Background: CSB, a member of the TC-NER pathway, is essential for UV light-induced DNA damage repair.
Results: CSB expression is reduced in TR4-deficient tissues/cells, and restored CSB expression rescues UV hypersensitivity of TR4-deficient cells.
Conclusion: TR4 modulates UV sensitivity via promoting the TC-NER DNA repair pathway through transcriptional regulation of CSB.
Significance: Our findings may provide new information for the treatment of UV light-sensitive syndromes, skin cancer, and aging.
Keywords: DNA Damage, DNA Repair, Nuclear Receptors, Signal Transduction, Transcriptional Regulation, CSB, TR4, UV Response
Abstract
UV irradiation is one of the major external insults to cells and can cause skin aging and cancer. In response to UV light-induced DNA damage, the nucleotide excision repair (NER) pathways are activated to remove DNA lesions. We report here that testicular nuclear receptor 4 (TR4), a member of the nuclear receptor family, modulates DNA repair specifically through the transcription-coupled (TC) NER pathway but not the global genomic NER pathway. The level of Cockayne syndrome B protein (CSB), a member of the TC-NER pathway, is 10-fold reduced in TR4-deficient mouse tissues, and TR4 directly regulates CSB at the transcriptional level. Moreover, restored CSB expression rescues UV hypersensitivity of TR4-deficient cells. Together, these results indicate that TR4 modulates UV sensitivity by promoting the TC-NER DNA repair pathway through transcriptional regulation of CSB. These results may lead to the development of new treatments for UV light-sensitive syndromes, skin cancer, and aging.
Introduction
Testicular nuclear receptor 4 (TR4)3 is a master transcriptional regulator of many signaling pathways (1, 2). Recent studies with TR4 gene knock-out mice (TR4−/−) have revealed that TR4 is essential for normal spermatogenesis, cerebellum development, glucose metabolism, and insulin resistance (3–7). Mouse embryonic fibroblasts (MEFs) from TR4−/− mice display a higher rate of apoptosis compared with wild-type MEFs when exposed to a high dose of UV irradiation (8). However, it remains unclear whether TR4 regulates the DNA damage response and DNA repair upon UV irradiation.
In mammalian cells, UV light-induced DNA damage is repaired by the nucleotide excision repair (NER) pathway (9). NER has two operational modes: the global genomic (GG) NER pathway, which operates throughout the genome without distinction, and the transcription-coupled (TC) NER (NER) pathway, which specifically targets transcribed DNA strands in transcriptionally active regions of the genome (10).
Cockayne syndrome B protein (CSB) is a member of the SWI2/SNF2 family, a group of DNA-dependent ATPases engaged in chromatin remodeling (11). Originally identified as a member of the TC-NER pathway, CSB has also been implicated in oxidative DNA damage repair (12), RNA polymerase II elongation (13), the hypoxic response (14), chromatin maintenance and remodeling (15), the recruitment of other repair factors, and the initiation of transcription after UV irradiation (16, 17). Despite recent progress, the exact roles of CSB in the TC-NER pathway and other processes are still unclear. CSB mutation in humans leads to Cockayne syndrome (18, 19), which is associated with a wide variety of clinical symptoms, including dwarfism, mental retardation, cataracts, and UV hypersensitivity (20). By studying the role of TR4 in UV light-induced cellular responses, we have now found that TR4 modulates transcription-coupled repair via CSB. Thus, this study provides evidence for a molecular link between TR4, CSB expression, and UV light-induced cellular responses, which may lead to new information on the treatment of UV light-sensitive syndromes, skin cancer, and aging.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfections
We separated and isolated mouse dermis-derived fibroblasts (MEFs) from the back skin of TR4+/+ and TR4−/− animals. The separated tissue fragments were minced with scissors and treated with 0.25% trypsin. MEF cells were isolated from day 14.5 embryos after removal of the heads and all internal organs; rinsed with PBS; and, after the addition of 5 ml of DMEM, passed through a 22-gauge needle several times to mince the tissues. The isolated cells were cultured at 37 °C at an atmospheric oxygen level (20%) in a humidified incubator. For knockdown of TR4 in cells, we used the pRetro-H1G retroviral delivery system (Cellogenetics) with AACGGGAGAAACCAAGCAATT as the targeted sequence.
