Abstract
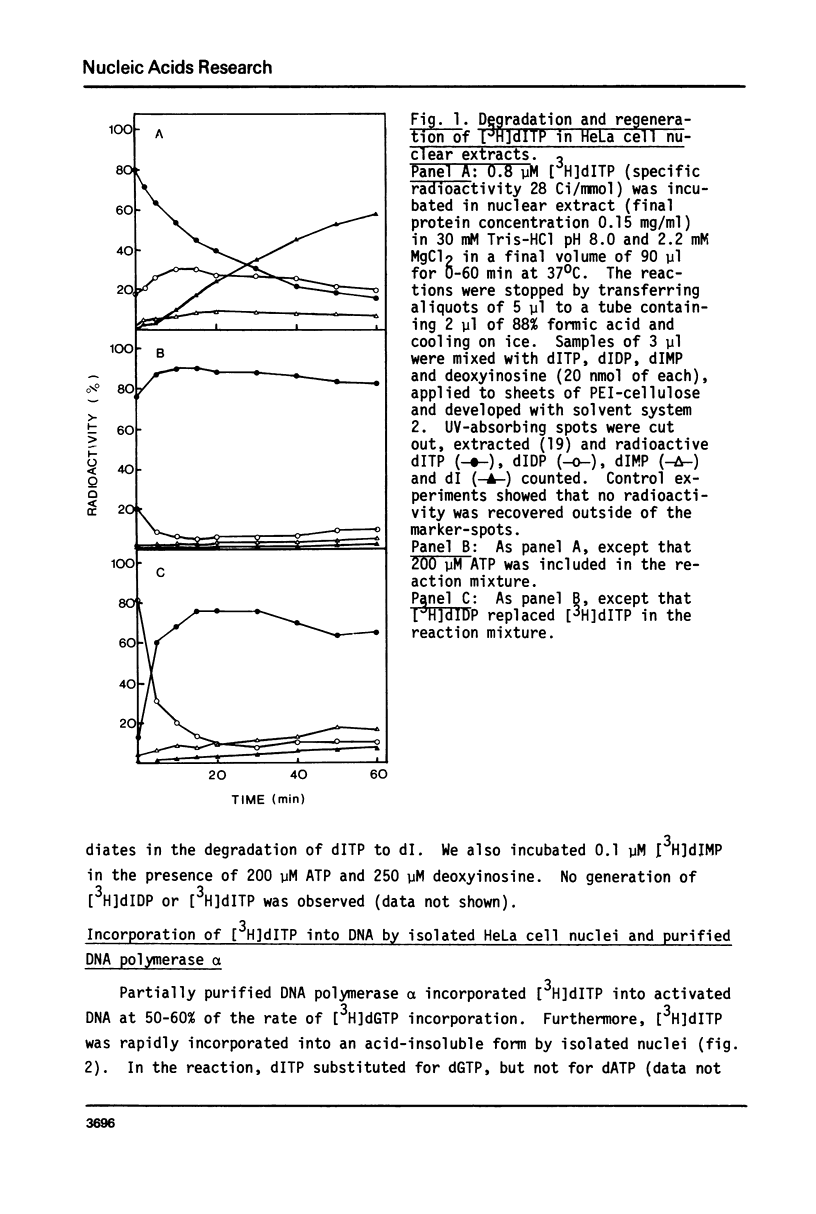
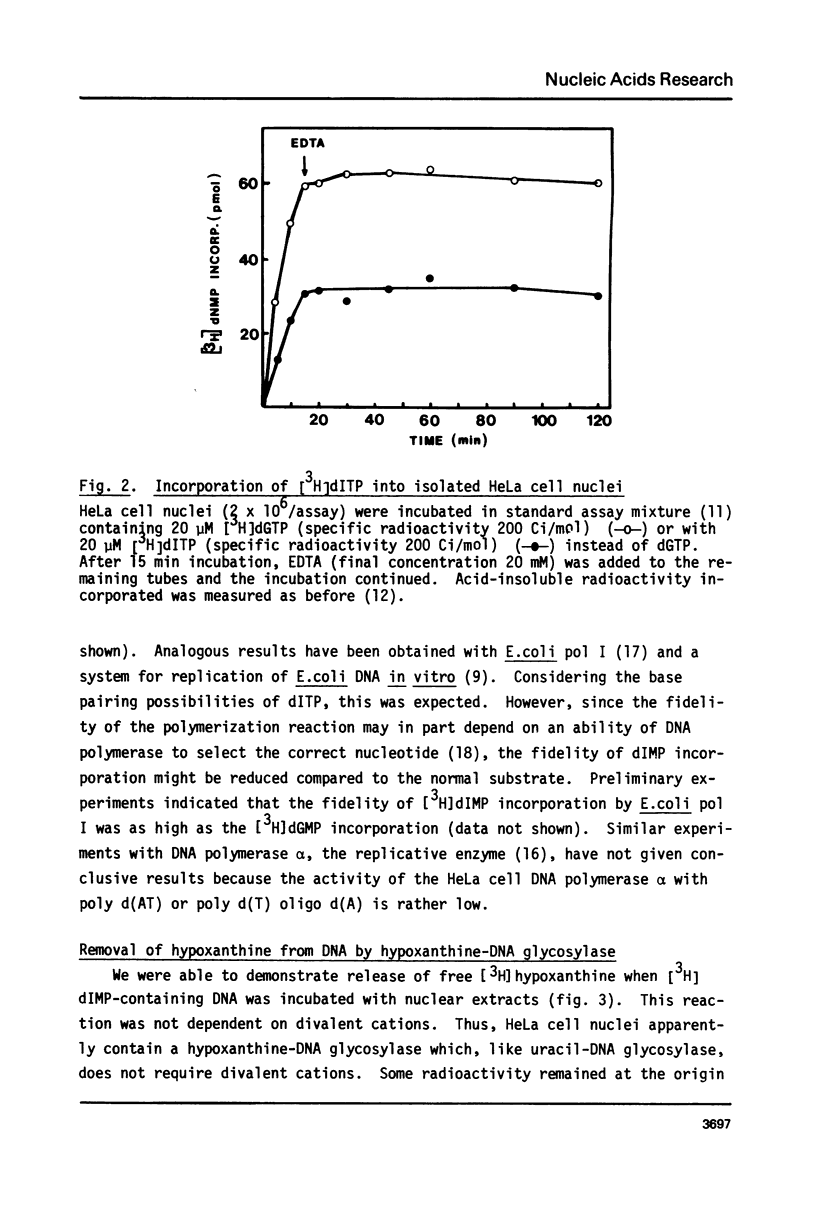
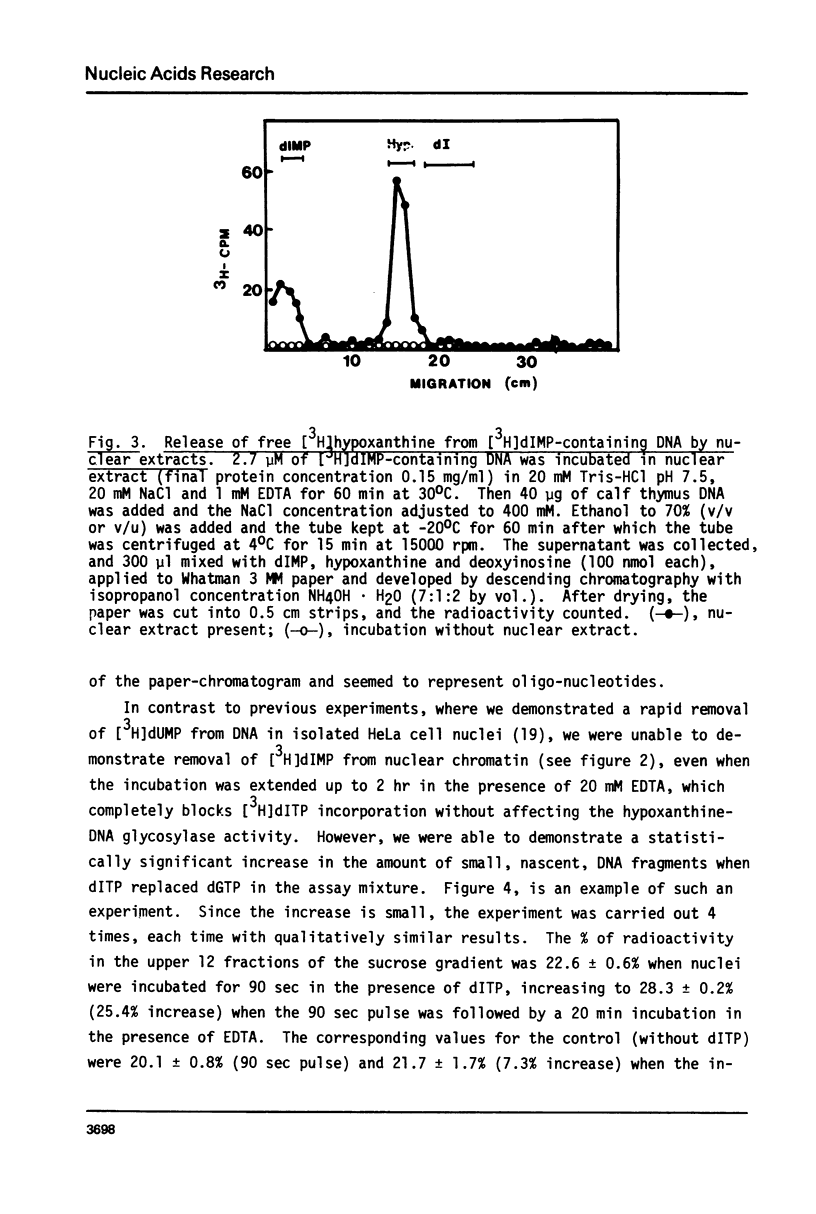
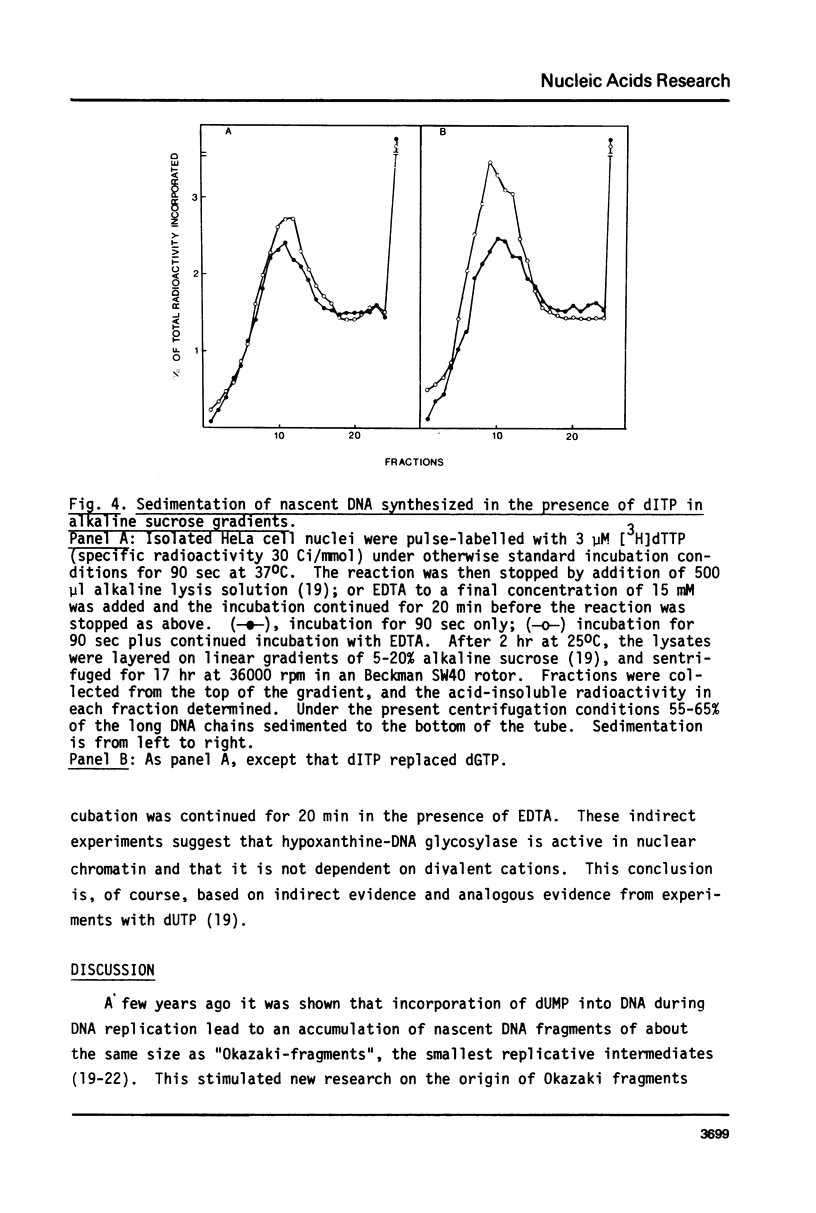
dITP may be generated from dATP by a slow, nonenzymatic hydrolysis. While [3H]dITP was degraded rapidly to [3H]deoxyinosine by HeLa cell nuclear extracts, no net degradation of [3H]dITP was observed in the presence of physiological concentrations of ATP, apparently because the extract contained deoxynucleoside diphosphate kinase activity that regenerated [3H]dITP from [3H]dIDP. Isolated HeLa cell nuclei, as well as partially purified DNA polymerase alpha, incorporated [3H]dITP into DNA at 50-60% of the rate of [3H]dGTP incorporation. No rapid release of the incorporated radioactivity was observed. The molecular weight of nascent DNA containing dIMP residues, however, decreased slightly after prolonged incubation in the presence of EDTA, suggesting that a repair process is initiated in dIMP-containing chromatin. Furthermore, release of free [3H]hypoxanthine from [3H]dIMP-containing DNA was detected after incubation with nuclear extracts in the presence of EDTA, suggesting the presence of hypoxanthine-DNA glycosylase activity in HeLa cell nuclei.