Abstract
The effects of a glycogen phosphorylase inhibitor (GPI) and metformin (MT) on hepatic glucose fluxes (μmol · kg−1 · min−1) in the presence of basal and 4-fold basal levels of plasma glucagon were investigated in 18-h fasted conscious dogs. Compared with the vehicle treatment, GPI infusion suppressed net hepatic glucose output (NHGO) completely (−3.8 ± 1.3 versus 9.9 ± 2.8) despite increased glucose 6-phosphate (G-6-P) neogenesis from gluconeogenic precursors (8.1 ± 1.1 versus 5.5 ± 1.1). MT infusion did not alter those parameters. In response to a 4-fold rise in plasma glucagon levels, in the vehicle group, plasma glucose levels were increased 2-fold, and NHGO was increased (43.9 ± 5.7 at 10 min and 22.7 ± 3.4 at steady state) without altering G-6-P neogenesis (3.7 ± 1.5 and 5.5 ± 0.5, respectively). In the GPI group, there was no increase in NHGO due to decreased glucose-6-phosphatase flux associated with reduced G-6-P concentration. A lower G-6-P concentration was the result of increased net glycogenesis without altering G-6-P neogenesis. In the MT group, the increment in NHGO (22.2 ± 4.4 at 10 min and 12.1 ± 3.6 at steady state) was approximately half of that of the vehicle group. The lesser NHGO was associated with reduced glucose-6-phosphatase flux but a rise in G-6-P concentration and only a small incorporation of plasma glucose into glycogen. In conclusion, the inhibition of glycogen phosphorylase a activity decreases basal and glucagon-induced NHGO via redirecting glucose 6-phosphate flux from glucose toward glycogen, and MT decreases glucagon-induced NHGO by inhibiting glucose-6-phospatase flux and thereby reducing glycogen breakdown.
Introduction
The liver produces glucose via glycogen breakdown and/or gluconeogenesis, and the relative contribution of each to total glucose production changes with altered nutritional and metabolic states. Several studies in dogs and humans have shown that increased delivery of gluconeogenic precursors, such as alanine (Diamond et al., 1988; Wolfe et al., 1988), glycerol (Jahoor et al., 1990), or lactate (Jenssen et al., 1990; Connolly et al., 1993), to the liver has no acute effect on the amount of glucose produced by that organ. Gluconeogenic precursors can alter hepatic glycogen metabolism by exerting regulatory effects on glycogen phosphorylase and synthase in addition to serving as substrates for glycogen synthesis (Youn and Bergman, 1990), of which glucose 6-phosphate (G-6-P), an intermediate at a central cross point between the metabolic pathways of glycogen metabolism and gluconeogenesis, has been shown in studies using isolated hepatocytes to regulate glycogen synthase (Ciudad et al., 1986) and phosphorylase activity within a physiological range (Aiston et al., 2003, 2004). The above data suggest the existence of autoregulatory control of glycogenolysis by gluconeogenesis within the liver, such that the desired rate of hepatic glucose output can be maintained regardless of the gluconeogenic precursor supply. In contrast, Staehr et al. (2007) reported that a galactose-induced increase in hepatic glycogenolysis resulted in a concomitant decrease in hepatic gluconeogenesis in 44-h fasted healthy humans. In keeping with this, we showed that a concomitant increase in hepatic gluconeogenesis resulted from an inhibition of hepatic glycogenolysis (Shiota et al., 1997), although this was not confirmed by others (Fosgerau et al., 2001). These findings suggest the existence of an autoregulatory mechanism between net glycogenolysis and gluconeogenesis within the liver to maintain the desired rate of hepatic glucose output. Furthermore, the flux from glucose to glycogen has two highly regulated steps, glucose phosphorylation by glucokinase and the formation of a glycoside bond between C1 of the activated glucose, UDP-glucose, and C4 of a terminal glucose residue of glycogen by glycogen synthase. It has been reported that increasing both glucokinase and glycogen synthase activity synergistically increases glycogen synthesis from glucose in cultured hepatocytes isolated from normal rats (Gomis et al., 2000; Hampson and Agius, 2005). Therefore, net glycogen flux may be tightly linked to fluxes in other pathways, including gluconeogenesis, glucose phosphorylation, and glucose 6-phosphate dephosphorylation. It is possible that alteration of net hepatic glucose output resulting from a modification in glycogenolytic flux involves a secondary change in other metabolic pathway(s).
In patients and animals with type 2 diabetes, the diabetic hyperglycemia is associated with inappropriately increased endogenous glucose production, a lesser suppression of endogenous glucose production and a blunted glucose disposal in response to increased plasma glucose and insulin (Firth et al., 1986; Consoli, 1992; Iozzo et al., 2003). The blunted response of hepatic glucose flux to raised insulin and glucose is associated with blunted response of net hepatic glycogen flux (Krssak et al., 2004). The normalization or reduction of net hepatic glycogenolysis has attracted attention as a potential therapeutic strategy. During the past decade, a specific inhibitor of glycogen phosphorylase that catalyzes glycogen breakdown to glucose 1-phosphate, a rate-limiting step of glycogenolysis, was generated to directly decrease glycogen breakdown. Treatment with the inhibitor has been shown to reduce hyperglycemia acutely in a model of type 2 diabetes (Treadway et al., 2001; Ogawa et al., 2003). Metformin (N,N-dimethylbiguanide; MT) has been used for decades to improve glycemic control in diabetic patients and is thought to decrease blood glucose levels by reducing hepatic glucose output (Bailey and Turner, 1996; Kirpichnikov et al., 2002). The most widely accepted mechanism of MT action is the inhibition of transcription of key gluconeogenic genes in the liver (Shaw et al., 2005; Viollet et al., 2009). On the other hand, it has been reported that MT reduces net hepatic glucose production acutely by decreasing glycogenolysis in the normal dog (Chu et al., 2000).
To verify the mechanism by which glycogen phosphorylase inhibition decreases net hepatic glucose output (NHGO), we examined whether any alteration of glucose phosphorylation, gluconeogenesis or glycogen synthesis, is associated with the inhibition of glycogen phosphorylase. Furthermore, to assess the mechanism by which MT decreases net glycogenolysis, we compared the alterations in liver glucose flux caused by MT with those caused by a glycogen phosphorylase inhibitor.
Materials and Methods
Animals and Surgical Procedures.
Experiments were performed on 15 overnight-fasted mongrel dogs (17.4–29.0 kg, mean 22.4 ± 1.1 kg) of either sex, which had been fed once daily a standard meat and chow diet (34% protein, 46% carbohydrate, 14% fat, and 6% fiber based on dry weight; Kal Kan, Vernon, CA; and Purina Lab Canine Diet 5006; Purina Mills, St. Louis, MO). The dogs were housed in a facility that met American Association for the Accreditation of Laboratory Animal Care guidelines, and the protocols were approved by the Vanderbilt University Medical Center Animal Care Committee. At least 16 days before an experiment, a laparotomy was performed under general endotracheal anesthesia (15 mg/kg b.wt. pentothal sodium presurgery and 1.0% isoflurane as an inhalation anesthetic during surgery), and catheters for blood sampling were placed into a femoral artery and the portal, a hepatic, a jejunal, and a splenic vein as described previously (Diamond et al., 1988; Connolly et al., 1993). A catheter for drug infusion was placed into the stomach as described previously (Moore et al., 1994). On the day of the experiment, the catheters were exteriorized under local anesthesia (2% lidocaine; Abbott Laboratories, Chicago, IL), their contents were aspirated, and they were flushed with saline. Angiocaths (20 gauge; Abbott Laboratories) were inserted into both cephalic veins for infusion of indocyanine green, radioactive tracers, and glucose and a saphenous vein for the infusion of somatostatin.
On the day before an experiment the leukocyte count and hematocrit were determined. Dogs were used for an experiment only if they had 1) a leukocyte count <18,000/mm3, 2) a hematocrit >38%, 3) a good appetite, and 4) normal stools.
Experimental Design.
