots using anti-mouse Ly-6G (alternate name Gr-1, 1:500, eBioscience, San Diego, CA) followed by HRP-conjugated goat anti-rat IgG (Cell Signaling Technology, Danvers, MA) as the secondary antibody. HRP-conjugated anti-mouse β-actin (Santa Cruz Biotechnology) was used to monitor levels of β-actin expression as an internal control. Protein bands detected with ECLTM Western blotting Detection Reagents (Millipore, Bedford, MA) were visualized on x-ray films, which were scanned and analyzed using ImageJ software to quantitate integrated density (pixels) of the bands. Density of Gr-1 bands in lung tissue lysates were normalized against β-actin.
Measurement of Lung Cytokines and Chemokines
Cytokine and chemokine levels in lung lysate supernatants were measured using Flex Set kits (BD Biosciences) for Th1 (IL-2, IFN-γ)/Th2 (IL-4, IL-5) cytokines (catalog no. 551287), IL-13 (catalog no. 558349), IL-17A (catalog no. 560283), and keratinocyte-derived chemokine (KC; catalog no. 558340) according to the manufacturer using the same amount of protein for each sample with a FACScan flow cytometer and FCAP ArrayTM Software (BD Biosciences). KC levels were expressed as pg/ml lung lysate, whereas levels of all other cytokines were expressed as pg cytokine/mg protein.
Induction of Pulmonary and Peritoneal Neutrophilic Inflammation
Peripheral blood was first collected from the facial vein of Mgat5−/− and WT mice to determine baseline differential cell counts. For pulmonary inflammation, mice were exposed to a single dose (10 μg in 50 μl of saline) of LPS (Sigma) administered intranasally (4). Three hours after LPS exposure, BALF and blood (by cardiac puncture) were collected, and post-challenge total and differential cells counts were determined. For peritoneal inflammation, mice were injected intraperitoneal with 1 ml of 3% thioglycollate (TG) (22), and the peritoneal cavity was lavaged with 5 ml of sterile PBS after 6 h to determine total and differential cell counts.
Mouse Eosinophils and Neutrophils
Mouse eosinophils were obtained from cultured BM of naïve Mgat5−/− and WT mice as described previously (23). Cells between day 11–13 of culture that were 99% Hema 3-positive (Fisher Scientific) and expressed both MBP (by confocal microscopy) and Siglec-F (by flow cytometry using phycoerythrin-conjugated rat anti-mouse Siglec-F at 5 μg/ml, BD Biosciences) were used in studies. Mature neutrophils were isolated from BM of naïve WT and Mgat5−/− mice by discontinuous Percoll gradient (82%/65%/55%) centrifugation. The 82%/65% interface of the gradient containing mature neutrophils was collected and demonstrated to be positive for Gr-1, a marker for murine neutrophils (24), using FITC-conjugated rat anti mouse Gr-1 (5 μg/ml, BD Biosciences) by flow cytometry. More than 85% of cells isolated were neutrophils as assessed by Hema 3 staining and cell morphology. Viability by trypan blue exclusion was >95%. PHA-L binding by eosinophils and neutrophils was assessed by flow cytometry as described in an earlier section.
Flow Chamber Studies
Rolling and adhesion of murine eosinophils and neutrophils from WT and Mgat5−/− mice on endothelial adhesion molecules under conditions of flow was evaluated in an in vitro parallel plate flow chamber as described in our previous studies (9). Coverslips treated with PBS were used to assess background rolling and adhesion. Interaction of eosinophils (1 × 106) with recombinant murine galectin-3 (10) and recombinant murine VCAM-1 (10) and of neutrophils (1 × 106) with recombinant murine P-selectin (R&D Systems) at a flow rate of 1 ml/min (wall shear stress, 1.0–2.0 dynes/cm2) was evaluated and analyzed as described previously (9). The number of adherent neutrophils is expressed as a fold increase in adhesion compared with background (adhesion to PBS-treated).
Static Adhesion Studies
WT and Mgat5−/− neutrophils were allowed to adhere to P-selectin-coated cover-slips for 5 min, washed, fixed, mounted in mounting medium with DAPI, and evaluated by confocal microscopy. The number of cells adhered in six fields were enumerated per coverslip for each cell type.
Expression of Adhesion Molecules
Expression of PSGL-1 by WT and Mgat5−/− neutrophils (4 × 105 cells) was assessed by flow cytometry with phycoerythrin-conjugated anti mouse PSGL-1 (5 μg/ml, BD Biosciences) after initially fixing the cells (4% paraformaldehyde) using FlowJo software for data analysis. Expression of α4 (CD49d), LFA-1 (CD11a), Mac-1 (CD11b), Siglec-F, and L-selectin (CD62L) by eosinophils was assessed using rat mAbs against murine α4 (PS/2) (25), CD11a (eBiosciences), CD11b (eBiosciences), Siglec-F (BD Biosciences), and CD62L (BD Biosciences). Depending on the mAb, rat IgG1, -2a, or -2b was used as the isotype-matched control.
Chemotaxis Assay and Expression of Chemokine Receptors
Eosinophils or neutrophils obtained from BM of WT and Mgat5−/− mice were suspended in DMEM and added to the upper wells (5 × 104/well in 50 μl) of 96-well Transwell® Chambers. Murine eotaxin (CCL11, 100 nm from PeproTech for eosinophil chemotaxis), murine KC (CXCL1, 100 nm from PeproTech for neutrophil chemotaxis), or medium alone was added to the lower wells, and the plates were incubated at 37 °C for 3 h. The number of cells that migrated in each case was evaluated under an Olympus CK2 inverted microscope using a 40× objective, and cells in three-seven different fields of each well were counted. The assay was performed two to three times in duplicate or triplicate, and results are expressed as the average number of cells/field. Expression of CXCR2 (CD182), the receptor for KC, in the absence and presence of KC (100 ng/ml for 5 min at 37 °C) was assessed on neutrophils from WT and Mgat5−/− mice by flow cytometry using PerCP/Cy5.5 anti-mouse CD182 (BioLegend, San Diego, CA) at 5 μg/ml with PerCP/Cy5.5-conjugated rat IgG2a as the isotype-matched control. Cells were fixed with 4% paraformaldehyde after KC treatment prior to incubation with anti-mouse CD182. Expression of CCR3 (CD193), the receptor for eotaxin-1, on WT and Mgat5−/− eosinophils was assessed using FITC-conjugated anti-mouse CCR3 (R&D Systems) with FITC-conjugated rat IgG2a as the isotype control.
