Abstract
Background
There is a lack of information from Canadian hospitals on the role of hospital characteristics such as procedure volume and teaching status on the survival of patients who undergo major cancer resection. Therefore, we chose to study these relationships using data from patients treated in Ontario hospitals.
Methods
We used the Ontario Cancer Registry from calendar years 1990–2000 to obtain data on patients who underwent surgery for breast, colon, lung or esophageal cancer or who underwent major liver surgery related to a cancer diagnosis between 1990 and 1995 in order to assess the influence of volume of procedures and teaching status of hospitals on in-hospital death rate and long-term survival. For each disease site and before observing patient outcomes data, volume cut-off points were selected to create volume groups with similar numbers of patients. Teaching hospitals were those directly affiliated with a medical school. Logistic regression and proportional hazards models were used to consider the clustering of data at the hospital level and to assess operative death and long-term survival. We also used 4 measures to gauge the degree of procedure regionalization across the province including (1) the number of hospitals performing a procedure; (2) the percentage of patients treated in teaching hospitals; (3) the percentage of rural patients treated in higher volume procedure hospitals; and (4) median distances travelled by patients to receive care.
Results
The number of patients in our cohorts who underwent resection of the breast, colon, lung, esophagus or liver was 14 346, 8398, 2698, 629 and 362, respectively. Surgery in a high-volume versus a low-volume hospital did not have a statistically significant influence on the odds of operative death for patients who underwent colon, liver, lung or esophageal cancer resection. The risk of long-term death was increased in low-volume versus high-volume hospitals for patients who underwent resection of the breast (hazard ratio [HR] 1.2, 95% confidence interval [95% CI] 1.0–1.4, p < 0.05), lung (HR 1.3, 95% CI 1.1–1.6, p < 0.01) and liver (HR 1.7, 95% CI 1.0–2.7, p = 0.04). There were no significant differences in the odds of operative (in-hospital) death or risk of long-term death among patients treated in teaching compared with nonteaching hospitals. There was more regionalization of liver, lung and esophageal operations versus breast and colon operations.
Conclusions
Increased hospital procedure volume correlated with improved long-term survival for patients in Ontario who underwent some, but not all, cancer resections, whereas hospital teaching status had no significant impact on patient outcomes. Across the province, further regionalization of care may help improve the quality of some cancer procedures.
Abstract
Contexte
On manque de données provenant des hôpitaux canadiens au sujet du rôle que jouent des caractéristiques hospitalières comme le volume des interventions et le statut d'établissement d'enseignement sur la survie des patients subissant une résection d'un cancer majeur. Nous avons donc décidé d'étudier ces liens à partir des données provenant de patients traités dans des hôpitaux de l'Ontario.
Méthodes
Nous avons utilisé le Registre du cancer de l'Ontario des années civiles 1990 à 2000 afin de réunir des données sur les patients qui ont subi une intervention chirurgicale pour un cancer du sein, du côlon, du poumon ou de l'œsophage, ou qui ont subi une chirurgie majeure du foie reliée à un diagnostic de cancer entre 1990 et 1995, afin d'évaluer l'influence du volume des interventions et du statut d'établissement d'enseignement des hôpitaux sur le taux de mortalité hospitalière et de survie à long terme. Pour chaque site morbide et avant d'observer les données sur les résultats chez les patients, on a choisi des points limites de volume afin de créer des groupes comptant des nombres semblables de patients. Les hôpitaux d'enseignement étaient ceux qui étaient affiliés directement à une faculté de médecine. On a utilisé des modèles de régression logistique et de danger proportionnel pour étudier le regroupement des données au niveau de l'hôpital et pour évaluer la mortalité opératoire et la survie à long terme. Nous avons aussi utilisé quatre paramètres pour mesurer le degré de régionalisation des interventions dans la province, soit les suivants : (1) le nombre d'hôpitaux qui pratiquent une intervention; (2) le pourcentage des patients traités dans des hôpitaux d'enseignement; (3) le pourcentage des patients ruraux traités dans des hôpitaux où le volume des interventions est plus élevé; (4) les distances médianes parcourues par les patients pour se faire traiter.