Each experiment consisted of a 100-min tracer and dye equilibration period (−140 to −40 min), a 40-min control period (−40 to 0 min), and two test periods: Test Period I (0–90 min) and Test Period II (90–210 min). A priming dose of [3-3H]glucose (41.7 μCi) was given at −140 min. Continuous infusions of [3-3H]glucose (0.34 μCi/min) and indocyanine green (0.1 mg/m2 · min−1) were also started at −140 min and were continued throughout the experiment. At −140 min, a peripheral infusion of somatostatin (0.8 μg · kg−1 · min−1) was started to inhibit endogenous insulin and glucagon secretion. Intraportal replacement infusions of insulin (0.25 mU · kg−1 · min−1) and glucagon (0.6 ng · kg−1 · min−1) were started simultaneously with initiation of the somatostatin infusion. Plasma glucose was then monitored every 5 min, and the rate of insulin infusion was adjusted until the level of plasma glucose was stabilized at a euglycemic value. Once stabilization was achieved, the insulin infusion rate was left unchanged. Three experimental protocols were used: 1) vehicle (placebo) group, an intragastric bolus (0.5 ml/kg) of polyethylene glycol (PEG)-500 (10%) was given at 0 min, and saline was infused into the portal vein at 7 μl · kg−1 · min−1 during the test periods. 2) GPI group, an intragastric bolus (10 mg/kg) of GPI, (3S,2R)-3-(5-chloroindole-2-carbonyl)amino-2-hydroxy-4-phenylbutyric acid N-methyl-N-methoxyamide (CP-316819; Pfizer, Inc., Groton, CT) (Treadway et al., 2001), with PEG-500 (0.5 ml/kg) was given at 0 min because of its low solubility in vehicle, and then saline was infused into the portal vein at 7 μl · kg−1 · min−1 during the test periods. 3) MT group, an intragastric bolus of PEG-500 (0.5 ml/kg) was given at 0 min, and MT was infused into the portal vein at 0.15 mg · kg−1 · min−1 with 7 μl of saline · kg−1 · min−1 during the test periods because MT causes diarrhea if given orally. Based on hepatic blood flow rate (approximately 30 ml · kg−1 · min−1), hematocrit (approximately 40%), and the infusion rate of MT (150 μg · kg−1 · min−1), hepatic sinusoidal plasma concentrations of MT were expected to be >8 μg/ml, which are 10 times higher than plasma concentrations of MT (in range of 0.5 to 2.0 μg/ml) seen when MT was given at 1 g per day in patients with type 2 diabetes (Scheen, 1996). After a 90-min test period, the infusion rate of glucagon was increased 4-fold in all groups for another 120 min.
Analytical Procedures.
Plasma glucose concentrations and plasma glucose radioactivity were determined as described previously (Fujimoto et al., 2006; Torres et al., 2009). Blood concentrations of gluconeogenic precursors (lactate, alanine, and glycerol) and plasma concentration of nonesterified fatty acids were determined according to the methods reported previously (Fujimoto et al., 2006; Torres et al., 2009). Individual blood amino acid (alanine, serine, threonine, and glycine) levels were assessed by high-performance liquid chromatography methods with an interassay coefficient of variation of 4% (Venkatakrishnan et al., 1996). Plasma arterial and hepatic vein indocyanine green concentrations were determined spectrophotometrically at 805 nm (Leevy et al., 1962). Immunoreactive plasma insulin, glucagon, and cortisol as well as plasma epinephrine and norepinephrine were determined as described previously (Wada et al., 1995).
Liver samples were obtained at the end of each experiment by euthanizing the dog with pentobarbital sodium, exposing the liver by laparotomy, and freeze-clamping approximately 5 g of liver sections from each lobe. The time elapsed from euthanization to freeze-clamping was less than 4 min. The entire liver was then removed from the dog and weighed. The frozen liver samples were stored at approximately −70°C for subsequent analysis. Glycogen and 3H in glycogen were determined as described previously (Fujimoto et al., 2006; Torres et al., 2009). Liver content of UDP-glucose and UDP-galactose were obtained through two sequential chromatographic separations, and the amount of 3H radioactivity in each fraction was measured as described previously (Fujimoto et al., 2006). Glycogen synthase and phosphorylase activities were measured as reported previously (Fujimoto et al., 2006; Torres et al., 2009). It has been reported that barbiturate derivatives do not have a direct effect on activity of liver phosphorylase in vitro (Brunner and Haugaard, 1965). However, Mikines et al. (1986) showed a time-dependent decrease in glycogen phosphorylase a activity in liver after anesthesia of rats with pentobarbital. Thus, there is a possibility that the activity of liver glycogen phosphorylase was altered to some extent during the time between euthanasia and freeze-clamping the tissue.
Materials.
[3-3H] glucose (PerkinElmer Life and Analytical Sciences, Waltham, MA) was used as the glucose tracer (500 μCi/0.005 mg). Indocyanine green (Hynson, Westcott, and Dunning, Baltimore, MD) was prepared in sterile water. Insulin (Squibb-Novo, Princeton, NJ), glucagon (Eli Lilly, Indianapolis, IN), and cyclic somatostatin (Bachem, Torrance, CA) were prepared with normal saline and contained 3% (v/v) of the dog's own plasma. Cortisol radioimmunoassay kits were obtained from Micromedic Systems (Horsham, PA). GPI (CP-316819; see Hoover et al., 2001), was synthesized at Pfizer Global Research and Development (Groton, CT).
Calculations.
Hepatic blood flow was assessed by measuring hepatic extraction of indocyanine green (Leevy et al., 1962). Based on data from Greenway and Stark (1971), the proportions of the hepatic blood supply provided by the hepatic artery and portal vein were assumed to be 28 and 72%, respectively, which conforms to data we obtained with Doppler flow probes during pancreatic clamps (Myers et al., 1991). These proportions were assumed to remain constant throughout all experiments because treatment did not significantly affect hepatic blood flow. Net hepatic substrate balance was calculated using the formula [H − (0.28A + 0.72P)] × HF, where A, P, and H are the arterial, portal vein, and hepatic vein substrate concentrations, respectively, and HF is the hepatic blood or plasma flow. When substrate levels in blood were measured, blood flow was used in the calculation, whereas plasma flow was used when plasma substrate levels were measured.
For calculation of minimal estimates of unidirectional hepatic glucose uptake (M-HGU), which does not include glucose taken up by liver and then returned (glucose cycling), the net hepatic [3-3H]glucose uptake was divided by average sinusoidal [3-3H]glucose-specific activity (SA). The sinusoidal [3-3H]glucose SA was calculated using the formula [(0.28 × [3-3H]SA in artery) + (0.72 × [3-3H]SA in portal vein)]. Minimal estimates of unidirectional hepatic glucose release (M-HGR), which does not contain glucose released via glucose cycling, was determined by adding M-HGU to net hepatic glucose balance, which is NHGO when the balance is positive and net hepatic glucose uptake (NHGU) when the balance is negative. Assuming that there is 100% conversion of gluconeogenic precursors taken up by the liver into G-6-P (Goldstein et al., 2002), the gluconeogenic flux to G-6-P (G-6-P neogenesis) was determined by summation of net hepatic uptake rates of the gluconeogenic precursors (alanine, glycine, serine, threonine, glycerol, and lactate), converting the sum to glucose equivalents and dividing by two to account for the incorporation of three carbon precursors into the six-carbon molecules. When lactate was released, net lactate uptake was calculated as zero. The fractional contribution of plasma glucose via the direct pathway to UDP-glucose flux was calculated as the ratio of [3-3H]SA in hepatic UDP-glucose to the liver sinusoidal [3-3H]glucose SA. The remaining G-6-P was formed via other pathways, mainly glycogenolysis and G-6-P neogenesis. Glucose-6-phosphatase (G-6-Pase) flux was calculated using the formula [M-HGR/[(1 − [3-3H]SA in hepatic UDP-glucose/[3-3H]SA in plasma glucose)]]. Glucose cycling was calculated as the difference between G-6-Pase flux rate and M-HGR.
Hepatic glycogen content was determined by multiplying the glycogen concentration (milligram per gram of liver) by liver weight. The amount of glycogen synthesized from glucose (the direct pathway) was calculated by dividing 3H radioactivity incorporated into liver glycogen by the average [3-3H]SA in arterial plasma glucose between 0 and 180 min. Net glycogenolysis was calculated as the difference between M-HGR and G-6-P neogenesis, with the assumption that all of the G-6-P derived from G-6-P neogenesis was converted to glucose.
Statistical Analysis.
Data are expressed as means ± S.E. Statistical comparisons were made using two-way analysis of variance with repeated-measures design. Post hoc analysis was performed using either paired t test or unpaired t test.
Results
Hematocrit, Hepatic Blood Flow, and Hormone Levels.
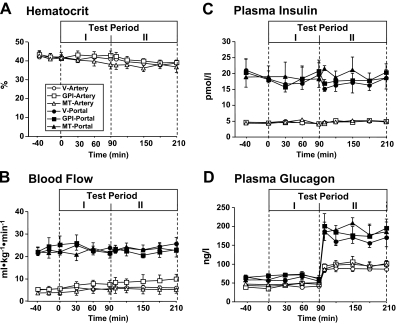
Arterial hematocrit (Fig. 1A) and hepatic arterial and portal vein blood flow (Fig. 1B) were not significantly different over time among the three groups. Portal and arterial insulin levels remained at basal levels throughout the study among all three groups (Fig. 1C). Arterial and portal glucagon levels were basal and unchanged in all protocols during the first two time periods, and then in the third period (Test Period II), they rose approximately 2- and 3-fold, respectively, when the glucagon infusion rate was increased (Fig. 1D). Arterial cortisol, norepinephrine, and epinephrine levels were not significantly changed with either GPI or MT treatment (Table 1).
