Background: α6β3*-Nicotinic receptors (nAChRs) are physiologically important but difficult to express heterologously.
Results: Influences of β3 subunits on α6β3*-nAChR function are impacted by α6 subunit N-terminal domain loop E residues.
Conclusion: There are unexpected roles for the complementary face of the nAChR α6 subunit in receptor function.
Significance: Novel medicinals acting at new sites on α6β3*-nAChRs could be useful antidepressants and/or smoking cessation aids.
Keywords: Ion Channels, Ligand-binding Protein, Neurotransmitter Receptors, Nicotinic Acetylcholine Receptors, Receptor Structure-Function
Abstract
Despite the apparent function of naturally expressed mammalian α6*-nicotinic acetylcholine receptors (α6*-nAChR; where * indicates the known or possible presence of additional subunits), their functional and heterologous expression has been difficult. Here, we report that coexpression with wild-type β3 subunits abolishes the small amount of function typically seen for all-human or all-mouse α6β4*-nAChR expressed in Xenopus oocytes. However, levels of function and agonist potencies are markedly increased, and there is atropine-sensitive blockade of spontaneous channel opening upon coexpression of α6 and β4 subunits with mutant β3 subunits harboring valine-to-serine mutations at 9′- or 13′-positions. There is no function when α6 and β2 subunits are expressed alone or in the presence of wild-type or mutant β3 subunits. Interestingly, hybrid nAChR containing mouse α6 and human (h) β4 subunits have function potentiated rather than suppressed by coexpression with wild-type hβ3 subunits and potentiated further upon coexpression with hβ3V9′S subunits. Studies using nAChR chimeric mouse/human α6 subunits indicated that residues involved in effects seen with hybrid nAChR are located in the α6 subunit N-terminal domain. More specifically, nAChR hα6 subunit residues Asn-143 and Met-145 are important for dominant-negative effects of nAChR hβ3 subunits on hα6hβ4-nAChR function. Asn-143 and additional residues in the N-terminal domain of nAChR hα6 subunits are involved in the gain-of-function effects of nAChR hβ3V9′S subunits on α6β2*-nAChR function. These studies illuminate the structural bases for effects of β3 subunits on α6*-nAChR function and suggest that unique subunit interfaces involving the complementary rather than the primary face of α6 subunits are involved.
Introduction
There are at least six different nicotinic acetylcholine receptor (nAChR)3 α subunits (α2–α7) and three nAChR β subunits (β2–β4) expressed in the mammalian central nervous system (1). nAChR α7 subunits are thought principally to form homopentameric receptors when expressed in heterologous expression systems, whereas the other indicated subunits are thought to assemble into heteropentameric structures containing various combinations of α and β subunits. nAChR β3 and α5 subunits are considered to be “wild cards,” as they do not form functional receptors when expressed alone or in binary complexes with any other single subunit. However, they seem capable of integrating as “accessory” subunits into complexes containing at least one other α and one other β subunit. α6*-nAChR (where the * indicates the known or possible presence of additional subunits in the complex) are expressed in the mammalian brain, predominantly in dopaminergic midbrain regions implicated in pleasure, reward, and drug (including nicotine) dependence; they modulate dopamine release and could be involved in schizophrenia and Parkinson disease (2–7). nAChR α6 and β3 subunit messages share very similar expression patterns, and studies using knock-out animal and α-conotoxin sensitivity assessments suggest that β3 subunit incorporation is important in the assembly and stability of mature α6*-nAChR, which also must have channel functions.
Mammalian α6*-nAChR are thought to naturally exist as combinations of α6 with β2 alone or with addition of β3 subunits and perhaps of α6 and β4 subunits (1, 7). However, nAChR with these subunit compositions are not easily recreated in functional forms in artificial expression systems (8). Coexpression of a human α6/α4 chimeric subunits with human β4 subunits in Xenopus oocytes or HEK-293 human embryonic kidney cells results in functional receptors, although expression of wild-type α6 subunits with β4 subunits did not produce ligand binding and functional α6*-nAChR (9). Chick or rat nAChR α6 subunits form functional channels when coexpressed with hβ4 subunits in Xenopus oocytes, but the α6 plus β2 subunit combination fails to form functional channels (10). Chick α6 subunits also formed functional receptors when coexpressed with chick β2 or β4 subunits in BOSC 23 cells (11). More recently, Kuryatov et al. (8) reported functional expression of human α6β4-nAChR in Xenopus oocytes and formation of ligand-binding (nonfunctional) aggregates of α6β2-nAChR. Their study also suggested that β3 subunits may facilitate α6*-nAChR trafficking to the cell membrane.
Recently (12), it was observed that coexpression with a large excess of human nAChR wild-type β3 subunits has a dominant-negative effect on the function of human α6*-nAChR, whereas coexpression with a large excess of human mutant β3 subunits (valine 273 to serine at position 9′ in the putative second transmembrane domain; β3V273S = β3V9′S) potentiates the function of human α6β2*- and α6β4*-nAChR. This observation is surprising, because knock-out studies strongly suggest that the naturally expressed murine α6*-nAChR containing β2 and β3 subunits are functional and that α6 and β3 subunits are needed to show sensitivity of these receptors to certain α-conotoxins (4–7).
To re-explore and expand on the prior findings, we further characterized human (h) or mouse (m) α6β3*-nAChR heterologously expressed in Xenopus oocytes. Further study employing hybrid nAChR containing subunits from different species, and use of chimeric subunits having sequences for a given subunit from different species to guide finer site-directed mutagenesis studies, led us to find unexpectedly that amino acid residues in the N-terminal domain of α6 subunits influence sometimes the dominant-negative effects of wild-type β3 subunits and are involved in gain-of-function effects of mutant β3V9′S subunits on α6*-nAChR function. Hence, these results suggest that coassembly of β3 with α6 and β2 subunits to form functional nAChR is determined by the α6 subunit N-terminal extracellular region. These results also suggest that a novel interface between nAChR subunits exists and can influence subunit assembly and receptor function.
EXPERIMENTAL PROCEDURES
Chemicals
All chemicals for electrophysiology were obtained from Sigma. Fresh agonist (acetylcholine or nicotine) and antagonist (atropine or mecamylamine) stock solutions were made daily in Ringer's solution and diluted as needed.
Wild Type, Chimeric, or Point Mutation nAChR Subunits
cDNAs corresponding to human nAChR α6 (hα6), β2 (hβ2), β3 (hβ3), or β4 (hβ4) subunits were excised from vectors containing them and subcloned into the oocyte expression vector pGEMHE. Similarly, cDNAs representing mouse nicotinic receptor α6 (mα6), β2 (mβ2), β3 (mβ3), or β4 (mβ4) subunits (kind gifts from Dr. Jerry A. Stitzel, Department of Integrative Physiology, Institute for Behavioral Genetics, University of Colorado, Boulder) were subcloned into pGEMHE. Fully synthetic nAChR hβ2 subunit with nucleotide sequences optimized for better heterologous expression (hβ2opt supplemental Fig. 1) was generated (GENEART, Burlingame, CA), subcloned into the pCI vector (Promega, San Luis Obispo, CA), and used in some studies, and although levels of function in complexes containing optimized β2 subunits were generally slightly higher, the source of β2 subunit did not materially affect outcomes.
Two chimeric nAChR subunits mα6(1–350)/hα6(351–494) and mα6(1–236)/hα6(237–494) were constructed as sketched in Fig. 1 and as described in detail in the supplemental material. Mutations in the mβ3 (V279S and V283S) or hβ3 (V273S and V277S) subunit transmembrane domain II 9′- (V9′S) or 13′ (V13′S)-position were introduced in the pGEMHE background using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) and also confirmed. Similarly, mutations in the N-terminal domain of the human nAChR α6 subunit (N91K, K94R, K114N, N143D, M145V, N91K/K94R, and N143D/M145V) were introduced using the QuikChange II site-directed mutagenesis kit. Primers used for mutagenesis are listed in supplemental Table S1. Construct integrity of all subunits was confirmed by sequencing.
FIGURE 1.
A, sequence alignment for mouse (m) or human (h) nAChR α6 subunits (GenBankTM NP_004189.1 (Homo sapiens) and NP_067344.2 (Mus musculus); single letter code, numbering begins at translation start methionine). Symbols below sequences indicate fully (*), strongly (:) or weakly (.) conserved residues, and the lack of a symbol indicates amino acid divergence, and boldface type in hα6 subunit indicates residues given prime attention in mutagenesis studies. Underlining in the hα6 subunit sequence indicates putative transmembrane domains. Underlined and italicized type in the hα6 subunit indicates putative domains involved in ligand binding (loops A–C), and boldface and italicized type in the mα6 subunit indicates junctions for chimeric subunits. B, schematic diagrams of wild-type, human, or mouse α6 subunits or chimeric subunits. Notations are: N = N-terminal domain; I, II, III, or IV = respective transmembrane domains; C-loop = cytoplasmic loop; C = C terminus.
In Vitro Transcription
All pGEMHE plasmids were linearized immediately downstream of the 3′-polyadenylation sequence. NheI was used to linearize mα6, mβ2, mβ3, mβ3V9′S, mβ3V13′S, mβ4, hα6, hβ3, hβ3V9′S, hβ3V13′S, hβ4, hα6N91K, hα6K94R, hα6K114N, hα6N143D, hα6M145V, hα6N91K+K94R, hα6N143D+M145V, mα6(1–350)/hα6(351–494) and mα6(1–236)/hα6(237–494) subunit containing plasmids. SbfI was used to linearize hβ2 subunits. Capped mRNA was transcribed from linearized plasmids in a reaction mixture (25 μl) containing 1× transcription buffer, 1.6 mm rNTPs (Promega, San Luis Obispo, CA), 0.5 mm 7m-CAP (New England Biolabs, Ipswich, MA), 1 μl of RNasin Plus (New England Biolabs), and 1 μl of T7 RNA polymerase (New England Biolabs) following standard protocols. Integrity and quality of the cRNA were checked by electrophoresis and UV spectroscopy.