Résultats
Le nombre de patients dans nos cohortes qui ont subi une résection du sein, du côlon, du poumon, de l'œsophage et du foie s'est établi à 14 346, 8398, 2698, 629 et 362 respectivement. Le fait que les interventions chirurgicales aient été pratiquées dans des hôpitaux à volume élevé plutôt que dans des hôpitaux à faible volume n'a pas eu d'influence statistiquement significative sur risque de décès pendant l'intervention pour les patients qui ont subi une résection d'un cancer du côlon, du foie, du poumon ou de l'œsophage. Le risque de décès à long terme a augmenté dans les hôpitaux à faible volume par rapport aux hôpitaux à volume élevé dans le cas des patients qui ont subi une résection d'un cancer du sein (taux de risque [TR] 1,2; intervalle de confiance à 95 % [IC à 95 %], 1,0–1,4, p < 0,05), du poumon (TR 1,3; IC à 95 %, 1,1–1,6, p < 0,01) et du foie (TR 1,7; IC à 95 %, 1,0–2,7, p = 0,04). Il n'y avait pas de différences significatives au niveau du risque de décès pendant l'intervention (à l'hôpital) ou du risque de décès à long terme chez les patients traités dans les hôpitaux d'enseignement comparativement aux autres. La régionalisation des opérations au foie, au poumon et à l'œsophage était plus importante que celle des opérations au sein et au côlon.
Conclusions
On a établi un lien entre le volume accru d'interventions dans les hôpitaux et l'amélioration de la survie à long terme chez les patients de l'Ontario qui ont subi une résection pour une partie mais non la totalité des cancers, tandis que le statut d'hôpital d'enseignement n'avait pas d'effet important sur l'évolution de l'état de santé des patients. Dans la province, une régionalisation plus poussée des soins pourrait contribuer à améliorer la qualité de certaines interventions contre le cancer.
Outcomes for certain cancer procedures are improved for patients who undergo their surgery in a hospital that performs a high volume compared with a low volume of the examined procedure — a positive volume–outcome relation.1–12 Other research has demonstrated better patient outcomes in teaching versus nonteaching hospitals.13,14 The finding of superior patient outcomes in high-volume versus low-volume or teaching versus nonteaching hospitals often results in calls to centralize or “regionalize” particular cancer operations.
Population-based studies of hospital characteristics and cancer surgery outcomes using data from Ontario (population 12 million) have typically focused on operative mortality, and few have considered the role of hospital teaching status.15–18 Surprisingly, most papers do not report a positive influence on operative mortality for increased hospital volume or teaching status, except for improved results after pancreatic cancer surgery in high-volume hospitals.17,18 Urbach and colleagues17 suggested that cancer surgery in Ontario is already highly regionalized, although they did not attempt to quantify the degree of regionalization.
We are also unaware of cancer studies using data from any Canadian jurisdiction that have considered the influence of hospital characteristics on long-term patient survival. Therefore, we wished to measure the impact of hospital procedure volume and teaching status on in-hospital operative mortality and long-term survival among patients in Ontario who underwent surgery for cancer of the colon, breast, lung, esophagus or liver. An ancillary objective was to quantify the degree of regionalization across the province for the 5 procedures. We speculated that this would allow a comparison of regionalization patterns and volume–outcome relations. For example, are highly regionalized procedures more or less likely to demonstrate positive volume–outcome relations?