In Vivo Migration Studies
To evaluate migration/recruitment of WT versus Mgat5−/− neutrophils or eosinophils in vivo, the TG-induced peritoneal inflammation model described earlier was used. BM collected from the femur and tibia of WT and Magt5−/− mice was exposed to hypotonic shock to remove red blood cells and used as a source of neutrophils. Carboxyfluorescein succinimidyl ester (Invitrogen) and CellTracker Orange (CMRA, Invitrogen) were used at 5 μm to label WT and Mgat5−/− cells, respectively, or vice versa for 15 min at room temperature. After washing, an equal number of labeled WT and Mgat5−/− neutrophils (2.3 × 107) were infused simultaneously via tail vein injection into WT recipient mice immediately after they were administered TG. Peritoneal fluid was collected from the TG-exposed mice after 6 h, and total and differential cell counts were determined. Cells in the peritoneal fluid were analyzed by flow cytometry after gating for neutrophils by staining with allophycocyanin-conjugated mAbs against mouse Gr-1 (BD Biosciences). To evaluate eosinophil recruitment, WT and Mgat5−/− eosinophils from BM cultures were labeled as described above for neutrophils and infused (1 × 107 cells) 13 h after administration of TG. Peritoneal fluid was collected after 2 h, i.e. 15 h after TG, based on previous studies that indicate that WT mice develop transient eosinophilic infiltration in the peritoneal cavity 12 to 24 h after TG injection (26). Cells were analyzed by flow cytometry after gating for eosinophils with mAbs against Siglec-F followed by allophycocyanin-conjugated anti-rat antibodies (Jackson ImmunoResearch Laboratories). The number of carboxyfluorescein succinimidyl ester- versus CMRA-labeled neutrophils or eosinophils in the peritoneal fluid in each case was determined.
Measurement of Intracellular Calcium [Ca2+]i Concentration
[Ca2+]i in BM-derived eosinophils and neutrophils was determined using the permeant Ca2+ indicator dye Fura-2 AM (Molecular Probes, Invitrogen) as described previously for airway smooth muscle cells (27) but with minor modifications. Briefly, eosinophils or neutrophils attached to poly-l-lysine-coated (10 μg/ml) glass coverslips were incubated with 5 μm Fura-2 AM for 30 min at 37 °C and 5% CO2. Coverslips were washed gently, perfused with HBSS containing 10 mm HEPES, 11 mm glucose, 2.5 mm CaCl2, and 1.2 mm MgCl2 (pH 7.4), and mounted on the stage of a Nikon Diaphot inverted microscope (Nikon Instruments, Inc.) in an open slide chamber. Fura-2 AM-loaded cells were alternately excited at 340 and 380 nm with a Lambda DG-4 high speed wavelength switcher (Sutter Instrument Co., Novato, CA). Basal [Ca2+]i was measured by real-time digital video fluorescence imaging using NIS-Elements imaging software (Nikon Instruments, Inc.). After 1 min, neutrophils were stimulated with 100 nm KC (Peprotech) and evaluated for an additional 2 min to assess agonist-induced [Ca2+]i responses.
Assessment of Neutrophil and Eosinophil Survival
Neutrophils obtained from the peritoneal cavity of TG-treated Mgat5−/− and WT mice as well as eosinophils cultured from BM of Mgat5−/− and WT mice were cultured in medium containing reduced serum (5%, to exclude effects of any growth factors) in 24-well plates (1 × 106 cells/ml/well) previously coated with poly-2-hydroxyethyl methacrylate (Sigma) to prevent cells from adhering as described previously (22). At various time points ranging from 2–24 h after culture, cells were assayed for apoptosis by flow cytometry based on FITC-annexin V binding by gated neutrophils or eosinophils using apoptosis detection kits according to the manufacturer's instructions (eBiosciences). Results are expressed as percent annexin V-positive cells.
Statistical Analysis
Results are expressed as mean ± S.E. Statistical significance was determined by one-tailed or two-tailed unpaired Student's t test. A p value <0.05 was considered as significant.
RESULTS
Allergen-challenged Mgat5−/− Mice Exhibit Reduced Eosinophilic Airway Inflammation
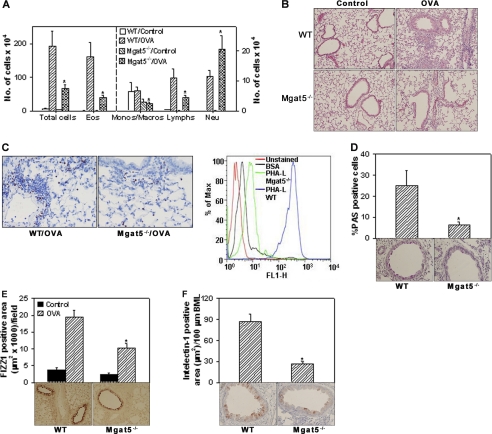
To examine the role of MGAT5-modified N-glycans in allergic inflammation, BALF and lung tissue from control and allergen-challenged Mgat5−/−and WT mice were evaluated for cellular infiltration. Significantly fewer cells were present in the BALF (Fig. 1A, left panel) and lung tissue (Fig. 1B) of allergen-challenged Mgat5−/− mice compared with WT counterparts. BALF differential cell counts indicated a significant reduction in the number of eosinophils (p < 0.01), monocytes/macrophages and lymphocytes (p < 0.05). Assessment of lung tissue eosinophils by immunohistology also revealed a decreased number of MBP-positive cells in allergen-challenged Mgat5−/− mice than in WT mice (Fig. 1C, left), with few to no MBP-positive cells in control mice of both groups (data not shown). BALF eosinophils from allergen-challenged WT mice exhibited strong binding to PHA-L compared with control (BSA), thus confirming surface expression of branched N-glycans, whereas residual eosinophils in BALF of allergen-challenged Mgat5−/− mice demonstrated only marginal binding to PHA-L relative to control (Fig. 1C, right). Evaluation of lung eotaxin-1 expression by immunohistology indicated no difference between allergen-challenged WT and Mgat5−/− mice (supplemental Fig. 1), suggesting that the reduced eosinophil recruitment observed in allergen-challenged Mgat5−/− mice is most likely regulated by factors other than eotaxin-1 levels.
FIGURE 1.
Allergen-induced airway inflammation is reduced in Mgat5−/− mice. A, BALF total and differential cell counts in control and allergen-challenged WT and Mgat5−/− mice (10–14 mice per group) by microscopic evaluation of Hema 3-stained cytocentrifuged slides. Combined data from experiments repeated at least three times is shown. Eos, eosinophils; Monos/Macros, monocytes/macrophages; Lymphs, lymphocytes; Neu, neutrophils. B, cellular infiltration of lung sections after H&E staining. Representative images (magnification, ×100) are shown (n = 5 mice per group). C, infiltration of lung tissue by eosinophils after staining with rat mAb against murine MBP. Representative images (magnification, ×200) are shown for allergen-challenged groups (left). Very few to no MBP-positive cells were detected in controls (n = 4 mice per group). Representative histogram of PHA-L binding by gated BALF eosinophils from allergen-challenged WT and Mgat5−/− mice by flow cytometry (right). WT and Mgat5−/− BALF eosinophils exhibited similar binding to BSA. Only WT BALF eosinophil binding to BSA is shown (n = 6 mice per group). D, mucus production in airways after PAS staining. PAS-positive cells in airways of relatively similar size were quantitated (4–20 airways/slide) by light microscopy and expressed as a percentage of the total number of epithelial cells per airway. FIZZ1 (E) and intelectin-1 (F) expression in lung sections by immunohistology. FIZZ1- and intelectin-1-positive area was quantitated by ImageJ analysis of captured images of 5–10 fields per slide (n = 4–5 mice per group in D–F). Control mice in each group showed little or no PAS and intelectin-1 staining. Representative microscopic images (magnification, ×200) of allergen-challenged groups are shown below bars in D–F. Data represent mean ± S.E. in A, D, E, and F. *, p < 0.05 for allergen-challenged Mgat5−/− versus WT mice.
