Abstract
Background
Fibrin sealants are used increasingly in surgery to reduce bleeding and improve wound healing. They have great potential as biocompatible, biodegradable drug delivery systems, because the sealant may adhere to the target tissue and allow controlled release of the drug over an extended period. We investigated the encapsulation, stability and controlled release of erythromycin and cefazolin from Beriplast fibrin sealants (Aventis Behring Canada).
Methods
Drug-loaded clots were cast in glass vials and allowed to set. We observed the clots for drug precipitation and aggregation, and we assessed the effect of drug encapsulation on clot strength. Drug stability and release from the clots in phosphate buffered saline (PBS) was quantified by ultraviolet and visible violet absorbance spectroscopy and high-performance liquid chromatography.
Results
Erythromycin was found to release slowly from the fibrin clots over the first 2 hours but then degrade rapidly. Cefazolin was found to be very stable in clots in PBS (97% stable at 2 d and 93% stable at 5 d). The drug released in a controlled manner over 2 days, with most being released during the first day. The dose of drug released could be varied by changing the amount placed in the thrombin solution. Clot thickness had no effect on the rate of cefazolin release.
Conclusion
Overall, the 2-day release profile and the excellent stability of the drug suggest that cefazolin-loaded fibrin sealants may offer an effective route of postoperative antibiotic delivery.
Abstract
Contexte
On utilise de plus en plus les agents de scellement à la fibrine en chirurgie afin de réduire le saignement de la plaie et d'en améliorer la guérison. Ces produits sont porteurs de grandes promesses comme systèmes biodégradables et biocompatibles de diffusion de médicaments, car l'agent de scellement peut adhérer au tissu cible et permettre la libération contrôlée du médicament pendant une période prolongée. Nous avons étudié l'encapsulation, la stabilité et la libération contrôlée de l'érythromycine et de la céfazoline par le Beriplast, agent de scellement à la fibrine (Aventis Behring Canada).
Méthodes
On a déposé des caillots chargés de médicament dans des fioles en verre, où on les a laissé coaguler. Nous avons observé les caillots pour déterminer la précipitation des médicaments et l'agrégation et nous avons évalué l'effet de l'encapsulation du médicament sur la résistance du caillot. On a quantifié la stabilité du médicament et sa libération des caillots dans une solution physiologique tamponnée au phosphate (PTP) par spectroscopie à absorbance des ultraviolets et du violet visible et par chromatographie liquide à haute performance.
Résultats
On a constaté que l'érythromycine se libère lentement des caillots de fibrine au cours des deux premières heures, mais qu'elle se dégrade ensuite rapidement. On a constaté que la céfazoline était très stable dans des caillots plongés dans une solution PTP (stable à 97 % au j 2 et à 93 % au j 5). Le médicament a été diffusé de façon contrôlée en deux jours, la majeure partie étant libérée au cours de la première journée. Il était possible de varier la dose de médicament libéré en modifiant la quantité déposée dans la solution de thrombine. L'épaisseur du caillot n'avait aucun effet sur le taux de libération de la céfazoline.
Conclusion
Dans l'ensemble, le profil de diffusion en deux jours et l'excellente stabilité du médicament indiquent que les agents de scellement à la fibrine chargés de céfazoline peuvent constituer une voie efficace de distribution d'antibiotiques après une intervention.
Antibiotics are generally given systemically to patients before and after many types of surgery. In orthopedic surgery, the administration of antibiotics is particularly important because the surgery often involves large external wound lengths and may be accompanied by the implantation of devices. Under such conditions, large doses of broad-spectrum antibiotics such as cefazolin are given for up to 48 hours postoperatively; an infection might be further complicated by an inflammatory response and the possible rejection of implanted materials. For example, following total hip arthroplasty, a common antibiotic regimen is 1 g of cefazolin at surgery and every 8 hours thereafter for 24–36 hours. These high doses are required to maintain therapeutic levels at the surgical site. The use of extended antibiotic treatments, however, may be associated with an increased danger of generating resistant strains, which are especially problematic in hospital settings.