Plasmid Constructs
The expression vectors for CSB (pCI-CSB) and the parental vector were kindly provided by Dr. Jan H. J. Hoeijmakers (Erasmus University Medical Center, Rotterdam, The Netherlands). Plasmids pCMX and pCMX-TR4 have been described previously (21). pRL-TK (thymidine kinase-Renilla luciferase), pGL2-SV40, and pRL-SV40 were purchased from Promega (Madison, WI). For the CSB promoter-luciferase (Luc) reporter, a total of 961 bp of the human CSB promoter sequence was ligated into the pGL3-Basic luciferase reporter vector (Promega), and the sequence of the construct was confirmed. The plasmid was designated pGL-CSB(−961). 5′-Deletion derivatives of the CSB promoter-Luc reporter were constructed by inserting different PCR-amplified DNA fragments (containing 661 and 301 bp of the human CSB upstream region) into pGL3-Basic using plasmid pGL-CSB(−961) as the DNA template.
Cell Survival Assay
After different doses of UVC (254 nm) exposure, cells were incubated in 96-well dishes (1.5 × 103 cells/well) for 24 h. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (Promega) was then added following the manufacturer's instructions. After a 4-h reaction, the absorbance of the converted dye was measured at a wavelength of 595 nm with background subtraction at 650 nm.
GG-NER Assay
This assay is similar to that described by Tran et al. (22). CV-1 cells were cotransfected with pBluescript plasmid that had been damaged before transfection by 5000 J/m2 UV irradiation, intact (undamaged) pGL3-Basic plasmids (used as the transfection efficiency control), and either pCMX-TR4 or pCMX vector control plasmid. 48 h after transfection, DNA was isolated from cells and subjected to quantitative real-time PCR. The GG-NER pathway-repaired plasmids were measured using T3 and T7 primers for pBluescript and GL2 and RV3 primers for pGL3-Basic. The relative amount of repaired pBluescript in cells was normalized to the pGL3-Basic PCR amount and then calculated by comparison with the vector control.
In Vitro Transcription Assay
MEF cells from TR4+/+ (WT) and TR4−/− (knock-out) animals were seeded and transfected with pRL-SV40 that had been damaged before transfection by 5000 J/m2 UV irradiation and intact pGal-SV40 plasmids (used as the transfection efficiency control). 48 h after transfection, cells were harvested, and luciferase and β-gal activities were measured. Luciferase activity was normalized to β-gal activity. (TR4 has no effect on undamaged pRL-SV40 luciferase activity (23).)
CV-1 cells were seeded and transfected with pRL-SV40 that had been damaged before transfection by 5000 J/m2 UV irradiation, intact pGal-SV40 plasmids (used as the transfection efficiency control), and either pCMX-TR4 or pCMX vector control. 48 h after transfection, cells were harvested, and luciferase and β-gal activities were measured. Luciferase activity was normalized to β-gal activity.
Measurement of RNA Synthesis
Transcription blockage upon UV exposure was checked by incorporation of [3H]uridine. Cells were exposed to 10 J/m2 UV irradiation and cultivated for different times as indicated. The medium was replaced with fresh medium containing [3H]uridine (10 μCi/ml, 43 Ci/mmol), and cells were incubated for 30 min at 37 °C. Thereafter, cells were washed two times with PBS and 6% trichloroacetic acid to remove unincorporated [3H]uridine. Lysis was performed by the addition of 2 ml of 0.1 n NaOH to the cells and overnight incubation. 0.5 ml of the lysate was mixed with 4 ml of scintillation mixture and counted in a liquid scintillation counter. The incorporated radioactivity of the non-UV light-exposed probe was set at 100% (24).
Transcription-coupled Repair Analysis
The removal of cyclobutane pyrimidine dimers from the transcribed or non-transcribed strand of the DHFR (dihydrofolate reductase) gene was examined following the standard method with minor modifications (25). For each time point, 30 μg of DNA restricted with EcoRI, which yields a restriction fragment spanning 20 kb of the central region of the DHFR gene, was split into two equal amounts. One sample was digested with T4 endonuclease V (T4 pyrimidine dimer glycosylase), which specifically cleaves UV light-induced DNA lesions; the other was mock-digested with buffer only. These samples were run in parallel on a 0.6% alkaline agarose gel under denaturing conditions and Southern-transferred onto Hybond N+ nylon membrane (Amersham Biosciences) (26). The membrane was hybridized with biotin-labeled strand-specific RNA probes and then hybridized with HRP-conjugated anti-streptavidin secondary antibody and developed with an ECL kit. RNA probes complementary to the transcribed or non-transcribed strand of the mouse DHFR gene were synthesized from plasmids pDKR25 and pDKR5 (kindly provided by Dr. Isabel Mellon, University of Kentucky), respectively (27). The quantity of DNA damage was determined by the ratio of the hybridization signal intensity in the T4 endonuclease V-treated DNA to the signal in the mock-treated DNA at each time point in each cell type.
