Gastrointestinal stromal tumours (GISTs) are rare sarcomas associated with the c-kit proto-oncogene. Because of the rarity of GISTs, and because until recently it was difficult to distinguish these tumours from other gastrointestinal mesenchymal tumours, only a few large case series have been reported that describe the clinical presentation and operative findings.1,2 Gain-of-function mutations have been shown in the c-kit transmembrane receptor, which has tyrosine kinase activity. The result can be uncontrolled cell division and neoplasia.3 The resulting clinical presentation, operative findings and prognosis of patients with this disease are highly variable. The varied presentation makes clinical suspicion an important element when diagnosing GISTs. Common anatomical sites of origin include the stomach and small intestine; however, these tumours can arise throughout the gastrointestinal tract. Although the prognosis associated with GISTs generally remains poor, patients with very large tumours can have positive outcomes. We present 5 examples that demonstrate the variable and often dramatic clinical, radiological and pathological presentations of this disease.
Case reports
Case 1
A 41-year-old man with multiple sclerosis and quadriplegia secondary to a motor vehicle accident was admitted with a large infected abdominal cyst. The cyst had been drained several times in the preceding 6 months in an effort to avoid surgical therapy because of the patient's associated comorbidities. On admission, the patient was febrile and had an elevated leukocyte count. Percutaneous drainage on this occasion revealed small-bowel contents, and pathological examination of a specimen of the cyst wall obtained by needle biopsy revealed a spindle cell neoplasm consistent with a GIST. Radiological investigations demonstrated a fistulous communication to the small bowel. After adequate metabolic and nutritional supplementation, laparotomy was performed, and an infected mesenteric cyst with a fistula tract to a Meckel's diverticulum was identified. A cystic mass measuring 22 × 21 × 9 cm was resected along with the Meckel's diverticulum (Fig. 1). Compromise of the mesenteric vessels in the mesocolon to the cecum and ascending colon necessitated a right hemicolectomy with primary ileocolic anastomosis. Pathological examination of the resected specimen showed a large GIST with a central cystic area, confirming the needle-biopsy diagnosis. The tumour was composed of a cellular proliferation of CD117 (c-kit)-positive spindle cells with moderate mitotic activity and areas of necrosis. Immunostains for neural (S-100 protein) and muscle markers were negative. Grossly, it appeared as though the tumour was arising from the Meckel's diverticulum, although this could not be confirmed histologically. The patient was discharged home on postoperative day 9 and was doing well 4 months after the operation at the postoperative clinic visit. Adjuvant chemotherapy was not instituted.
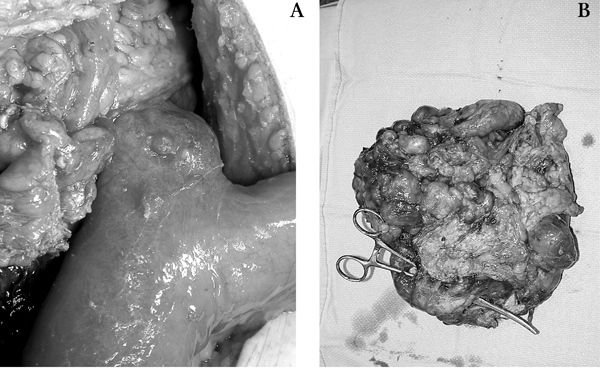
FIG. 1. Case 1: (A) Meckel's diverticulum and (B) the resected tumour.
