Abstract
Introduction
This study was performed to evaluate the safety and short-term efficacy of laparoscopic Roux-en-Y gastric bypass (LRYGB) for morbid obesity and to describe the relation between learning curve and short-term outcomes.
Methods
We collected a prospective database on the first 201 consecutive patients who underwent LRYGB by a single university-based, experienced bariatric surgeon over 24 months. We divided patients into 3 consecutive groups of 67 patients for analysis (Group 1, Group 2 and Group 3).
Results
The mean patient age was 37 (standard deviation [SD] 9) years; mean body mass index (BMI) was 49.2 (SD 8.3) kg/m2. BMI was similar in Groups 1 and 2 (mean 47.1, SD 5.9 and mean 48.7, SD 8.9 kg/m2) but increased in Group 3 (mean 52, SD 9.7 kg/m2, p < 0.01). Operative time decreased from 145 (SD 30) minutes in Group 1 to 114 (SD 24) minutes in Group 2 (p < 0.01) and was maintained at 119 (SD 23) minutes in Group 3. Early and late complication rates were 14.9% and 12.4%, respectively. Leak rates decreased from 6.0% in the first group to 1.5% in Groups 2 and 3, but they did not reach statistical significance. Anastomotic stricture rates decreased from 11.9% in Group 1 to 3.0% in Group 2 (p < 0.01). Overall excess weight loss for the entire series was 31.5% (SD 11.9%), 54.5% (SD 14.1%), 77.1% (SD 18.5%) and 82.1% (SD 17.5%) at 3, 6, 12 and 18 months, respectively.
Conclusion
LRYGB can be performed with acceptable morbidity and short-term results during the learning curve. In our series, operative time and anastomotic stricture rates decreased with experience, despite an increase in mean BMI.
Abstract
Introduction
Cette étude visait à évaluer la sécurité et l'efficacité à court terme de la dérivation gastrique isolée dite de Roux en Y (DGIRY) effectuée par laparoscopie pour traiter l'obésité morbide et à décrire le lien entre la courbe d'apprentissage et les résultats à court terme.
Méthodes
Nous avons recueilli une base de données prospective au sujet des 201 premiers patients consécutifs ayant subi dans un centre universitaire une intervention de DGIRY effectuée par un seul chirurgien spécialiste de l'obésité au cours d'une période de 24 mois. Aux fins d'analyse, nous avons réparti les patients en trois groupes consécutifs de 67 patients (Groupe 1, Groupe 2 et Groupe 3).
Résultats
L'âge moyen des patients était de 37 ans (écart type [ET] de 9); l'indice de masse corporelle moyen (IMC), de 49,2 kg/m2 (ET de 8,3). L'IMC était semblable entre les Groupes 1 et 2 (moyenne de 47,1 et ET de 5,9; moyenne de 48,7 et ET de 8,9 kg/m2) mais était plus élevé chez les patients du Groupe 3 (moyenne de 52 et ET de 9,7 kg/m2, p < 0,01). La durée de l'intervention est passée de 145 minutes (ET de 30) pour le Groupe 1 à 114 minutes (ET de 24) pour le Groupe 2 (p < 0,01), tandis qu'elle s'est maintenue à 119 minutes (ET de 23) pour le Groupe 3. Les taux de complications précoces et tardives se sont établies à 14,9 % et 12,4 %, respectivement. Les taux de fuites ont chuté, passant de 6,0 % dans le premier groupe à 1,5 % dans les Groupes 2 et 3, mais sans toutefois atteindre un degré statistiquement significatif. Les taux de constriction anastomotique sont passés de 11,9 % dans le Groupe 1 à 3,0 % dans le Groupe 2 (p < 0,01). La perte de poids globale pour toute la série s'est établie à 31,5 % (ET de 11,9 %), 54,5 % (ET de 14,1 %), 77,1 % (ET de 18,5%) et 82,1 % (ET de 17,5 %) à 3, 6, 12 et 18 mois, respectivement.
Conclusion
On peut effectuer la DGIRY avec des résultats acceptables sur le plan de la morbidité et à court terme au cours de la courbe d'apprentissage. Dans notre série, la durée de l'intervention et les taux de constriction anastomotique ont diminué en fonction de l'expérience, malgré une hausse de l'IMC moyen.