Oocyte Preparation and cRNA Injection
Female Xenopus laevis (Xenopus I, Ann Arbor, MI) were anesthetized using 0.2% Tricaine methanesulfonate (MS-222). Ovarian lobes were surgically removed from the frogs and placed in an incubation solution that consisted of (in mm) 82.5 NaCl, 2.5 KCl, 1 MgCl2, 1 CaCl2, 1 Na2HPO4, 0.6 theophylline, 2.5 sodium pyruvate, 5 HEPES, 50 mg/ml gentamycin, 50 units/ml penicillin, and 50 μg/ml streptomycin, pH 7.5. The frogs were allowed to recover from surgery before being returned to the incubation tank. The lobes were cut into small pieces and digested with 0.08 Wunsch units/ml liberase Blendzyme 3 (Roche Applied Science) with constant stirring at room temperature for 1.5–2 h. The dispersed oocytes were thoroughly rinsed with incubation solution. Stage VI oocytes were selected and incubated at 16 °C before injection. Micropipettes used for injection were pulled from borosilicate glass (Drummond Scientific, Broomall, PA) using a Sutter P87 horizontal puller, and the tips were broken with forceps to ∼40 μm in diameter. cRNA was drawn up into the micropipette and injected into oocytes using a Nanoject microinjection system (Drummond Scientific) at a total volume of ∼60 nl. To express nAChR in oocytes, about 4 ng of cRNA corresponding to each nAChR subunit was injected.
Oocyte Electrophysiology
Two to 7 days after injection, oocytes were placed in a small volume chamber and continuously perfused with oocyte Ringer solution, which consisted of (in mm) 92.5 NaCl, 2.5 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES, pH 7.5. The chamber was grounded through an agarose bridge. The oocytes were voltage-clamped at −70 mV (unless otherwise noted) to measure agonist-induced currents using Axoclamp 900A and pClamp 10.2 software (Axon Instruments, Sunnyvale, CA). The current signal was low-pass filtered at 10 Hz with the built in low-pass Bessel filter in the Axoclamp 900A and digitized at 20 Hz with Axon Digidata 1440A and pClamp10.2. Electrodes contained 3 m KCl and had a resistance of 1–2 megohms. Drugs (agonists and antagonists) were prepared daily in bath solution. Drug was applied using a ValveLink 8.2 perfusion system (Automate scientific, Berkeley, CA). 1 μm atropine was always coapplied for acetylcholine (ACh)-based recordings to eliminate muscarinic AChR (mAChR) responses. All electrophysiological measurements were conducted or checked in at least two batches of oocytes.
Experimental Controls
Injection of cRNA corresponding to one subunit alone or pairwise combinations of β3 or β3V9′S or β3V13′S subunits with either a α subunit or β2 or β4 or chimeric subunits (10–12 ng of total cRNA) did not result in the expression of functional nAChR. Current responses to 100 μm nicotine were less than 5–20 nA (data not shown).
Data Analyses
Raw data were collected and processed in part using pClamp 10.2 (Molecular Devices, Sunnyvale, CA) and a spreadsheet (Excel; Microsoft, Bellevue, WA), using peak current amplitudes as measures of functional nAChR expression and results pooled across experiments (mean ± S.E. for data from at least three oocytes). Assessment of true Imax values for different nAChR subunit combinations was made based on complete concentration-response relationships, in which mean peak current amplitudes at specified ligand concentrations were fit to the Hill equation or its variants using Prism 4 (GraphPad Software, San Diego). F-tests (p < 0.05 to define statistical significance) were carried out to compare the best fit values of log molar EC50 values across specific nAChR subunit combinations. There are limitations in the ability to compare levels of functional nAChR expression, even though we injected similar amounts of RNA for all constructs (13, 14). We made no attempt to measure or control for subunit combination-specific effects, but whenever preliminary studies revealed possible differences in peak current amplitudes, the findings were further confirmed across different subunit combinations using the same batch of oocytes and the same time between cRNA injection and recording. Whenever we make statements about results comparing ligand potencies and efficacies across subunit combinations, the observations are clear and significant (one-way analyses of variance followed by Tukey's multiple comparison tests).
RESULTS
Wild-type or Mutant nAChR β3 Subunits from Either Humans or Mice Incorporate into α6β4*-nAChR
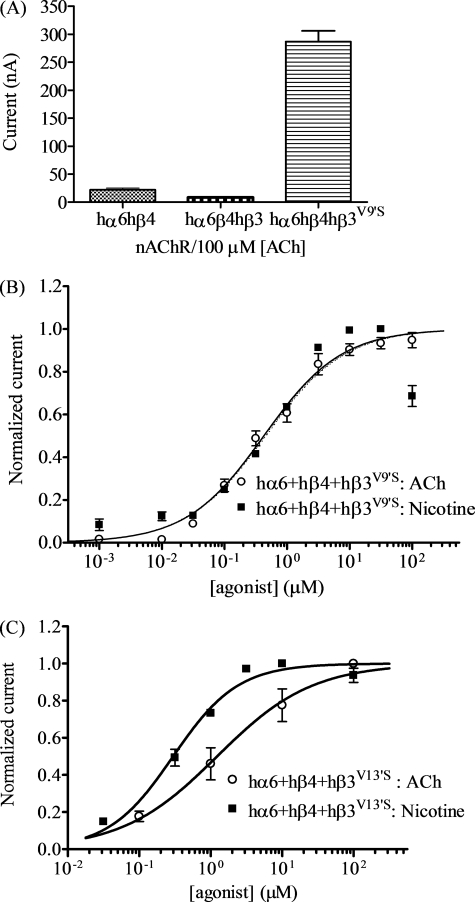
In initial studies (Fig. 2A), coexpression of nAChR wild-type hα6 and hβ4 subunits produced nicotinic responses in only about 3–5% of injected oocytes, and functional responses to 100 μm ACh when present were very modest (22 ± 3 nA; Table 1). Responses to nicotine were less reliable (Table 1). Coexpression with wild-type hβ3 subunits suppressed the modest responses to 100 μm ACh (8.4 ± 1.0 nA; Fig. 2A). By contrast, coexpression with mutant hβ3V9′S subunits significantly increased nicotinic responses to 100 μm ACh (290 ± 19 nA), and nearly every oocyte injected with nAChR hα6, hβ4, and mutant hβ3V9′S subunits expressed functional nAChR (Fig. 2A and Table 1).
FIGURE 2.
Functional properties of hα6hβ4*-nAChR. A, mean peak inward current amplitude (±S.E.; abscissa; nA) elicited by oocytes expressing the indicated human nAChR subunit combinations in response to application of 100 μm ACh. The level of nAChR function in oocytes expressing hα6 and hβ4 subunits is reduced by addition of wild-type hβ3 subunits but increased in the presence of hβ3V9′S subunits (p < 0.05). B and C, results averaged across experiments were used to produce concentration-response curves (ordinate − mean normalized current ± S.E.; abscissa − ligand concentration in log μm) for responses to ACh (○) or nicotine (■) as indicated for oocytes expressing nAChR hα6 and hβ4 subunits with either hβ3V9′S (B) or hβ3V13′S (C) subunits. Concentration-response curves for ACh and nicotine are almost superimposable for oocytes expressing hα6, hβ4, and hβ3V9′S subunits, but EC50 values for ACh and nicotine are different (p < 0.0001) for oocytes expressing hα6, hβ4, and hβ3V13′S subunits (see Table 1 for parameters).
TABLE 1.
Parameters for agonist action at nAChR containing human or mouse α6 subunits
Potencies (micromolar EC50 values with 95% confidence intervals), Hill coefficients (nH ± S.E.), mean ± S.E. efficacies (Imax in nA), and concentrations where maximal peak current amplitudes (Imax) are achieved (in μm) are provided for the indicated agonist (ACh or nicotine) acting at nAChR composed of the indicated subunits derived from the specified species and from the indicated number of independent experiments (n) based on studies as shown in the figures. ↑ or ↓ indicates a significant (p < 0.05) increase or decrease, respectively, in potency or efficacy of the indicated agonist at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit; ▴ or ▾ indicates a significant increase or decrease, respectively, in indicated agonist potency or efficacy at the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits, and △ or ▽ indicates a significant increase or decrease, respectively, in potency or efficacy of the indicated agonist at the indicated nAChR containing β3V13′S subunits relative to the same complex containing β3V9′S subunits. Note that no responses or very rare and then small responses were seen for the following subunit combinations (n = 9 each) to ACh or nicotine: hα6 + hβ2 alone or with hβ3 or hβ3V9′S or hβ3V13′S; and mα6 + mβ2 alone or with mβ3 or mβ3V9′S or mβ3V13′S. − indicates that absent or inconsistent functional responses in two-electrode voltage clamp studies precluded determination of the parameter of interest.
