Abstract
Background
The mass media and clinical journals have reported lengthy waiting times after surgery before initiation of radiation therapy (RT) for cancer across Canada. We aimed to describe the length of time between the last date of surgery or biopsy or chemotherapy and first date of RT.
Methods
This is a population-based study measuring waiting times for RT in Ontario among all patients with potentially curable cancer of the cervix, tonsil and larynx and a random sample of women who had had breast cancer resection, whose first date of RT fell between Sept. 1, 2001, and Aug. 31, 2002. Abstraction of original health care records provided each patient's demographics, cancer stage and cancer treatment (last surgery, consultation, simulation, first RT). Last dates of chemotherapy before RT were obtained from abstraction or from Ontario Health Insurance Plan (OHIP) files, and last dates of surgery before RT were compared with dates in the Canadian Institute for Health Information (CIHI) Discharge Abstract Database.
Results
Waiting times between the last date of surgery or chemotherapy and the first date of RT varied significantly among the health regions of Ontario. Increasing age, but not the presence of comorbidity, was associated with longer waiting times. Women who did not receive postoperative chemotherapy before RT for breast cancer waited significantly longer than all others.
Conclusion
Measurement of waiting times for cancer RT must discount time during which adjuvant intravenous chemotherapy is administered after surgery and before RT. There appears to be a formal or informal process by which those at highest risk begin RT most rapidly.
Abstract
Contexte
Les médias de masse et les journaux cliniques ont signalé de longs délais d'attente après une intervention chirurgicale avant le début de la radiothérapie (RT) contre le cancer, partout au Canada. Nous voulions décrire la longueur de la période écoulée entre la date de la plus récente intervention chirurgicale, biopsie ou chimiothérapie et la date d'administration du premier traitement de RT.
Méthodes
Cette étude représentative mesure les temps d'attente pour la RT en Ontario chez tous les patients atteints d'un cancer du col, des amygdales et du larynx qui pourrait être guérissable et un échantillon aléatoire de femmes ayant subi une exérèse d'un cancer du sein et qui ont reçu leur premier traitement de RT entre le 1er septembre 2001 et le 31 août 2002. Le résumé des dossiers de santé originaux a produit les caractéristiques démographiques de chaque patient, le stade du cancer et le traitement contre le cancer (dernière intervention chirurgicale, consultation, planification de la RT, premier traitement de RT). On a tiré les dernières dates de la chimiothérapie avant la RT du résumé ou des dossiers du Régime d'assurance-maladie de l'Ontario (RAMO) et l'on a comparé les dernières dates de la chirurgie avant la RT aux dates tirées de la base de données sur les congés des patients de l'Institut canadien d'information sur la santé (ICIS).
Résultats
Les temps d'attente entre la date de la dernière chirurgie ou de la chimiothérapie et celle du premier traitement de RT ont varié considérablement entre les régions de santé de l'Ontario. On a établi un lien entre l'âge, mais non la présence d'une comorbidité, et des temps d'attente plus longs. Les femmes qui n'ont pas reçu de chimiothérapie postopératoire avant la RT contre un cancer du sein ont attendu beaucoup plus longtemps que toutes les autres.
Conclusion
La mesure des temps d'attente pour des traitements de RT contre le cancer doit tenir compte du temps pendant lequel on administre une chimiothérapie intraveineuse d'appoint après la chirurgie et avant la RT. Il semble y avoir un processus officiel ou officieux en résultat duquel ce sont les patients exposés au risque le plus élevé qui commencent des traitements de RT plus rapidement.
Adjuvant radiation therapy (RT) after surgery for breast cancer is a common application of RT. Over the past 2 decades, indications for adjuvant RT have increased because of the evidence-based adoption of breast-conserving surgery requiring postoperative radiation and an evidence-based revival of indications for postmastectomy RT. In addition, the age-adjusted incidence of breast cancer has increased.
RT is one of the curative modalities of therapy for cancer of the cervix, larynx and tonsil, among others. For many women with cervical cancer, RT offers the only possibility of cure. For cases of larynx or tonsil cancer, RT may offer the only possibility of cure with preservation of voice or the avoidance of major disfigurement. Cancers of the cervix, larynx and tonsil are understood to have rapid growth of clonogenic tumour cells. Theoretically, delays in initiation of RT might allow the population of these cells to exceed the ability of RT to cure these cancers. The impact of delaying RT on the risk of local recurrence remains controversial.1,2,3,4
RT capacity has not increased proportionally with demand, resulting in increasingly long delays across Canada.5,6,7 Ontario adopted interim measures between 1998 and 2002 to reduce delays through a system of re-referral within the province, to the United States or to a publicly funded, privately managed RT service (Canadian Radiation Oncology Services).