Assessment of mucus production by goblet cells exhibited significantly increased number of PAS-positive mucus-producing cells in the airways of allergen-challenged WT mice compared with the small number observed in Mgat5−/− counterparts (Fig. 1D). Little to no positive PAS staining was observed in lung sections of control mice (data not shown). Expression of FIZZ1, which is associated with early stage remodeling events in allergic airways (28), by type II alveolar and airway epithelial cells was also markedly reduced in allergen-challenged Mgat5−/− mice compared with WT mice (Fig. 1E). Furthermore, expression of intelectin-1, another proinflammatory molecule induced during allergic inflammation (29), was significantly inhibited in secretory cells of the airway epithelium in allergen-challenged Mgat5−/− mice (Fig. 1F). No expression of intelectin-1 was observed in control mice (data not shown). These data suggest that MGAT5 deficiency results in attenuated inflammatory responses that promote allergen-induced airway eosinophilic inflammation and remodeling.
Mgat5−/− Eosinophils Exhibit Reduced Trafficking and Survival
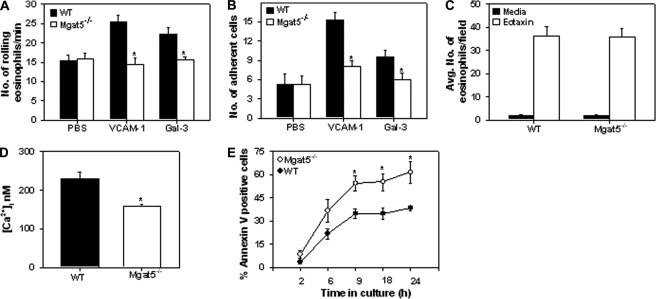
MGAT5 is involved in the generation of N-glycan ligands for galectin-3, which supports eosinophil trafficking and recruitment (9, 10). Furthermore, eosinophils are known to roll and adhere on VCAM-1 (30), a key adhesion molecule up-regulated during allergic inflammation. Therefore, we evaluated the interaction of BM-cultured eosinophils from WT and Mgat5−/− mice with immobilized galectin-3 and VCAM-1 under conditions of physiologic flow in vitro. Expression of branched N-glycans by cultured WT eosinophils and the lack of N-glycan expression by Mgat5−/− eosinophils was first confirmed based on PHA-L binding (supplemental Fig. 2). Rolling and adhesion of Mgat5−/− eosinophils on galectin-3 and VCAM-1 was significantly inhibited (to baseline levels) compared with WT eosinophils (p < 0.05) (Fig. 2, A and B). Flow cytometry revealed a small difference in expression of LFA-1 and Mac-1 but no difference in expression of α4 or Siglec-F between WT and Mgat5−/− eosinophils. However, a reduction in L-selectin expression by a population of Mgat5−/− eosinophils was observed (supplemental Fig. 3A). Although lung eotaxin-1 levels were similar in allergen-challenged Mgat5−/− and WT mice (supplemental Fig. 1), we investigated whether the ability of Mgat5−/− eosinophils to migrate in response to this chemokine was compromised. WT and Mgat5−/− eosinophils exhibited comparable migration toward eotaxin (Fig. 2C). Furthermore, there was no difference in the level of expression of the eotaxin receptor CCR3 between WT and Mgat5−/− eosinophils (supplemental Fig. 3B). The reduced adhesive interactions of Magt5−/− eosinophils was also associated with significantly lower basal intracellular Ca2+ ([Ca2+]i) levels compared with WT eosinophils (p < 0.05) (Fig. 2D). Interestingly, Mgat5−/− eosinophils cultured up to 24 h demonstrated significantly increased apoptosis from 9 h onwards compared with WT eosinophils (p < 0.05) (Fig. 2E). These data indicate an important role for MGAT5-modifed N-glycans not only in supporting eosinophil rolling and adhesion but also survival. Thus, in allergen-challenged Mgat5−/− mice, despite their ability to undergo normal chemotaxis, reduced adhesive interactions with the vascular endothelium may result in decreased recruitment of eosinophils to the airways (Fig. 1). In addition, the decreased survival of eosinophils may contribute to reduced airway eosinophilia in allergen-challenged Mgat5−/− mice.
FIGURE 2.
MGAT5-modified N-glycans are essential for eosinophil trafficking and survival. Rolling (A) and adhesion (B) of WT and Mgat5−/− eosinophils on immobilized VCAM-1 or galectin-3 (Gal-3) under conditions of flow. Rolling on PBS was considered as background. C, chemotaxis of WT and Mgat5−/− eosinophils in response to eotaxin (100 nm) or medium alone (control) in vitro. Combined data of two independent experiments performed in triplicate for each condition with eosinophils from two different mice for each group is shown for A–C. D, basal [Ca2+]i levels in WT and Mgat5−/− eosinophils by digital videofluorescence imaging using the Ca2+ indicator dye Fura-2 AM. Combined data of three pooled experiments with Mgat5−/− and WT eosinophils (n = 270–340 cells) from three different mice. E, eosinophil survival evaluated by flow cytometry based on FITC-annexin V binding by gated eosinophils. Combined data of three experiments with eosinophils from three different mice for each group is shown. Data represent mean ± S.E. in A–E. *, p < 0.05 for comparison of WT versus Mgat5−/− eosinophils.
Th2 Cytokines Are Decreased but Proinflammatory Cytokines Remain Elevated in Lungs of Allergen-challenged Mgat5−/− Mice
Although allergen challenge-induced expression of IL-4, IL-5, and IL-13 in lungs of WT and Mgat5−/− mice relative to corresponding control mice (Table 1), the levels of each of these Th2 cytokines were significantly lower in allergen-challenged Mgat5−/− mice compared with WT mice. With Th1 cytokines, there was no change in IL-2 levels in response to allergen challenge, whereas IFNγ levels increased in WT and Mgat5−/− mice compared with respective control mice but without any significant difference between the allergen-challenged groups. Allergen challenge also induced IL-17A and TNFα in WT and Mgat5−/− mice, albeit with no statistically significant difference between the two groups. Overall, although allergen-challenged Mgat5−/− mice exhibit a significant reduction in Th2 cytokines compared with WT mice, the levels of proinflammatory cytokines, IFNγ, IL-17A, and TNFα remain elevated and are nearer to the levels observed in allergen-challenged WT mice.