The laparoscopic approach to Roux-en-Y gastric bypass for the treatment of morbid obesity was first introduced by Whitegrove in 1994.1 Since that time, the number of bariatric procedures performed worldwide has risen exponentially. Last year in the United States, over 100 000 operations for morbid obesity were performed, up from approximately 160 000 per year in the early 1990s.2 Laparoscopic Roux-en-Y gastric bypass is now offered in some Canadian centres, and demand for this procedure is increasing. LRYGB is a technically challenging operation requiring such advanced skills as dissection, stapling, suturing and working in different areas of the abdominal cavity. Other authors have stated that this procedure is associated with a steep learning curve, but the effect of this learning curve has yet to be defined.3–5 The purpose of this study was to evaluate the safety and efficacy of LRYGB for the treatment of morbid obesity, as performed by an experienced bariatric surgeon (part of a dedicated bariatric surgery team) and to describe the relation between learning curve and early outcomes.
Methods
Study design
This case series included the first 201 consecutive patients to undergo laparoscopic Roux-en-Y gastric bypass by a single university-based (McGill University Health Centre) dedicated bariatric surgeon, from February 2002 until January 2004 (23 months). We collected data from a prospective database and supplemented it with a chart review. To determine the effects of the learning curve on outcomes, we divided patients into 3 consecutive groups of 67 patients each for analysis (Group 1 = patients 1–67, Group 2 = patients 68–134 and Group 3 = patients 135–201). We defined early complications as complications occurring in the first 30 postoperative days and late complications as those occurring after 30 days.
Surgeon experience
The surgeon who did the laparoscopic procedures completed over 1000 open bariatric operations over the last 20 years and had no previous laparoscopic training or experience in performing advanced laparoscopic surgery. In preparation for LRYGB, the surgeon took a formal laparoscopic bariatric course in Pittsburgh, US, with Dr. Phil Schauer, as well as 3-day preceptorships with Dr. M. Gagnon in New York and Dr. K. Higa in California. Three months of training in intracorporeal suturing techniques were then spent with an inanimate surgical trainer box. Next, components of the Roux-en-Y gastric bypass were performed laparoscopically on 10 informed patients and were verified by converting to the open technique with subsequent completion of the operation. Satisfied that the procedure was safe, the surgical team completed the first LRYGB without conversion. Every case was videotaped and reviewed by the surgeon for quality control.
Surgical technique
A modification of Higa's laparoscopic Roux-en-Y gastric bypass technique was used.6 We chose this technique because it uses a vertical gastric pouch orientation that duplicates our open technique. All patients received 2 g sodium cephazolin (unless allergic to cephalosporins) and 7500 U subcutaneous unfractionated heparin before induction of anesthesia. Pneumoperitoneum was achieved with a verous needle inserted in the left upper quadrant. Access to the peritoneal cavity was established to the left of the midline, 2 hand-breadths below the xiphoid process, with a 12-mm optical trocar (Endopath Optiview; Ethicon Endosurgery Inc.). Three additional 12-mm radially expanding trocars were used: 2 in the left upper quadrant and 1 in the right upper quadrant. A 5-mm subxiphoid stab incision was used for the liver retractor. After retracting the omentum superiorly, the ligament of Treitz was identified and the lesser sac was entered via a window created in the transverse mesocolon. The jejunum and its mesentery were transected with a stapler 30 cm distal to the ligament of Treitz. The alimentary limb was measured at 100 cm (150 cm for individuals with extreme obesity). A side-to-side jejunojejunostomy was created with a stapler, and the enterotomy was closed with a single layer of 3–0 Vicryl. The alimentary limb was passed into the lesser sac in a retrocolic fashion. The mesenteric defect, Pederson's defect and transverse colon mesenteric defect were all closed with a running silk suture. The lesser sac was entered via the gastrocolic ligament and medial to the lesser curvature of the stomach. Any posterior gastric attachments were divided. A 15-mL gastric pouch was created with an Ewald orogastric tube as a sizer. This was accomplished with a single transverse staple line 7 cm distal to the GE junction along the lesser curve, followed by sequential longitudinal firings toward the Angle of His. A 1.2-cm 2-layered hand-sewn gastrojejunostomy was created with running 3–0 Vicryl sutures. Anastomotic integrity was tested with a methylene blue dye test and an underwater air bubble test. Routine use of drains and postoperative contrast studies were not employed.