The measurement and evaluation of waiting times for RT is complex. We have described delays in RT and associated factors as closely as possible to real time.
Methods
This is a population-based study of all patients with potentially curable cancer of the cervix, tonsil and larynx, as well as a random sample of patients with breast cancer who began to receive RT between Sept. 1, 2001, and Aug. 31, 2002, in Ontario. RT was provided by 8 regional cancer centres, 1 acute care hospital (Princess Margaret Hospital, Toronto) and the publicly funded, privately managed after-hours RT program managed by Canadian Radiation Oncology Services.
Lists of eligible patients were obtained directly from the RT providers' health record departments and from Cancer Care Ontario. Patients who were aged 18 years or older at the start of RT and had a valid Ontario Health Insurance Plan (OHIP) number were eligible. The number of patients residing outside Canada who receive RT in Ontario is negligible and would be excluded by their lack of a valid OHIP number. All patients with cervix or tonsil and larynx cancers were included, plus a random sample of 25% of patients with breast cancer who were starting RT in the same time period.
Original health records from RT facilities were abstracted to obtain data on patients' sociodemographic characteristics (age and postal codes), treatment history (dates and descriptions of surgeries, pathological and staging information) and dates of key events in the process of RT (consultation, simulation [planning], first date of RT). Dates of referral to an RT provider were frequently unavailable. Abstractors photocopied RT records that were later reviewed and abstracted by an experienced radiation therapist.
Data on all primary cancer– directed surgeries (surgeries, excisions, re-excisions, biopsies — except for needle biopsies) during the year before RT were obtained from chart abstraction. From abstracted data, the Discharge Abstract Database of the Canadian Institute for Health Information (CIHI) and OHIP, we determined the date of most recent cancer surgery up to 1 year before RT. Information on chemotherapy administered between the last surgery or biopsy and the first RT was obtained from chart abstraction and OHIP. Seven patients with cancer who did not have a valid date of surgery or biopsy were assigned the date of the referral for RT as a surrogate for the date of last surgery before RT.
Stage of disease was assigned according to the criteria of the American Joint Committee on Cancer.8 Less than 2% of patients had missing information concerning staging, and their missing stage was assigned by the principal investigator, based on abstracted data and pathology reports. Eighteen stage 0 patients (11 with breast cancer and 7 with tonsil and larynx cancer) were included with stage I patients in our analyses.
The date of first consultation with a radiation oncologist after the last surgery or biopsy was obtained from chart abstraction and OHIP files. The date of RT simulation was obtained from chart abstraction. We combined the chart and CIHI information and calculated a modified (Deyo–Charlson with all cancers removed)9,10 comorbidity index with higher scores reflecting patients with pre-existing conditions (e.g., diabetes, asthma, liver disease).
The information on patients' sex, age and postal code was confirmed by the Registered Persons Database containing contact and administrative data for all OHIP beneficiaries (i.e., all permanent residents). We used census data from 2001 to label each case with the health region (n = 7) in which the patient resided and to link to an ecological indicator of socioeconomic status (SES). Patients were divided into 2 groups based on their residence geographic code: (1) those who lived in areas with 15% or more low-income families and (2) those living in areas with less than 15% low-income families.
Ethics review and approval of the study was obtained from the Sunnybrook and Women's College Health Sciences Centre, and the study was then approved by each RT facility in Ontario.
The quality and consistency of abstracted data was assessed during training and re-established twice during the study. A randomly selected sample of charts that had been previously abstracted were re-abstracted by another abstractor. Assessment of data consistency was performed for 16 important variables such as the dates of surgery or biopsy, histological confirmation, referrals and consultations, and start of RT.
The definition of the primary outcome is the time period from the date of last surgery or biopsy or chemotherapy to the date of first RT. We calculated time intervals between the last surgery or biopsy and first radiation oncology consultation, between first consultation and simulation, and between simulation and the date of the first RT. Data are presented using medians and the 25th and 75th percentiles by cancer type. This presentation was chosen to avoid drawing attention to unexplainable outliers and to draw attention to the central tendency, as well as the shortest and longest quartile of waiting. Patients with final surgery after first RT consultation were excluded from these calculations, but included in overall calculations. The interval between the date of last surgery or chemotherapy and the first date of RT was modelled using linear regression.