TABLE 1.
Th1, Th2, and proinflammatory cytokines in lung tissue from control and OVA-challenged WT and Mgat5−/− mice
ND indicates not detectable (n = 4–6 per group).
| Cytokine | WT mice (mean ± S.E.) |
Mgat5−/− mice (mean ± S.E.) |
||
|---|---|---|---|---|
| Control | OVA | Control | OVA | |
| pg/mg protein | pg/mg protein | |||
| IL-4 | 4.97 ± 0.21 | 9.27 ± 1.04a | 4.39 ± 0.12 | 6.64 ± 0.33a,b |
| IL-5 | 4.18 ± 0.3 | 14.33 ± 1.42a | 3.86 ± 0.19 | 7.03 ± 0.35a,b |
| IL-13 | 3.81 ± 0.87 | 49.41 ± 9.72a | 3.85 ± 0.36 | 14.78 ± 2.65a,b |
| IFNγ | 0.44 ± 0.16 | 12.29 ± 2.26a | 0.01 ± 0.01 | 9.08 ± 3.16a |
| IL-2 | 9.0 ± 0.99 | 7.02 ± 0.98 | 10.48 ± 1.16 | 7.42 ± 0.48 |
| IL-17A | ND | 60.63 ± 21.58a | ND | 41.89 ± 2.94a |
| TNFα | 15.73 ± 1.45 | 207.38 ± 35.39a | 24.81 ± 7.07 | 144.75 ± 17.46a |
a p < 0.05 control versus OVA-challenged groups for WT and Mgat5−/− mice.
b p < 0.05 OVA-challenged Mgat5−/− mice versus allergen-challenged WT mice.
Allergen-challenged Mgat5−/− Mice Exhibit Increased AHR and Neutrophilic Inflammation
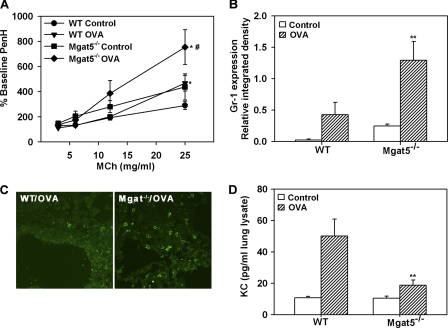
Despite the significant reduction in eosinophilic inflammation (Fig. 1) and Th2 cytokine levels (Table 1) in OVA-challenged Mgat5−/− mice, these mice displayed elevated AHR to inhaled MCh (25 mg/ml) relative to OVA-challenged WT mice (p < 0.05) as assessed by whole body plethysmography (Fig. 3A). Interestingly, airway responsiveness to MCh in control Mgat5−/− mice was higher than in control WT mice, although the difference between these two groups was not found to be statistically significant (p = 0.1 at 25 mg/ml MCh). Immunohistology studies to detect smooth muscle hypertrophy revealed no difference in airway α-smooth muscle actin between control and allergen-challenged WT and Mgat5−/− mice (data not shown), suggesting that the increased airway reactivity in Mgat5−/− mice is not likely due to exaggerated smooth muscle hypertrophy.
FIGURE 3.
Allergen-challenged Mgat5−/− mice exhibit increased AHR and develop lung neutrophilia. A, AHR in control and allergen-challenged WT and Mgat5−/− mice assessed by whole body plethysmography. The enhanced pause in breathing after MCh was expressed as a percentage of baseline enhanced pause with saline (n = 8 mice per group). B, Gr-1 expression in lung tissue lysates of control and allergen-challenged mice by densitometry of Western blots normalized against β-actin expression (n = 4–5 per group). C, representative images of lung tissue neutrophils in allergen-challenged WT and Mgat5−/− mice by confocal microscopy after staining with mAb NIMP-R14 (magnification, ×600). No NIMP-R14-positive cells were detected in control lungs from both groups (n = 4–5 per group). D, KC levels in lung tissue lysates of control and allergen-challenged WT and Mgat5−/− mice by flow cytometry (n = 4–6 per group). Data represent mean ± S.E. in A, C, and E. *, p < 0.05 versus respective control; and #, p < 0.05 between allergen-challenged groups for A; **, p < 0.05 versus WT OVA in B and D.
However, allergen-challenged Mgat5−/− mice exhibited a significant increase (2-fold) in the number of neutrophils recruited to the airways compared with WT mice (p < 0.05) (Fig. 1A), which was even more profound when expressed as a percentage of total cells (3-fold higher, p < 0.01) (supplemental Fig. 4A). This was further examined in lung tissue as tissue neutrophilia could potentially contribute to the increased AHR. Lung tissue of allergen-challenged Mgat5−/− mice demonstrated a significant increase in Gr-1 expression, a marker for neutrophils, not only compared with control Mgat5−/− mice, but also allergen-challenged WT mice (p < 0.05), which mount only a modest neutrophil response compared with corresponding unchallenged mice (Fig. 3B). Interestingly, lung tissue Gr-1 expression in control Mgat5−/− mice was higher than in control WT mice and may explain the higher AHR observed in these mice compared with WT counterparts (Fig. 3A). Confocal microscopy with another neutrophil-specific mAb (NIMP-R14) also demonstrated increased neutrophils in lung sections of allergen-challenged Mgat5−/− mice compared with WT counterparts (Fig. 3C). Surprisingly, despite increased neutrophils in BALF and lung tissue, KC levels in the lungs of allergen-challenged Mgat5−/− mice were substantially lower than in WT mice (p < 0.05) (Fig. 3D), suggesting that the increased airway neutrophilia observed in these mice may not be driven by KC levels.