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Arima T., Akiyoshi H., Fujii S. A new deoxyuridine-5'-triphosphatase in Yoshida sarcoma cells involved in deoxyuridine 5'-triphosphate metabolism. Cancer Res. 1977 Jun;37(6):1598–1601. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Miller J. H. Mutagenic deamination of cytosine residues in DNA. Nature. 1980 Oct 9;287(5782):560–561. doi: 10.1038/287560a0. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Couch J. L., Glaser D. A. Mutator activity of a short Okazaki fragment mutant of Escherichia coli. J Bacteriol. 1978 Jun;134(3):1192–1194. doi: 10.1128/jb.134.3.1192-1194.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstrom R. H., Tseng B. Y., Goulian M. The incorporation of uracil into animal cell DNA in vitro. Cell. 1978 Sep;15(1):131–140. doi: 10.1016/0092-8674(78)90089-2. [DOI] [PubMed] [Google Scholar]
- Grindey G. R., Nichol C. A. Mammalian deoxyuridine 5'-triphosphate pyrophosphatase. Biochim Biophys Acta. 1971 Jun 30;240(2):180–183. doi: 10.1016/0005-2787(71)90655-1. [DOI] [PubMed] [Google Scholar]
- KOTAKA T., BALDWIN R. L. EFFECTS OF NITROUS ACID ON THE DAT COPOLYMER AS A TEMPLATE FOR DNA POLYMERASE. J Mol Biol. 1964 Aug;9:323–339. doi: 10.1016/s0022-2836(64)80210-2. [DOI] [PubMed] [Google Scholar]