Statistical analysis
We expressed categorical data as percentages and continuous data as either mean (and standard deviation) or median (range). We analyzed categorical variables with the chi-square test or Fisher's exact test, where appropriate. Continuous variables were analyzed with 1-way analysis of variance. Additionally, anastomotic stenosis rates between groups were compared with a time-to-failure analysis (Kaplan–Meier survival curves). A p value of less than or equal to 0.05 was considered statistically significant.
Results
Patient demographics
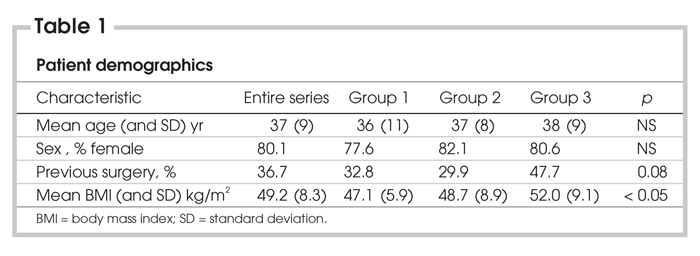
LRYGB was attempted on 201 patients over 23 months. Mean age for the entire series was 37 (SD 9) years, and 80.1% of patients were women; 36.7% of patients had prior abdominal surgery. Neither age, sex, nor incidence of prior abdominal surgery differed between the 3 groups. Mean body mass index (BMI) was 49.2 (SD 8.3) kg/m2 for the entire series. BMI was similar in Groups 1 and 2 (47.1, SD 5.9 and 48.7, SD 8.9 kg/m2) but increased in the last 67 patients (52, SD 9.7 kg/m2, p < 0.01). Patient demographics are listed in Table 1.
Table 1
Operative data
There were 3 conversions (1.5%): 1 as a result of severe steatohepatosis with liver injury, 1 due to inadequate instrument length and 1 for an inadvertent esophagotomy at the gastroesophageal (GE) junction. Median length of stay was 3 days (range 1–64 d). Neither conversion rates nor length of stay differed significantly between groups.
Mean operative time, defined as time taken from the initial incision made to the final dressing placed, was 126 (SD 29) minutes. Operative time decreased significantly from 145 (SD 30) minutes in the first group to 115 (SD 24) minutes in the second group (p < 0.01) and was maintained at 118 (SD 23) minutes in the final group (Fig. 1).
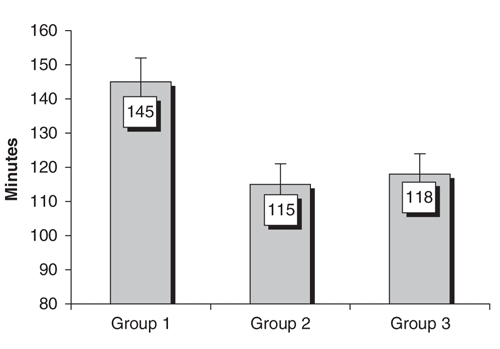
FIG. 1. Operative time.
Mortality
There was a single perioperative death (mortality rate 0.5%). The patient had severe nonalcoholic steatohepatitis and was converted to the open technique because of a liver injury from the laparoscopic liver retractor. Subcutaneous heparin was withheld postoperatively due to the risk of bleeding. On the fourth postoperative day, the patient died suddenly as a result of a massive pulmonary embolism.