MGAT5 Deficiency Enhances Neutrophilic Inflammation in Response to LPS and TG
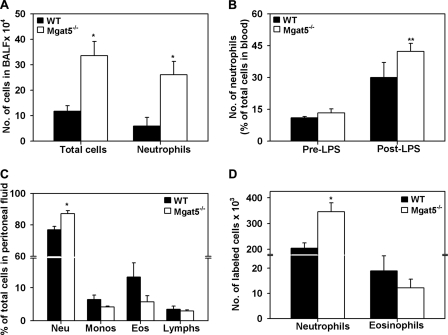
In two different models of inflammation that predominantly induce a neutrophilic response, Mgat5−/− mice demonstrated significantly increased neutrophil recruitment compared with WT mice. In a model of LPS-induced airway inflammation, total cells and number of neutrophils in BALF of Mgat5−/− mice were significantly higher than in WT mice (Fig. 4A). Furthermore, LPS exposure increased peripheral blood neutrophils in WT and Mgat5−/− mice compared with pre-exposure levels, but the increase in blood neutrophils was significantly higher only in Mgat5−/− mice (p < 0.05) not in WT mice (Fig. 4B). Similarly, in a model of TG-induced peritonitis, Mgat5−/− mice demonstrated significantly increased peritoneal recruitment of neutrophils at 6 h compared with WT mice (p < 0.05) (Fig. 4C). Previous studies have demonstrated decreased total leukocyte recruitment to the peritoneum in Mgat5−/− mice after exposure to TG (31). However, it is important to note that the end point of the study as well as the mouse strain were different compared with the current study (3 h versus 6 h), and strain-related differences are known to exist during induced neutrophilia in mice (32). In the TG-exposed Mgat5−/− mice in our study, we did not observe a significant reduction in eosinophil recruitment compared with WT counterparts (p = 0.1), although the trend in reduction paralleled the BALF eosinophils in the allergen challenge model (Fig. 1). These findings suggest that Mgat5−/− mice have a greater propensity to develop neutrophilia during conditions of inflammation at different tissue sites. Finally, when Mgat5−/− and WT neutrophils and eosinophils were infused into TG-exposed WT recipient mice, there was significantly increased recruitment of the transferred Mgat5−/− neutrophils to the peritoneal cavity compared with WT neutrophils (p < 0.05) (Fig. 4D). On the other hand, the number of transferred Mgat5−/− eosinophils recruited to the peritoneum of TG-exposed WT mice was not significantly reduced compared with WT eosinophils (p = 0.19). These findings are similar to that observed in TG-exposed Mgat5−/− versus WT mice (Fig. 4C) and further indicate a differential role for MGAT5-modified N-glycans in eosinophil versus neutrophil recruitment.
FIGURE 4.
MGAT5 deficiency enhances neutrophilic inflammation in response to LPS and TG. BALF cell counts (A) and peripheral blood neutrophils (B) in LPS-exposed WT and Mgat5−/− mice after Hema 3 staining. C, differential cell counts in the peritoneal fluid of TG-exposed WT and Mgat5−/− mice. Neu, neutrophils; Monos, monocytes; Lymphs, lymphocytes. D, number of labeled cells in the peritoneal fluid of TG-exposed WT mice 6 h after transfer for neutrophils and 2 h after transfer for eosinophils (Eos). Combined data of three experiments is shown in each case (n = 4 mice per group in A and B and n = 3 mice per group in C and D). Data represent mean ± S.E. *, p < 0.05 for Mgat5−/− versus WT in A, C, and D. **, p < 0.05 for post-LPS versus pre-LPS in Mgat5−/− mice in B.
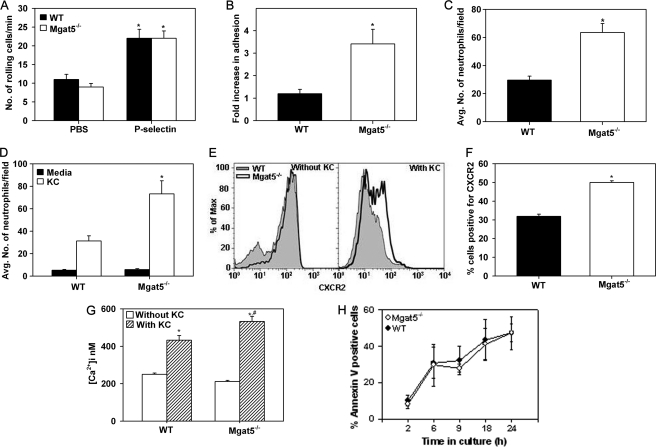
Mgat5−/− Neutrophils Exhibit Increased Adhesion and Migration along with Elevated CXCR2 Expression
The basis for the increased recruitment of neutrophils in Mgat5−/− mice was further evaluated. First, WT BM neutrophils exhibited strong binding to FITC-PHA-L compared with the control (FITC-BSA), indicating the expression of branched N-glycans. Mgat5−/− neutrophils also exhibited PHA-L binding relative to control but to a much lesser extent compared with WT cells (supplemental Fig. 4B). This residual binding of Mgat5−/− neutrophils to PHA-L relative to the control could be due to interaction of PHA-L with N-glycans other than MGAT5-modified N-glycans expressed by neutrophils. Next, unlike the decreased rolling observed with Mgat5−/− eosinophils, rolling of Mgat5−/− neutrophils on immobilized P-selectin was similar to that of WT neutrophils under conditions of flow (Fig. 5A). However, contrary to the decreased adhesion of Mgat5−/− eosinophils (Fig. 2B), Mgat5−/− neutrophils demonstrated a 4-fold increase in adhesion to P-selectin, whereas WT neutrophils demonstrated only a 1.3-fold increase compared with baseline adhesion under flow conditions (Fig. 5B). Confocal microscopy studies confirmed increased adhesion of Mgat5−/− neutrophils to P-selectin even under static conditions (Fig. 5C), although no difference was detected between Mgat5−/− and WT neutrophils in the expression of PSGL-1 by flow cytometry (supplemental Fig. 4C). Indeed, an increased number of neutrophils were found to be adhered to the wall of blood vessels in lung sections from allergen-challenged Mgat5−/− mice as opposed to WT mice by immunohistology (data not shown). In addition to increased adhesion, Mgat5−/− neutrophils also demonstrated significantly increased migration toward KC compared with WT neutrophils (p < 0.05) (Fig. 5D). Analysis of CXCR2 (KC receptor) expression by flow cytometry indicated that after treatment with KC (agonist-induced), Mgat5−/− neutrophils demonstrated increased expression of CXCR2 compared with WT neutrophils (Fig. 5E, right panel) and a significantly larger percentage of Mgat5−/− neutrophils were positive for CXCR2 expression after treatment with KC compared with WT neutrophils (Fig. 5F), correlating with their increased ability to migrate toward KC. Furthermore, although stimulation with KC resulted in an increase in [Ca2+]i levels in WT and Mgat5−/− neutrophils, stimulated [Ca2+]i in Mgat5−/− neutrophils was significantly higher than in WT neutrophils (p < 0.05) (Fig. 5G). Finally, in contrast to the increased apoptosis of Mgat5−/− eosinophils, annexin V staining demonstrated no difference in apoptosis between WT and Mgat5−/− neutrophils from TG-exposed mice up to 24 h in culture (Fig. 5H), suggesting that MGAT5-elaborated N-glycans are not critical for survival of recruited neutrophils.
FIGURE 5.