An alternative approach might be to administer the antibiotics locally so that the drug is targeted to the surgical site and released in a controlled manner over time. Such a method should allow for high initial drug concentrations to eliminate any existing bacteria at the surgical site, followed by the extended release of the antibiotic to prevent reinfection. Controlled release requires the drug to be encapsulated in a material or device that delays its release at the site. However, the use of any non-autologous controlled-release biomaterials for this purpose may generate an inflammatory response and rejection of implanted devices.
Fibrin sealants are topical hemostatic materials derived from plasma coagulation proteins that are being used increasingly in surgical procedures.1–4 Fibrin sealants have great potential for the delivery of antibiotics,2,5–8 chemotherapy9,10 and even growth factors11 at surgical sites. They are biocompatible and degrade by normal fibrinolysis within days or weeks of application, depending on the site. Most fibrin-based sealants use a 2-component system that mixes thrombin with fibrinogen to form a fibrin clot at the site of application. Although the material has been available in Europe and Japan for 20 years, it was only recently approved by the Federal Drug Administration in the United States.3 The main use of these sealants has been in cardiovascular, thoracic, dental, and plastic and reconstructive surgery. More recently, orthopedic procedures, such as total knee arthroplasty or hip replacement, have also been shown to benefit from the use of fibrin sealants.3 Although the main application of these sealants is to reduce bleeding, they may also be used to augment the action of sutures and aid in wound healing.
Clearly, the compatibility of these materials with surgical wound sites makes fibrin sealants logical candidates for use as controlled-release carriers for local antibiotic delivery. It has been shown that antibiotics with low water solubility, such as tetracycline base, are particularly suited to this system,12 presumably because the precipitated drug dissolves and diffuses slowly from the fibrin clot. Even more water-soluble antibiotics such as gentamicin and ciprofloxacin have been shown to release from fibrin over 5–7 days, although more than 66% was released in the first 2 days.13–15 However, in many surgical applications, especially “dirty” operations or those involving the implantation of devices, the most important criterion is to localize high concentrations of the antibiotic at the site for the first day after the operation. Fibrin-sealed Dacron grafts used in vascular procedures were shown to release vancomycin16 or sisomicin17 rapidly and establish high localized antibiotic concentrations during the first postoperative day. Further, no infections were observed in 10 patients treated with sisomycin-loaded fibrin sealant applied directly to Dacron grafts in vascular prosthetic reconstruction.17 In orthopedic surgery, 2 commonly used, systemically administered antibiotics are cefazolin and erythromycin. The purpose of this study was to investigate the encapsulation and release kinetics of these drugs from fibrin sealant clots in vitro.
Methods
Materials
Beriplast fibrin sealant was supplied by Aventis Behring Canada. Cefazolin and erythromycin were obtained from Sigma Chemical (St. Louis, Mo.).
Manufacture of fibrin clot–drug matrices
An appropriate amount of erythromycin or cefazolin was weighed into a 20-mL flat-bottomed glass bottle. Both the thrombin and fibrinogen vials were reconstituted in injection media but were not mixed together. For most experiments, one-tenth the volume of the thrombin solution was added to the drug in the vial, and the mixture was sonicated and vortexed lightly to ensure the homogeneous dispersion/dissolution of the drug. In experiments designed to investigate the effect of clot thickness on drug release, a larger or smaller volume of the thrombin solution was added to the drug. An equal volume of the fibrinogen mixture was then added to the drug containing thrombin solution, and the mixture was further mixed by light swirling. By this method, the composition set to a solid clot within 1 minute.
Quantification of drug concentrations
Both drugs have characteristic spectra by UV-VIS (ultraviolet and visible light) absorption spectroscopy with peaks at 280 nm and 270 nm for erythromycin and cefazolin, respectively. With drug standards ranging from 1 to 200 μg/mL, absorbance calibration curves obtained at these peak wavelengths gave linear graphs with correlation coefficients greater than 0.98. High performance liquid chromatography (HPLC) methods were used to further investigate cefazolin stability and release from fibrin clots. This method separates the drug from other components in the drug release system, such as degradation products, which may not be apparent in UV-VIS determinations. HPLC was performed with a Waters chromatography system with Millennium software control. The chromatography system used a Novapak C18 column (1998), a 20-μL injection volume, detection at 270 nm and a mobile phase composed of 90% pH 3.4 phosphate buffer and 10% acetonitrile, at a flow rate of 1 mL/min. Calibration graphs were linear in the 1–200 μg/mL concentration range.