| Drug | nAChR subunit combinations | Potency |
Peak response |
||||
|---|---|---|---|---|---|---|---|
| n | EC50 (95% CI) | nH ± S.E. | n | Mean Imax ± S.E. | Imax concentration | ||
| μm | nA | μm | |||||
| ACh | hα6 + hβ4 | 9 | – | – | 3 | 22 ± 3 | 100 |
| hα6 + hβ4 + hβ3 | 9 | – | – | 9 | – | – | |
| hα6 + hβ4 + hβ3V9′S | 9 | 0.43 (0.34–0.54) ↑▴ | 0.69 ± 0.04 | 11 | 2100 ± 220 ↑▴ | 10 ↑▴ | |
| hα6 + hβ4 + hβ3V13′S | 3 | 1.2 (0.66–2.3) ↑ ▴ ▽ | 0.65 ± 0.10 | 3 | 910 ± 150 ↑▴ ▽ | 10 ↑▴ | |
| mα6 + mβ4 | 3 | 38 (25–58) | 0.72 ± 0.09 | 3 | 65 ± 25 | 1000 | |
| mα6 + mβ4 + mβ3 | 9 | – | – | 9 | – | – | |
| mα6 + mβ4 + mβ3V9′S | 4 | 0.42 (0.25–0.71) ↑▴ | 0.57 ± 0.08 | 3 | 2100 ± 190 ↑▴ | 100 ↑▴ | |
| mα6 + mβ4 + mβ3V13′S | 4 | 0.30 (0.22–0.41) ↑▴ | 0.71 ± 0.07 | 4 | 2400 ± 420 ↑▴ | 100 ↑▴ | |
| Nicotine | hα6 + hβ4 | 9 | – | – | 9 | – | – |
| hα6 + hβ4 + hβ3 | 9 | – | – | 9 | – | – | |
| hα6 + hβ4 + hβ3V9′S | 5 | 0.42 (0.26–0.68) ↑▴ | 0.70 ± 0.19 | 8 | 1900 ± 340 ↑▴ | 10 ↑▴ | |
| hα6 + hβ4 + hβ3V13′S | 4 | 0.30 (0.24–0.37) ↑▴ | 0.96 ± 0.08 | 4 | 1600 ± 54 ↑▴ | 10 ↑▴ | |
| mα6 + mβ4 | 3 | 26 (18–38) | 0.65 ± 0.13 | 5 | 27 ± 7 | 1000 | |
| mα6 + mβ4 + mβ3 | 9 | – | – | 9 | – | – | |
| mα6 + mβ4 + mβ3V9′S | 6 | 0.07 (0.04–0.10) ↑ ▴ | 0.89 ± 0.14 | 7 | 2500 ± 250 ↑▴ | 10 ↑▴ | |
| mα6 + mβ4 + mβ3V13′S | 4 | 0.26 (0.19–0.36) ↑▴ ▽ | 0.79 ± 0.08 | 4 | 3000 ± 360 ↑▴ | 10 ↑▴ | |
Although the small amplitudes of current in the few oocytes that yielded functional receptors when injected with cRNA encoding nAChR hα6 and hβ4 subunits plus wild-type hβ3 subunits confounded detailed analyses, studies done comparing nicotine and ACh efficacies and apparent potencies done on the same day after injection of the same batch of oocytes indicated that these agonists were equally efficacious (p > 0.05) (Table 1) at hα6hβ4hβ3V9′S-nAChR. Also, ACh and nicotine were equally potent at hα6hβ4hβ3V9′S-nAChR, yielding EC50 values of 0.43 and 0.42 μm, respectively (Fig. 2B and Table 1). Our studies also demonstrated that there also was emergence of receptor function when oocytes were injected with cRNA for nAChR hα6 and hβ4 subunits along with hβ3V13′S subunits (Fig. 2C and Table 1). Apparent potency and efficacy for nicotine did not differ across 9′ and 13′ β3 subunit mutations (EC50 values of 0.42 and 0.30 μm, respectively). However, the EC50 value for ACh of 1.2 μm at hα6hβ4hβ3V13′S-nAChR differs significantly (p = 0.0011) from that of 0.43 μm at hα6hβ4hβ3V9′S-nAChR, and average ACh efficacy also was lower for the former set of receptors (Table 1).
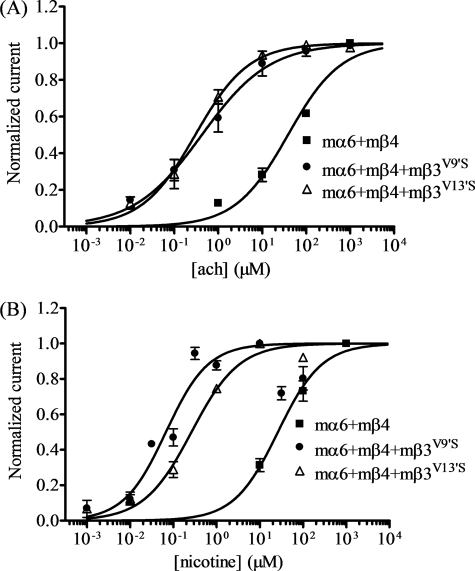
Difficulties in expressing functional α6*-nAChR from human subunits gave us pause, and so we undertook studies of α6*-nAChR heterologously expressed from mouse subunits, because the literature strongly suggests that naturally expressed mouse α6*-nAChR are functional (2–7). Many oocytes injected with cRNA encoding nAChR wild-type mα6 and mβ4 subunits yielded functional nicotinic responses (Fig. 3), but coinjection of wild-type mβ3 subunits failed to yield oocytes that responded to nicotinic agonists (Table 1). As with human subunits, oocytes expressing nAChR mα6 and mβ4 with mβ3V9′S or mβ3V13′S subunits yielded relatively large responses to nicotinic agonists (Fig. 3 and Table 1). Agonist concentration-response curves (Fig. 3) reveal that ACh and nicotine sensitivities are 100–400-fold higher for mα6mβ4mβ3V9′S- or mα6mβ4mβ3V13′S-nAChR than for mα6mβ4-nAChR (Fig. 3).
FIGURE 3.
Functional properties of mα6mβ4*-nAChR. Results averaged across experiments were used to produce concentration-response curves (ordinate − mean normalized current ± S.E.; abscissa − ligand concentration in log μm) for responses to ACh (A) or nicotine (B) as indicated for oocytes expressing nAChR mα6 and mβ4 subunits alone (■) or with either mβ3V9′S (●) or mβ3V13′S (△) subunits. Leftward shifts in agonist concentration-response curves are evident for functional nAChR containing mβ3V9′S or mβ3V13′S subunits (p < 0.0001; ∼91- and ∼130-fold, respectively, for ACh and ∼370- and 100-fold, respectively for nicotine). See Table 1 for parameters.
These results indicated that both wild-type β3 and mutant β3V9′S or β3V13′S subunits incorporate into at least some complexes containing α6 and β4 subunits. Incorporation of β3 subunits has a dominant-negative effect, reflected by lowering of levels of functional receptors (again, assuming that peak current amplitudes are legitimate proxies for functional nAChR expression levels, with the caveats about this interpretation mentioned under “Experimental Procedures: Data Analyses.” By contrast, incorporation of the nAChR β3V9′S or β3V13′S subunit produces a gain-of-function effect reflected by an increase in agonist sensitivity and in absolute levels of functional receptor expression.
There was no functional expression represented by nicotinic agonist-induced current responses in oocytes expressing nAChR α6 and β2 subunits alone, in combination with wild-type β3 subunits, or in combination with either mutant β3V9′S or β3V13′S subunits from either human or mouse (Table 1). This confounded the ability to interpret results, but they do indicate that coexpression with mutant β3V9′S or β3V13′S subunits does not have a gain-of-function effect on α6β2*-nAChR from either species, contrary to effects on α6β4*-nAChR.
Studies Using Hybrid nAChR Containing Subunits from Different Species Indicate Forms of These α6*-nAChR into Which nAChR Wild-type or Mutant β3 Subunits Can Incorporate
For a given nAChR subunit (α6, β2, β4, and β3) across species, amino acid residues are nearly perfectly matched in transmembrane domains, but there are some differences in other regions of the N terminus, first extracellular domain, and in the second, large cytoplasmic loop. We hypothesized that switching between mouse and human α6 plus β2 or β4 subunits would lead to formation of functional α6*-nAChR and/or α6*-nAChR with function influenced by β3 subunits.
Human nAChR α6 Subunits Coexpressed With mβ4 (but Not With mβ2) and hβ3V9′S Subunits Produce Functional Receptors
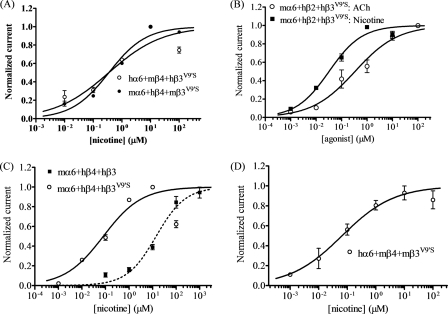
Oocytes expressing hα6 plus either mβ2 or mβ4 subunits alone or additionally coexpressing hβ3 subunits did not respond appreciably to nicotinic agonists nor were there responses in oocytes coexpressing hα6, mβ2, and hβ3V9′S subunits (Table 2). However, coexpression of hα6, mβ4, and mutant hβ3V9′S subunits yielded oocytes with functional nicotinic responses (∼40-nA peak response, see Fig. 4A and Table 2).
TABLE 2.
Parameters for drug action at hybrid α6* nAChR
Potencies (micromolar EC50 values with 95% confidence intervals for ACh or nicotine effects), Hill coefficients (nH ± S.E.), mean ± S.E. peak response (Imax in nA), and concentrations where maximal peak current amplitudes (Imax) are achieved (in μm) are provided for the indicated agonist (ACh or nicotine) acting at nAChR composed of the indicated subunits derived from the specified species and from the indicated number of independent experiments (n) based on studies as shown in the figures. ↑ or ↓ indicates a significant (p < 0.05) increase or decrease, respectively, in potency of or peak response elicited by the indicated agonist at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit. ▴ or ▾ indicates a significant increase or decrease, respectively, in indicated agonist potency or peak response at the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits. Note that no or very rare and then small responses were seen for the following subunit combinations (n = 9 each) to nicotine: hα6 + mβ2 alone or with mβ3, hβ3, hβ3V9′S, or mβ3V9′S; hα6 + mβ4 alone or with hβ3 or mβ3; mα6 + hβ2 alone or with mβ3, hβ3, or mβ3V9′S; mα6 + hβ4 alone or with mβ3. − indicates that absent or inconsistent functional responses in two-electrode voltage clamp studies precluded determination of the parameter of interest.
| Drug | nAChR subunit combinations | Potency |
Peak response |
||||
|---|---|---|---|---|---|---|---|
| n | EC50 (95% CI) | nH ± S.E. nH | n | Mean Imax ± S.E.) | Imax concentration | ||
| μm | nA | μm | |||||
| Nicotine | mα6 + hβ2 + hβ3V9′S | 4 | 0.03 (0.02–0.05) ↑▴ | 0.67 ± 0.07 | 4 | 280 ± 50 ↑▴ | 1 ↑▴ |
| hα6 + mβ4 + hβ3V9′S | 3 | 0.27 (0.1–0.73) ↑▴ | 0.46 ± 0.09 | 3 | 40 ± 2↑▴ | 10↑▴ | |
| hα6 + mβ4 + mβ3V9′S | 5 | 0.06 (0.03–0.15) ↑▴ | 0.48 ± 0.08 | 5 | 600 ± 120↑▴ | 10↑▴ | |
| mα6 + hβ4 + hβ3 | 7 | 14 (9.6–21) ↑ | 0.7 ± 0.08 | 8 | 57 ± 7 ↑ | 1000 ↑ | |
| mα6 + hβ4 + hβ3V9′S | 3 | 0.08 (0.03–0.02) ↑▴ | 0.53 ± 0.14 | 3 | 1600 ± 98 ↑▴ | 10 ↑▴ | |
| mα6 + hβ4 + mβ3V9′S | 3 | 0.42 (0.28–0.64) ↑▴ | 0.69±.08 | 3 | 1000 ± 12↑▴ | 10↑▴ | |
| ACh | mα6 + hβ2 + hβ3V9′S | 4 | 0.34 (0.18–0.62) ↑▴ | 0.52 ± 0.07 | 4 | 300 ± 90 ↑▴ | 100 ↑▴ |
FIGURE 4.