Mgat5−/− neutrophils exhibit increased adhesion and migration along with elevated CXCR2 expression. Rolling (A) and adhesion (B) of WT and Mgat5−/− neutrophils on immobilized P-selectin under conditions of flow. Adhesion is expressed as fold increase over background (adhesion to PBS). Combined data of three independent experiments performed in duplicate for each condition are shown for A and B. C, quantitation of WT and Mgat5−/− neutrophils adhered to P-selectin under static conditions. Adherent cells in six fields were counted for each cell type, and combined data of two independent experiments are shown. D, migration of WT and Mgat5−/− neutrophils in response to KC (100 nm) or medium alone (control). Combined data of two experiments performed in triplicate for each condition with neutrophils from two different mice for each group is shown. E, surface expression of CXCR2 (CD182) on WT and Mgat5−/− neutrophils by flow cytometry with PerCP/Cy5.5 anti-mouse CD182 (5 μg/ml) before and after activation with KC (100 ng/ml). Histograms shown are representative of three independent experiments. F, relative expression of CXCR2 on WT versus Mgat5−/− neutrophils by flow cytometry after activation with KC as percent cells positive for CXCR2. Combined data of three independent experiments with neutrophils from three different mice for each group is shown. G, basal and KC-induced [Ca2+]i levels in WT and Mgat5−/− neutrophils by digital videofluorescence imaging with Fura-2 AM. Combined data of three pooled experiments with Mgat5−/− and WT neutrophils (∼500 cells for each group) from three different mice are shown. H, neutrophil survival evaluated flow cytometry based on FITC-annexin V binding by gated neutrophils. Combined data of three independent experiments with neutrophils from three different mice are shown. Data represent mean ± S.E. in A–D and F–H. *, p < 0.05 for Mgat5−/− versus WT neutrophils in B, C, D, and F, and versus respective control in A (rolling on PBS) and G (without KC). #, p < 0.05 for Mgat5−/− versus WT neutrophils with KC treatment in G.
DISCUSSION
Whereas the role of eosinophils in the pathogenesis of allergic airway inflammation including asthma is more established (2, 33), several lines of evidence suggest that neutrophils also accumulate in the airways in asthmatic patients with more severe airflow obstruction and in airway secretions during acute severe asthma exacerbations (2) with neutrophil numbers correlating with severity of the disease (3). However, factors regulating the recruitment of eosinophils relative to neutrophils during allergen-induced airway inflammation are unclear. Our studies demonstrate an important role for MGAT5 synthesized N-glycans in regulation of allergen-induced airway inflammation by exerting opposing effects on eosinophil versus neutrophil recruitment. Exposure of Mgat5−/− mice to allergen challenge resulted in attenuation of eosinophilic inflammation, lung Th2 cytokines, airway mucus secretion, and expression of proinflammatory molecules. However, AHR remained elevated in these mice with aberrant N-glycan expression and was associated with increased airway neutrophilia, which may be a contributory factor for the increased airway reactivity.
Galectin-3, which binds to N-glycan ligands elaborated by MGAT5 activity (14), has been shown to mediate eosinophil rolling and adhesion by binding to α4 integrins in a carbohydrate-dependent manner (9). Integrins contain multiple MGAT5-modified N-linked glycosylation sites that play a role in regulating integrin clustering, adhesion, and migration (34–37). Alterations in glycan expression on α4 integrins in the absence of MGAT5 may not alter the level of expression of α4 but affect binding of α4 to VCAM-1 or galectin-3 resulting in reduced eosinophil rolling, adhesion, and infiltration of Mgat5−/− eosinophils in allergen-challenged airways. Because LFA-1 and Mac-1 do not play a role in mediating rolling and/or adhesion on VCAM-1, the small difference observed between WT and Mgat5−/− eosinophils in expression of these receptors is not likely to impact rolling and adhesion on VCAM-1 but may modulate eosinophil trafficking in vivo. Likewise, the reduced expression of L-selectin on Mgat5−/− eosinophils could also lead to decreased trafficking and recruitment in vivo. Along with this, levels of [Ca2+]i, an important regulator of integrins and cytoskeleton structure in the stabilization of cell adhesion (38), were significantly lower in non-activated Mgat5−/− eosinophils. Finally, decreased survival of Mgat5−/− eosinophils could also contribute to the reduced airway eosinophilia.
Decreased eosinophilic inflammation in allergen-challenged Mgat5−/− mice was associated with decreased lung Th2 cytokines (IL-4, IL-5, and IL-13). This is most likely due to the decreased recruitment of lymphocytes to the airways of allergen-challenged Mgat5−/− mice compared with WT mice (Fig. 1A) leading to inhibition of Th2 responses. Furthermore, mucus secretion and expression of proinflammatory molecules (FIZZ1 and intelectin-1) were also reduced in these mice. Despite this, AHR remained significantly elevated in allergen-challenged Mgat5−/− mice compared with WT mice. With no evidence of exaggerated smooth muscle hypertrophy that could account for the increased airway reactivity in these mice, an alternate and conceivable explanation may be the increased presence of airway neutrophils along with elevated levels of the proinflammatory cytokines IFNγ, IL-17A, and TNFα. The increased neutrophilia in allergen-challenged Mgat5−/− mice was reproducible in other models of neutrophilic inflammation induced by LPS or TG, suggesting that overall MGAT5-elaborated N-glycans may negatively regulate neutrophil recruitment.
Neutrophilia in allergen-challenged Mgat5−/− mice can be attributed to increased adhesion of Mgat5−/− neutrophils to P-selectin. Although the level of expression of PSGL-1 in Mgat5−/− neutrophils was similar to WT neutrophils, cell surface distribution of PSGL-1 or signaling events upon activation may be altered in Mgat5−/− neutrophils and may cause increased adhesion. Alternatively, residual PHA-L binding observed in Mgat5−/− neutrophils in contrast to eosinophils is suggestive of the expression of non-Mgat-5 modified N-glycans in the former, thereby promoting neutrophil over eosinophil recruitment. Increased neutrophilia in allergen-challenged Mgat5−/− mice may also be a consequence of the increased ability of Mgat5−/− neutrophils to migrate in response to KC compared with WT neutrophils. Moreover, enhanced migration may be facilitated by the significant increase in KC-induced calcium flux observed in Mgat5−/− neutrophils relative to WT neutrophils. The calcium-signaling pathway plays an important role in neutrophil adhesion and is critical for inflammatory recruitment (38). Increased chemotaxis of Mgat5−/− neutrophils in response to KC may be facilitated by the activation-induced cell surface expression and potentially retention of CXCR2 compared with WT neutrophils. Inhibition of MGAT5 gene expression by siRNA results in retention of EGF receptor, a glycoprotein with multiple putative N-glycosylation sites, at the cell surface (39). It is conceivable that aberrant glycosylation of CXCR2, which contains three putative N-glycosylation sites in its extracellular domain (40), due to MGAT5 deficiency could lead to a similar retention of CXCR2 on the cell surface of Mgat5−/− neutrophils. Interestingly, CCR3 does not contain any consensus sequence for N-glycosylation sites (41), which could explain the lack of a difference in CCR3 expression between WT and Mgat5−/− eosinophils and comparable eotaxin-induced migration.