Measurement of drug stability
Standard solutions of erythromycin or cefazolin in phosphate buffered saline (PBS), pH 7.4, were placed in an oven in the dark at 37ºC. At given time intervals, the solutions were removed and the UV-VIS spectra were monitored. The absorbances at 280 nm or 270 nm for erythromycin and cefazolin were measured at each time point. When the clots had sat for 15 minutes, they were observed for integrity and general strength.
Drug-release studies
The drug-loaded clots were placed in the 20 mL glass vials after inspection. Ten mL of PBS were added to the vials, which were capped and placed in an incubator at 37°C in the dark, with orbital shaking at 50 rpm. At appropriate times, the vials were removed from the incubator, the drug-containing solutions were fully removed from the clot and fresh PBS (10 mL) was pipetted back onto the clot. The vials were then returned to the incubator. Drug-containing solutions were assayed by either UV-VIS absorbance or HPLC methods. Pilot studies were performed with UV-VIS spectroscopy to investigate whether any breakdown products eluting from fibrin clot might interfere with the drug absorbance values. More detailed studies on cefazolin release and stability were performed with HPLC methods.
Results
Drug-loaded clot formation
The addition of any concentration of the 2 drugs to the fibrin clot mixtures had no detrimental effect on the time to clot or the final apparent strength of the clots. There was no evidence of drug precipitation or particle aggregation for either drug in the clots.
Drug stability in aqueous solution
Cefazolin calibration standards gave essentially unchanged UV-VIS spectra after incubation for 48 hours. Peak absorbances decreased by less than 5% after 48 hours and by 10% at 5 days. HPLC analysis confirmed the uniform absorbance values and retention times of the drug, establishing the stability of the drug over the 48-hour period. By HPLC analysis, the potency (percentage of drug remaining) of cefazolin was 96.8% at 48 hours and 89.8% at 5 days. Erythromycin was found to be stable in aqueous solution for only a few hours. After that time, the shape of UV-VIS spectra and the absorbance peak intensity at 280 nm decreased rapidly. Encapsulation in the fibrin clot did not protect the drug from hydrolytic degradation; preliminary drug release studies showed that the drug was released as a degradation product.
Drug-release studies
Drug-release experiments on the erythromycin-loaded clots showed slow release at 2 hours but significant hydrolytic degradation after 2 hours. Detailed drug-release studies were performed on cefazolin-loaded films only, with HPLC quantification. In all experiments, cefazolin was found to release in a controlled manner with a sharp burst phase of release over the first 6–8 hours, followed by a sustained release over the following 16 hours. This amount was between approximately 1% and 9% of the total load of the drug in the discs. Generally, a small but measurable amount of drug was released in the 24- to 48-hour period, but there was no detectable drug release after that time. Because the graphs show cumulative drug release, the errors involved in the drug analysis are also cumulative. Therefore, the mean values showing more than 100% drug release reflect these cumulative errors in drug analysis. (As an experimental control, between 3.7 and 15 mg of cefazolin was weighed into sample tubes and PBS was added. After 15 minutes at room temperature, 1 mL of the PBS was centrifuged [to remove particulate drug] and analyzed for drug content. In all tubes, 100% of the drug had dissolved after 15 minutes.)
The rate of drug release was unaffected by the amount of drug loaded into the fibrin clots (Fig. 1). There was no difference in the release rate of the cefazolin from the Beriplast when the same amount of drug was loaded into clots of different thickness (Fig. 2). The 0.6 mL and 1 mL terms refer to the volume of Beriplast components used to make a single clot in a vial. The 0.6 mL and 1 mL volumes gave clots of approximately 0.3 mm and 0.5 mm thickness, respectively.