Results
The importance of distinguishing patients who have received chemotherapy between surgery and RT from those who have not is illustrated in Table 1. Henceforth, all data for breast cancer will be stratified by the use of intravenous (IV) chemotherapy or not between surgery and RT, and only the intervals between the dates of last chemotherapy and first RT will be presented for those who received chemotherapy. This has face validity, whereas the interval between last date of surgery and first date of RT is grossly misleading among this subgroup, because patients with breast cancer will normally wait until chemotherapy is finished before starting radiation.
Table 1
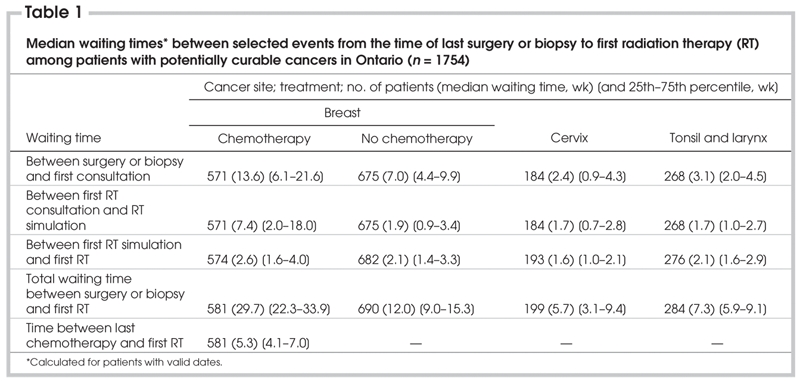
There is remarkable variation in the interval between last surgery and first radiation oncology consultation. However, the interval is 2–6 times longer among women who received IV chemotherapy between surgery and RT and is irrelevant among this subgroup. The shortest interval is observed among those patients whose cancers are potentially curable by RT (cervix, larynx and tonsil). Similarly, the interval between first radiation oncology consultation and simulation varies 4-fold. For those patients whose cancers were potentially and/or exclusively curable by RT (cervix, larynx and tonsil) the median interval was less than 2 weeks.
Table 2 displays the variation in median intervals and interquartile ranges among the 7 health regions, which is substantially larger than the minor variations among patients grouped by SES, cancer stage and comorbidity. Among the health region and SES, cancer stage and comorbidity subgroups, the overall and discrete subcomponent intervals of waiting were shortest for the potentially and/or exclusively curable cancers. After discounting time on chemotherapy among women with breast cancer, we can see that women who did not receive IV chemotherapy between surgery and RT for breast cancer have the longest waits.
Table 2
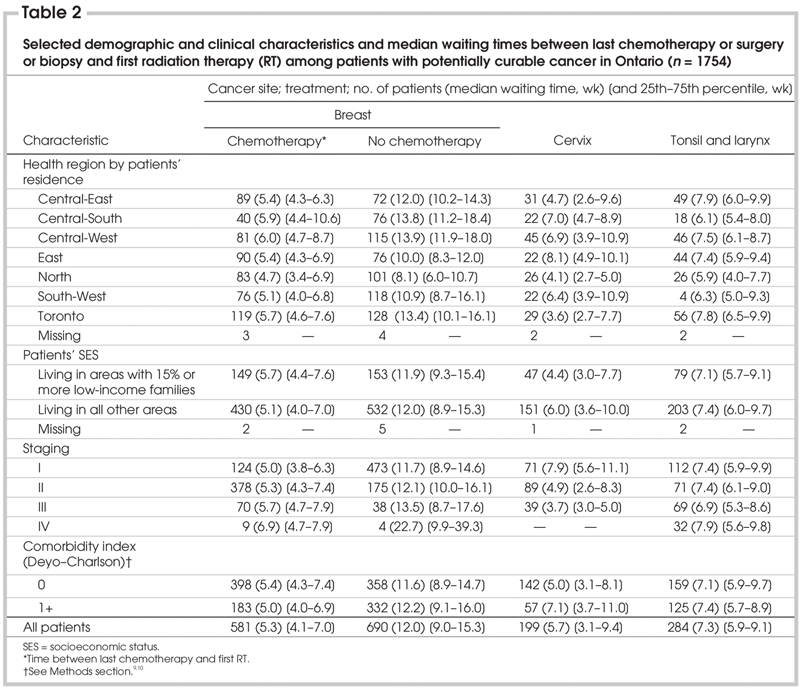
Table 3 shows the results of multiple linear regression analysis on the waiting time between the last date of surgery or IV chemotherapy and the first date of RT for the entire study population. The parameter estimates are simultaneously adjusted for the other variables in the model and for SES and comorbidity. Cancer stage did not improve the model; this is probably because the breast cancer with chemotherapy group substantially overlaps with stage 2 cancer or higher, and because the waiting times for patients with those cancers that are potentially or exclusively curable by RT show little variation.