Fig. 1.
Arterial blood hematocrit (A), hepatic arterial and portal blood flows (B), arterial and portal plasma levels of insulin (C), and glucagon (D) before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs. The animals received intragastric bolus injection of vehicle or GPI (10 mg/kg) at 0 min, continuous infusion of metformin, or vehicle into the portal vein (0.15 mg · kg−1 · min−1) from 0 min, and a 4-fold increase in intraportal glucagon brought about from 90 min. Data are means ± S.E. for five experiments.
TABLE 1.
Arterial plasma levels of norepinephrine, epinephrine and cortisol before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs
Values are means ± S.E. for five experiments. V, vehicle.
| Hormones | Groups | Control (0 min) | Study Periods |
|||
|---|---|---|---|---|---|---|
| Test Period I |
Test Period II |
|||||
| 60 min | 90 min | 150 min | 210 min | |||
| Norepinephrine (pM) | V | 673 ± 59 | 682 ± 118 | 532 ± 127 | 751 ± 136 | 790 ± 129 |
| GPI | 523 ± 138 | 489 ± 142 | 488 ± 105 | 519 ± 175 | 689 ± 189 | |
| MT | 761 ± 227 | 592 ± 184 | 697 ± 245 | 767 ± 250 | 871 ± 291 | |
| Epinephrine (pM) | V | 345 ± 144 | 383 ± 160 | 237 ± 110 | 315 ± 101 | 535 ± 230 |
| GPI | 332 ± 152 | 658 ± 373 | 509 ± 157 | 350 ± 157 | 624 ± 317 | |
| MT | 824 ± 277 | 527 ± 215 | 713 ± 248 | 589 ± 204 | 520 ± 245 | |
| Cortisol (nM) | V | 62 ± 12 | 41 ± 5 | 54 ± 18 | 58 ± 8 | 46 ± 4 |
| GPI | 79 ± 16 | 101 ± 31 | 83 ± 19 | 96 ± 22 | 95 ± 24 | |
| MT | 69 ± 6 | 87 ± 19 | 84 ± 12 | 94 ± 22 | 97 ± 10 | |
Hepatic Glucose Balance.
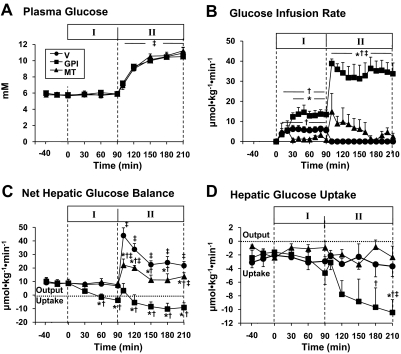
Plasma glucose levels were maintained at basal during the control period and Test Period I (Fig. 2A). During the control period, there were no significant differences in NHGO and M-HGU among the groups (Fig. 2, C and D). During Test Period I, in the vehicle group, glucose infusion (5.6 ± 1.6 μmol · kg−1 · min−1) was necessary to maintain euglycemia (Fig. 2B). Net hepatic balance of glucose and hepatic glucose uptake changed minimally (Fig. 2, C and D). In the GPI group, glucose needed to be infused at 13.3 ± 2.6 μmol · kg−1 · min−1 to maintain euglycemia in Test Period I. NHGO was completely suppressed by 60 min (−1.5 ± 2.3 μmol · kg−1 · min−1) and, thereafter, switched to NHGU (3.8 ± 1.3 μmol · kg−1 · min−1) by 90 min (Fig. 2C). Hepatic glucose uptake more than doubled in response to the GPI. In the MT group, small amount of glucose (2.3 ± 0.6 μmol · kg−1 · min−1) needed to be infused to maintain euglycemia. NHGO tended to decrease from basal (8.9 ± 0.9 μmol · kg−1 · min−1) to 7.6 ± 1.3 μmol · kg−1 · min−1 and was not significantly different from that of the vehicle group (Fig. 2C). Hepatic glucose uptake was not altered by MT treatment.
Fig. 2.
Arterial plasma glucose levels (A), glucose infusion rates (B), and changes in net hepatic glucose (C), and [3-3H]glucose balance (D) before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs. The animals received intragastric bolus injection of vehicle or GPI (10 mg/kg) at 0 min, continuous infusion of metformin, or vehicle into the portal vein (0.15 mg · kg−1 · min−1) from 0 min, and a 4-fold increase in intraportal glucagon brought about from 90 min. Values are means ± S.E. for five experiments. *, significantly different from the corresponding value in vehicle group (P < 0.05). †, significantly different from the control period with the same group (P < 0.05). ‡, significantly different from the corresponding value at 90 min in the Test Period I with the same group (P < 0.05).
In response to the rise in plasma glucagon levels, in the vehicle group, the plasma glucose level rose from 5.8 ± 0.2 to 11.1 ± 1.0 mM by the end of the study (Fig. 1A). NHGO increased by 42 ± 6 μmol · kg−1 · min−1 in 10 min and then fell but remained elevated relative to the previous period (Fig. 2C), and there was no change in hepatic glucose uptake (Fig. 2D).
In the GPI group, the plasma glucose levels were completely matched to the levels seen in the vehicle group by infusing glucose at 35 ± 5 μmol · kg−1 · min−1 (Fig. 2, A and B). NHGO was not observed in response to the rise in plasma glucagon (Fig. 2C). Net and absolute hepatic uptake of glucose was increased in parallel (Fig. 2, C and D). In the MT group, NHGO was increased by 21 ± 5 μmol · kg−1 · min−1 within 10 min, after which it returned close to the rate evident in the previous period (Fig. 2C), although the increment of NHGO was significantly smaller than that in the vehicle group (Fig. 2C). Hepatic glucose balance uptake did not change significantly in response to MT treatment.
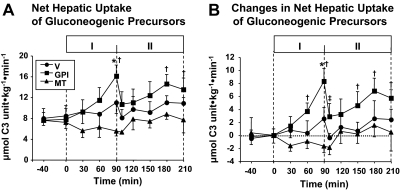
Net Hepatic Balances of Gluconeogenic Precursors.
As shown in Tables 2 and 3, the arterial level and net hepatic balance of all of measured gluconeogenic precursors were not significantly changed from control values by treatment with vehicle or by the rise in glucagon in the presence of vehicle (Test Period I). On the other hand, treatment with GPI during Test Period I caused markedly decreased net hepatic lactate release and brought about a switch to hepatic lactate uptake (Table 3). The sum of net hepatic gluconeogenic precursors uptake increased significantly (Fig. 3). The rise in glucagon in the presence of GPI treatment brought about an immediate decrease in the sum of net hepatic gluconeogenic precursor uptake, which was due to a sudden decrease in net hepatic lactate uptake. Treatment with metformin tended to decrease net hepatic gluconeogenic precursor uptake (Fig. 3; Table 3). In response to the rise in plasma glucagon and glucose, in all of the three groups, arterial amino acids levels tended to decrease (Table 2) with a tendency toward an increase in net hepatic uptake and fractional extraction (data not shown) of these precursors (Table 3; Fig. 3).
TABLE 2.
Arterial levels of lactate, alanine, serine, threonine, glycine, glycerol, and free fatty acids (FFAs) before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs
Blood (BL) and plasma concentration (PL) of metabolites are described in micromolars. Values are means ± S.E. for five experiments. V, vehicle.