Case 2
A 75-year-old woman was admitted following a 3-day history of fever, chills and diffuse abdominal pain. Her past medical history was significant for diverticulitis requiring Hartmann's resection and subsequent reversal. Computed tomography (CT) showed 2 separate intra-abdominal masses (6 × 6 × 12 cm and 3 × 7 × 8 cm) leading to a provisional diagnosis of probable retroperitoneal sarcoma. An image-guided needle-biopsy specimen obtained by interventional radiology revealed a spindle cell tumour. At laparotomy, diffuse metastatic lesions involving the small bowel, sigmoid colon, rectum, mesentery and abdominal wall were identified, and the operation consisted of diagnostic tissue biopsy following lysis of adhesions. The tumour masses were unresectable because of fixation in the retroperitoneum. The metastatic deposits were diffusely disseminated, teardrop-shaped, white nodules ranging from 1 to 2 cm in diameter. Pathological examination of the biopsy specimen showed a spindle cell neoplasm with low mitotic activity and CD117 (c-kit)-positive and muscle marker–negative immunophenotype. The primary site of origin of this GIST was uncertain, but the most probable location was the sigmoid colon.
The patient's postoperative course was complicated by a prolonged ileus, difficulty with pain control, and nausea and vomiting. Twenty-two months after her first admission, the patient continued to be followed in the cancer clinic. A trial of STI571 (imatinib) was not tolerated because of side effects and was abandoned. The latest CT scan revealed a complex mass (18 × 17 × 15 cm ) in the abdomen, with additional masses in the pelvis and liver.
Case 3
An otherwise healthy 71-year-old-woman presented to her family physician after a 1-week history of fatigue and was found to have a hemoglobin level of 50 g/L. This had dropped from a hemoglobin level of 109 g/L measured 2 weeks earlier at her annual visit. Upper gastrointestinal endoscopy demonstrated a large non-bleeding gastric ulcer on which a biopsy was performed, and the pathology was consistent with a GIST. A CT scan showed an associated large gastric mass with central necrosis that appeared to be resectable (Fig. 2). At laparotomy, on gross inspection of the abdomen, the tumour was seen as a baseball-sized exophytic lesion arising from the lesser curvature of the stomach, with serosal neovascularization. Three small nodules in both lobes of the liver were also identified, and biopsies were performed. Frozen section of the liver lesions showed an undifferentiated malignant neoplasm. A palliative partial distal gastrectomy and Billroth II anastomosis was performed. Pathological examination revealed a GIST (9 × 6 × 6 cm) arising within the gastric wall with liver metastases. This large gastric tumour demonstrated multiple mitotic figures per single high-power field and was strongly CD117 (c-kit)-positive by immunohistochemistry.
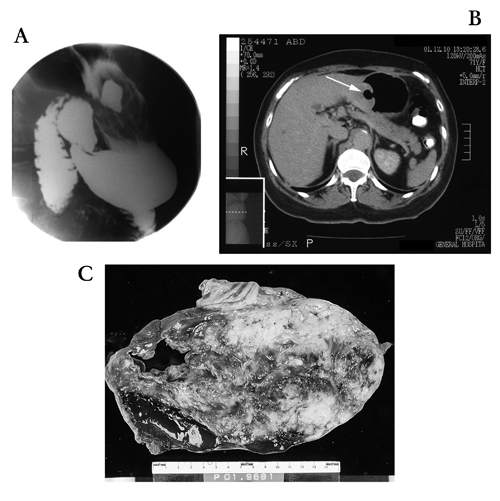
FIG. 2. Case 3: (A) Barium swallow shows a large collection of fluid within the ulcer cavity. (B) Abdominal computed tomography shows an impressive “collar button” lesion (arrow) arising from the stomach. (C) Gross section of the tumour shows a necrotic ulcer.
The postoperative course was uneventful, and the patient was discharged home on postoperative day 8. She has been treated with STI571 (imatinib), 400 mg daily, and is doing well with minimal side effects at 6 months postoperatively.