Table 3
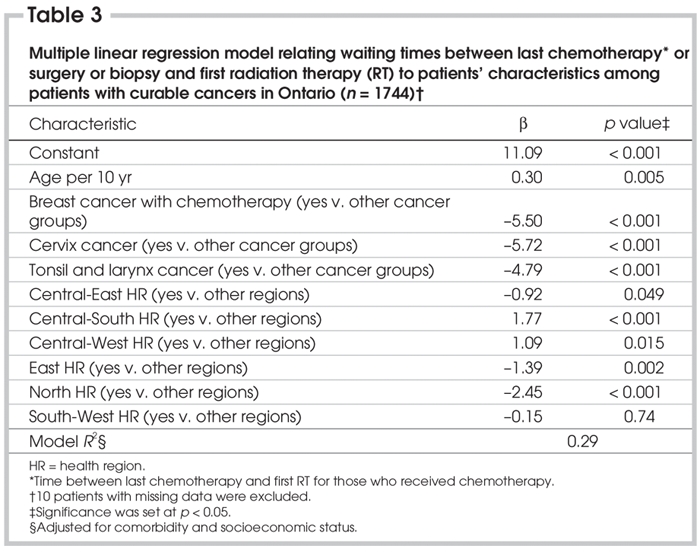
The estimates for the groups by type of cancer listed are substantially influenced by the inclusion of women who did not receive IV chemotherapy for breast cancer in the „other cancer” groups (and the interval between their last date of surgery and the first date of RT), and the estimates for health regions are substantially influenced by the inclusion of the most populous health region (City of Toronto, population 2.5 million, and its waiting times between the last date of surgery or chemotherapy and the first date of RT) in the „other regions” group.
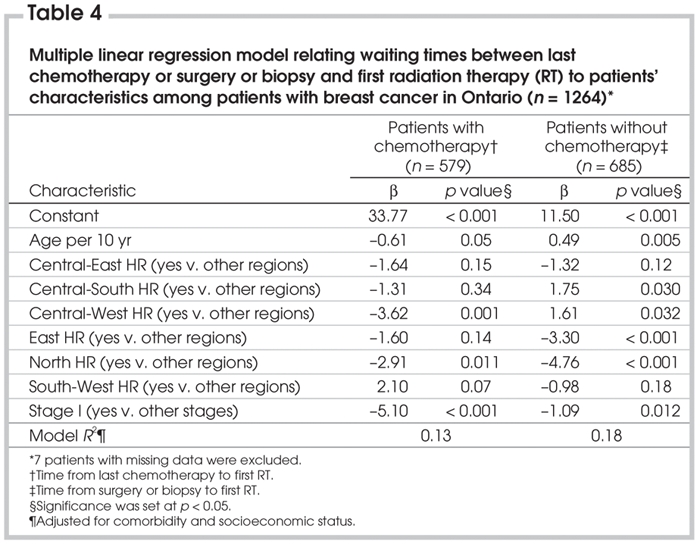
Table 4 shows linear regression models for the 2 subgroups of women with breast cancer. Stage does contribute to each model and, in each case, stage I patients had shorter waiting times. This may reflect less intensive therapy for women whose cancers were detected by screening, which would possibly be associated with fewer surgical or chemotherapy complications, depending on the subgroup. Although only 2 health regions vary from the others in the model for women who received chemotherapy, the model for women who did not receive chemotherapy showed that the interval between the last date of breast cancer surgery and the first date of RT varied significantly among most health regions.
Table 4
Discussion
This study had adequate power to compare waiting times for women with breast cancer who did receive chemotherapy with those for women who did not. Most earlier publications excluded women who had received chemotherapy or did not identify the use of chemotherapy.3,11,12 We have shown that it is most important to measure the interval between the last date of IV chemotherapy and the first date of RT in order to estimate intervals between treatment events with face validity and relevance.
The flow of patients appears to be consistent with (1) either a formal or informal process to ensure that those with the highest risk of recurrence or those with cancer potentially and/or exclusively curable by RT move through the processes to initiation of RT more rapidly than others or (2) it may be that the women who did not receive chemotherapy for breast cancer included more cases with lower stage and less aggressive cancer but that this group was not exclusively comprised of them. The longer interval from last surgery to RT consultation may reflect less urgency in the perception of the surgeon or the patient (selection bias) or a limitation on the inflow rate of such women into the facilities of the RT providers.