Methods
We obtained data from the Ontario Cancer Registry (OCR) from calendar years 1990–2000 for patients newly diagnosed with cancer of the breast, colon, lung or esophagus, or patients who underwent major liver surgery related to a cancer diagnosis. This registry uses probability linkage strategies to identify and collect information on every newly diagnosed case of cancer in the province. Data sources include the Canadian Institute for Health Information (CIHI) and the Ontario Registered Persons Database (RPDB). The CIHI database contains information on every patient discharged from an Ontario hospital, including patient demographics (age, sex, postal code or place of residence), major diagnoses and procedures, and outcomes such as discharge status (dead or alive). The RPDB contains information on all births and deaths in the province and, thus, can provide information on long-term survival. In addition, by law, hospitals and laboratories in Ontario must forward to the OCR copies of all pathology reports containing a cancer diagnosis, although data from these reports are not systematically abstracted. There are published reports on OCR linkage strategies, which detail high rates of case capture.19,20
We used data from patients newly diagnosed with cancer of the breast, colon or lung in 1991–1993 and with cancer of the esophagus or who underwent any major liver procedure related to a cancer diagnosis in 1990–1995. For the latter 2 sites, more years of data were captured because of low annual case volumes. We created patient cohorts by linking diagnosis codes contained in theclinical modification of the International Classification of Diseases, 9th revision (ICD-9-CM)21 to Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures (CCP) codes.22 For the 5 disease sites, we included major extirpative procedures for which it was reasonable to assume that the surgical intent was patient cure or long-term palliation. Thus, linkages were breast cancer to partial or total mastectomy, colon cancer to large-bowel resection, lung cancer to pneumonectomy or lobectomy, esophageal cancer to resection resulting in an esophageal anastomosis, or any cancer diagnosis to liver lobectomy. Patients with previous diagnoses of cancer were excluded, with the exception of those with liver cancer, as were those younger than 20 years. For breast surgery, because many patients undergo more than 1 procedure to definitively deal with their cancer, we used the hospital admission with the most major breast operation in a 4-month period. The order of selection was mastectomy, partial mastectomy and excisional biopsy.
Calculations for hospital volume for a procedure considered the administrative merging of institutions over time. For each disease site volume cut-off points were selected to create volume groups with about equal numbers of patients.23 Because of the small numbers of patients involved, only 2 volume groups were created for liver procedures, whereas 4 volume groups were created for the others. A teaching hospital was 1 of the 21 hospitals in the province directly affiliated with a medical school.
We used the modification of a comorbidity index validated by Deyo and associates24 for the ICD-9-CM database to define comorbidity for individual patients: only diagnoses coded during the hospital admission of interest were considered. We used household income as a marker of socioeconomic status. A conversion file that incorporates Statistics Canada census data from 1991 allowed the linking of postal codes to household-size-adjusted household income and the determination of rural versus urban place of residence.25
Stage of disease is an important prognostic marker among patients with cancer. To assess whether patients with similar stages of cancer were treated in the various hospital groups, we abstracted data from random samples of pathology reports for 979 breast, 960 colon, 692 lung, 301 esophageal and 354 liver lobectomies. This provided such pathological information as T and N category, but not M category.26 T and N categories are not relevant to liver surgery, so we measured the number of lesions removed and the percentage of lesions that were greater than 5 cm in diameter.27 We hypothesized that the distribution among hospital groups of the T and N category measures would parallel the distribution of M category measures and, thus, overall stage.
The patient outcomes of interest were in-hospital operative mortality and long-term survival from the date of admission for major surgery. Follow-up data were available to Dec. 31, 2000. We measured in-hospital operative mortality because patients who may die of operative or perioperative complications can do so after an arbitrary time point such as 30 days. Data on operative death were censored before the survival analyses were carried out.
We defined regionalization of cancer surgery as a tendency for patients to undergo surgery in fewer locations. Previous research has shown that centres performing a high volume of the procedure are more likely to be teaching hospitals and to be located in urban centres.16,18 In addition, distances travelled by patients to receive care should be greater for more regionalized procedures. We therefore quantified regionalization of surgical care using the following 4 measures: the number of hospitals performing a procedure; the percentage of patients treated in teaching hospitals; the percentage of rural patients treated in high-volume hospitals; and the median distances travelled by patients to receive care. Patient and treating hospital postal codes allowed the calculation of median distances travelled.
We used descriptive, univariate and multivariate analyses. All hypothesis tests were 2-sided and considered statistically significant at p < 0.05. Logistic regression and proportional hazards multilevel models were designed to consider patient clustering at the hospital level when measuring operative mortality and long-term survival.27,28 All models included the explanatory variables of hospital teaching status (yes or no), hospital volume group (high, medium–high, low–medium or low), and various patient characteristics including age, sex, comorbidity score, place of residence (rural v. urban) and socioeconomic status (high-income, medium-income or low-income level). Patient age was treated as a continuous variable.