Unlike Th2 cytokines, IL-17A and TNFα protein levels remain elevated after allergen challenge in Mgat5−/− mice similar to levels observed in WT counterparts. Sustained levels of IL-17A could result in increased granulopoiesis and chemotaxis of neutrophils (42), whereas TNFα can support neutrophil adhesion and migration through induction of vascular adhesion molecule expression (43). Furthermore, neutrophils themselves could be a potential source of IL-17A (44) and TNFα (45). Neutrophils are the first inflammatory cells to be recruited to the airways in response to allergen challenge in humans (46) and mice (47). However, the early recruitment of neutrophils following allergen challenge is considered transient and is subsequently followed by an influx of eosinophils and lymphocytes (48). Because neutrophil survival after recruitment is not affected by Mgat5 deficiency, it is more likely their increased ability to adhere and migrate that leads to increased recruitment during inflammation. Once recruited, neutrophils are able to release a wide range of inflammatory molecules such as lipids (LTB4, PAF), cytokines, proteases (elastase, collagenase, MMP-9), reactive oxygen intermediates, and nitric oxide (49). Neutrophil elastase has been shown to induce airway constriction and AHR (50), whereas nitric oxide contributes to allergen-induced AHR (51). Indeed, neutrophils from asthmatic subjects have been found to exhibit increased migration and superoxide generation, whereas their secretory products increase bronchial responsiveness (43).
Overall, deficiency of MGAT5 results in attenuation of eosinophilic inflammation and Th2 cytokines but enhanced neutrophilic inflammation with AHR. The latter correlated with elevated IL-17A and TNFα levels in the lungs as well as increased adhesion and CXCR2 expression by MGAT5-deficient neutrophils, supporting their increased ability to migrate. As the deficiency of MGAT5 is not limited to leukocytes in Mgat5−/− mice, it is possible that other cell types may also contribute to the attenuated airway inflammation observed in these mice. However, differences in rolling and adhesion on endothelial adhesion molecules in vitro together with the differential recruitment of infused Mgat5−/− neutrophils and eosinophils to the peritoneal cavity of recipient WT mice compared with WT cells in vivo clearly indicate that N-glycans expressed on eosinophils and neutrophils play a significant role in mediating trafficking and migration of these cells during inflammation. Furthermore, these studies provide novel insights into the role of N-glycans in the development of allergic airway inflammation and asthma.
Supplementary Material
Acknowledgments
We thank Cari M. Calhoun for excellent technical assistance and Dr. Riaz I. Zuberi (Torrey Pines Institute for Molecular Studies, San Diego, CA) for helpful discussions.
This work was supported by National Institutes of Health Grants AI35796, U19-AI70535, and HL0793041 (to P. Sriramarao) and AI053331 (to Michael Demetriou).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
- used: MGAT5
- UDP-N-acetylglucosamine:α-6-d-mannoside β1,6-N-acetylglucosaminyltransferase V
- MCh
- methacholine
- MBP
- major basic protein
- PHA-L
- Phaseolus vulgaris leucoagglutinin-L
- OVA
- ovalbumin
- AHR
- airway hyperresponsiveness
- TG
- thioglycollate
- KC
- keratinocyte-derived chemokine
- PAS
- periodic acid-Schiff
- VCAM
- vascular cell adhesion molecule
- BM
- bone marrow.
REFERENCES
- 1. Galli S. J., Tsai M., Piliponsky A. M. (2008) Nature 454, 445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fahy J. V. (2009) Proc. Am. Thorac Soc. 6, 256–259 [DOI] [PubMed] [Google Scholar]
- 3. Alcorn J. F., Crowe C. R., Kolls J. K. (2010) Annu. Rev. Physiol. 72, 495–516 [DOI] [PubMed] [Google Scholar]
- 4. Broide D. H., Miller M., Castaneda D., Nayar J., Cho J. Y., Roman M., Ellies L. G., Sriramarao P. (2002) Am. J. Physiol. Lung Cell Mol. Physiol. 282, L259–266 [DOI] [PubMed] [Google Scholar]
- 5. Sperandio M., Gleissner C. A., Ley K. (2009) Immunol. Rev. 230, 97–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanino Y., Coombe D. R., Gill S. E., Kett W. C., Kajikawa O., Proudfoot A. E., Wells T. N., Parks W. C., Wight T. N., Martin T. R., Frevert C. W. (2010) J. Immunol. 184, 2677–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J. M., Yu S. Y., Kawashima H., Saito H., Ohtsubo K., Marth J. D., Khoo K. H., von Andrian U. H., Lowe J. B., Fukuda M. (2007) Nat. Immunol. 8, 409–418 [DOI] [PubMed] [Google Scholar]
- 8. Sriramarao P., von Andrian U. H., Butcher E. C., Bourdon M. A., Broide D. H. (1994) J. Immunol. 53, 4238–4246 [PubMed] [Google Scholar]
- 9. Rao S. P., Wang Z., Zuberi R. I., Sikora L., Bahaie N. S., Zuraw B. L., Liu F. T., Sriramarao P. (2007) J. Immunol. 179, 7800–7807 [DOI] [PubMed] [Google Scholar]
- 10. Ge X. N., Bahaie N. S., Kang B. N., Hosseinkhani M. R., Ha S. G., Frenzel E. M., Liu F. T., Rao S. P., Sriramarao P. (2010) J. Immunol. 185, 1205–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dennis J. W., Nabi I. R., Demetriou M. (2009) Cell 139, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grigorian A., Torossian S., Demetriou M. (2009) Immunol. Rev. 230, 232–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mkhikian H., Grigorian A., Li C. F., Chen H. L., Newton B., Zhou R. W., Beeton C., Torossian S., Tatarian G. G., Lee S. U., Lau K., Walker E., Siminovitch K. A., Chandy K. G., Yu Z., Dennis J. W., Demetriou M. (2011) Nat. Commun. 2, 334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dennis J. W., Pawling J., Cheung P., Partridge E., Demetriou M. (2002) Biochim. Biophys. Acta 1573, 414–422 [DOI] [PubMed] [Google Scholar]