Functional properties of hybrid α6*-nAChR. Concentration (abscissa; log μm)-response (ordinate − mean normalized current ± S.E.) curves are shown for responses as follows. A, to nicotine for oocytes expressing hα6mβ4hβ3V9′S- (○) or mα6hβ4mβ3V9′S- (●) nAChR; B, to ACh (○) or nicotine (■) for oocytes expressing mαhβ2hβ3V9′S-nAChR; C, to nicotine for oocytes expressing nAChR mα6 and hβ4 subunits with either hβ3 (■) or hβ3V9′S (○) subunits; or D, to nicotine for oocytes expressing nAChR hα6, mβ4, and mβ3V9′S subunits. A leftward shift in the nicotine concentration-response curve is evident for functional nAChR containing hβ3V9′S subunits relative to nAChR containing wild-type hβ3 subunits (p < 0.0001; ∼173-fold). See Table 2 for parameters.
Mouse nAChR α6 Subunits Coexpressed with hβ4 (but Not with hβ2) and mβ3V9′S Subunits Produce Functional Receptors
Coexpression with mβ3V9′S subunits significantly increased nicotinic responses in oocytes also expressing mα6 and hβ4 subunits (∼1022-nA peak response, Fig. 4A, Table 2), although there was not formation of functional mα6hβ4- or mα6hβ4mβ3-nAChR. Also, there was no appreciable function in oocytes expressing mα6 plus hβ2 subunits alone or in the additional presence of wild-type mβ3 or mβ3V9′S subunits (Table 2).
Mouse nAChR α6 Subunits Coexpressed with hβ2 and hβ3V9′S (but Not with hβ3) Subunits Produce Functional Receptors
There is functional nAChR expression in oocytes expressing nAChR mα6, hβ2, and mutant hβ3V9′S subunits (Fig. 4B and Table 2) but not for hybrid mα6hβ2hβ3-nAChR. mα6hβ2hβ3V9′S-nAChR have maximal responses of ∼300 nA at 100 μm ACh and at 1 μm nicotine, and EC50 values are 0.34 μm for ACh and 0.03 μm for nicotine (Fig. 4B and Table 2). Concentration-response curves show little to no evidence of what would be expected to be low efficacy, low agonist sensitivity responses that could be attributed to mα6hβ2-nAChR (Fig. 4B). These studies suggest that the presence of mα6 instead of hα6 subunits is key to the function of mα6hβ2hβ3V9′S-nAChR.
Mouse nAChR α6 Subunits Coexpressed with nAChR hβ4 and hβ3 Subunits Produce Functional Receptors with Increased Agonist Sensitivity and Responsiveness
Hybrid nAChR produced in oocytes expressing mα6, hβ4, and hβ3 subunits have functional responses to nicotinic agonists that are elevated relative to the negligible to no levels of function observed for mα6hβ4-nAChR (Table 2 and Fig. 4C). Furthermore, coexpression with mutant hβ3V9′S subunits significantly increases nicotinic responses of oocytes also expressing mα6 and hβ4 subunits (Fig. 4C and Table 2), as is the case for oocytes expressing hα6, hβ4, and hβ3V9′S subunits or mα6, mβ4, and mβ3V9′S subunits. Pairwise comparisons show that differences in amplitudes of responses to nicotine are statistically significant (p < 0.001) between mα6hβ4hβ3- and mα6hβ4hβ3V9′S-nAChR (∼60 and ∼1600 nA, respectively; Table 2). Moreover, nicotine potency is increased for mα6hβ4hβ3V9′S-nAChR (EC50 = 0.08 μm) relative to that for mα6hβ4hβ3-nAChR (EC50 = 14 μm; Table 2). These results indicate that both wild-type β3 and mutant hβ3V9′S subunits incorporate into at least some complexes containing mα6 and hβ4 subunits. However, although mutant hβ3V9′S subunits have the reasonably expected gain-of-function effect, wild-type hβ3 subunits have potentiation rather than an expected, dominant-negative effect. These studies again suggest importance of mα6 instead of hα6 subunits in these effects.
Human nAChR α6 Subunits Coexpressed with nAChR mβ4 (but Not with mβ2) and mβ3V9′S Subunits Produce Functional Receptors
There is no appreciable function of nAChR in oocytes expressing hα6 plus either mβ2 or mβ4 subunits alone or in the additional presence of mβ3 subunits nor does coexpression with mβ3V9′S subunits produce functional nAChR in oocytes also expressing hα6 and mβ2 subunits (Table 2). However, coexpression with mutant mβ3V9′S subunits significantly increases nicotinic responses in oocytes also expressing hα6 and mβ4 subunits (∼600-nA peak response, nicotine EC50 = 0.06 μm; Fig. 4D and Table 2).
Evidence for Spontaneous Opening of α6*-nAChR Containing Mutant β3V9′S or β3V13′S Subunits
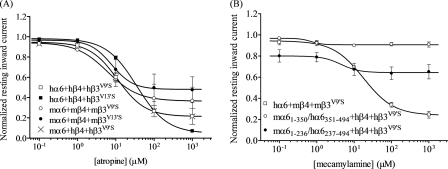
To eliminate possible contributions of muscarinic AChRs to responses in oocytes expressing nAChR α6 and other subunits, ACh always was applied in the presence of 1 μm atropine, a muscarinic receptor antagonist that at higher concentrations also can noncompetitively block nAChR function. To help define specificity of agonist action, additional studies involved regular assessment of effects on nAChR function of the nAChR antagonist, mecamylamine, which also acts noncompetitively through the open channel block. Effects of atropine or mecamylamine alone were absent when assessed using oocytes expressing any combination of wild-type nAChR subunits from any species (data not shown). However, exposure to atropine or mecamylamine alone resulted in what seemed to be outward currents in oocytes expressing α6*-nAChR containing β3V9′S or β3V13′S subunits (Table 3). These effects were reversible, in that effects on currents ceased when atropine or mecamylamine were removed. They also were typically concentration-dependent, in that the magnitudes of the apparent outward currents were largest at the highest concentrations of atropine or mecamylamine (Fig. 5).
TABLE 3.
Parameters for antagonist action at nAChR containing α6 subunits
Potencies (micromolar IC50 values with 95% confidence intervals), Hill coefficients (nH ± S.E.), mean ± S.E. efficacies (Imax in nA), and concentrations where maximal peak current amplitudes (Imax) are achieved (in μm) are provided for the indicated antagonist (atropine or mecamylamine) acting at nAChR composed of the indicated subunits derived from the specified species and from the indicated number of independent experiments (n) based on studies as shown in figures.  indicates a significant decrease in potency or efficacy of the indicated antagonist at the indicated nAChR containing β3V13′S subunits relative to the same complex containing β3V9′S subunits. − indicates that absent or inconsistent functional responses in two-electrode voltage clamp studies precluded determination of the parameter of interest.
indicates a significant decrease in potency or efficacy of the indicated antagonist at the indicated nAChR containing β3V13′S subunits relative to the same complex containing β3V9′S subunits. − indicates that absent or inconsistent functional responses in two-electrode voltage clamp studies precluded determination of the parameter of interest.
| Drug | nAChR subunit combinations | Potency |
Peak response |
Spontaneous opening | ||||
|---|---|---|---|---|---|---|---|---|
| n | IC50 (95% CI) | nH ± S.E. | n | Mean Imax ± S.E. | Imax concentration | |||
| μm | nA | μm | % total | |||||
| Atropine | hα6 + hβ4 + hβ3V9′S | 5 | 11 (5.7–21) | −1.2 ± 0.62 | 5 | 500 ± 76 | 1000 | 19 |
| hα6 + hβ4 + hβ3V13′S | 4 | 35 (30–41)
|
−1.1 ± 0.06 | 3 | 630 ± 81 | 1000 | 41 | |
| mα6 + mβ4 + mβ3V9′S | 3 | 6.1 (1–36) | −1.1 ± 0.91 | 3 | 450 ± 210 | 1000 | 15 | |
| mα6 + mβ4 + mβ3V13′S | 3 | 7.8 (1.3–48) | −1.6 ± 2.8 | 3 | 120 ± 56
|
1000 | 4 | |
| mα6 + hβ2 + hβ3V9′S | 3 | – | – | 3 | 42 ± 6 | 1000 | 13 | |
| mα6 + hβ4 + hβ3V9′S | 3 | 11 (5.7–21) | −1.20 ± 0.13 | 3 | 230 ± 7.9 | 1000 | 13 | |
| Mecamylamine | hα6 + mβ4 + mβ3V9′S | 4 | 18 (12.6–25) | −1.07 ± 0.17 | 4 | 300 ± 110 | 1000 | 33 |
| mα6(1–350)/hα6(351–494) + hβ4 + hβ3V9′S | 3 | – | – | 3 | 16 ± 1.6 | 100 | 10 | |
| mα6(1–236)/hα6(237–494) + hβ4 + hβ3V9′S | 3 | 4.4 (0.06–309) | −1.8 ± 3.62 | 3 | 46 ± 14 | 100 | 8 | |
FIGURE 5.
Evidence for spontaneous opening of α6β4*-nAChR containing mutant β3V9′S or β3V13′S subunits. A, concentration-response curves (ordinate − mean normalized current ± S.E.; abscissa − ligand concentration in log μm) are shown for apparent outward current responses as follows: A, to atropine for oocytes expressing hα6hβ4hβ3V9′S- (○); hα6hβ4hβ3V13′S- (●); mα6mβ4mβ3V9′S- (○); mα6mβ4mβ3V13′S- (●); or mα6hβ4hβ3V9′S- (×) nAChR (note that curves are superimposable for oocytes expressing hα6hβ4hβ3V9′S- or mα6hβ4hβ3V9′S-nAChR), or B, to mecamylamine for oocytes expressing hα6mβ4mβ3V9′S- (□); (mα6(1–350)/hα6(351–494))hβ4hβ3V9′S- (○) or (mα6(1–236)/hα6(237–494))hβ4hβ3V9′S- (●) nAChR. The interpretation is that coexpression with mutant β3 subunits produces nAChR that open spontaneously, producing a stable inward current that is progressively blocked in the presence of increasing concentrations of antagonists acting as open channel blockers. See Table 3 for parameters.