- Karran P., Lindahl T. Enzymatic excision of free hypoxanthine from polydeoxynucleotides and DNA containing deoxyinosine monophosphate residues. J Biol Chem. 1978 Sep 10;253(17):5877–5879. [PubMed] [Google Scholar]
- Karran P., Lindahl T. Hypoxanthine in deoxyribonucleic acid: generation by heat-induced hydrolysis of adenine residues and release in free form by a deoxyribonucleic acid glycosylase from calf thymus. Biochemistry. 1980 Dec 23;19(26):6005–6011. doi: 10.1021/bi00567a010. [DOI] [PubMed] [Google Scholar]
- Krokan H., Bjorklid E., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Optimalization of the system and characterization of the product. Biochemistry. 1975 Sep 23;14(19):4227–4232. doi: 10.1021/bi00690a012. [DOI] [PubMed] [Google Scholar]
- Krokan H., Wist E., Krokan R. H. Aphidicolin inhibits DNA synthesis by DNA polymerase alpha and isolated nuclei by a similar mechanism. Nucleic Acids Res. 1981 Sep 25;9(18):4709–4719. doi: 10.1093/nar/9.18.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Wittwer C. U. Uracil DNa-glycosylase from HeLa cells: general properties, substrate specificity and effect of uracil analogs. Nucleic Acids Res. 1981 Jun 11;9(11):2599–2613. doi: 10.1093/nar/9.11.