Case 4
A 69-year-old woman with Parkinson's disease was initially assessed by the gynecology service following a 3-month history of weight loss, fatigue and increasing abdominal distension. A CT scan showed a mass measuring 8 × 12 × 22 cm in the lesser sac. This mass effaced the normal anatomy of the stomach, pancreas, superior vena cava and inferior vena cava. The patient was referred to the general surgery service, and an antrectomy with en bloc resection of the lesser sac mass was performed with Billroth II reconstruction. Her postoperative course was complicated by an ileus and difficulty with nutrition, but she was eventually discharged home on postoperative day 11. Pathological examination of the surgical specimen showed an ill-defined lesser sac tumour weighing 2572 g that was adherent to the gastric antrum. The cut surface of this large GIST showed patchy hemorrhage and a central cavity that communicated via a small sinus tract with the gastric antral lumen. Immunostaining of this spindle cell neoplasm showed diffuse, intense CD117 (c-kit) labelling, with patchy, weak S-100 staining and negative muscle markers. No mitotic activity was identified. Gastric resection margins and regional lymph nodes were negative for tumour. One year after resection the patient was doing well, with stable weight and good appetite. Adjuvant therapy was not deemed necessary.
Case 5
A 69-year-old man was admitted to hospital for elective partial gastrectomy to treat a CT-demonstrated abdominal mass (7 × 11 × 8 cm) arising from the stomach. Significant past medical history included Canadian Cardiovascular Society (CCS) class II angina, chronic obstructive pulmonary disease, hypertension and hyperlipidemia. He had previously been investigated for early satiety, and abdominal ultrasonography suggested a possible pancreatic mass, which was not found on CT. Upper endoscopy revealed a distorted gastric luminal contour consistent with extrinsic tumour compression but no mucosal-based gastric neoplasm. The CT scan demonstrated no lymphadenopathy, nor evidence for metastatic disease. At surgery, a protuberant, lobulated cystic mass associated with posterior greater curvature of the stomach was resected. Histology of the rim of solid tumour tissue surrounding the large central cystic zone showed an epitheloid-cell neoplasm demonstrating intense CD117 immunohistochemical staining (Fig. 3). This GIST had moderate mitotic activity and central cystic cavitation secondary to necrosis and hemorrhage. This patient remained in hospital for 9 days postoperatively because of pulmonary edema secondary to fluid overload and poor blood pressure control. He is currently doing well 1 month postoperatively.
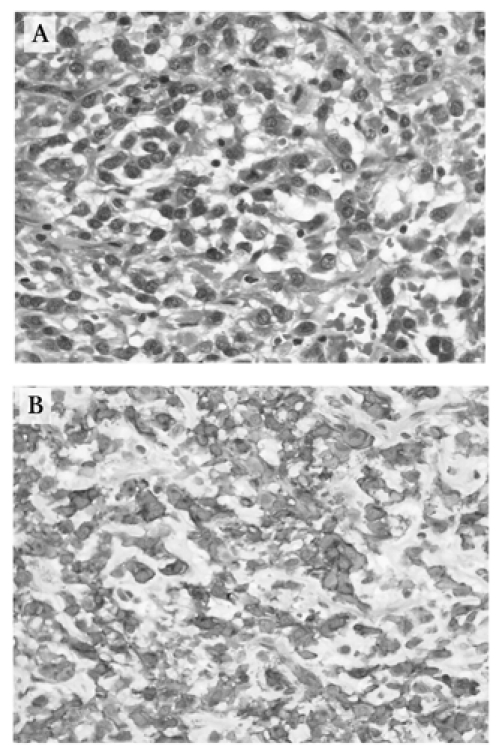
FIG. 3. Case 5: (A) Histology reveals the epithelioid morphology seen in 30% of gastrointestinal stromal tumours. More commonly, the cells take on more of a spindle cell appearance (hematoxylin–phloxine–saffron stain, original magnification × 200). 3 (B) This specimen stained strongly for CD117 (polyclonal rabbit anti-human antibody, DakoCytomation, original magnification × 200). The concentration of stain in the cell membrane occurs because the c-kit protein is a transmembrane receptor.