| Metabolites | Group | Control | Study Period |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test Period I |
Test Period II |
||||||||||
| 30 | 60 | 90 | 100 | 120 | 150 | 180 | 210 | ||||
| Lactate | V | BL | 495 ± 30 | 504 ± 64 | 448 ± 26 | 465 ± 61 | 632 ± 49 | 577 ± 53 | 506 ± 51 | 511 ± 39 | 609 ± 87 |
| GPI | BL | 810 ± 150 | 608 ± 93 | 578 ± 112 | 471 ± 129 | 567 ± 111 | 662 ± 174 | 587 ± 156 | 523 ± 100 | 508 ± 117 | |
| MT | BL | 830 ± 111 | 822 ± 125 | 849 ± 115 | 903 ± 121 | 981 ± 159 | 1042 ± 159 | 952 ± 125 | 1030 ± 224 | 986 ± 187 | |
| Alanine | V | BL | 507 ± 47 | 553 ± 28 | 503 ± 39 | 521 ± 58 | 556 ± 32 | 543 ± 39 | 487 ± 32 | 426 ± 61 | 394 ± 43 |
| GPI | BL | 509 ± 74 | 457 ± 73 | 398 ± 71 | 388 ± 65 | 408 ± 92 | 428 ± 123 | 369 ± 104 | 308 ± 84 | 311 ± 87 | |
| MT | BL | 556 ± 26 | 577 ± 36 | 540 ± 23 | 544 ± 38 | 582 ± 83 | 611 ± 53 | 567 ± 64 | 563 ± 42 | 501 ± 66 | |
| Serine | V | BL | 198 ± 19 | 187 ± 10 | 194 ± 19 | 180 ± 15 | 165 ± 12 | 154 ± 14 | 152 ± 12 | 142 ± 8 | 127 ± 8 |
| GPI | BL | 174 ± 14 | 174 ± 22 | 184 ± 29 | 177 ± 14 | 148 ± 20 | 133 ± 13 | 144 ± 20 | 128 ± 14 | 132 ± 14 | |
| MT | BL | 188 ± 26 | 174 ± 26 | 176 ± 27 | 181 ± 25 | 135 ± 35 | 165 ± 17 | 155 ± 19 | 153 ± 18 | 136 ± 21 | |
| Threonine | V | BL | 276 ± 32 | 264 ± 36 | 287 ± 39 | 269 ± 31 | 242 ± 22 | 228 ± 15 | 234 ± 23 | 212 ± 24 | 190 ± 14 |
| GPI | BL | 277 ± 36 | 270 ± 51 | 288 ± 58 | 262 ± 31 | 248 ± 45 | 216 ± 32 | 224 ± 42 | 203 ± 32 | 213 ± 42 | |
| MT | BL | 303 ± 47 | 287 ± 36 | 296 ± 29 | 299 ± 38 | 197 ± 53 | 298 ± 33 | 255 ± 14 | 269 ± 23 | 242 ± 34 | |
| Glycine | V | BL | 267 ± 27 | 253 ± 21 | 263 ± 26 | 250 ± 19 | 233 ± 19 | 214 ± 19 | 207 ± 20 | 193 ± 16 | 171 ± 15 |
| GPI | BL | 253 ± 32 | 257 ± 39 | 264 ± 48 | 269 ± 39 | 221 ± 33 | 189 ± 22 | 210 ± 42 | 176 ± 26 | 183 ± 27 | |
| MT | BL | 285 ± 30 | 278 ± 30 | 281 ± 24 | 278 ± 24 | 212 ± 56 | 262 ± 12 | 229 ± 15 | 230 ± 18 | 195 ± 22 | |
| Glycerol | V | PL | 124 ± 14 | 112 ± 12 | 104 ± 10 | 110 ± 15 | 127 ± 15 | 98 ± 5 | 73 ± 10 | 93 ± 15 | 125 ± 26 |
| GPI | PL | 164 ± 14 | 195 ± 24 | 155 ± 13 | 180 ± 10 | 180 ± 12 | 144 ± 23 | 158 ± 19 | 149 ± 15 | 157 ± 23 | |
| MT | PL | 171 ± 29 | 152 ± 19 | 156 ± 25 | 149 ± 18 | 158 ± 18 | 156 ± 17 | 131 ± 16 | 139 ± 15 | 132 ± 14 | |
| FFAs | V | PL | 285 ± 75 | 244 ± 62 | 297 ± 51 | 225 ± 51 | 222 ± 56 | 221 ± 61 | 164 ± 47 | 158 ± 33 | 271 ± 93 |
| GPI | PL | 315 ± 82 | 304 ± 73 | 311 ± 61 | 383 ± 72 | 355 ± 58 | 283 ± 69 | 229 ± 48 | 211 ± 76 | 204 ± 87 | |
| MT | PL | 331 ± 85 | 254 ± 53 | 258 ± 68 | 239 ± 35 | 224 ± 59 | 203 ± 58 | 144 ± 17 | 153 ± 29 | 211 ± 47 | |
TABLE 3.
Net hepatic balance of lactate, alanine, serine, threonine, glycine, glycerol and free fatty acids (FFAs) before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs
Net hepatic balance of metabolites are described as μmol · kg−1 · min−1. Values are means ± S.E. for five experiments. Negative values indicate net hepatic uptake. V, vehicle.
| Metabolites | Group | Control | Study Period |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test Period I |
Test Period II |
|||||||||
| 30 min | 60 min | 90 min | 100 min | 120 min | 150 min | 180 min | 210 min | |||
| Lactate | V | 7.64 ± 2.81 | 8.19 ± 2.75 | 6.88 ± 2.93 | 5.26 ± 2.05 | 8.22 ± 1.28 | 8.09 ± 1.28 | 6.62 ± 2.02 | 5.48 ± 1.74 | 6.48 ± 2.40 |
| GPI | 9.39 ± 3.99 | 3.49 ± 3.64 | −5.02 ± 1.77* | −4.87 ± 2.58* | −0.21 ± 3.73* | 2.82 ± 4.23 | 1.31 ± 3.78 | −0.40 ± 3.76 | 0.71 ± 3.99 | |
| MT | 7.00 ± 2.31 | 5.35 ± 1.25 | 3.62 ± 1.44 | 4.20 ± 1.04 | 8.87 ± 2.99 | 6.23 ± 2.23 | 7.84 ± 2.20 | 4.02 ± 1.44 | 3.15 ± 2.53 | |
| Alanine | V | −2.87 ± 0.71 | −6.04 ± 1.25 | −3.81 ± 1.71 | −4.56 ± 1.20 | −3.19 ± 0.64 | −5.08 ± 1.60 | −5.84 ± 1.46 | −5.13 ± 0.78 | −7.02 ± 0.95 |
| GPI | −2.59 ± 0.77 | −3.04 ± 0.52 | −2.69 ± 0.79 | −4.40 ± 1.09 | −4.19 ± 0.89 | −6.40 ± 2.41 | −4.93 ± 2.04 | −5.86 ± 1.61 | −6.58 ± 2.19 | |
| MT | −3.11 ± 0.48 | −2.76 ± 0.83 | −1.84 ± 0.6 | −3.26 ± 0.74 | −2.35 ± 0.5 | −2.31 ± 0.5 | −3.39 ± 0.83 | −4.9 ± 0.51 | −3.41 ± 1.13 | |
| Serine | V | −1.48 ± 0.27 | −1.09 ± 0.23 | −1.40 ± 0.36 | −1.43 ± 0.33 | −1.16 ± 0.15 | −1.17 ± 0.14 | −0.96 ± 0.3 | −1.53 ± 0.19 | −1.24 ± 0.13 |
| GPI | −0.79 ± 0.35 | −0.97 ± 0.23 | −0.92 ± 0.25 | −1.11 ± 0.23 | −1.09 ± 0.31 | −1.25 ± 0.62 | −1.40 ± 0.3 | −1.57 ± 0.33 | −0.97 ± 0.24 | |
| MT | −0.85 ± 0.24 | −0.77 ± 0.22 | −0.85 ± 0.28 | −0.51 ± 0.21 | −0.71 ± 0.11 | −0.79 ± 0.46 | −0.85 ± 0.24 | −0.80 ± 0.24 | −0.82 ± 0.23 | |
| Threonine | V | −1.13 ± 0.4 | −0.21 ± 0.35 | −0.79 ± 0.48 | −1.15 ± 0.35 | −0.60 ± 0.28 | −1.14 ± 0.41 | −1.15 ± 0.39 | −1.29 ± 0.48 | −0.91 ± 0.15 |
| GPI | −0.72 ± 0.33 | −0.93 ± 0.32 | −0.99 ± 0.25 | −1.21 ± 0.50 | −0.69 ± 0.21 | −0.81 ± 0.14 | −0.69 ± 0.49 | −0.79 ± 0.36 | −0.42 ± 0.15 | |
| MT | −0.63 ± 0.26 | −0.48 ± 0.09 | −0.72 ± 0.26 | −0.34 ± 0.17 | −0.22 ± 0.23 | −0.72 ± 0.32 | −0.64 ± 0.15 | −0.47 ± 0.36 | −0.36 ± 0.19 | |
| Glycine | V | −1.69 ± 0.39 | −1.10 ± 0.48 | −1.60 ± 0.33 | −2.26 ± 0.58 | −1.36 ± 0.27 | −1.47 ± 0.17 | −0.90 ± 0.35 | −1.80 ± 0.37 | −1.45 ± 0.16 |
| GPI | −0.88 ± 0.41 | −1.14 ± 0.39 | −1.13 ± 0.45 | −1.53 ± 0.42 | −0.95 ± 0.33 | −0.70 ± 0.29 | −1.36 ± 0.46 | −1.20 ± 0.38 | −1.10 ± 0.31 | |
| MT | −1.21 ± 0.50 | −0.75 ± 0.19 | −1.13 ± 0.50 | −0.57 ± 0.29 | −0.77 ± 0.29 | −1.29 ± 0.69 | −1.32 ± 0.21 | −1.14 ± 0.25 | −1.09 ± 0.18 | |
| Glycerol | V | −0.83 ± 0.49 | −1.52 ± 0.81 | −1.09 ± 0.21 | −1.54 ± 0.25 | −1.64 ± 0.66 | −0.83 ± 0.36 | −1.00 ± 0.27 | −1.27 ± 0.31 | −0.91 ± 0.19 |
| GPI | −1.80 ± 0.38 | −2.20 ± 0.51 | −1.77 ± 0.61 | −2.55 ± 0.71 | −1.76 ± 0.48 | −1.11 ± 0.41 | −1.09 ± 0.47 | −1.37 ± 0.42 | −1.55 ± 0.29 | |
| MT | −1.59 ± 0.49 | −1.25 ± 0.43 | −1.91 ± 0.66 | −1.29 ± 0.18 | −1.28 ± 0.33 | −1.57 ± 0.31 | −1.18 ± 0.34 | −1.44 ± 0.35 | −1.01 ± 0.31 | |
| FFAs | V | −3.13 ± 0.77 | −1.96 ± 0.42 | −2.60 ± 0.83 | −2.44 ± 0.69 | −2.48 ± 0.64 | −2.11 ± 0.61 | −1.73 ± 0.33 | −1.82 ± 0.33 | −2.28 ± 0.36 |
| GPI | −3.08 ± 1.23 | −3.05 ± 0.95 | −2.69 ± 0.63 | −3.44 ± 1.01 | −3.80 ± 0.95 | −1.88 ± 0.62 | −1.62 ± 0.30 | −2.60 ± 0.81 | −2.71 ± 1.05 | |
| MT | −2.76 ± 1.18 | −2.58 ± 0.43 | −2.97 ± 0.74 | −2.11 ± 0.39 | −2.20 ± 0.40 | −1.96 ± 0.95 | −1.60 ± 0.36 | −1.68 ± 0.26 | −2.76 ± 1.09 | |
Significantly different from the corresponding value in vehicle group (P < 0.05).