Inward currents elicited by nicotinic agonists from oocytes expressing nAChR α6 and β2 or β4 subunits in the presence of mutant β3 subunits were actually reversed to apparent outward currents (relative to base-line levels) in the presence of added atropine or mecamylamine, even when agonist was first applied alone prior to application of agonist in the presence of antagonist. These phenomena made it evident that atropine and mecamylamine were in fact blocking not just inward current responses to agonists but that they also were blocking resting inward currents rather than inducing outward currents. Our interpretation of these results is that α6*-nAChR also containing mutant β3 subunits and that had large functional responses to nicotinic agonists has a finite level of spontaneous channel opening that contributes to a resting inward current. This inward current can be ceased in the presence of adequately high concentrations of atropine or mecamylamine thanks to their open channel blocking abilities. It is not uncommon for gain-of-function mutations in second transmembrane domains of nAChR subunits to confer spontaneous channel opening to nAChR that contain those subunits (1). This means that atropine or mecamylamine potencies when acting alone (Table 3) actually are measures of ligand antagonist IC50 values, and additional studies assessing effects of these agents in block of agonist-induced responses yield similar IC50 values (data not shown). Antagonist potency determinations (IC50 values; typically ∼10 μm for both ligands) for atropine or mecamylamine are summarized in Table 3.
The results also can be used to estimate the number of nAChR that are spontaneously open. For example, at maximally efficacious concentrations, the atropine-alone sensitive component of its effect plus the inward current response to a full agonist (42 nA/(42 + 280 nA); Tables 2 and 3) indicate that about 13% of mα6hβ2hβ3V9′S-nAChR is spontaneously open even in the absence of agonist. Similar calculations indicate that 4–41% of α6*-nAChR containing β3V9′S or β3V13′S subunits are spontaneously open (Tables 1–4).
TABLE 4.
Parameters for drug action at nAChR containing chimeric mouse/human α6 subunits and human β2 or β4 subunits alone or in combination with wild-type or mutant human β3 subunits
Potencies (micromolar EC50 values with 95% confidence intervals for nicotine effects or micromolar IC50 values with 95% confidence intervals for mecamylamine effects), Hill coefficients (nH ± S.E.), mean ± S.E. peak response (Imax in nA) and concentrations where maximal peak current amplitudes (Imax) are achieved (in μm) are provided for the indicated agonist (nicotine) or antagonist (mecamylamine) acting at nAChR composed of the indicated subunits derived from the specified species and from the indicated number of independent experiments (n) based on studies as shown in figures. ↑ or ↓ indicates a significant (p < 0.05) increase or decrease, respectively, in potency of or peak response elicited by the indicated agonist at the indicated nAChR subtype relative to nAChR containing the same subunits but in the absence of the indicated β3 subunit. ▴ or ▾ indicates a significant increase or decrease, respectively, in indicated agonist potency or peak response at the indicated nAChR subtype relative to nAChR containing the same subunits in the presence wild-type β3 subunits. Note that no response or very rare and then small responses were seen for the following subunit combinations (n = 4–9 each) to nicotine: mα6(1–350)/hα6(351–494) + hβ2 alone or with hβ3 or hβ3V9′S; mα6(1–236)/hα6(237–494) + hβ2 alone or with hβ3 or hβ3V9′S subunits. − indicates that absent or inconsistent functional responses in two-electrode voltage clamp studies precluded determination of the parameter of interest.
| Drug | nAChR subunit combinations | Potency |
Peak response |
||||
|---|---|---|---|---|---|---|---|
| n | EC50 or IC50 (95% CI) | nH ± S.E. nH | n | Mean Imax ± S.E. | Imax concentration | ||
| μm | nA | μm | |||||
| Nicotine | mα6(1–350)/hα6(351–494) + hβ4 | 9 | – | – | 9 | – | – |
| mα6(1–350)/hα6(351–494) + hβ4 + hβ3 | 4 | 12 (8.6–15) ↑ | 1 ± 0.4 | 4 | 33 ± 14↑ | 100 | |
| mα6(1–350)/hα6(351–494) + hβ4 + hβ3V9′S | 4 | 1.8 (1.3–2.6) ↑▴ | 1.4 ± 0.14 | 4 | 140 ± 48↑▴ | 100 | |
| mα6(1–236)/hα6(237–494) + hβ4 | 3 | – | – | 3 | – | – | |
| mα6(1–236)/hα6(237–494) + hβ4 + hβ3 | 5 | 15 (9.5–22) ↑ | 1.5 ± 0.48 | 5 | 120 ± 26↑ | 100 | |
| mα6(1–236)/hα6(237–494) + hβ4 + hβ3V9′S | 4 | 1.6 (0.97–2.6) ↑▴ | 0.74 ± 0.11 | 4 | 500 ± 160↑▴ | 100 | |
Contributions of nAChR α6 Subunit N-terminal Domain to Effects of nAChR Wild-type or Mutant β3 Subunits on Function of α6*-nAChR
Deviating from the predominant, dominant-negative effect of nAChR β3 subunits on any function of α6β4-nAChR, there is facilitation of function by nAChR hβ3 subunits in mα6hβ4hβ3-nAChR (Table 2 and Fig. 4). Moreover, deviating from the predominant absence of function for α6β2-, α6β2β3-, and α6β2β3V9′S- or α6β2β3V13′S-nAChR, nAChR hβ3V9′S subunits exert a gain-of-function effect in mα6hβ2hβ3V9′S-nAChR (Table 2 and Fig. 4). These effects could only be attributed to the presence of nAChR mα6 instead of hα6 subunits in these receptors. Furthermore, the strongest indication of a gain-of-function effect of mutant β3 subunits on α6β2*-nAChR function comes from studies of hybrid receptors containing mα6, hβ2, and hβ3V9′S subunits and are not seen for complexes instead containing hα6 subunits. To understand the bases for these effects, we extended our studies to work using chimeric mouse/human α6 nAChR subunits.
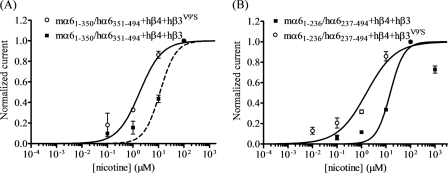
Coexpression of Chimeric mα6(1–350)/hα6(351–494) nAChR Subunits in Oocytes with nAChR hβ4 and Either Wild-type hβ3 or Mutant hβ3V9′S Subunits Produces Functional nAChR
There is functional expression represented by nicotinic agonist-induced current responses in oocytes expressing nAChR chimeric mα6(1–350)/hα6(351–494) subunits in combination with hβ4 and hβ3 or hβ3V9′S subunits (Fig. 6 and Table 4). These results indicate that the nAChR α6 subunit region from cytoplasmic loop residue Pro-350 through to the C terminus does not strongly influence effects of wild-type β3 subunits and has a limited influence on the effects of mutant β3 subunits on function of α6β4*-nAChR. Reciprocally, the results suggested that the region N-terminal to Pro-350 is likely to account for differences in effects of wild-type or mutant β3 subunits on function of hα6hβ4*-nAChR relative to effects on function of mα6hβ4*-nAChR.
FIGURE 6.
Functional properties of nAChR containing chimeric α6 subunits. A, concentration-response curves (ordinate − mean normalized current ± S.E.; abscissa − ligand concentration in log μm) are shown for responses to nicotine for oocytes expressing nAChR chimeric mα6(1–350)/hα6(351–494) subunits (A) or mα6(1–236)/hα6(237–494) subunits (B) with hβ4 subunits along with either hβ3 (■) or hβ3V9′S (○) subunits. A leftward shift in the nicotine concentration-response curve is evident for functional nAChR containing hβ3V9′S subunits relative to nAChR containing wild-type hβ3 subunits (p < 0.0001; ∼7–9-fold). See Table 4 for parameters.
Coexpression of nAChR Chimeric mα6(1–236)/hα6(237–494) Subunits in Oocytes with hβ4 and Either nAChR Wild-type hβ3 or Mutant hβ3V9′S Subunits Produces Functional Receptors
To further narrow the search for the region of the nAChR α6 subunit important for interactions with wild-type or mutant β3 subunits, we assessed function of nAChR-containing chimeric mα6(1–236)/hα6(237–494) subunits that link the N-terminal domain of the mα6 subunit to the transmembrane domains, cytoplasmic loops, and C terminus of the hα6 subunit. There is functional expression represented by nicotinic agonist-induced current responses in oocytes expressing nAChR mα6(1–236)/hα6(237–494) and hβ4 subunits in combination with wild-type nAChR hβ3 subunits (Fig. 6 and Table 4). The potentiation rather than the dominant-negative suppression of α6β4*-nAChR function in the presence of wild-type β3 subunits thus seems to be influenced by α6 subunit residues in the N-terminal extracellular domain. Although not as strong from the perspectives of nicotine potency and levels of functional expression, the N-terminal extracellular domain of α6 subunits also influences gain-of-function effects of mutant β3V9′S subunits on α6β4*-nAChR, including susceptibility to spontaneous opening sensitive to open channel block (Table 3).
Region(s) Apart from the N-terminal Domain and First Three Transmembrane Domains of nAChR α6 Subunits Seem to Be Required for the nAChR hβ3V9′S Subunit to Exert Gain-of-Function Effects on mα6hβ2*-nAChR
Oocytes coexpressing nAChR chimeric mα6(1–236)/hα6(237–494) or mα6(1–350)/hα6(351–494) subunits with hβ2 subunits alone or in the presence of wild-type hβ3 or mutant hβ3V9′S subunits do not respond to nicotinic agonists (Table 4). These results indicate that the ability of nAChR hβ3V9′S subunits to exert gain-of-function effects on mα6hβ2*-nAChR (Table 2), unlike effects on α6β4*-nAChR, must be influenced by regions aside from those in the N-terminal domains and first three transmembrane domains of nAChR α6 subunits.