Quantitative Real-time PCR (qPCR) Analysis of Gene Expression
For RT-PCR and qPCR analysis of TR4 and CSB mRNA expression, total RNA was isolated from TR4+/+ and TR4−/− mouse tissues or from cells using TRIzol® reagent (Invitrogen). The relative abundance of target mRNA was quantified relative to control β-actin gene expression from the same reaction. The sequences for sense and antisense strand PCR primer are as follows: TR4, 5′-CATATTCACCACCTCGGACAAC-3′ (sense) and 5′-TGACGCCACAGACCACAC-3′ (antisense); CSB, 5′-CCACTCAAGTCAAACTCAGGAG-3′ (sense) and 5′-ATCTGATGTCGGTCGATGTGC-3′ (antisense); and β-actin, 5′-TGTGCCCATCTACGAGGGGTATGC-3′ (sense) and 5′-GGTACATGGTGGTGCCGCCAGACA-3′ (antisense).
qPCR amplifications of reverse-transcribed first-strand DNA samples were performed using the iCycler iQTM PCR cycler (Bio-Rad). Relative quantification of PCR products was based upon value differences between the target and β-actin control using the 2−ΔΔCT method (28). Each sample was analyzed in triplicate in assays performed three separate times.
Luciferase Assay
CV-1 cells were cultured in 24-well plates and cotransfected with plasmid pGL-CSB-Luc, internal control plasmid pRL-TK, and either pCMX-TR4 or pCMX vector. After 24 h, the cells were harvested, and luciferase assays were performed using the Dual-Luciferase kit (Promega). Firefly luciferase activity (pGL-CSB) was normalized to Renilla luciferase activity (pRL-TK).
H1299 cells were cultured in 24-well plates and cotransfected with plasmid pGL-CSB, internal control plasmid pRL-TK, and either pRetro-siTR4 or a scrambled control. After 24 h, the cells were harvested, and luciferase assays were performed using the Dual-Luciferase kit. Firefly luciferase activity (pGL-CSB) was normalized to Renilla luciferase activity (pRL-TK).
For UV irradiation-induced luciferase activity assay, cells were seeded and transfected as described above. 24 h after transfection, cells were exposed to 30 J/m2 UVC light (254 nm). After different time intervals, cells were harvested, and luciferase assays were performed as described above for other luciferase assays. Western blotting and ChIP assays were carried out as described previously (5, 29).
Statistical Analysis
The data are presented as means ± S.D. Student's t test was used for comparisons between groups. A p value of <0.05 was considered statistically significant.
RESULTS
TR4 Is Involved in Regulating the Response to UV Light in Different Types of Cells
To investigate whether TR4 is involved in the UV light-induced cellular response in different cell types, we first compared cell survival following UV irradiation of WT (TR4+/+) versus TR4 knock-out (TR4−/−) MDFs. Compared with TR4+/+ MDFs, TR4−/− cells showed increased sensitivity to UV light-induced growth inhibition (supplemental Fig. S1A). We confirmed these results in H1299 lung cells, which express high levels of endogenous TR4. Cells expressing siRNA targeting TR4 or a scrambled control were exposed to different doses of UV irradiation, and the numbers of viable cells were measured 3 days later. Knockdown of TR4 again resulted in increased sensitivity to UV irradiation (supplemental Fig. S1B). Together, these results indicate a role for TR4 in modulating UV sensitivity in different types of cells.