Fig. 3.
Net hepatic gluconeogenesis precursor uptake (A) before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) and changes in net hepatic gluconeogenesis precursor uptake from that in the control period (B) in 18-h fasted conscious dogs. The animals received intragastric bolus injection of vehicle or GPI (10 mg/kg) at 0 min, continuous infusion of metformin, or vehicle into the portal vein (0.15 mg · kg−1 · min−1) from 0 min, and a 4-fold increase in intraportal glucagon brought about from 90 min. The rate was obtained by summation of net hepatic uptake rates of lactate, glycerol, pyruvate, and all gluconeogenic amino acids. Lactate uptake was considered as zero when lactate was released by the liver. Values are means ± S.E. for five experiments. *, significantly different from the corresponding value in the vehicle group (P < 0.05). †, significantly different from the control period (P < 0.05). ‡, significantly different from the corresponding value at 90 min in the Test Period I with the same group (P < 0.05).
Arterial Nonesterified Fatty Acids and Net Hepatic Balance.
Compared with the control period, arterial levels and net hepatic uptake of Nonesterified Fatty Acids did not change significantly during Test Period I with either GPI or MT treatment. Likewise, the rise in plasma glucagon levels that occurred during Test Period II (Tables 2 and 3) had no effect. Likewise, there were no changes in lipolysis as indicated by the stability in plasma glycerol levels.
Intermediary Metabolism in the Liver.
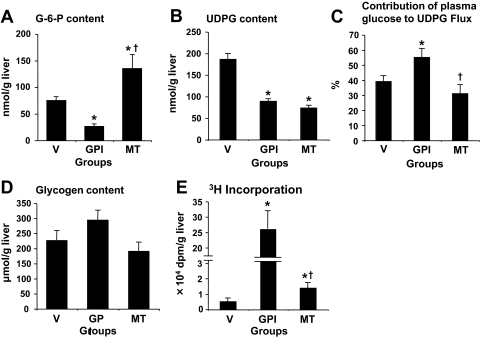
At the end of Test Period II, compared with the vehicle group, the MT group had a 2-fold higher G-6-P content but 50% lower UDP-glucose content (Fig. 4, A and B). The ratio of 3H-specific activity of UDP-glucose to that of plasma glucose, reflecting the percentage contribution of plasma glucose to UDP-glucose flux, was similar (Fig. 4C). Hepatic glycogen content was similar, and the amounts of 3H in hepatic glycogen were similarly very low but slightly elevated with metformin (Fig. 4, D and E). In the GPI group compared with the vehicle group, both G-6-P and UDP-glucose contents were markedly lower. The percentage contribution of plasma glucose to UDP-glucose flux was increased by 40% (55 ± 6% in GPI versus 42 ± 2% in vehicle). The hepatic glycogen content was higher by 30%, and the incorporated amount of [3-3H]glucose into hepatic glycogen was 100-times greater (Fig. 4, D and E).
Fig. 4.
Hepatic content of G-6-P (A), UDP-glucose (B), and glycogen content (D), percentage contribution of plasma glucose to UDP-glucose flux (C), and amount of [3-3H]glucose incorporated into glycogen (E) at the end of the Test Period II. Data are means ± S.E. for five experiments. *, significantly different from the corresponding value in the vehicle group (P < 0.05). +, significantly different from the control period (P < 0.05).
Glycogen Phosphorylase and Synthase Activity.
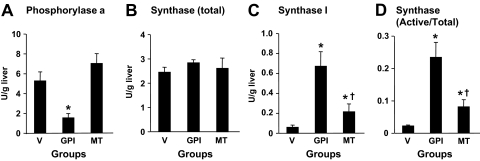
Compared with the vehicle group, as shown in Fig. 5, in the GPI group, glycogen phosphorylase a activity was markedly lower, and in contrast, glycogen synthase I activity and the ratio of active form (I) to total activity were higher. In the MT group, glycogen phosphorylase a activity was similar to that in the vehicle group. However, the active form of glycogen synthase and the ratio of active to total activity were slightly but significantly higher.
Fig. 5.
Phosphorylase activity (A), total (B), and active form (C) activities, and the ratio of active form to total activity in the liver at the end of the Test Period II. Data are means ± S.E. for five experiments. *, significantly different from the corresponding value in the vehicle group (P < 0.05). †, significantly different from the control period (P < 0.05).
Estimated Hepatic Glucose Fluxes.
During the control period, there were no significant differences in NHGO, M-HGU, M-HGR, G-6-P neogenesis, net glycogenolytic rate, and percentage contribution of gluconeogenesis and glycogenolysis to M-HGR among the groups (Table 4). During Test Period I, in the vehicle group, NHGO and M-HGR tended to reduce with significant decrease in the rate and the contribution of glycogenolysis to M-HGR from that in the control period. On the other hand, G-6-P neogenesis was not changed, and the percentage contribution of gluconeogenesis to M-HGR rose relative to that in the control period. In the GPI group, GPI treatment switched NHGB from NHGO to NHGU, with a marked decrease in M-HGR and net glycogenolytic rate and an increase in the gluconeogenic rate. In the MT group, NHGO, M-HGR, net glycogenolysis, and G-6-P neogenesis tended to decrease from that in the control period. The percentage contribution of glycogenolysis and gluconeogenesis to M-HGR remained.
TABLE 4.
Hepatic glucose and intermediate fluxes before (control) and after drug treatment (Test Period I) and subsequent increase of plasma glucagon (Test Period II) in 18-h fasted conscious dogs
Data are mean ± S.E.; n = 5 dogs for each group. Negative values for NHGO represent net uptake. V, vehicle.