In an effort to test the robustness of model results, volume cut-off points were changed to divide the patient cohorts into thirds or fifths, with respective groupings again containing roughly equal numbers of patients. With respect to the liver models, only thirds were created because of the small numbers of patients. We also measured interactions between hospital procedure volume and teaching status. We also reran the survival models without censoring the data for operative mortality. Finally, operative mortality and survival models were run using the respective subset cohorts with pathology data. In addition to the covariables already mentioned, T-category and N-category measures were included for all sites except the liver. For the liver model, pathology covariables were number and size of lesions. Hierarchical models were created using MLWin (version 1.1, Centre for Multilevel Modelling, Institute of Education, London, UK) and S-Plus (version 6.1, Insightful Corporation, Seattle Wash.).
Results
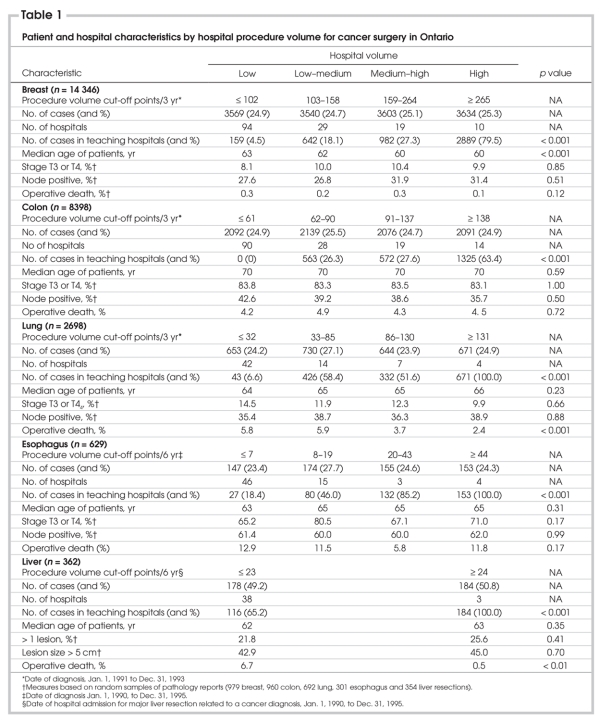
The numbers of patients who underwent resection for breast, colon, lung, esophageal or liver cancer were 14 346, 8398, 2698, 629 and 362, respectively (Table 1). Patients operated on in high-volume hospitals were more likely to be treated in teaching centres (p < 0.001 for all). Median age was lowest for patients with breast cancer (age 61 yr) and highest for patients with colon cancer (age 70 yr). Among the hospital volume groups, for the liver site there was no significant differences in the percentage of patients with more than 1 lesion removed or with lesions larger than 5 cm. For the other 4 sites there were no significant differences in the distribution of T and N category measures. The distribution of pathology measures was also similar in teaching and nonteaching hospitals (data not shown).
Table 1
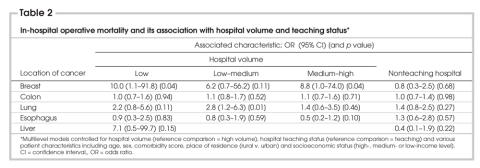
The mean provincial postoperative death rate was 0.2% for breast procedures, 4.5% for colon procedures, 3.6% for liver procedures, 4.5% for lung procedures and 10.5% for esophageal procedures. Among the 5 surgical sites, the unadjusted operative death rates (Table 1) were significantly greater in low-volume hospitals for lung and liver procedures (p < 0.01 for both). In multivariable models, with the incorporation of patient and hospital variables, hospital procedure volume did not affect the odds of postoperative death in low-volume versus high-volume centres, except for breast cancer surgery (odds ratio [OR] 10.0, 95% confidence interval [95% CI] 1.1–91.8, p = 0.04) (Table 2). The wide CI and, thus, the instability of this result probably reflects the small number of operative deaths after breast resection (29 patients over 3 years). For lung procedures, the odds of operative death were lower in high–medium than in low-volume hospitals (OR 2.8, 95% CI 1.2–6.3, p = 0.01), and there was a trend for better results in high-volume hospitals (OR 2.2, 95% CI 0.8–5.6, p = 0.11). There was a similar trend for better results in high-volume institutions for liver procedures (OR 7.1, 95% CI 0.5–99.7, p = 0.15).