Residues in the nAChR α6 Subunit That Influence Function of α6*-nAChR
Studies described above using hybrid α6*-nAChR and chimeric α6 subunits suggested that residues in the highly conserved N-terminal domain of nAChR α6 subunits influence effects of β3 subunits on α6β4*-nAChR function. It is thought that productive agonist binding occurs at selected interfaces between specific subunits in nAChR assemblies, more specifically in a pocket formed by loops A, B, and C on the + or primary face of α subunits except for α5 and loops D, E, and F on the − or complementary face of neighboring subunits (β2 or β4 in the central or autonomic nervous systems as partners to α2, α3, α4, or α6 subunits, α7 in α7-nAChR homomers, and γ or δ subunits as partners to α1 subunits in muscle-type nAChR). Consideration of the alignment of mouse and human α6 subunit amino acid sequences (Fig. 1) pointed us to residues in loop A (hα6 Lys-114) but also in loops D (hα6 Asn-91 and Lys-94) and E (hα6 Asn-143 and Met-145). Each of these residues in the nAChR hα6 subunit was mutated to their counterpart in the nAChR mα6 subunit individually or in specific combinations (i.e. hα6N91K, hα6K94R, hα6K114N, hα6N143D, hα6M145V, hα6N91K+K94R, and hα6N143D+M145V; see Fig. 1).
Residues at Positions 143 and 145 in the N-terminal Domain of nAChR α6 Subunits Influence Effects of nAChR β3 Subunits on α6β4*-nAChR Function
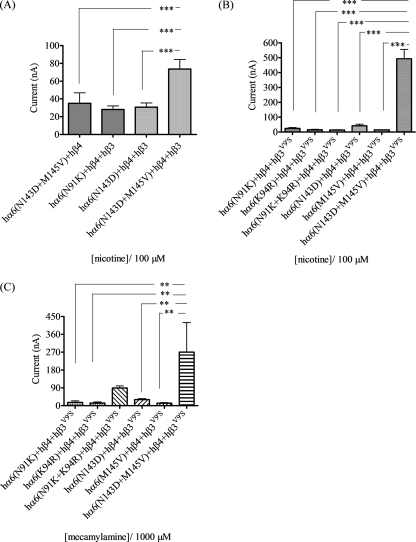
Coexpression of the double mutant hα6N143D/M145V subunit (but not any other single point mutations or the hα6N91K/K94R double mutation) with hβ4 subunits yields oocytes responding to 100 μm nicotine (mean ± S.E. peak response = 35 ± 12 nA; Fig. 7A). When the same set of mutant hα6 subunits are coexpressed with nAChR hβ4 and hβ3 subunits, significant and reproducible peak current responses (±S.E.) to 100 μm nicotine are obtained only for hα6N91Khβ4hβ3-nAChR (28 ± 4 nA), hα6N143Dhβ4hβ3-nAChR (31 ± 5 nA), and double mutant hα6N143D/M145Vhβ4hβ3-nAChR (74 ± 11 nA; Fig. 7A). However, only nAChR containing the double mutant hα6N143D/M145V subunit show the peak current response potentiation by coexpression with β3 subunits seen for mα6hβ4hβ3-nAChR.
FIGURE 7.
Effects of nAChR α6 subunit N-terminal domain amino acid substitutions on functional responsiveness of α6β4β3-nAChR to nicotinic ligands. A and B, mean (±S.E.) peak inward current responses upon exposure (10 s) to 100 μm nicotine (10 s exposure; ordinate) from oocytes (n = 4) voltage-clamped at −70 mV and heterologously expressing the indicated nAChR subunits. C, mean (±S.E.) apparent peak outward current responses upon exposure (10 s) to 1000 μm mecamylamine (10 s exposure; ordinate) from oocytes (n = 4) voltage-clamped at −70 mV and heterologously expressing the indicated nAChR subunits. *, p < 0.05; **, p < 0.01; ***, p < 0.001. hα6(N143D) subunits are present in hα6hβ4*-nAChR that have the largest amplitude responses to nicotine and the largest amplitude for mecamylamine-sensitive spontaneous openings, whether in the presence of wild-type or mutant hβ3 subunits.
There is some level of functional response (peak ± S.E.) to 100 μm nicotine in oocytes coexpressing nAChR hβ4 and hβ3V9′S subunits in combination with hα6N91K (25 ± 6 nA), hα6(K94R) (16 ± 3 nA), hα6N143D (42 ± 12 nA), hα6M145V (15 ± 1 nA), or hα6N91K+K94R (15 ± 1 nA) subunits (Fig. 7B), but none of these combinations yields responses like those seen for mα6hβ4hβ3V9′S-nAChR (∼1600 nA) or even (mα6(1–236)/hα6(237–494))hβ4hβ3V9′S-nAChR (∼140 nA) or (mα6(1–350)/hα6(351–494))hβ4hβ3V9′S-nAChR (∼500 nA). Curiously, coexpression of nAChR hβ4 and hβ3V9′S with nAChR hα6K114N subunits yields oocytes giving outward current responses to 100 μm nicotine (data not shown). Significantly, only coexpression of double mutant hα6N143D/M145V subunits in combination with hβ4 and hβ3V9′S subunits yields oocytes giving peak current responses (490 ± 63 nA) to 100 μm nicotine that rival those of mα6hβ4hβ3V9′S- or (mα6(1–236)/hα6-(237–494))hβ4hβ3V9′S-nAChR.
Upon coexpression with hβ4 and hβ3V9′S subunits, exposure to 1 mm mecamylamine elicits apparently outward currents in oocytes also injected with hα6N91K (17 ± 8 nA), hα6K94R (14 ± 5 nA), hα6N143D (90 ± 11 nA), hα6M145V (31 ± 5 nA), or hα6N91K/K94R (13 ± 3 nA) subunit cRNA (Fig. 7C). However, the largest apparently outward current is mediated by hα6N143D/M145Vhβ4hβ3V9′S-nAChR (270 + 150 nA; Fig. 7C), with an amplitude comparable with that seen for atropine action on mα6hβ4hβ3V9′S-nAChR and ∼5-fold higher than that seen for mecamylamine action at (mα6(1–236)/hα6-(237–494))hβ4hβ3V9′S-nAChR. This indicates that there is some spontaneous opening of receptors containing the indicated subunits.
These findings indicate that nAChR α6 subunit residues at positions 143 and 145 influence the ability to form functional α6β4-, α6β4β3-, and α6β4β3V9′S-nAChR, dictate whether β3 subunits have dominant-negative suppression or gain-of-function effects, and are involved in gain-of-function effects of mutant β3V9′S subunits on α6β4*-nAChR. Clearly, wild-type or mutant β3 subunits are incorporated into functional α6β4*-nAChR complexes when residues at positions 143 and 145 are the same as those in the nAChR mα6 subunit.
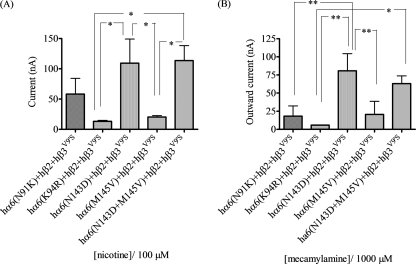
Residues at Positions 91 and 143 Are among Those in the N-terminal Domain of nAChR α6 Subunits That Influence Effects of nAChR β3V9′S Subunits on α6β2*-nAChR Function
Although studies with chimeric nAChR α6 subunits suggested that residues C-terminal to the third transmembrane domain influenced assembly of functional α6β2β3V9′S-nAChR, we also examined the effects of mα6 subunit-like mutations in the N-terminal extracellular domain of the hα6 subunit on the function of nAChR produced in oocytes upon coexpression with hβ2 subunits alone or in addition to hβ3 or hβ3V9′S subunits. Coexpression of hα6N91K, hα6K94R, hα6N143D, hα6M145V, hα6N91K+K94R, or hα6N143D+M145V subunits with nAChR hβ2 subunits alone or in combination with wild-type hβ3 subunits does not result in consistent production of function upon exposure to 100 μm nicotine (data not shown). However, and surprisingly, coexpression of hα6N91K, hα6K94R, hα6N143D, hα6M145V, or hα6N143D/M145V subunits with hβ2 and hβ3V9′S subunits resulted in production of oocytes giving inward current responses to 100 μm nicotine or apparently outward current responses to 1 mm mecamylamine (Fig. 8). Oocytes coexpressing hα6N91K+K94R subunits with hβ2 and hβ3V9′S subunits failed to produce consistent response to 100 μm nicotine or outward current responses to 1 mm mecamylamine (data not shown). These results suggest that the indicated trinary complexes are formed and have some function, although levels of functional expression and spontaneous channel opening are less than 1/3rd of that seen for mα6hβ2hβ3V9′S-nAChR.
FIGURE 8.
Effects of nAChR α6 subunit N-terminal domain amino acid substitutions on functional responsiveness of α6β2β3-nAChR to nicotinic ligands. A, mean (±S.E.) peak inward current responses upon exposure (10 s) to 100 μm nicotine (10 s exposure; ordinate) from oocytes (n = 4) voltage-clamped at −70 mV and heterologously expressing the indicated nAChR subunits. B, mean (±S.E.) apparent peak outward current responses upon exposure (10 s) to 1000 μm mecamylamine (10 s exposure; ordinate) from oocytes (n = 4) voltage-clamped at −70 mV and heterologously expressing the indicated nAChR subunits. *, p < 0.05; **, p < 0.01; ***, p < 0.001. hα6(N143D) subunits are present in hα6hβ2*-nAChR that have the largest amplitude responses to nicotine and the largest amplitude for mecamylamine-sensitive spontaneous openings, whether in the presence of wild-type or mutant hβ3 subunits.