TR4 Modulates DNA Repair Specifically through the TC-NER (but Not GG-NER) Pathway
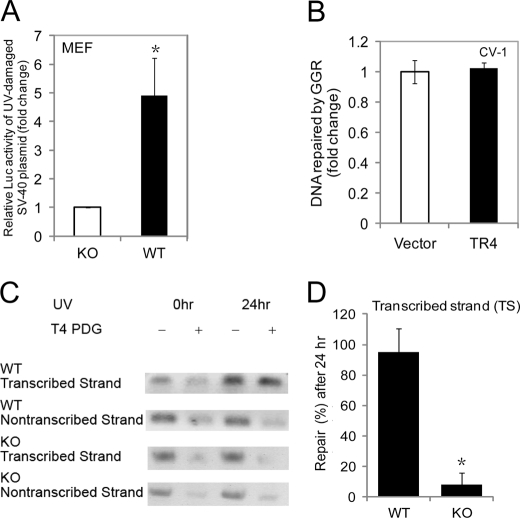
We next investigated whether TR4 modulates the UV light-induced cellular response through DNA repair. We first used an in vivo DNA repair assay (22) that monitors reactivation of promoter activity upon repair of previously UV light-damaged reporter plasmid DNA. TR4+/+ and TR4−/− MEFs were transfected with a UV light-damaged reporter plasmid (pRL-SV40). TR4+/+ MEFs displayed a greater reactivation of SV40 promoter-dependent luciferase activity compared with TR4−/− MEFs (Fig. 1A), suggesting that TR4 promotes DNA repair upon UV irradiation. Similarly, CV-1 cells that were cotransfected with the UV light-damaged pRL-SV40 plasmid and the pCMX-TR4 plasmid also displayed a higher reactivation of SV40 promoter-dependent luciferase activity than cells cotransfected with the empty pCMX vector (supplemental Fig. S2A). Interestingly, a GG-NER assay (22), bacterial pBluescript vector DNA, which is transcriptionally inactive in mammalian cells, showed a similar repair efficiency by the GG-NER pathway as measured by qPCR in CV-1 cells overexpressing TR4 or the vector control (Fig. 1B). These results suggest that TR4 is involved in TC-NER but not GG-NER. We then analyzed directly the integrity of TC-NER function in MEFs isolated from TR4+/+ and TR4−/− mice. The difference in repair kinetics between the preferentially repaired transcribed strand and the non-transcribed strand of the housekeeping gene DHFR was evident in the TR4+/+ MEFs, as 24 h after irradiation, DNA lesions in the DHFR gene were rapidly removed from the transcribed strand by TC-NER, whereas the non-transcribed strand was slowly repaired by the GG-NER pathway (Fig. 1C, first and second panels, and D) (30). However, the transcribed strand was not repaired efficiently in TR4−/− MEFs (Fig. 1C, third panel, and D), similar to the non-transcribed strand in both TR4+/+ and TR4−/− MEFs (Fig. 1C, second and fourth panels), consistent with previous studies on the rate of repair of the non-transcribed strand (30). These results further confirm that TR4 is specifically involved in TC-NER. Because TC-NER deficiency is often associated with transcription arrest (17), we also examined the recovery of RNA synthesis after UV exposure in MEFs. We found that 24 h after UV exposure, the recovery of RNA synthesis in TR4−/− MEFs was lower than in TR4+/+ cells (supplemental Fig. S2B). Thus, our data suggest that TR4 modulates UV light-induced DNA repair specifically via the TC-NER pathway but not via the GG-NER pathway.
FIGURE 1.
TR4 regulates DNA repair specifically through the TC-NER pathway but not the GG-NER pathway. A, TR4+/+ and TR4−/− MEF cells were transfected with the pRL-SV40 luciferase reporter plasmid that had been damaged before transfection by 5000 J/m2 UV irradiation. 48 h after transfection, cells were harvested, and luciferase activities were measured. The mean ± S.D. from triplicate samples was calculated and is plotted. *, p < 0.05 versus the control. KO, knock-out. B, CV-1 cells were cotransfected with pBluescript plasmid that had been damaged before transfection by 5000 J/m2 UV irradiation together with pCMX-TR4 or pCMX vector control. 48 h after transfection, DNA was isolated from cells and subjected to qPCR. The mean ± S.D. from triplicate samples was calculated and is plotted. Similar results were found at 72 h after transfection (data not shown). GGR, global genomic repair. C and D, representative chemiluminescent blots (C) and quantified graphical presentation (D) of transcribed strand-specific repair of the DHFR gene in TR4+/+ and TR4−/− MEFs. (Repair at 0 h was set as 0%.) Results shown in C are representative of three independent experiments. TR4+/+ and TR4−/− fibroblasts were irradiated with 10 J/m2 UV light and allowed to repair for the indicated times. Genomic DNA was EcoRI-digested, treated (+) or mock-treated (−) with T4 endonuclease V (T4 pyrimidine dimer glycosylase (PDG), which specifically cleaves UV light-induced DNA lesions), and electrophoresed under denaturing conditions. Southern blot analysis was performed using probes specific for the transcribed or non-transcribed strand of the DHFR gene. After measurement of the amount of chemiluminescence intensity, the percentage of repair was calculated. The mean ± S.D. from three independent experiments was calculated and is plotted. *, p < 0.05 versus the control. Note that 24 h after irradiation, transcribed strand-specific repair of the DHFR gene by the TC-NER pathway was absent in TR4−/− MEFs, whereas transcribed strand-specific repair was complete in TR4+/+ MEFs; no significant repair of the non-transcribed strand was observed in either TR4−/− or TR4+/+ MEFs. 0hr, immediately after irradiation; 24hr, 24 h after irradiation.