Table 2
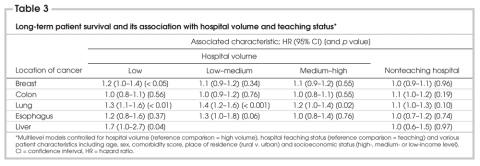
Hospital procedure volume did not affect the risk of long-term death for patients who underwent colon or esophageal resection (Table 3). However, hazard ratios (HRs) were significantly increased in low-volume versus high-volume hospitals for patients who underwent breast (HR 1.2, 95% CI 1.0–1.4, p < 0.05), lung (HR 1.3, 95% CI 1.1–1.6, p < 0.01) and liver (HR 1.7, 95% CI 1.0–2.7, p = 0.04) resection, respectively. For all sites, there were no significant differences in the odds of operative death or hazard of long-term death among patients treated in teaching compared with nonteaching hospitals (Table 2, Table 3).
Table 3
The model results were robust to changing volume groups from halves to thirds for liver lobectomy, and from quarters to thirds or fifths for the other sites. For all sites, there were no significant interactions between hospital volume and teaching status. Including in the survival models patients who suffered operative death did not change the direction or size of HRs. The inclusion of pathology variables did not change the direction or size of survival HRs for any of the sites, but the associations between low hospital volume and worse survival became nonsignificant for breast (HR 1.3, 95% CI 0.8–1.9, p = 0.27), lung (HR 1.1, 95% CI 0.7–1.5, p = 0.76) and liver (HR 1.6, 95% CI 0.9–3.0, p = 0.13) resections, respectively.
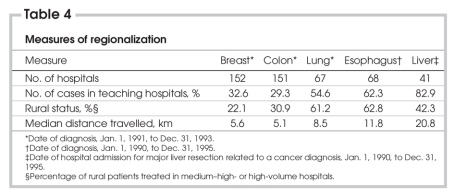
The number of hospitals providing breast and colon operations was greater than the number providing lung, esophageal and liver operations (Table 4). A minority of breast and colon resections and most liver, lung and esophageal resections were provided in teaching centres. Across the 5 disease sites, the percentage of patients with a rural status ranged from 14.7% to 17.7%. Surgery in high-volume and medium–high-volume centres occurred for a majority of rural patients with lung or esophageal cancer (61.2% and 62.8% respectively) and a minority of rural patients with breast or colon cancer (22.1% and 30.9% respectively). The median distance travelled by patients for breast, colon, lung, esophageal and liver cancer procedures was 5.6, 5.1, 8.5, 11.8 and 20.8 km, respectively. These measures likely demonstrate a propensity to provide breast and colon resection close to patients' homes (i.e., less regionalized care), and liver, lung and esophageal procedures in high-volume or teaching centres (i.e., more regionalized care).
Table 4
Discussion
In this series of patients in Ontario who underwent major cancer procedures, we found that surgery in a high-volume versus a low-volume hospital did not have a statistically significant influence on the odds of operative mortality, with the exception of breast surgery, although this finding must be interpreted cautiously because there were so few operative deaths. There was also a trend for lower odds of operative death with increased procedure volume for both lung and liver procedures. Increased hospital procedure volume did correlate with improved long-term survival for patients who underwent breast, liver and lung cancer surgery, although these results became nonsignificant for subsets of patients for whom pathology data were available. This probably reflected a decreased power to detect differences because of smaller cohort sizes. Of interest is that the teaching status of the treating institution had no influence on the odds of operative death or long-term survival. A positive influence of increased hospital volume on patient survival was noted in more regionalized (liver and lung) and less regionalized (breast) cancer procedures. This suggests that positive volume–outcome relations may persist following successful efforts to regionalize a particular cancer operation.