DISCUSSION
Patterns of nAChR α6 and β3 subunit coexpression in primates or rodents in vivo (15–17) and clear evidence of the functional importance of native α6*-nAChR in the same species (2–7, 18, 19) suggest that α6β3*-nAChR exist in functional forms that should be evident upon heterologous expression. However, some of the prior studies of heterologous expression of α6*-nAChR indicated that hybrid receptors containing combinations of chicken and human nAChR subunits exhibit some level of function, although all-human or all-chicken α6*-nAChR did not (10). Moreover, and although these studies were done using oocytes manipulated to express disproportionate ratios of subunits, incorporation of nAChR hβ3 subunits present in presumed excess have a dominant-negative effect on the function of all-human α2β2*-, α2β4*-, α3β2*-, α3β4*-, α4β2*-, or α4β4*-nAChR that is reversed upon substitution of mutant, hβ3V9′S for wild-type β3 subunits (12). Similar effects are mentioned (but not described in detail) of effects of wild-type or mutant β3 subunits on function of α6β2*- and α6β4*-nAChR (11). Interestingly, other studies using chimeric (α6/α3) subunits (containing the N-terminal domain of the nAChR α6 subunit substituting for that of the otherwise α3 subunit) instead of wild-type α6 subunits showed potentiating effects of wild-type β3 subunit coexpression on α6*-nAChR (8). Here, we have extended these lines of studies to make novel and sometimes surprising findings that help to crystallize our cumulative understanding of α6β3*-nAChR function.
One of the conclusions from this work is that nAChR wild-type or mutant β3 subunits can incorporate into heterologously expressed α6β4*-nAChR, where they predominantly exert dominant-negative (wild-type β3 subunits) or gain-of-function (mutant β3V9′S or β3V13′S subunits) effects, respectively. The abilities of wild-type mβ3 subunits to mimic dominant-negative effects of wild-type hβ3 subunits suggests that β3 subunits from either species have the same features needed for negative dominance or gain-of-function. These findings are of interest for nAChR structure-function relationships (see below), but their physiological significance is tempered because there are few brain regions in rodents where all three subunits are expressed, although the circumstance may be different in primates, which might express higher levels of β4 subunits and do so more broadly (15, 17–19).
It is clear from a variety of studies, including those done using knock-out animals and nicotinic agonist-activated neurotransmitter release assays (2, 4), that naturally expressed mouse nAChR containing α6, β3, and β2 subunits seem to be functional and sensitive to blockade by specific α-conotoxins (7), but there are fewer indications that human α6β2β3*-nAChR are functional when naturally or heterologously expressed. Thus, we wondered whether there simply might be species-specific differences in the ability to heterologously express α6β2β3*-nAChR, and so we chose to see if murine α6β2β3*-nAChR could be functionally expressed when equivalent human receptors could not. However, we realized very similar outcomes in our studies of all-human or all-mouse α6β2β3*-nAChR, even when β3 subunits had gain-of-function mutations and despite success of the gain-of-function strategy when applied to α6β4β3*-nAChR. The inability of wild-type or mutant β3 subunits to influence function of α6β2*-nAChR confounds the ability to make inferences about assembly of the indicated subunits. Also, the lack of function of hα6hβ2hβ3V9′S- or hα6hβ2hβ3V13′S-nAChR observed in this work is in contrast to the observation by Broadbent et al. (12) that human α6β2β3V9′S-nAChR are functional and to our findings that hα6hβ4hβ3V9′S- or hα6hβ4hβ3V13′S-nAChR are functional.
We tried to solve this problem by using two different approaches. One approach involved switching sequences between mβ2 and hβ2 subunits, but this did not influence the ability of wild-type or mutant β3 subunits to affect function of α6β2*-nAChR, although hα6mβ4hβ3V9′S- and mα6hβ4mβ3V9′S-nAChR were functional. The other approach entailed switching sequences between mα6 and hα6 subunits and surprisingly showed that mα6hβ2hβ3V9′S- and mα6hβ4hβ3- nAChR are functional.
The persisting lack of function seen for all-human or all-mouse α6β2- or α6β2β3-nAChR suggests that native and physiologically relevant receptors likely contain an additional assembly partner, perhaps α4 or α3 subunits. Another possibility that would be much more difficult to test is that oocytes, but not the right kinds of nerve cells, lack chaperones that facilitate assembly and functional expression of α6β2β3*-nAChR.
However, success in formation of functional, hybrid mα6hβ2hβ3V9′S- and mα6hβ4hβ3-nAChR indicated that features of α6 subunits influence the function or functional assembly of α6β3*-nAChR. Chimeric α6 subunits containing different length segments of the mα6 subunit fused to otherwise hα6 subunits localized key determinants involved in effects of β3 subunits on α6β4*-nAChR function to the N-terminal extracellular domain of the nAChR α6 subunit but gave little initial indication that the same region influenced effects of β3 subunits on α6β2*-nAChR function. Our studies of α6β2*-nAChR also suggest that the cytoplasmic loop (and perhaps, although less likely, the fourth transmembrane domain and the C-terminal tail) of the α6 subunit can influence functional expression of receptors and their interactions with β3 subunits, perhaps consistent with the observations by Kuryatov et al. (8) who used chimeric subunits possessing intracellular domains of the α3 instead of α6 subunit.
Site-directed mutagenesis work, simplified to some extent by the remarkable homology between human and mouse nAChR α6 subunits, indicated that the function like that seen in hybrid receptors occurring for hα6N143D/M145Vhβ4-nAChR is potentiated in the presence of wild-type hβ3 subunits and is further potentiated upon substitution of mutant hβ3V9′S for wild-type hβ3 subunits. These findings indicated that amino acid residues 143 and 145 in the α6 subunit are key determinants influencing effects of β3 subunits on α6β4*-nAChR function. mα6 differs from hα6 by having a negatively charged rather than a polar side chain at position 143 and a residue with a slightly higher hydrophobicity and smaller side chain volume at position 145. These differences might enhance interactions between α6 and β3 and/or β4 subunits. These residues are in loop E on the − or complementary face of the α6 subunit that would be involved in presumed interactions with residues on the + or primary faces of the neighboring β3 subunit and/or of the neighboring β4 subunit in a complex that has the presumed (counterclockwise when viewed from the extracellular space) arrangement as follows: −β3+:−α6+:−β4+:−α6+:−β4+. Neighboring (−) face residues in loop E at consensus positions 139, 141, 147, and 149 (Leu, Lys, Thr, and Thr, respectively, preserved across mammalian α6 subunits, although chickens have Pro-141; see Fig. 9) have been implicated in agonist binding based largely on mutagenesis or structural studies of muscle-type or α7-nAChR (20). It is unexpected that agonist binding would occur at β3+:−α6 or β4+:−α6 subunit interfaces, as it would be expected to be confined to α6+:−β4 (or α6+:−β2) interfaces (but see Moroni et al. (21) for evidence that nAChR β2+:−α4 interfaces are engaged in allosteric effects of Zn2+). It is possible that β3−:+α6 and/or β4−:+α6 subunit interfaces in the vicinity of loop E are important for subunit assembly leading to closure of functional cell-surface expression of α6β4β3-nAChR complexes, and distal involvement of β3−:+α6 and β4−:+α6 subunit interfaces in ligand binding or transduction of ligand binding to channel gating cannot be discounted. It is notable that mouse and chicken α6 subunits share the presence of aspartate at position 143 as opposed to the human asparagine, perhaps more deeply implicating that residue in the function of hybrid α6*-nAChR.
FIGURE 9.
Multiple sequence alignment of nAChR α6 subunit proteins from several species (GenBankTM accession number NP_004189.1 (human, Homo sapiens), NP_001029266.1 (chimpanzee, Pan troglodytes), XP_001099152.1 (monkey. Macaca mulatta), NP_476532.1 (rat, Rattus norvegicus), NP_067344.2 (mouse, Mus musculus), XP_584902.3 (cow, Bos taurus), NP_990695.1 (chicken, Gallus gallus), and NP_001036149.1 (zebrafish, Danio rerio)). Numbering begins at translation start methionine of human nAChR α6 subunit protein and is shown in the N-terminal domain region of interest. Symbols below sequences indicate fully (*), strongly (:) or weakly (.) conserved residues, and underlining in boldface indicates numbered residues given prime attention in human nAChR α6 subunit mutagenesis studies.
Although studies using chimeric mouse/human α6 subunits did not initially implicate the N-terminal region in interactions with β3 subunits, consistent with the site-directed mutagenesis work at some of the N-terminal domain residues investigated, coexpression of nAChR hα6N91K or hα6N143D subunits together with nAChR hβ2 and hβ3V9′S subunits resulted in production of functional receptors as evident by inward current responses to nicotine and apparently outward current responses to mecamylamine. The hα6N143D single mutation also accounted for effects when it was coupled with the more conservative, hα6M145V mutation. Although absolute levels of function were not particularly high and were just 1/3rd of those for hybrid mα6hβ2hβ3V9′S-nAChR, again suggesting as did chimeric subunit studies that residues C-terminal to the third transmembrane domain play a role in influencing effects of β3 subunits on α6β2*-nAChR function, these studies implicate sites involved in formation of functional α6β2β3-nAChR. Aside from the considerations already described above about residue 143 in the E loop of the α6 subunit engaging in interactions with neighboring β3 or, in this case, β2 subunits, there are potential influences of the introduction of a positively charged instead of a polar side chain at position 91 that could influence effects of β3 subunits on α6β2*-nAChR. Interestingly, residue 91 is in loop D and on the − face of the α6 subunit, slightly C-terminal to residues at positions 85 and 87 (Trp and Arg, respectively, preserved across mammalian, chicken, and fish α6 subunits). Residue 91 is an asparagine in primates, cows, and chickens but is a lysine in rats and mice (Fig. 9). It, as opposed to residue 143, might not account for differences between chicken and human α6 subunits in hybrid receptors, but it seems to contribute to differences between mouse and human α6 subunits. Perhaps the incomplete (relative to hybrid α6β2β3V9′S-nAChR) potentiation of α6β2*-nAChR function is because the changes in position 143 reflect influences on β3+:−α6 and β4+:−α6 subunit interactions but not β2+:−α6 subunit interactions. Reciprocally, changes in position 91 might have effects only because they influence β2+:−α6 and not β4+:−α6 subunit interactions. Again, given that it would be unexpected for agonist binding to occur at β3+:−α6 or β2+:−α6 subunit interfaces, as opposed to at α6+:−β2 interfaces, changes in residues in loops D and E probably affect assembly and closure of functional α6β2β3-nAChR pentamers, although they could have allosteric effects on ligand binding and/or coupling to channel opening (e.g. see Ref. 21), maybe even serving as coagonist-binding sites.