The most obvious interval variations, apart from those among the cancer subgroups, are among the health regions. There are multiple potential explanations, none of which can be proven, and probably multiple factors are simultaneously operating in any health region. The efficiency or inefficiency of the processes and/or the capacity to provide RT (staff, equipment) may vary among the regions. Variations in types of breast cancer surgery or in breast cancer incidence (and influences upon it, such as screening) are unlikely to be large enough to explain the differences among the health regions, and there are no obvious disease-related or treatment-related factors to explain differences among the health regions for cervix, larynx and tonsil cancers. After adjustment for comorbidity, increasing age is associated with longer waiting times among the entire study population, except for women who received IV chemotherapy for breast cancer. Table 2 shows that the variation among health regions is the most likely explanation for the huge discrepancies, such as 25% of patients with cancer of the cervix waiting less than 3.1 weeks from date of last surgery to first RT but 25% waiting longer than 9.4 weeks.
The method we used to estimate waiting times has been validated by a review of original health records showing concordance with administrative data. In Ontario, all data points are found in the OHIP and hospital databases, with the first date of RT available at Cancer Care Ontario. Except for the tiny area of Ontario where surgeons do not bill OHIP, it is possible to monitor these intervals using only OHIP and Cancer Care Ontario data within 2 or 3 months of the end of each quarter of the year. This population-based patient group should serve as an inception cohort, among which to measure recurrence of cancer within the RT-treated anatomical part within 3 years from the time of writing of this manuscript. The study has sufficient power to detect variation in recurrence rates associated with variation in waiting times, adjusting for important covariates and confounders, especially treatment with chemotherapy and cancer stage.
Acknowledgments
This research was funded by a Canadian Institutes of Health Research (CIHR) operating grant to the primary author (V.B.) and by the Institute for Clinical Evaluative Sciences (ICES).
This article is dedicated to the memory of our late coauthor, Dr. Veronique Benk.
Competing interests: None declared.
Correspondence to: Dr. Lawrence Paszat, Institute for Clinical Evaluative Sciences (ICES), 2075 Bayview Ave., Toronto ON M4N 3M5; fax 416 480-6048; lawrence.paszat@ices.on.ca
References
- 1.Huang J, Barbera L, Brouwers M, et al. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol 2003;21:555-63. [DOI] [PubMed]
- 2.Benk V, Joseph L, Fortin P, et al. Effect of delay in initiating radiotherapy for patients with early stage breast cancer. Clin Oncol (R Coll Radiol) 2004;16:6-11. [DOI] [PubMed]
- 3.Froud PJ, Mates D, Jackson JS, et al. Effect of time interval between breast-conserving surgery and RT on ipsilateral breast recurrence. Int J Radiat Oncol Biol Phys 2000;46:363-72. [DOI] [PubMed]
- 4.Recht A, Come SE, Henderson IC, et al. The sequencing of chemotherapy and RT after conservative surgery for early-stage breast cancer. N Engl J Med 1996;334:1356-61. [DOI] [PubMed]
- 5.Mackillop WJ, Zhou S, Groome P, et al. Changes in the use of radiotherapy in Ontario 1984-1995. Int J Radiat Oncol Biol Phys 1999;44:355-62. [DOI] [PubMed]
- 6.Mackillop WJ, Groome PA, Zhang-Solomons J, et al. Does a centralized radiotherapy system provide adequate access to care? J Clin Oncol 1997;15:1261-71. [DOI] [PubMed]
- 7.Mackillop WJ, Zhou Y, Quirt CF. A comparison of delays in the treatment of cancer with radiation in Canada and the United States. Int J Radiat Oncol Biol Phys 1995;32:531-9. [DOI] [PubMed]
- 8.American Joint Committee on Cancer (AJCC). Cancer staging manual. 6th ed. Chicago: AJCC; 2002.
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [DOI] [PubMed]
- 10.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [DOI] [PubMed]
- 11.Clarke DH, Le MG, Sarrazin D, et al. Analysis of local-regional relapses in patients with early breast cancers treated by excision and radiotherapy: experience of the Institut Gustave-Roussy. Int J Radiat Oncol Biol Phys 1985;11:137-45. [DOI] [PubMed]
- 12.Nixon AJ, Recht A, Neuberg D, et al. The relation between the surgery-radiotherapy interval and treatment outcome in patients treated with breast-conserving surgery and radiation therapy without systemic therapy. Int J Radiat Oncol Biol Phys 1994;30:17-21. [DOI] [PubMed]