Early complications
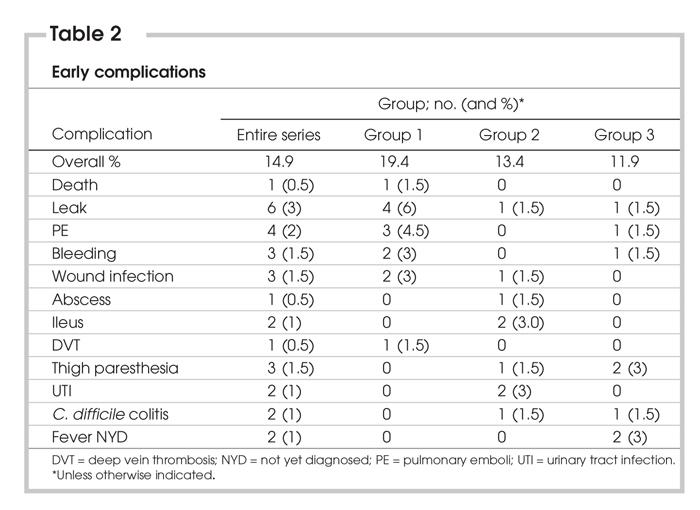
Thirty early complications occurred in 26 patients, for an overall early complication rate of 14.9%. Early complication rates did not differ significantly between groups. There were 6 anastomotic leaks (3.0%): 4 in the first group and 1 in each of the second and third groups (p = ns). Four leaks were detected before postoperative day 4. All of these patients required laparotomy, with 2 undergoing omentoplasty and 2 undergoing anastomotic revision. The other 2 leaks were detected at postoperative day 8 (treated by laparoscopically placed drains) and postoperative day 16 (treated by percutaneous drainage). Three patients (1.5%) suffered clinically significant postoperative hemorrhage (2 upper gastrointestinal [GI] bleeds and 1 mesenteric hematoma); all resolved with conservative management. Pulmonary emboli were detected in 4 patients (2.0%), and 1 resulted in death (see above); the other 3 resolved with anticoagulation therapy. There were 3 wound infections, all of which occurred in patients who underwent laparotomy, either as a result of conversion (1) or reoperation for a complication (2). Additional early complications are included in Table 2.
Table 2
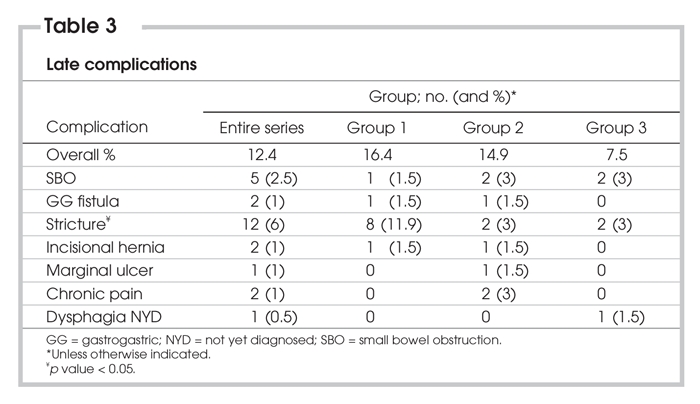
Late complications
Twenty-five late complications occurred in 25 patients, for an overall rate of 12.4%. Again, there was no significant difference in overall late complication rates between the 3 groups. Stricture at the gastrojejunostomy occurred in 12 patients (6.0%), and stricture rates decreased from 11.9% in Group 1, to 3.0% in both Group 2 and Group 3 (p < 0.05). Kaplan–Meier analysis also demonstrated a significant difference in stricture rates between the initial 67 patients, and the subsequent 134 patients (Fig. 2).
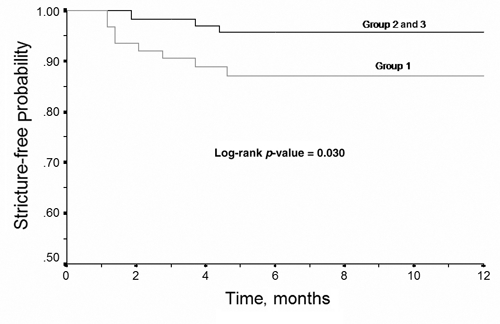
FIG. 2. 12-month time-to-failure (stricture) analysis.
Five small bowel obstructions occurred in the series (2.5%), with no statistically significant difference between the groups. Three were secondary to obstruction at the transverse mesocolon, 1 was a jejunal obstruction at Peterson's defect, and 1 was secondary to twisting of the alimentary limb. All were treated operatively — 3 by laparotomy and 2 by laparoscopy. Additional late complications are listed in Table 3.
Table 3
Weight loss
The overall percentage of excess weight loss for the entire series was 31.5 (SD 11.9), 54.5 (SD 14.1), 77.1 (SD 18.5), 82.1 kg/ m2 (SD 17.5) at 3, 6, 12 and 18 months, respectively. Because of the short length of follow-up in this study (mean follow-up 8.1, SD 5.9 months), weight loss results were not compared among the 3 groups. Weight loss is detailed in Figure 3.