Finally, effects of atropine or mecamylamine interpreted as that of α6*-nAChR showing gain-of-function have a significant probability of spontaneous channel opening that is abated by atropine- or mecamylamine-mediated open channel block. Gain-of-function effects due to mutations occurring at the 9′- or 13′-positions in the β3 subunit second transmembrane domain have largely similar effects but in some cases yield functional receptors with different agonist sensitivities, suggesting subtleties in coupling between ligand binding and channel opening.
In conclusion, our results provide evidence that wild-type β3 or mutant β3V9′S or β3V13′S subunits can incorporate into and either suppress/abolish or enhance function of α6β4*-nAChR but not α6β2*-nAChR. These observations, along with the demonstration that β3 subunits can form functional receptors with α7 subunits (22) and that β3V273T subunits participate in formation of α3β4*-nAChR (23, 24), help to define nAChR subtypes capable of containing β3 subunits, thus providing insights into roles of β3*-nAChR in nicotinic signaling. However, there remain puzzles about the makeup of functional, all-human or all-mouse α6*-nAChR, especially α6β3*-nAChR. Nevertheless, our results provide further evidence that wild-type and/or mutant β3 subunits not only form functional receptors in combination with α6 subunits but also influence α6*-nAChR function. We also show for the first time that dominant-negative suppression or potentiation of α6β4*-nAChR upon heterologous coexpression with wild-type β3 subunits is influenced in hybrid receptors and in the presence of chimeric α6 subunits in ways affected by selected residues that unexpectedly are found on the N-terminal extracellular domain − face of α6 subunits. These and additional residues influence effects of mutant β3 subunits on function of α6β2*-nAChR. The current findings suggest that the molecular description of functional nAChR is incomplete and that novel interfaces (i.e. other than the consensus interface thought to be involved in productive agonist binding, α6+:−β2 or α6+:−β4) between nAChR subunits play heretofore unappreciated roles in receptor assembly, ligand recognition, and/or function. Finally, the development of oocytes that express α6β3*-nAChR and the prospect that cell lines also containing the same assemblies in functional forms provide potentially useful tools for development of α6*-nAChR-selective or -specific ligands, with the caveat that any α6*-nAChR-containing subunits with gain-of-function properties or chimeric subunits may have different sensitivities for agonists or perhaps other types of ligands than α6*-nAChR composed of fully wild-type subunits. This is important due to the growing interest in functional α6β3*-nAChR based on their demonstrated or perceived importance in locomotion, reward and reinforcement behavior, schizophrenia, and Parkinson disease (2, 15, 25). Perhaps of high significance is the association of single nucleotide polymorphisms in the genes (CHRNA6 and CHRNB3) encoding α6 and β3 subunits with nicotine dependence, number of quit attempts, and subjective responses to nicotine (26–30). Studies as described here are essential for an improved understanding of structure and function and ultimately of biological roles of α6β3*-nAChR.
Supplementary Material
Acknowledgments
We thank Dr. Jerry A. Stitzel (Dept. of Integrative Physiology, University of Colorado, Boulder) for providing the mouse nAChR subunits. We also thank Dr. Paul Whiteaker (Barrow Neurological Institute) for comments about the project and manuscript and Dr. Jianliang Zhang for technical advice and assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DA015389. This work was also supported by the External Research Program of Philip Morris U. S. A. Inc. and Philip Morris International and by endowment and capitalization funds from the Men's and Women's Boards of the Barrow Neurological Foundation. Portions of this work have been presented in abstract form (Dash, B., Zhang, J., Chang, Y., and Lukas, R. J. (2007) Soc. Neurosci. Abstr. 33, 39.19; Dash, B., Bhakta, M., Whiteaker, P., Stitzel, J. A., Chang, Y., and Lukas, R. J. (2009) Soc. Neurosci. Abstr. 35, 34.7).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures,” Table S1, and Fig. S1.
- nAChR
- nicotinic acetylcholine receptor
- ACh
- acetylcholine
- h
- human
- m
- mouse.
REFERENCES
- 1. Lukas R. J., Changeux J. P., Le Novère N., Albuquerque E. X., Balfour D. J., Berg D. K., Bertrand D., Chiappinelli V. A., Clarke P. B., Collins A. C., Dani J. A., Grady S. R., Kellar K. J., Lindstrom J. M., Marks M. J., Quik M., Taylor P. W., Wonnacott S. (1999) Pharmacol. Rev. 51, 397–401 [PubMed] [Google Scholar]
- 2. Cui C., Booker T. K., Allen R. S., Grady S. R., Whiteaker P., Marks M. J., Salminen O., Tritto T., Butt C. M., Allen W. R., Stitzel J. A., McIntosh J. M., Boulter J., Collins A. C., Heinemann S. F. (2003) J. Neurosci. 23, 11045–11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Drenan R. M., Grady S. R., Whiteaker P., McClure-Begley T., McKinney S., Miwa J. M., Bupp S., Heintz N., McIntosh J. M., Bencherif M., Marks M. J., Lester H. A. (2008) Neuron 60, 123–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kulak J. M., Nguyen T. A., Olivera B. M., McIntosh J. M. (1997) J. Neurosci. 17, 5263–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Champtiaux N., Han Z. Y., Bessis A., Rossi F. M., Zoli M., Marubio L., McIntosh J. M., Changeux J. P. (2002) J. Neurosci. 22, 1208–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pons S., Fattore L., Cossu G., Tolu S., Porcu E., McIntosh J. M., Changeux J. P., Maskos U., Fratta W. (2008) J. Neurosci. 28, 12318–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Azam L., Maskos U., Changeux J. P., Dowell C. D., Christensen S., De Biasi M., McIntosh J. M. (2010) FASEB J. 24, 5113–5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuryatov A., Olale F., Cooper J., Choi C., Lindstrom J. (2000) Neuropharmacology 39, 2570–2590 [DOI] [PubMed] [Google Scholar]
- 9. Evans N. M., Bose S., Benedetti G., Zwart R., Pearson K. H., McPhie G. I., Craig P. J., Benton J. P., Volsen S. G., Sher E., Broad L. M. (2003) Eur. J. Pharmacol. 466, 31–39 [DOI] [PubMed] [Google Scholar]
- 10. Gerzanich V., Kuryatov A., Anand R., Lindstrom J. (1997) Mol. Pharmacol. 51, 320–327 [PubMed] [Google Scholar]
- 11. Fucile S., Matter J. M., Erkman L., Ragozzino D., Barabino B., Grassi F., Alemà S., Ballivet M., Eusebi F. (1998) Eur. J. Neurosci. 10, 172–178 [DOI] [PubMed] [Google Scholar]
- 12. Broadbent S., Groot-Kormelink P. J., Krashia P. A., Harkness P. C., Millar N. S., Beato M., Sivilotti L. G. (2006) Mol. Pharmacol. 70, 1350–1357 [DOI] [PubMed] [Google Scholar]
- 13. Groot-Kormelink P. J., Luyten W. H., Colquhoun D., Sivilotti L. G. (1998) J. Biol. Chem. 273, 15317–15320 [DOI] [PubMed] [Google Scholar]
- 14. Groot-Kormelink P. J., Boorman J. P., Sivilotti L. G. (2001) Br. J. Pharmacol. 134, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Novère N., Zoli M., Changeux J. P. (1996) Eur. J. Neurosci. 8, 2428–2439 [DOI] [PubMed] [Google Scholar]
- 16. Azam L., Winzer-Serhan U. H., Chen Y., Leslie F. M. (2002) J. Comp. Neurol. 444, 260–274 [DOI] [PubMed] [Google Scholar]
- 17. Klink R., de Kerchove d'Exaerde A., Zoli M., Changeux J. P. (2001) J. Neurosci. 21, 1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bordia T., Grady S. R., McIntosh J. M., Quik M. (2007) Mol. Pharmacol. 72, 52–61 [DOI] [PubMed] [Google Scholar]
- 19. Lai A., Parameswaran N., Khwaja M., Whiteaker P., Lindstrom J. M., Fan H., McIntosh J. M., Grady S. R., Quik M. (2005) Mol. Pharmacol. 67, 1639–1647 [DOI] [PubMed] [Google Scholar]
- 20. Unwin N. (2005) J. Mol. Biol. 346, 967–989 [DOI] [PubMed] [Google Scholar]
- 21. Moroni M., Vijayan R., Carbone A., Zwart R., Biggin P. C., Bermudez I. (2008) J. Neurosci. 28, 6884–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palma E., Maggi L., Barabino B., Eusebi F., Ballivet M. (1999) J. Biol. Chem. 274, 18335–18340 [DOI] [PubMed] [Google Scholar]
- 23. Boorman J. P., Groot-Kormelink P. J., Sivilotti L. G. (2000) J. Physiol. 529, 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boorman J. P., Beato M., Groot-Kormelink P. J., Broadbent S. D., Sivilotti L. G. (2003) J. Biol. Chem. 278, 44033–44040 [DOI] [PubMed] [Google Scholar]
- 25. Bencherif M., Schmitt J. D. (2002) Curr. Drug Targets CNS Neurol. Disord. 1, 349–357 [DOI] [PubMed] [Google Scholar]
- 26. Greenbaum L., Kanyas K., Karni O., Merbl Y., Olender T., Horowitz A., Yakir A., Lancet D., Ben-Asher E., Lerer B. (2006) Mol. Psychiatry 11, 312–322 [DOI] [PubMed] [Google Scholar]
- 27. Bierut L. J. (2007) Pharmacogenomics 8, 881–883 [DOI] [PubMed] [Google Scholar]
- 28. Saccone S. F., Hinrichs A. L., Saccone N. L., Chase G. A., Konvicka K., Madden P. A., Breslau N., Johnson E. O., Hatsukam i D., Pomerleau O., Swan G. E., Goate A. M., Rutter J., Bertelsen S., Fox L., Fugman D., Martin N. G., Montgomery G. W., Wang J. C., Ballinger D. G., Rice J. P., Bierut L. J. (2007) Hum. Mol. Genet. 16, 36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoft N. R., Corley R. P., McQueen M. B., Schlaepfer I. R., Huizinga D., Ehringer M. A. (2009) Neuropsychopharmacology 34, 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zeiger J. S., Haberstick B. C., Schlaepfer I., Collins A. C., Corley R. P., Crowley T. J., Hewitt J. K., Hopfer C. J., Lessem J., McQueen M. B., Rhee S. H., Ehringer M. A. (2008) Hum. Mol. Genet. 17, 724–734 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.