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Springgate C. F., Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974 Sep;34(9):2311–2321. [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. Deoxyuridine triphosphatase of Escherichia coli. Purification, properties, and use as a reagent to reduce uracil incorporation into DNA. J Biol Chem. 1978 May 10;253(9):3305–3312. [PubMed] [Google Scholar]
- Thomas K. R., Manlapaz-Ramos P., Lundquist R., Olivera B. M. Formation of Okazaki pieces at the Escherichia coli replication fork in vitro. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):231–237. doi: 10.1101/sqb.1979.043.01.028. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wist E., Krokan H. Preservation by glycerol of DNA synthetic capacity of isolated HeLa S(3) cell nuclei during long term storage at low temperature. Exp Cell Res. 1978 Oct 15;116(2):313–316. doi: 10.1016/0014-4827(78)90453-6. [DOI] [PubMed] [Google Scholar]
- Wist E. Partial purification of deoxyuridine triphosphate nucleotidohydrolase and its effect on DNA synthesis in isolated HeLa cell nuclei. Biochim Biophys Acta. 1979 Nov 22;565(1):98–106. doi: 10.1016/0005-2787(79)90085-6. [DOI] [PubMed] [Google Scholar]
- Wist E., Unhjem O., Krokan H. Accumulation of small fragments of DNA in isolated HeLa cell nuclei due to transient incorporation of dUMP. Biochim Biophys Acta. 1978 Sep 27;520(2):253–270. doi: 10.1016/0005-2787(78)90225-3. [DOI] [PubMed] [Google Scholar]