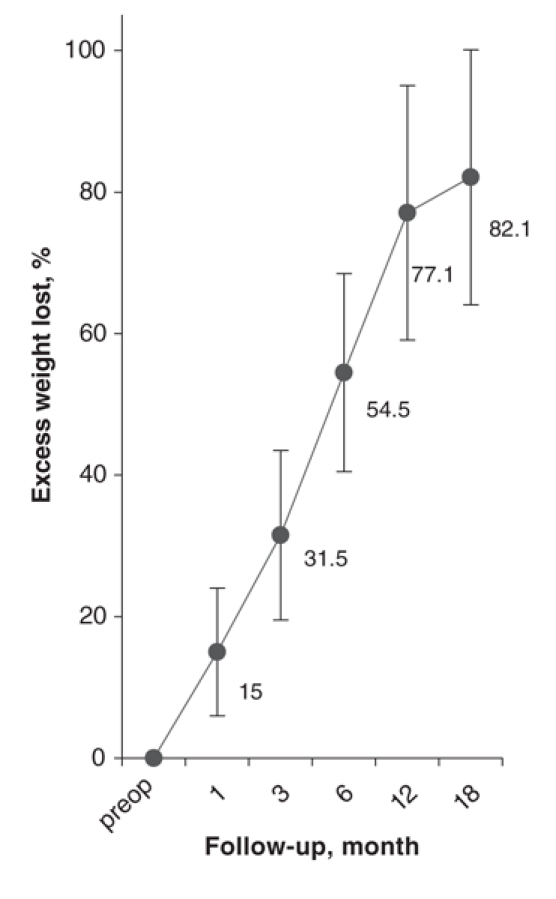
FIG. 3. Weight loss.
Discussion
Morbid obesity is a worldwide public health threat.7 Second only to smoking as a leading cause of preventable, premature death in the United States, morbid obesity is a known risk factor for development of type II diabetes, coronary artery disease, hypertension and dyslipidemia.8–10 Canada is no exception. In 1998, the prevalence of obesity in Canada was 14.8%, up from 9.2% in 1990.11 To date, the only consistently effective method of weight loss is surgery.12–14 Several studies have demonstrated that bariatric surgery improves obesity related comorbid conditions,15–19 and 2 recent studies have demonstrated a significant reduction in mortality after morbid obesity surgery.20,21 It is clear that, in the appropriate hands, not only can patients' quality of life be improved after bariatric surgery, but longevity can be increased as well.
We are already seeing increasing demand for bariatric surgery in Canada — a demand that can only be met by training more surgeons in the treatment of patients with morbid obesity. Laparoscopic Roux-en-Y gastric bypass is a technically challenging operation. Previous studies have suggested that the learning curve for this operation is anywhere from 50 to 150 cases.3–5,22 Kligman and colleagues5 analyzed their learning curve by dividing their first 160 LRYGB cases into quartiles for analysis. Their operative time continued to improve even after 120 cases. Nguyen and others22 evaluated 150 consecutive cases and found that operative time, hospital stay and major complications all improved as surgeon experience increased past 75 procedures. Perhaps most significantly, Flum and Dellinger demonstrated that early mortality after LRYGB is directly linked to surgeon experience and that patients of less experienced surgeons were nearly 5 times more likely to die within a month of the procedure than were patients of more experienced surgeons.21
Our study demonstrates a learning curve effect with LRYGB. Both operative time and anastomotic stricture rates plateaued after approximately 70 cases. Although statistical significance was not reached in the reduction of anastomotic leak rates after the first 67 cases, this is likely a result of the relative infrequency of this event. The only death occurred near the beginning of our experience with LRYGB. The overall excess weight loss of 82.1% at 18 months is comparable with that recorded in other large case series.3,4 We did not compare weight loss results between the groups because the follow-up time in the latter half of the series was too short to draw reliable conclusions.
As demand for this procedure increases, it is imperative that appropriate training and credentialing be implemented for those surgeons who plan to treat patients with morbid obesity. This has prompted both the American Society for Bariatric Surgery23 and the Society of American Gastrointestinal Endoscopic Surgeons24 to publish specific recommendations regarding experience, training and proctoring to safely perform laparoscopic bariatric surgery. With any new surgical procedure, some learning curve effects must be accepted; however, these effects may be minimized by specific training in both bariatric surgery and laparoscopic surgery, as well as by proctoring during the early part of the learning curve.