| Groups | Control |
Test Period I |
Test Period II |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V | GPI | MT | V | GPI | MT | V | GPI | MT | ||
| NHGO (μmol · kg−1 · min−1) | 8.8 ± 1.2 | 8.9 ± 2.8 | 8.9 ± 1.3 | 6.8 ± 1.1 | −3.8 ± 1.3* | 7.6 ± 1.3 | 21.7 ± 3.1# | −8.9 ± 3.6* | 13.8 ± 2.7*†# | |
| M-HGU (μmol · kg−1 · min−1) | 3.3 ± 0.8 | 2.4 ± 1.0 | 2.4 ± 0.9 | 2.9 ± 0.7 | 4.7 ± 1.7 | 0.9 ± 0.9 | 3.7 ± 1.5 | 12.5 ± 2.7*# | 3.4 ± 1.1† | |
| G-6-P neogenesis | 4.2 ± 0.9 | 3.9 ± 0.8 | 3.6 ± 0.6 | 5.5 ± 1.1 | 8.1 ± 1.1 | 2.8 ± 0.5* | 5.5 ± 0.5 | 6.8 ± 1.1 | 3.9 ± 1.2 | |
| Net glycogenolysis | 7.9 ± 1.8 | 7.4 ± 2.1 | 7.7 ± 1.7 | 4.2 ± 1.4 | −7.2 ± 1.1* | 5.7 ± 1.1 | 19.9 ± 1.9# | −3.2 ± 0.9*# | 13.3 ± 1.5*†# | |
| M-HGR (μmol · kg−1 · min−1) | 12.1 ± 1.8 | 11.3 ± 2.3 | 11.3 ± 2.2 | 9.7 ± 1.8 | 0.9 ± 1.7* | 8.5 ± 1.6 | 25.4 ± 2.5# | 3.6 ± 1.6* | 17.2 ± 2.5*†# | |
| Percentage contribution of GNG | 35 ± 2 | 35 ± 4 | 32 ± 2 | 57 ± 4‡ | 0 | 33 ± 3* | 22 ± 2# | 0 | 23 ± 3 | |
| Percentage contribution of glycogenolysis | 65 ± 4 | 65 ± 5 | 68 ± 5 | 43 ± 2‡ | 100 | 67 ± 4* | 78 ± 4# | 100 | 77 ± 3 | |
| GK flux (μmol · kg−1 · min−1) | 21.9 ± 2.2 | 16.6 ± 3.2 | 14.4 ± 4.2 | |||||||
| Glucose cycle | 18.2 ± 1.7 | 4.1 ± 2.0* | 11.0 ± 3.2*† | |||||||
| G-6-Pase flux (μmol · kg−1 · min−1) | Total | 43.6 ± 3.7 | 7.7 ± 3.6* | 28.2 ± 4.1*† | ||||||
| Percentage contribution of glycogenolysis | 45 ± 3 | 47 ± 4 | ||||||||
| Percentage contribution of GNG | 13 ± 2 | 14 ± 2 | ||||||||
| Percentage contribution of glucose cycling | 42 ± 4 | 39 ± 3 | ||||||||
GNG, conversion of G-6-P derived from gluconeogenic precursors to glucose.
Significant difference from the corresponding values of the placebo group in the identical test period (P < 0.05).
Significant difference from the corresponding values of the GPI group in the identical test period (P < 0.05).
Significant difference from the corresponding values of the control period in the identical group (P < 0.05).
Significant difference from the corresponding values of the test period I in the identical group (P < 0.05).
At the end Test Period II, in the vehicle group, NHGO was increased 3-fold, which was associated with a 3-fold increase in M-HGR that was accompanied by increased net glycogenolysis without an alteration of G-6-P neogenesis. GPI treatment markedly decreased glucagon-induced increase in NHGO, M-HGR, and net glycogenolysis. G-6-Pase flux and glucose cycling rate were markedly lower in the GPI group compared with that in the vehicle group. On the other hand, M-HGU was markedly increased. G-6-P neogenesis and GK flux in the GPI group were not significantly different from that in the vehicle group. MT treatment reduced glucagon-induced increase in NHGO, M-HGR, and net glycogenolysis by approximately half (by 58, 44, and 52%, respectively). G-6-Pase flux, GK flux, and glucose cycling in the MT group were lower by 40% compared with that in the vehicle group. On the other hand, the percentage contribution of net glycogenolysis and gluconeogenesis to M-HGR and the percentage contribution of net glycogenolysis, gluconeogenesis, and glucose cycling to G-6-Pase flux were similar to that of the vehicle group.
Discussion
This study shows that decreased basal and glucagon-induced NHGO by inhibiting glycogen phosphorylase is associated with decreased G-6-Pase flux because of lower G-6-P concentrations, which in turn resulted from an activation of net glycogenesis. On the other hand, MT decreases G-6-Pase activity, promoting glycogen sparing via increased G-6-P content, leading up to decreased NHGO.
Effect of GPI and MT on Basal Hepatic Glucose Flux.
GPI-induced suppression of NHGO was associated with a marked decrease in M-HGR (from 12.3 to 0.9 μmol · kg−1 · min−1). Yet, the increased rate of G-6-P neogenesis (4–8 μmol of C6 unit · kg−1 · min−1) exceeded M-HGR (0.9 μmol · kg−1 · min−1), which indicated that the suppression of M-HGR by GPI treatment was associated with abolished net glycogenolysis and also redirected G-6-P flux from glucose release toward other pathway(s), such as glycogen synthesis and glycolysis. In addition, with GPI treatment, there was a shift of net hepatic lactate balance from production to uptake, and with a rapid equilibration between the plasma lactate pool and the intracellular lactate and pyruvate pools (Wolfe et al., 1988), this shift in lactate balance may have reflected a shift from pyruvate generation to pyruvate consumption in the net sense. Intracellular pyruvate is generated by glycolysis and deamination of amino acids mediated by transaminases. Because net hepatic amino acid uptake was not decreased by GPI, the switch of hepatic lactate flux from production to uptake probably resulted from decreased net glycolytic flux. This would support that GPI treatment redirects G-6-P from glucose release and glycolysis toward glycogen synthesis.
An inhibition of hepatic glycogenolysis using isopropyl 4-(2-chlorophenyl)-1-ethyl-2-methyl-5-oxo-1,4,5,7-tetrahydro-furo [3,4-b]pyridine-3-carboxylate (BAY R3401) resulted in a concomitant increase in hepatic gluconeogenesis, in agreement with our previous study in dogs (Shiota et al., 1997). In contrast, this was not observed by Fosgerau et al. (2001) using 1,4-diderexy-1,4-imino-d-arabinitol, which can induce a small degree of phosphorylation of phosphorylase in hepatocytes, thus leading to inhibition of glycogen synthase (Latsis et al., 2002). However, 1,4-diderexy-1,4-imino-d-arabinitol had no effect on glycogen synthesis in hepatocytes (Andersen et al., 1999; Latsis et al., 2002). On the other hand, a 1,4-dihydroxy-pyridine-2,3-dicarboxylate derivative (BAY R3401) and an indole 2-carboxamide (CP-316819), which were used in the previous (Shiota et al., 1997) and present studies, respectively, cause conversion of phosphorylase a into phosphorylase b and activation of glycogen synthase and, as a result, promote net glycogenesis (Shiota et al., 1997; Bergans et al., 2000); thereby, BAY R3401 and CP-316819 might pull gluconeogenic flux by increasing G-6-P flux toward glycogen via stimulation of net glycogenesis. Therefore, the observed differences in the effects of GPI on gluconeogenesis between Fosgerau et al. (2001) and us (Shiota et al., 1997; Bergans et al., 2000) may be explained as a difference in the mechanism of action among the compounds used.
MT tended to decrease NHGO and M-HGR that was associated with a decrease in both net glycogenolysis and gluconeogenesis. The percentage contribution of gluconeogenesis and glycogenolysis to M-HGR was not changed by MT. Therefore, it is likely that decreased NHGO by MT result from an inhibition of common site in the metabolic pathway where glucose is produced via glycogenolysis and gluconeogenesis.
Effect of Glucagon on Hepatic Glucose Flux in the Presence of GPI.
The increment of NHGO brought about by glucagon was associated exclusively with increased net glycogenolysis in the placebo group. Glucagon failed to increase NHGO in the presence of GPI. Net hepatic glucose flux is a balance between glucose phosphorylation rate mediated by glucokinase and G-6-P dephosphorylation rate mediated by G-6-Pase. A failure of glucagon to increase NHGO in the presence of GPI was associated with a failure to increase in G-6-Pase flux, because glucokinase flux was not altered. Hepatic content of G-6-P, a substrate for G-6-Pase, was less than half of that in the vehicle group, implying that the failure of glucagon to increase G-6-Pase flux arose from the failure of the hormone to maintain or increase the G-6-P pool. The rate of glucose phosphorylation (GK flux) or G-6-P neogenesis was not significantly altered; therefore, the decreased G-6-P content resulted from greater conversion of G-6-P into glycogen.
Glucagon increases net glycogenolysis via activation of glycogen phosphorylase and by inhibition of glycogen synthesis via inactivation of glycogen synthase. Compared with the vehicle group, glycogen phosphorylase a activity was significantly lower, and glycogen synthase activity was higher with GPI treatment. Activation of glycogen phosphorylase via protein kinase A and phosphorylase kinase results in enhanced phosphorylation of the inactive form (glycogen phosphorylase b), which promotes the formation of an active enzyme complex (glycogen phosphorylase a) (Bollen et al., 1998). The GPI, CP-316819, has been reported to bind the indole inhibitor site of glycogen phosphorylase by enhancing the dephosphorylation of the glycogen phosphorylase a complex, converting it to an inactive form (glycogen phosphorylase b) (Treadway et al., 2001). This is thought to occur as a result of a binding-induced conformational change that makes glycogen phosphorylase a better substrate for the regulatory phosphatase (protein phosphatase 1) (Kasvinsky et al., 1981) and/or interference with the binding of phosphorylase a to the C-terminal domain of the glycogen-targeting subunit of protein phosphatase 1 (Kelsall et al., 2007; Pautsch et al., 2008). The indole-2-carboxamide (CP-91149; Pfizer, Inc.) was shown to counteract the phosphorylation caused by glucagon (Latsis et al., 2002). Therefore, the treatment with GPI may interfere with the action of glucagon by activation of protein phosphatase 1.