CSB Expression Is Dependent on TR4
To understand the molecular mechanisms by which TR4 modulates the NER pathway, we examined the expression of a battery of NER genes in TR4+/+ and TR4−/− mouse tissues and cells. We found that CSB, a member of the TC-NER pathway, but not other NER genes, including CSA, XPD, XPC, XPF, XPG, ERCC1, and DDB2, was significantly reduced in TR4−/− mouse skeletal muscle compared with the TR4+/+ control (Fig. 2A). CSB mRNA was also reduced by 10-fold in TR4−/− MDFs and MEFs compared with TR4+/+ controls (Fig. 2B). We also examined CSB protein levels in C2C12 myoblast cells transfected with TR4 siRNA or a scrambled vector. As in mouse fibroblasts and tissues, the expression level of CSB protein was lower in TR4 knockdown cells (Fig. 2C). These results suggest that TR4 regulates CSB expression and are consistent with a role for TR4 in the TC-NER pathway.
FIGURE 2.
Expression of CSB is reduced in TR4-deficient tissues and cells. A, mRNAs extracted from skeletal muscle tissues from TR4−/− mice and their TR4+/+ littermates were subsequently subjected to qPCR. The relative mRNA levels compared with TR4+/+ tissues were calculated. **, p < 0.01 versus TR4+/+. The β-actin mRNA level was used as the internal control. KO, knock-out. B, mRNAs purified from TR4+/+ and TR4−/− MEFs and MDFs were subjected to qPCR. The relative CSB mRNA level compared with TR4+/+ cells was calculated. **, p < 0.01 versus TR4+/+. The β-actin mRNA level was used as the internal control. C, protein extracted from C2C12 cells expressing TR4 siRNA (si-TR4) or a scrambled control (Ctrl) was subjected to Western blot analysis of TR4 (middle panel) and CSB (upper panel). Tubulin served as a loading control (lower panel). The mean ± S.D. from triplicate samples was calculated and is plotted in A and B.
CSB Is a Direct Target Gene of TR4
To dissect further the mechanism by which TR4 mediates CSB expression, we tested whether TR4, a transcription factor, directly regulates the promoter activity of the CSB gene. When we cotransfected the CSB(−961)-Luc vector along with either the pCMX vector or increasing amounts of pCMX-TR4 into CV-1 cells, we found that TR4 activated CSB(−961)-Luc transcription by up to 16-fold in a dose-dependent manner (Fig. 3A).
FIGURE 3.
CSB is a direct target gene of TR4. A, increasing amounts of the pCMX-TR4 expression plasmid were cotransfected with the pGL3-CSB(−961)-Luc reporter into CV-1 cells. After 48 h, cells were harvested, and luciferase activities were measured. Error bars represent the means ± S.D. of three independent experiments. *, p < 0.05 versus the control. B, schematic diagram of 5′-serial deletions of the CSB promoter-reporter constructs (left panel). The CSB promoter-reporter constructs were cotransfected with pCMX-TR4 or empty vector into CV-1 cells. After 48 h, cells were harvested, and luciferase activities were measured (right panel). *, p < 0.05; **, p < 0.01. C, ChIP assay using a TR4-specific antibody in H1299 cells. PCR amplification of the human CSB promoter covers regions −641 to −392 and −874 to −622. First lane, input control; second lane, control immunoprecipitation with normal mouse IgG; third lane, immunoprecipitation with a TR4-specific monoclonal antibody (No. 15).
We next identified the region of the CSB 5′-promoter that is essential for the response to TR4 by constructing two deletion mutants of the CSB promoter-reporter plasmid, CSB(−661)-Luc and CSB(−301)-Luc (Fig. 3B, left panel). The results showed that TR4-dependent transcriptional activity was reduced by 50% with CSB(−661)-Luc and almost totally lost with CSB(−301)-Luc compared with full-length CSB(−961)-Luc (Fig. 3B, right panel). Thus, the region between nucleotides −961 and −301 of the CSB gene is important for TR4-induced transcriptional activity. We then applied a ChIP assay to test whether TR4 binds directly to the CSB gene promoter and found that region −874 to −622 of the CSB promoter was efficiently recovered from immunoprecipitates using anti-TR4 antibodies but not from immunoprecipitates using control IgG (Fig. 3C), suggesting that TR4 directly binds to the CSB promoter. Together, these results strongly suggest that CSB is a direct target gene of TR4.