Conclusion
After appropriate proctoring and dedication to the field of bariatrics, laparoscopic Roux-en-Y gastric bypass can be performed with acceptable morbidity and short-term results during the learning curve. In our series, operative time and anastomotic stricture rates decreased with experience, despite an increase in mean BMI.
Presented at the Canadian Surgery Forum, September, 2004, Ottawa, Ont.
Competing interests: None declared.
Correspondence to: Dr. Christopher G. Andrew, Department of Surgery, University of Manitoba, Z3043 409 Tache Ave., St. Boniface General Hospital, Winnipeg MB R2H 2A6; fax 204 237-3429; candrew@sbgh.mb.ca
References
- 1.Wittgrove AC, Clark GW, Tremblay U. Laparoscopic gastric bypass, Roux-en-Y: preliminary report of five cases. Obes Surg 1994;4:353-7. [DOI] [PubMed]
- 2.Steinbrook R. Surgery for severe obesity. N Engl J Med 2004;350:1075-9. [DOI] [PubMed]
- 3.DeMaria EJ, Sugerman HJ, Kellum JM, et al. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Ann Surg 2002;235:640-5. [DOI] [PMC free article] [PubMed]
- 4.Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg 2000;232:515-29. [DOI] [PMC free article] [PubMed]
- 5.Kligman MD, Thomas C, Saxe J. Effect of the learning curve on the early outcomes of laparoscopic Roux-en-Y gastric bypass. Am Surg 2003;69:304-9. [PubMed]
- 6.Higa KD, Boone KB, Ho T, et al. Laparoscopic Roux-en-Y gastric bypass for morbid obesity: technique and preliminary results of our first 400 patients. Arch Surg 2000;135:1029-33. [DOI] [PubMed]
- 7.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1-253. [PubMed]
- 8.Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA 2004;291:1238-45. [DOI] [PubMed]
- 9.Kral JG. Morbidity of severe obesity. Surg Clin North Am 2001;81:1039-61. [DOI] [PubMed]
- 10.Overweight, obesity, and health risk. National Task Force on the prevention and treatment of obesity. Arch Intern Med 2000;160:898-904. [DOI] [PubMed]
- 11.Katzmarzyk PT. The Canadian obesity epidemic, 1985-1998. CMAJ 2002;166:1039-40. [PMC free article] [PubMed]
- 12.National Institutes of Health Consensus Development Panel. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991;115:956-61. [PubMed]
- 13.Fobi MA, Lee H, Holness R, et al. Gastric bypass operation for obesity. World J Surg 1998;22:925-35. [DOI] [PubMed]
- 14.Long-term pharmacotherapy in the management of obesity. National Task Force on the prevention and treatment of obesity. JAMA 1996;276:1907-15. [DOI] [PubMed]
- 15.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724-37. [DOI] [PubMed]
- 16.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 2003;238:467-84. [DOI] [PMC free article] [PubMed]
- 17.Sugerman HJ, Wolfe LG, Sica DA, et al. Diabetes and hypertension in severe obesity and effects of gastric bypass-induced weight loss. Ann Surg 2003;237:751-6. [DOI] [PMC free article] [PubMed]
- 18.Sjostrom CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res 1999;7:477-84. [DOI] [PubMed]
- 19.MacDonald KG Jr, Long SD, Swanson MS, et al. The gastric bypass operation reduces the progression and mortality on non-insulin-dependent diabetes mellitus. J Gastrointest Surg 1997;1:213-20. [DOI] [PubMed]
- 20.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004;240:416-23. [DOI] [PMC free article] [PubMed]
- 21.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg 2004;199:543-51. [DOI] [PubMed]
- 22.Nguyen NT, Rivers R, Wolfe BM. Factors associated with operative outcomes in laparoscopic gastric bypass. J Am Coll Surg 2003;197:548-55. [DOI] [PubMed]
- 23.American Society for Bariatric Surgery. Guidelines for granting privileges in bariatric surgery. Gainesville (FL): the Society; 2003. Available: www.asbs.org/html/guidelines.html [accessed 2006 Oct 12]. [DOI] [PubMed]
- 24.The Society of American Gastrointestinal Endoscopic Surgeons. Guidelines for the clinical application of laparoscopic bariatric surgery. Los Angeles (CA): the Society; 2003. Available: www.sages.org/sagespublication.php?doc=30 [accessed 2006 Oct 12].