The inactivation of glycogen synthase is mediated by enhancing the phosphorylation of the active form (glycogen synthase I) and converting it to an inactive form (glycogen synthase D) via activation of protein kinase A and mediated by protein phosphatase 1 (Bollen et al., 1998). Glycogen phosphorylase a may inhibit protein phosphatase 1 allosterically through the binding of the enzyme to the C-terminal domain of the glycogen-targeting subunit of protein phosphatase 1 (Armstrong et al., 1998). The failure of glucagon to inactivate glycogen synthase in the presence of GPI probably results from a defective activation of glycogen phosphorylase by this hormone.
Insulin inactivates glycogen phosphorylase a by activation of protein phosphatase 1 via activation of protein kinase B/Akt (Aiston et al., 2006). It has been reported that inhibition of phosphorylase activity is critical to activate glycogen synthase (glycogen synthesis) by elevation of glucose and insulin in cultured hepatocytes isolated from normal rats (Aiston et al., 2003b). Therefore, it is likely that acute alteration of net glycogen flux in the liver by glucagon and insulin is primarily mediated by regulating glycogen phosphorylase activity.
Effect of Glucagon on Hepatic Glucose Flux in the Presence of MT.
Partial reduction of the glucagon-induced increase in NHGO in the presence of MT was also associated with decreased G-6-Pase flux without an alteration of GK flux. In contrast to the decreased G-6-Pase flux in the GPI group with a marked reduction in metabolites in the GPI group, the decreased G-6-Pase flux with MT treatment was accompanied by a markedly greater content of G-6-P compared with the vehicle group. This increase in the G-6-P pool occurred without an increase in glucose phosphorylation (GK flux), G-6-P neogenesis, or glycogenolysis. Furthermore, the lower G-6-Pase flux was associated with equally lower rates of gluconeogenesis, glucose cycling, and glycogenolysis without changes in the percentage contribution of glycogenolysis, gluconeogenesis, and glucose cycling to G-6-Pase flux. These results suggest that the reduced glucagon-induced NHGO brought about by treatment with MT resulted primarily from an inhibition of G-6-Pase activity by MT.
Decreased glucagon-induced glycogenolysis in the presence of MT was brought about without decreased phosphorylase a activity. On the other hand, glycogen synthase activity and incorporation of glucose into glycogen were slightly but significantly higher in the MT group compared with that in the vehicle group. The greater glycogen synthase activity in the MT group might have arisen due to the increased G-6-P content, because the latter is a strong activator of glycogen synthase. Our results are consistent with the report by Mithieux et al. (2002) that the suppression of hepatic glucose production by chronic treatment with MT in insulin-resistant high-fat diet fed rats is accompanied by increased content of G-6-P in liver. Therefore, it is likely that inhibition of G-6-Pase activity by MT decreased net glycogenolysis by promoting glycogen sparing through increased G-6-P content.
The glucose-lowering effects of MT are mainly a consequence of reduced hepatic glucose output through inhibition of both gluconeogenesis and glycogenolysis in patients with type 2 diabetes (Hundal et al., 2000; Kirpichnikov et al., 2002). The in vitro studies using isolated hepatocytes (Argaud et al., 1993) or perfused liver (Radziuk et al., 1997) demonstrated the acute effect of MT in inhibiting gluconeogenesis. Although its primary action has been proposed to be mitochondrial function, the exact mechanism through which MT reduces hepatic gluconeogenesis remains unclear (Kirpichnikov et al., 2002). The present study demonstrates that the primary action of MT to reduce net glycogenolysis may not be through glycogen phosphorylase inhibition. Glucose production via glycogenolysis and gluconeogenesis shares one common step in their metabolic pathway, dephosphorylation of G-6-P by G-6-Pase. Therefore, the present data suggest that G-6-Pase is one of the primary sites of MT's action.
This work was supported by a grant from Pfizer, Inc. (to M.S.). The hormone assay core laboratory at Vanderbilt University Medical Center was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK20593].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.177899.
- G-6-P
- glucose 6-phosphate
- GPI
- glycogen phosphorylase inhibitor
- MT
- metformin
- NHGO
- net hepatic glucose output
- PEG
- polyethylene glycol
- NHGU
- net hepatic glucose uptake
- M-HGR
- minimal estimate of unidirectional hepatic glucose release
- CP-316819
- (3S,2R)-3-(5-chloroindole-2-carbonyl)amino-2-hydroxy-4-phenylbutyric acid N-methyl-N-methoxyamide
- SA
- specific activity
- M-HGU
- minimal estimate of unidirectional hepatic glucose uptake
- GK
- glucokinase
- G-6-Pase
- glucose-6-phosphatase
- BAY R3401
- isopropyl 4-(2-chlorophenyl)-1-ethyl-2-methyl-5-oxo-1,4,5,7-tetrahydro-furo[3,4-b]pyridine-3-carboxylate.
Authorship Contributions
Participated in research design: Shiota and Treadway.
Conducted experiments: Torres, Sasaki, Lacy, Donahue, and Shiota.
Contributed new reagents or analytic tools: Treadway.
Performed data analysis: Shiota and Cherrington.
Wrote or contributed to the writing of the manuscript: Shiota, Printz, and Cherrington.
References
- Aiston S, Andersen B, Agius L. (2003a) Glucose 6-phosphate regulates hepatic glycogenolysis through inactivation of phosphorylase. Diabetes 52:1333–1339 [DOI] [PubMed] [Google Scholar]
- Aiston S, Coghlan MP, Agius L. (2003b) Inactivation of phosphorylase is a major component of the mechanism by which insulin stimulates hepatic glycogen synthesis. Eur J Biochem 270:2773–2781 [DOI] [PubMed] [Google Scholar]
- Aiston S, Green A, Mukhtar M, Agius L. (2004) Glucose 6-phosphate causes translocation of phosphorylase in hepatocytes and inactivates the enzyme synergistically with glucose. Biochem J 377:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiston S, Hampson LJ, Arden C, Iynedjian PB, Agius L. (2006) The role of protein kinase B/Akt in insulin-induced inactivation of phosphorylase in rat hepatocytes. Diabetologia 49:174–182 [DOI] [PubMed] [Google Scholar]
- Andersen B, Rassov A, Westergaard N, Lundgren K. (1999) Inhibition of glycogenolysis in primary rat hepatocytes by 1,4-dideoxy-1,4-imino-d-arabinitol. Biochem J 342:545–550 [PMC free article] [PubMed] [Google Scholar]
- Argaud D, Roth H, Wiernsperger N, Leverve XM. (1993) Metformin decreases gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes. Eur J Biochem 213:1341–1348 [DOI] [PubMed] [Google Scholar]
- Armstrong CG, Doherty MJ, Cohen PT. (1998) Identification of the separate domains in the hepatic glycogen-targeting subunit of protein phosphatase 1 that interact with phosphorylase a, glycogen and protein phosphatase 1. Biochem J 336:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ, Turner RC. (1996) Metformin. N Engl J Med 334:574–579 [DOI] [PubMed] [Google Scholar]
- Bergans N, Stalmans W, Goldmann S, Vanstapel F. (2000) Molecular mode of inhibition of glycogenolysis in rat liver by the dihydropyridine derivative, BAY R3401: inhibition and inactivation of glycogen phosphorylase by an activated metabolite. Diabetes 49:1419–1426 [DOI] [PubMed] [Google Scholar]
- Bollen M, Keppens S, Stalmans W. (1998) Specific features of glycogen metabolism in the liver. Biochem J 336:19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner EA, Haugaard N. (1965) The effect of thiopental on hepatic glycogen phosphorylase. J Pharmacol Exp Ther 150:99–104 [PubMed] [Google Scholar]
- Ciudad CJ, Carabaza A, Guinovart JJ. (1986) Glucose 6-phospate plays a central role in the activation of glycogen synthase by glucose in hepatocytes. Biochem Biophys Res Commun 141:1195–1200 [DOI] [PubMed] [Google Scholar]
- Chu CA, Wiernsperger N, Muscato N, Knauf M, Neal DW, Cherrington AD. (2000) The acute effect of metformin on glucose production in the conscious dog is primarily attributable to inhibition of glycogenolysis. Metabolism 49:1619–1626 [DOI] [PubMed] [Google Scholar]