Reconstitution of CSB in TR4 Knock-out Cells Alleviates UV Hypersensitivity
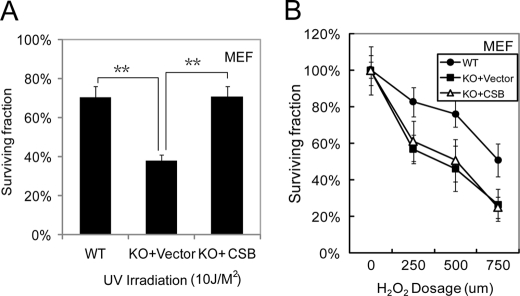
The results shown above suggest that TR4 mediates UV sensitivity via regulation of the DNA repair protein CSB. To test whether reduction of CSB levels in TR4−/− cells is the key factor responsible for UV hypersensitivity in these cells, a CSB cDNA-expressing plasmid (pCI-CSB) was transfected into TR4−/− cells, and cellular UV responses in TR4+/+, TR4−/−/empty vector, and TR4−/−/CSB MEFs were examined. As shown in Fig. 4A, reconstitution of CSB increased the cell survival rate of TR4−/− MEFs upon UV exposure compared with TR4−/−/empty vector MEFs. This result strongly implies that the reduction in CSB levels in TR4−/− cells leads to UV hypersensitivity. It also explains the higher apoptotic rate of TR4−/− cells upon UV overexposure because CSB-deficient cells show increased apoptosis (31).
FIGURE 4.
Reconstitution of CSB in TR4-deficient cells alleviates UV hypersensitivity. A, TR4+/+, TR4−/−/vector, and TR4−/−/CSB MEF cells were exposed to 10 J/m2 UV light. 72 h after UV exposure, cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The percentages of surviving cells compared with controls without UV exposure were calculated and are plotted. **, p < 0.05 versus the control. B, TR4+/+, TR4−/−/vector, and TR4−/−/CSB MEF cells were exposed to H2O2 for 30 min at different doses as indicated. 48 h after H2O2 treatment, cell viability was measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The percentages of surviving cells compared with controls without H2O2 treatment were calculated and are plotted. The mean ± S.D. from triplicates is shown in A and B. KO, knock-out.
Because CSB may also be involved in oxidative damage repair (32), and TR4−/− cells are less resistant to oxidative stress (33), we investigated whether restoration of CSB would confer oxidative stress resistance to TR4−/− cells. However, no difference in survival rate after H2O2 challenge was observed in TR4−/−/vector and TR4−/−/CSB cells (Fig. 4B), indicating that CSB alone might not be sufficient to rescue the deficiency in the oxidative stress defense system in TR4−/− cells. Thus, TR4 specifically modulates UV sensitivity through the DNA repair protein CSB.
DISCUSSION
The TR4 nuclear receptor has been found to play an essential role in cellular signal transduction when activated by upstream activators/ligands, including polyunsaturated fatty acids, or higher concentrations of vitamin A (34–36). We have demonstrated here that TR4 modulates cellular UV sensitivity through a member of the TC-NER pathway, CSB. UV irradiation is one of the major external insults to cells and is clearly linked to skin cancer (37). Despite the great improvement in understanding the NER machinery that repairs UV light-induced DNA damage (18, 38), some questions still remain. For example, how exactly does CSB function upon UV exposure? How is CSB function regulated? Our data demonstrated that TR4 directly modulates CSB transcription and thus provides a novel pathway in CSB functional regulation.
Our results might also add one more dimension of mechanism to our previous finding that TR4 regulates the apoptotic response upon UV overexposure via Bcl-2 (8) because CSB-deficient cells also have increased apoptosis (31). p53 has been previously linked to the cellular UV response (39), and p53 up-regulation was observed in CSBm/m mice (40), but the TR4-regulated UV response is p53-independent because TR4 functions in the p53-null cell line H1299 (supplemental Fig. S1B). Thus, we have identified a new p53-independent signaling pathway in the cellular UV response.
TR4 has been implicated in the oxidative response working as a FOX3a downstream mediator (33), and TR4−/− cells have higher reactive oxygen species levels (41). A handful of studies also reveal the involvement of CSB in oxidative DNA damage repair (42, 43). However, the hypersensitivity to oxidative stress was not alleviated in CSB-restored TR4-deficient cells (Fig. 4B), indicating that reduction of CSB levels alone is not solely responsible for the oxidative stress hypersensitivity in TR4-deficient cells.