Is further regionalization of cancer surgery needed in Ontario? There are reasons to support this for liver, lung and esophageal procedures. First, long-term survival rates for the patients who had liver lobectomy or lung resection were superior in high-volume hospitals. Because the influence of operative mortality was removed from these analyses, processes of care provided outside the operating room, such as superior patient selection, were likely responsible for this finding. Second, for patients who underwent esophageal resection, operative death rates in low-volume and high-volume hospitals were 12.9% and 11.8% respectively. Hence, there is a need to improve surgical performance across the province, something more readily done by concentrating care in a small number of hospitals. Third, regionalization would likely result in the more efficient use of scarce resources and personnel, which is especially important for procedures with low annual case volumes. Fourth, the minimal differences in median distance travelled by patients for liver, lung and esophageal procedures versus breast and colon resections suggests that most patients would not be geographically inconvenienced by more regionalized care. With regard to breast cancer, it is probably impractical to regionalize the large annual volumes of these procedures despite the better survival for patients treated in high-volume centres. It may be more effective to identify the processes that contribute to better patient outcomes in high-volume breast cancer treatment centres and implement their wider adoption.
The goal of any change in cancer surgery practice or organization in the province should be to improve patient outcomes. This underscores the need for ongoing data monitoring. It would make no sense to transfer a patient requiring a lung resection to a centre with poor outcomes, simply because the centre was already a high-volume institution. Furthermore, whereas teaching hospitals typically act as regional referral centres and thus are well positioned to increase case volumes if greater regionalization is implemented, the results of this study indicate that in Ontario this would not guarantee improved patient outcomes. For procedures where regionalization may not be justified (colon surgery) or practical (breast surgery), or for hospitals that are designated to become referral centres, ongoing data monitoring and the sharing of such information with surgeons may assist in ensuring improved surgical care or best practice by individual surgeons and hospitals. This would further assist in determining whether regionalization will lead to improved patient outcomes.
The limitations of this study include the use of retrospective administrative data. Despite our efforts to adjust results using available variables and to consider the clustering of data at the hospital level, there is still the potential for unforeseen confounding. For example, we lacked full tumour staging data. However, among the hospital groups, sub-samples of patients for whom pathology reports were available had very similar distributions of relevant pathology measures. There is also evidence that the databases used in this study have a high degree of accuracy for coding procedures, major diagnoses and major outcomes, such as operative mortality.29,30 Another drawback was the use of inception cohorts from the early 1990s. But this was necessary to obtain adequate follow-up for long-term survival analysis. We also could not isolate surgeons' volume of procedures, or the use of hormone therapy or chemotherapy. This last point may be important in explaining hospital-volume survival differences observed for patients who underwent breast surgery.
Surgery for cancer in a high-versus low-volume hospital did not have a statistically significant influence on the odds of in-hospital operative death for patients in Ontario who underwent colon, liver, lung or esophageal cancer surgery but did correlate with improved long-term survival for patients who underwent liver, lung or breast cancer surgery. Hospital teaching status had no significant impact on patient outcomes. There was more regionalization of liver, lung and esophageal procedures than breast and colon procedures, and further regionalization of the former group should be considered. Efforts to improve the quality of surgical care, whether through increased regionalization or hospital level projects, should include ongoing data monitoring.
Acknowledgments
We thank Janie Freud, Cancer Registry Analyst, Division of Preventive Oncology, Cancer Care Ontario, for her review of pathology reports. We also thank Nelson Chong, Senior Research Associate, and Darlene Dale, former Manager of Operations, Division of Preventive Oncology, Cancer Care Ontario, for data acquisition.
Competing interests: None declared.