Discussion
Recently, the probable cell of origin in GISTs has been identified as the interstitial cell of Cajal.4 The expression of CD117 is seen in nearly all of these tumours. This transmembrane protein is the receptor for stem cell factor (mast cell factor) and is also expressed by the interstitial cells of Cajal, melancytes, germ cells and mast cells.5 These cells sometimes express muscle actins, but rarely express desmin or the S-100 protein, which helps to distinguish them from leiomyoma and neurofibroma.6 As our experience with these tumours accumulates, preoperative histopathological diagnosis becomes easier. Three of these cases were diagnosed with only needle biopsies.
The present series represents a spectrum of clinical presentations for GISTs. The presentations included infected mesenteric cyst; sepsis with fever, chills and abdominal pain; gastric ulcer with anemia; weight loss, fatigue and abdominal distension; and early satiety. The anatomical location for the primary tumour was also varied, and the clinical presentation varied based on the location. Tumours were located in a Meckel's diverticulum, different parts of the stomach and, presumably, the sigmoid colon. In their series of 200 patients with GIST, DeMatteo and colleagues1 reported that 38% of GISTs were found in the stomach, 32% in the small intestine, 10% in the rectum, 5% in the large intestine, 5% unspecified and 9% in other sites, including the omentum, esophagus, mesentery, diaphragm and intra-abdominal sites. Two of the GISTs in our series were cystic in nature in contrast to sarcomas, which tend to be solid tumours.
The outcome from surgery and prognosis is also varied for our patients. Patients with completely resected tumours were not offered adjuvant therapy, whereas the 2 patients with metastatic disease were treated with palliative medical therapy using STI571 (imatinib). Although GIST has been recognized as a distinct entity for a decade, until the recent introduction of STI571 (imatinib), a receptor tyrosine kinase inhibitor, there has been little effective treatment for recurrent or metastatic GISTs. Two of our patients had a complete resection with no evidence of metastasis and are doing well as of the most recent follow-up. One patient was found to have diffuse metastases, but was unable to tolerate imatinib. At 22 months since diagnosis, her prognosis is poor. A second patient was also found to have metastatic disease, which indicates poor prognosis, but 6 months from surgery she was responding well to imatinib. Her liver metastases had not changed in size on MRI, and her only complaint had been eye puffiness. The final patient had disease-free margins on resection, but the prognosis remains uncertain. Prognosis for patients with GISTs is difficult to predict. The most important predictors of tumour behaviour are size and mitotic rate. Intestinal tumours with a maximal diameter greater than 5 cm or more than 5 mitoses per 50 high-power fields are considered probably malignant. Gastric tumours with maximal diameter greater than 10 cm or more than 5 mitoses per 50 high-power fields are considered probably malignant.7 The overall 5-year survival rate for patients with GIST has been reported as 28%–35%.1,2
Conclusions
GISTs can present in different ways, making clinical suspicion an important element when making this diagnosis. Although the prognosis for these tumours remains poor, patients with large tumours can have positive outcomes.
Competing interests: None declared.
Correspondence to: Dr. Dale Mercer, Department of Surgery, Victory 3, Rm. 352, Kingston General Hospital, 76 Stuart St., Kingston ON K7L 2V7; fax 613 546-4854; mercerd@kgh.kari.net
References
- 1.DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [DOI] [PMC free article] [PubMed]
- 2.Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68-77. [DOI] [PMC free article] [PubMed]
- 3.Lux M, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumours. Am J Pathol 2000;156:791-5. [DOI] [PMC free article] [PubMed]
- 4.Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PMC free article] [PubMed]
- 5.Fletcher CDM, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumours: a consensus approach. Hum Pathol 2002;33:459-65. [DOI] [PubMed]
- 6.Miettinen M, Sarloma-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding their biology. Hum Pathol 1999;30:1213-20. [DOI] [PubMed]
- 7.Miettinen M, El-Rifah W, Sobin LH, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002;33:478-83. [DOI] [PubMed]