- Connolly CC, Stevenson RW, Neal DW, Wasserman DH, Cherrington AD. (1993) The effects of lactate loading on alanine and glucose metabolism in the conscious dog. Metabolism 42:154–161 [DOI] [PubMed] [Google Scholar]
- Consoli A. (1992) Role of liver in pathophysiology of NIDDM. Diabetes Care 15:430–441 [DOI] [PubMed] [Google Scholar]
- Diamond MP, Rollings RC, Steiner KE, Williams PE, Lacy WW, Cherrington AD. (1988) Effect of alanine concentration independent of changes in insulin and glucagon on alanine and glucose homeostasis in the conscious dog. Metabolism 37:28–33 [DOI] [PubMed] [Google Scholar]
- Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. (1986) Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest 77:1525–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosgerau K, Mittelman SD, Sunehag A, Dea MK, Lundgren K, Bergman RN. (2001) Lack of hepatic “interregulation” during inhibition of glycogenolysis in a canine model. Am J Physiol 281:E375–E383 [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Torres TP, Donahue EP, Shiota M. (2006) Glucose toxicity is responsible for the development of impaired regulation of endogenous glucose production and hepatic glucokinase in Zucker diabetic fatty rats. Diabetes 55:2479–2490 [DOI] [PubMed] [Google Scholar]
- Goldstein RE, Rossetti L, Palmer BA, Liu R, Massillon D, Scott M, Neal D, Williams P, Peeler B, Cherrington AD. (2002) Effects of fasting and glucocorticoids on hepatic gluconeogenesis assessed using two independent methods in vivo. Am J Physiol Endocrinol Metab 283:E946–E957 [DOI] [PubMed] [Google Scholar]
- Gomis RR, Ferrer JC, Guinovart JJ. (2000) Shared control of hepatic glycogen synthesis by glycogen synthase and glucokinase. Biochem J 351:811–816 [PMC free article] [PubMed] [Google Scholar]
- Greenway CV, Stark RD. (1971) Hepatic vascular bed. Physiol Rev 51:23–65 [DOI] [PubMed] [Google Scholar]
- Hampson LJ, Agius L. (2005) Increased potency and efficacy of combined phosphorylase inactivation and glucokinase activation in control of hepatocyte glycogen metabolism. Diabetes 54:617–623 [DOI] [PubMed] [Google Scholar]
- Hoover DJ, Hulin B, Martin WH, Treadway JL. (2001), inventors; Pfizer Inc., assignee. Substituted N-(indole-2-carbonyl-)amides and derivatives as glycogen phosphorylase inhibitors. U.S. Patent 6,297,269 2001 Oct 2
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, et al. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo P, Hallsten K, Oikonen V, Virtanen KA, Kemppainen J, Solin O, Ferrannini E, Knuuti J, Nuutila P. (2003) Insulin-mediated hepatic glucose uptake is impaired in type 2 diabetes: evidence for a relationship with glycemic control. J Clin Endocrinol Metab 88:2055–2060 [DOI] [PubMed] [Google Scholar]
- Jahoor F, Peters EJ, Wolfe RR. (1990) The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol 258:E288–E296 [DOI] [PubMed] [Google Scholar]
- Jenssen T, Nurjhan N, Consoli A, Gerich JE. (1990) Failure of substrate-induced gluconeogenesis to increase overall glucose appearance in normal humans. Demonstration of hepatic autoregulation without a change in plasma glucose concentration. J Clin Invest 86:489–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasvinsky PJ, Fletterick RJ, Madsen NB. (1981) Regulation of the dephosphorylation of glycogen phosphorylase a and synthase b by glucose and caffeine in isolated hepatocytes. Can J Biochem 59:387–395 [DOI] [PubMed] [Google Scholar]
- Kelsall IR, Munro S, Hallyburton I, Treadway JL, Cohen PT. (2007) The hepatic PP1 glycogen-targeting subunit interaction with phosphorylase a can be blocked by C-terminal tyrosine deletion or an indole drug. FEBS Lett 581:4749–4753 [DOI] [PubMed] [Google Scholar]
- Kirpichnikov D, McFarlane SI, Sowers JR. (2002) Metformin: an update. Ann Intern Med 137:25–33 [DOI] [PubMed] [Google Scholar]
- Krssak M, Brehm A, Bernroider E, Anderwald C, Nowotny P, Dalla Man C, Cobelli C, Cline GW, Shulman GI, Waldhäusl W, et al. (2004) Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 53:3048–3056 [DOI] [PubMed] [Google Scholar]
- Latsis T, Andersen B, Agius L. (2002) Diverse effects of two allosteric inhibitors on the phosphorylation state of glycogen phosphorylase in hepatocytes. Biochem J 368:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevy CM, Mendenhall CL, Lesko W, Howard MM. (1962) Estimation of hepatic blood flow with indocyanine green. J Clin Invest 41:1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Richter EA, Galbo H. (1986) Route of administration of pentobarbital affects activity of liver glycogen phosphorylase. J Appl Physiol 61:804–806 [DOI] [PubMed] [Google Scholar]
- Mithieux G, Guignot L, Bordet JC, Wiernsperger N. (2002) Intrahepatic mechanisms underlying the effect of metformin in decreasing basal glucose production in rats fed a high-fat diet. Diabetes 51:139–143 [DOI] [PubMed] [Google Scholar]
- Moore MC, Pagliassotti MJ, Swift LL, Asher J, Murrell J, Neal D, Cherrington AD. (1994) Disposition of a mixed meal by the conscious dog. Am J Physiol 266:E666–E675 [DOI] [PubMed] [Google Scholar]
- Myers SR, McGuinness OP, Neal DW, Cherrington AD. (1991) Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest 87:930–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa AK, Willoughby CA, Bergeron R, Ellsworth KP, Geissler WM, Myers RW, Yao J, Harris G, Chapman KT. (2003) Glucose-lowering in a db/db mouse model by dihydropyridine diacid glycogen phosphorylase inhibitors. Bioorg Med Chem Lett 13:3405–3408 [DOI] [PubMed] [Google Scholar]
- Pautsch A, Stadler N, Wissdorf O, Langkopf E, Moreth W, Streicher R. (2008) Molecular recognition of the protein phosphatase 1 glycogen targeting subunit by glycogen phosphorylase. J Biol Chem 283:8913–8918 [DOI] [PubMed] [Google Scholar]
- Radziuk J, Zhang Z, Wiernsperger N, Pye S. (1997) Effects of metformin on lactate uptake and gluconeogenesis in the perfused rat liver. Diabetes 46:1406–1413 [DOI] [PubMed] [Google Scholar]
- Scheen AJ. (1996) Clinical pharmacokinetics of metformin. Clin Pharmacokinet 30:359–371 [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310:1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota M, Jackson PA, Bischoff H, McCaleb M, Scott M, Monohan M, Neal DW, Cherrington AD. (1997) Inhibition of glycogenolysis enhances gluconeogenic precursor uptake by the liver of conscious dogs. Am J Physiol 273:E868–E879 [DOI] [PubMed] [Google Scholar]
- Staehr P, Hother-Nielsen O, Beck-Nielsen H, Roden M, Stingl H, Holst JJ, Jones PK, Chandramouli V, Landau BR. (2007) Hepatic autoregulation: response of glucose production and gluconeogenesis to increased glycogenolysis. Am J Physiol Endocrinol Metab 292:E1265–E1269 [DOI] [PubMed] [Google Scholar]
- Torres TP, Catlin RL, Chan R, Fujimoto Y, Sasaki N, Printz RL, Newgard CB, Shiota M. (2009) Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zucker diabetic fatty rats. Diabetes 58:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway JL, Mendys P, Hoover DJ. (2001) Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs 10:439–454 [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A, Abel MJ, Campbell RA, Donahue EP, Uselton TC, Flakoll PJ. (1996) Whole blood analysis of gluconeogenic amino acids for estimation of de novo gluconeogenesis using pre-column o-phthalaldehyde derivatization and high-performance liquid chromatography. J Chromatogr B Biomed Appl 676:1–6 [DOI] [PubMed] [Google Scholar]
- Wada M, Connolly CC, Tarumi C, Neal DW, Cherrington AD. (1995) Hepatic denervation does not significantly change the response of the liver to glucagon in conscious dogs. Am J Physiol 268:E194–E203 [DOI] [PubMed] [Google Scholar]
- Wolfe RR, Jahoor F, Miyoshi H. (1988) Evaluation of the isotopic equilibration between lactate and pyruvate. Am J Physiol 254:E532–E535 [DOI] [PubMed] [Google Scholar]
- Youn JH, Bergman RN. (1990) Enhancement of hepatic glycogen by gluconeogenic precursors: substrate flux or metabolic control? Am J Physiol 258:E899–E906 [DOI] [PubMed] [Google Scholar]