- 15. Lau K. S., Partridge E. A., Grigorian A., Silvescu C. I., Reinhold V. N., Demetriou M., Dennis J. W. (2007) Cell 129, 123–134 [DOI] [PubMed] [Google Scholar]
- 16. Demetriou M., Granovsky M., Quaggin S., Dennis J. W. (2001) Nature 409, 733–739 [DOI] [PubMed] [Google Scholar]
- 17. Morgan R., Gao G., Pawling J., Dennis J. W., Demetriou M., Li B. (2004) J. Immunol. 173, 7200–7208 [DOI] [PubMed] [Google Scholar]
- 18. Lee S. U., Grigorian A., Pawling J., Chen I. J., Gao G., Mozaffar T., McKerlie C., Demetriou M. (2007) J. Biol. Chem. 282, 33725–33734 [DOI] [PubMed] [Google Scholar]
- 19. Miller M., Sung K. L., Muller W. A., Cho J. Y., Roman M., Castaneda D., Nayar J., Condon T., Kim J., Sriramarao P., Broide D. H. (2001) J. Immunol. 167, 2292–2297 [DOI] [PubMed] [Google Scholar]
- 20. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Biophotonics International 11, 36–42 [Google Scholar]
- 21. Zuberi R. I., Ge X. N., Jiang S., Bahaie N. S., Kang B. N., Hosseinkhani R. M., Frenzel E. M., Fuster M. M., Esko J. D., Rao S. P., Sriramarao P. (2009) J. Immunol. 183, 3971–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coxon A., Rieu P., Barkalow F. J., Askari S., Sharpe A. H., von Andrian U. H., Arnaout M. A., Mayadas T. N. (1996) Immunity 5, 653–666 [DOI] [PubMed] [Google Scholar]
- 23. Dyer K. D., Moser J. M., Czapiga M., Siegel S. J., Percopo C. M., Rosenberg H. F. (2008) J. Immunol. 181, 4004–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abbitt K. B., Cotter M. J., Ridger V. C., Crossman D. C., Hellewell P. G., Norman K. E. (2009) J. Leukoc. Biol. 85, 55–63 [DOI] [PubMed] [Google Scholar]
- 25. Miyake K., Medina K., Ishihara K., Kimoto M., Auerbach R., Kincade P. W. (1991) J. Cell Biol. 114, 557–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Louahed J., Zhou Y., Maloy W. L., Rani P. U., Weiss C., Tomer Y., Vink A., Renauld J., Van Snick J., Nicolaides N. C., Levitt R. C., Haczku A. (2001) Blood 97, 1035–1042 [DOI] [PubMed] [Google Scholar]
- 27. Deshpande D. A., Dogan S., Walseth T. F., Miller S. M., Amrani Y., Panettieri R. A., Kannan M. S. (2004) Am. J. Respir. Cell Mol. Biol. 31, 36–42 [DOI] [PubMed] [Google Scholar]
- 28. Dong L., Wang S. J., Camoretti-Mercado B., Li H. J., Chen M., Bi W. X. (2008) J. Asthma 45, 648–653 [DOI] [PubMed] [Google Scholar]
- 29. Gu N., Kang G., Jin C., Xu Y., Zhang Z., Erle D. J., Zhen G. (2010) Am. J. Physiol. Lung Cell Mol. Physiol. 298, L290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sriramarao P., DiScipio R. G., Cobb R. R., Cybulsky M., Stachnick G., Castaneda D., Elices M., Broide D. H. (2000) Blood 95, 592–601 [PubMed] [Google Scholar]
- 31. Partridge E. A., Le Roy C., Di Guglielmo G. M., Pawling J., Cheung P., Granovsky M., Nabi I. R., Wrana J. L., Dennis J. W. (2004) Science 306, 120–124 [DOI] [PubMed] [Google Scholar]
- 32. Marley S. B., Hadley C. L., Wakelin D. (1994) Infect. Immun. 62, 4304–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee J. J., Dimina D., Macias M. P., Ochkur S. I., McGarry M. P., O'Neill K. R., Protheroe C., Pero R., Nguyen T., Cormier S. A., Lenkiewicz E., Colbert D., Rinaldi L., Ackerman S. J., Irvin C. G., Lee N. A. (2004) Science 305, 1773–1776 [DOI] [PubMed] [Google Scholar]
- 34. Demetriou M., Nabi I. R., Coppolino M., Dedhar S., Dennis J. W. (1995) J. Cell Biol. 130, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Granovsky M., Fata J., Pawling J., Muller W. J., Khokha R., Dennis J. W. (2000) Nat. Med. 6, 306–312 [DOI] [PubMed] [Google Scholar]
- 36. Gu J., Isaji T., Sato Y., Kariya Y., Fukuda T. (2009) Biol. Pharm Bull 32, 780–785 [DOI] [PubMed] [Google Scholar]
- 37. Janik M. E., Lityńska A., Vereecken P. (2010) Biochim. Biophys. Acta 1800, 545–555 [DOI] [PubMed] [Google Scholar]
- 38. Simon S. I., Sarantos M. R., Green C. E., Schaff U. Y. (2009) Clin. Exp. Pharmacol. Physiol. 36, 217–224 [DOI] [PubMed] [Google Scholar]
- 39. Guo H. B., Johnson H., Randolph M., Lee I., Pierce M. (2009) Glycobiology 19, 547–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Suzuki H., Prado G. N., Wilkinson N., Navarro J. (1994) J. Biol. Chem. 269, 18263–18266 [PubMed] [Google Scholar]
- 41. Combadiere C., Ahuja S. K., Murphy P. M. (1995) J. Biol. Chem. 270, 16491–16494 [DOI] [PubMed] [Google Scholar]
- 42. Ouyang W., Kolls J. K., Zheng Y. (2008) Immunity 28, 454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas P. S. (2001) Immunol. Cell Biol. 79, 132–140 [DOI] [PubMed] [Google Scholar]
- 44. Li L., Huang L., Vergis A. L., Ye H., Bajwa A., Narayan V., Strieter R. M., Rosin D. L., Okusa M. D. (2010) J. Clin. Invest. 120, 331–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Feiken E., Rømer J., Eriksen J., Lund L. R. (1995) J. Invest. Dermatol. 105, 120–123 [DOI] [PubMed] [Google Scholar]
- 46. Nocker R. E., Out T. A., Weller F. R., Mul E. P., Jansen H. M., van der Zee J. S. (1999) Int. Arch. Allergy Immunol. 119, 45–53 [DOI] [PubMed] [Google Scholar]
- 47. Taube C., Dakhama A., Takeda K., Nick J. A., Gelfand E. W. (2003) Chest 123, 410S–411S [DOI] [PubMed] [Google Scholar]
- 48. Tomkinson A., Cieslewicz G., Duez C., Larson K. A., Lee J. J., Gelfand E. W. (2001) Am. J. Respir. Crit Care Med. 163, 721–730 [DOI] [PubMed] [Google Scholar]
- 49. Monteseirín J. (2009) J. Investig. Allergol. Clin. Immunol. 19, 340–354 [PubMed] [Google Scholar]
- 50. Suzuki T., Wang W., Lin J. T., Shirato K., Mitsuhashi H., Inoue H. (1996) Am. J. Respir. Crit Care Med. 153, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 51. Eynott P. R., Groneberg D. A., Caramori G., Adcock I. M., Donnelly L. E., Kharitonov S., Barnes P. J., Chung K. F. (2002) Eur. J. Pharmacol. 452, 123–133 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.