Correspondence to: Dr. Marko Simunovic, Juravinski Cancer Centre, 699 Concession St., Hamilton ON L8V 5C2; fax 905 575-6343; marko.simunovic@hrcc.on.ca
References
- 1.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [DOI] [PubMed]
- 2.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg 2003;138:721-5. [DOI] [PubMed]
- 3.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [DOI] [PubMed]
- 4.Roohan PJ, Bickell NA, Baptiste MS, et al. Hospital volume differences and five-year survival from breast cancer. Am J Public Health 1998;88:454-7. [DOI] [PMC free article] [PubMed]
- 5.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg 1999;230:404-11. [DOI] [PMC free article] [PubMed]
- 6.Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst 2003;95:708-16. [DOI] [PubMed]
- 7.Glasgow RE, Showstack JA, Katz PP, et al. The relationship between hospital volume and outcomes of hepatic resection for hepatocellular carcinoma. Arch Surg 1999;134:30-5. [DOI] [PubMed]
- 8.Choti MA, Bowman HM, Pitt HA, et al. Should hepatic resections be performed at high-volume referral centers? J Gastrointest Surg 1998;2:11-20. [DOI] [PubMed]
- 9.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001;345:181-8. [DOI] [PubMed]
- 10.Dimick JB, Pronovost PJ, Cowan JA, et al. Surgical volume and quality of care for esophageal resection: do high-volume hospitals have fewer complications? Ann Thorac Surg 2003;75:337-41. [DOI] [PubMed]
- 11.Patti MG, Corvera CU, Glasgow RE, et al. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg 1998;2:186-92. [DOI] [PubMed]
- 12.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA 2000;284:3028-35. [DOI] [PubMed]
- 13.Holm T, Johansson H, Cedermark B, et al. Influence of hospital-and surgeon-related factors on outcome after treatment of rectal cancer with or without preoperative radiotherapy. Br J Surg 1997;84:657-63. [PubMed]
- 14.Taylor DH Jr, Whellan DJ, Sloan FA. Effects of admission to a teaching hospital on the cost and quality of care for Medicare beneficiaries. N Engl J Med 1999;340:293-9. [DOI] [PubMed]
- 15.Chaudhry R, Goel V, Sawka C. Breast cancer survival by teaching status of the initial treating hospital. CMAJ 2001;164:183-8. [PMC free article] [PubMed]
- 16.Simunovic M, To T, Baxter N, et al. Hospital procedure volume and teaching status do not influence treatment and outcome measures of rectal cancer surgery in a large general population. J Gastrointest Surg 2000;4:324-30. [DOI] [PubMed]
- 17.Urbach DR, Bell CM, Austin PC. Differences in operative mortality between high-and low-volume hospitals in Ontario for 5 major surgical procedures: estimating the number of lives potentially saved through regionalization. CMAJ 2003;168:1409-14. [PMC free article] [PubMed]
- 18.Simunovic M, To T, Theriault M, et al. Relation between hospital surgical volume and outcome for pancreatic resection for neoplasm in a publicly funded health care system. CMAJ 1999;160:643-8. [PMC free article] [PubMed]
- 19.Holowaty EJ, Marrett LD, Fehringer G. Cancer incidence in Ontario, trends and regional variations. Toronto: The Ontario Cancer Treatment and Research Foundation; 1995.
- 20.Robles SC, Marrett LD, Clarke EA, et al. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol 1988;41:495-501. [DOI] [PubMed]
- 21.U.S. Department of Health and Human Services. ICD-9-CM: International classification of diseases, clinical modification, 9th Revision. 5th ed. Los Angeles: Practice Management Information Corporation (PMIC); 1999.
- 22.Statistics Canada. Canadian classification of diagnostic, therapeutic and surgical procedures. Ottawa: Ministry of Industry, Science, and Technology; 1993.
- 23.Hong D, Tandan VR, Goldsmith CH, et al. Users' guide to the surgical literature: how to use an article reporting population-based volume-outcome relationships in surgery. Can J Surg 2002;45:109-15. [PMC free article] [PubMed]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [DOI] [PubMed]
- 25.Statistics Canada. Postal Code Conversion File Plus (PFFC +), June 2001. Ottawa: Geography Division, Statistics Canada; 2001.
- 26.Greene FL, Page DL, Fleming ID, et al, editors. AJCC (American Joint Committee on Cancer) cancer staging manual. 6th ed. New York: Springer-Verlag; 2002.
- 27.Goldstein H. Multilevel statistical models. 2nd ed. London: Edward Arnold; 1995.
- 28.Panageas KS, Schrag D, Riedel E, et al. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med 2003;139:658-65. [DOI] [PubMed]
- 29.Williams J, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams JI, Anderson GM, et al, editors. Patterns of health care in Ontario: the ICES practice atlas. 2nd ed. Ottawa: Canadian Medical Association; 1996.
- 30.Simunovic M, To T, Langer B. Influence of hospital volume on mortality following major cancer surgery. JAMA 1999;281:1374-5. [PubMed]