Other than regulating CSB transcription by directly binding to the CSB promoter (Fig. 3C), TR4 might also mediate CSB activity through an indirect mechanism. The luciferase reporter assay showed that the CSB promoter region between residues −661 and −301 contributes to the induction of CSB transcriptional activity by TR4 (CSB(−661)-Luc versus CSB(−301)-Luc) (Fig. 3B), whereas the ChIP assay indicated no direct binding of TR4 to this region (Fig. 3C). Further investigation is needed to determine the pathway involved in this indirect mechanism. Interestingly, TR4−/− mice demonstrate a number of the phenotypes observed in Cockayne syndrome patients, including growth retardation (3), demyelination (44), and neuron loss (4). Does CSB deficiency contribute to these defects in TR4−/− mice? Further investigation is needed to answer these questions. Our findings illuminate a new aspect of TR4 function and reveal a new pathway contributing to cellular UV sensitivity and a new mechanism of CSB regulation, which may help to delineate a clearer and more complete picture of the cellular UV response and may aid in the development of new treatments for UV light-sensitive syndromes and skin cancer.
Supplementary Material
Acknowledgments
We thank Dr. Jan H. J. Hoeijmakers for providing the pCI-CSB plasmid and Dr. Isabel Mellon for the pDKR5 and pDKR25 plasmids. We thank Dr. Louise Silver-Morse for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK073414 and CA127548. This work was also supported by the George Whipple Professorship Endowment and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 (to the China Medical University, Taiwan).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- TR4
- testicular nuclear receptor 4
- MEF
- mouse embryonic fibroblast
- NER
- nucleotide excision repair
- GG
- global genomic
- TC
- transcription-coupled
- CSB
- Cockayne syndrome B protein
- Luc
- luciferase
- qPCR
- quantitative real-time PCR
- MDF
- mouse dermis-derived fibroblast.
REFERENCES
- 1. Tanabe O., Shen Y., Liu Q., Campbell A. D., Kuroha T., Yamamoto M., Engel J. D. (2007) Genes Dev. 21, 2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Young W. J., Smith S. M., Chang C. (1997) J. Biol. Chem. 272, 3109–3116 [DOI] [PubMed] [Google Scholar]
- 3. Collins L. L., Lee Y. F., Heinlein C. A., Liu N. C., Chen Y. T., Shyr C. R., Meshul C. K., Uno H., Platt K. A., Chang C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15058–15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Y. T., Collins L. L., Uno H., Chang C. (2005) Mol. Cell. Biol. 25, 2722–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu N. C., Lin W. J., Kim E., Collins L. L., Lin H. Y., Yu I. C., Sparks J. D., Chen L. M., Lee Y. F., Chang C. (2007) Diabetes 56, 2901–2909 [DOI] [PubMed] [Google Scholar]
- 6. Kim E., Liu N. C., Yu I. C., Lin H. Y., Lee Y. F., Sparks J. D., Chen L. M., Chang C. (2011) Diabetes 60, 1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kang H. S., Okamoto K., Kim Y. S., Takeda Y., Bortner C. D., Dang H., Wada T., Xie W., Yang X. P., Liao G., Jetten A. M. (2011) Diabetes 60, 177–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim E., Ma W. L., Lin D. L., Inui S., Chen Y. L., Chang C. (2007) Biochem. Biophys. Res. Commun. 361, 323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cleaver J. E., Lam E. T., Revet I. (2009) Nat. Rev. Genet. 10, 756–768 [DOI] [PubMed] [Google Scholar]
- 10. Nouspikel T. (2009) Cell. Mol. Life Sci. 66, 994–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Licht C. L., Stevnsner T., Bohr V. A. (2003) Am. J. Hum. Genet. 73, 1217–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuo J., Jaruga P., Rodriguez H., Dizdaroglu M., Bohr V. A. (2002) J. Biol. Chem. 277, 30832–30837 [DOI] [PubMed] [Google Scholar]
- 13. Selby C. P., Sancar A. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 11205–11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Filippi S., Latini P., Frontini M., Palitti F., Egly J. M., Proietti-De-Santis L. (2008) EMBO J. 27, 2545–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Newman J. C., Bailey A. D., Weiner A. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fousteri M., Mullenders L. H. (2008) Cell Res. 18, 73–84 [DOI] [PubMed] [Google Scholar]
- 17. Proietti-De-Santis L., Drané P., Egly J. M. (2006) EMBO J. 25, 1915–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanawalt P. C., Spivak G. (2008) Nat. Rev. Mol. Cell Biol. 9, 958–970 [DOI] [PubMed] [Google Scholar]
- 19. Horibata K., Iwamoto Y., Kuraoka I., Jaspers N. G., Kurimasa A., Oshimura M., Ichihashi M., Tanaka K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15410–15415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mallery D. L., Tanganelli B., Colella S., Steingrimsdottir H., van Gool A. J., Troelstra C., Stefanini M., Lehmann A. R. (1998) Am. J. Hum. Genet. 62, 77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim E., Yang Z., Liu N. C., Chang C. (2005) Biochem. Biophys. Res. Commun. 328, 85–90 [DOI] [PubMed] [Google Scholar]
- 22. Tran H., Brunet A., Grenier J. M., Datta S. R., Fornace A. J., Jr., DiStefano P. S., Chiang L. W., Greenberg M. E. (2002) Science 296, 530–534 [DOI] [PubMed] [Google Scholar]
- 23. Yang Y., Wang X., Dong T., Kim E., Lin W. J., Chang C. (2003) J. Biol. Chem. 278, 7709–7717 [DOI] [PubMed] [Google Scholar]
- 24. Christmann M., Tomicic M. T., Origer J., Aasland D., Kaina B. (2006) Nucleic Acids Res. 34, 6530–6539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mellon I., Rajpal D. K., Koi M., Boland C. R., Champe G. N. (1996) Science 272, 557–560 [DOI] [PubMed] [Google Scholar]
- 26. Spivak G., Pfeifer G. P., Hanawalt P. (2006) Methods Enzymol. 408, 223–246 [DOI] [PubMed] [Google Scholar]
- 27. Plosky B., Samson L., Engelward B. P., Gold B., Schlaen B., Millas T., Magnotti M., Schor J., Scicchitano D. A. (2002) DNA Repair 1, 683–696 [DOI] [PubMed] [Google Scholar]
- 28. Livak K. J., Schmittgen T. D. (2001) Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 29. Liu N. C., Lin W. J., Yu I. C., Lin H. Y., Liu S., Lee Y. F., Chang C. (2009) Endocrine 36, 211–217 [DOI] [PubMed] [Google Scholar]
- 30. van der Horst G. T., van Steeg H., Berg R. J., van Gool A. J., de Wit J., Weeda G., Morreau H., Beems R. B., van Kreijl C. F., de Gruijl F. R., Bootsma D., Hoeijmakers J. H. (1997) Cell 89, 425–435 [DOI] [PubMed] [Google Scholar]
- 31. Balajee A. S., Proietti De Santis L., Brosh R. M., Jr., Selzer R., Bohr V. A. (2000) Oncogene 19, 477–489 [DOI] [PubMed] [Google Scholar]
- 32. Gorgels T. G., van der Pluijm I., Brandt R. M., Garinis G. A., van Steeg H., van den Aardweg G., Jansen G. H., Ruijter J. M., Bergen A. A., van Norren D., Hoeijmakers J. H., van der Horst G. T. (2007) Mol. Cell. Biol. 27, 1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li G., Lee Y. F., Liu S., Cai Y., Xie S., Liu N. C., Bao B. Y., Chen Z., Chang C. (2008) Endocrinology 149, 3490–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou X. E., Suino-Powell K. M., Xu Y., Chan C. W., Tanabe O., Kruse S. W., Reynolds R., Engel J. D., Xu H. E. (2011) J. Biol. Chem. 286, 2877–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsai N. P., Huq M., Gupta P., Yamamoto K., Kagechika H., Wei L. N. (2009) Biochim. Biophys. Acta 1789, 734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie S., Lee Y. F., Kim E., Chen L. M., Ni J., Fang L. Y., Liu S., Lin S. J., Abe J., Berk B., Ho F. M., Chang C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 13353–13358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corona R. (1996) Ann. Ist. Super. Sanita 32, 37–42 [PubMed] [Google Scholar]
- 38. Anindya R., Mari P. O., Kristensen U., Kool H., Giglia-Mari G., Mullenders L. H., Fousteri M., Vermeulen W., Egly J. M., Svejstrup J. Q. (2010) Mol. Cell 38, 637–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaulian E., Schreiber M., Piu F., Beeche M., Wagner E. F., Karin M. (2000) Cell 103, 897–907 [DOI] [PubMed] [Google Scholar]
- 40. Laposa R. R., Huang E. J., Cleaver J. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1389–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee Y. F., Liu S., Liu N. C., Wang R. S., Chen L. M., Lin W. J., Ting H. J., Ho H. C., Li G., Puzas E. J., Wu Q., Chang C. (2011) Am. J. Physiol. Endocrinol. Metab. 301, E91–E98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tuo J., Jaruga P., Rodriguez H., Bohr V. A., Dizdaroglu M. (2003) FASEB J. 17, 668–674 [DOI] [PubMed] [Google Scholar]
- 43. Stevnsner T., Nyaga S., de Souza-Pinto N. C., van der Horst G. T., Gorgels T. G., Hogue B. A., Thorslund T., Bohr V. A. (2002) Oncogene 21, 8675–8682 [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y., Chen Y. T., Xie S., Wang L., Lee Y. F., Chang S. S., Chang C. (2007) Mol. Endocrinol. 21, 908–920 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.