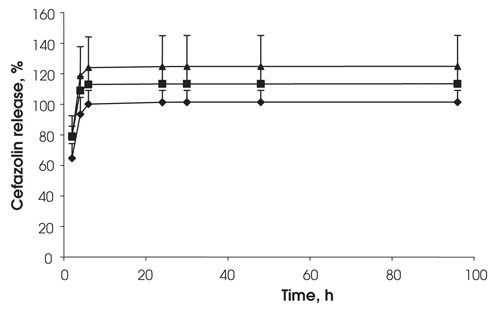
FIG. 1. Effect of drug loading on cefazolin release from fibrin sealant. Drug loadings were 15 mg (diamonds), 7.5 mg (squares) and 3.7 mg (triangles) in 0.6 mL sealant (clot thickness 0.32 mm), n = 3.
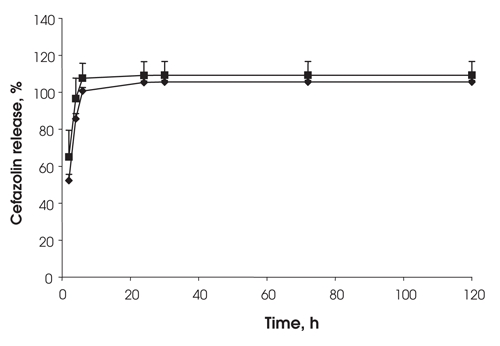
FIG. 2. Effect of fibrin clot thickness on the release of cefazolin — 30 mg of cefazolin loaded into 1 mL fibrin sealant (diamonds) (0.53 mm clot thickness) or 0.6 mL fibrin sealant (squares) (0.32 mm clot thickness), n = 3.
When 15 mg of cefazolin was loaded into the larger 1 mL (0.5 mm thick) clots, the release rate was slightly slower at 2 and 5 hours than for identical clots loaded with 7.5 mg of cefazolin (Fig. 3). However, after that time, both formulations released the drug in a similar manner. At the end of all drug-release experiments, all of the discs were fragmented with sonication and vortexing and were centrifuged. No residual drug was found in any clots, indicating that all of the drug had released from the disc.
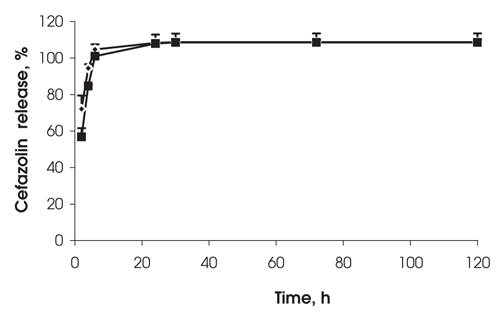
FIG. 3. Effect of drug loading on cefazolin release from 0.53 mm thick clots (1 mL of fibrin sealant). Drug loadings were 15 mg (squares) and 7.5 mg (diamonds), n = 3.
Discussion
Fibrin sealants have been used successfully in numerous surgical procedures in Europe and Japan over the last 20 years. In a recent study, the incidence of adverse effects due to the use of fibrin sealants was approximately 1 in 100 000 applications,18 establishing their safety. The main use of these sealants is to limit bleeding and postoperative hemorrhage. A secondary, but very important, application lies in the sealing of tissue surfaces. Although these products are typically applied with a dual syringe as liquids that mix and clot at the required site, they may also be sprayed onto an area to give a broader, more uniform covering. Such a spray technique may suit larger orthopedic surgical sites, whereas more focused surgery such as vascular operations may require a larger localized deposition of the sealant.19 In 1998, the FDA approved the commercial forms of sealant for use in the US, increasing the global use of these products and expanding the interest in alternative clinical applications.
The use of dry fibrin sealant in surgical gauze has been shown to reduce bleeding in a ballistic injury model.20 Fibrin sealants have obvious applications in all surgery performed on patients taking antithrombotic drugs.3,20 Sealants have been used in minimally invasive surgery by injection through catheters and have even been used as embolic agents to block blood vessels. Sealants have been used in conjunction with vascular stent and graft applications.20 However, these products also possess great potential as biodegradable carriers for the controlled release of drugs.2
The material is biocompatible and induces no inflammatory response. It adheres well to moist tissue surfaces and may be applied as a liquid solution of a drug or as an aerosol dispersion to form a solid matrix that holds the drug depot adjacent to the target tissue. Although these sealants have been used to deliver anticancer drugs to tumour sites,9,10 the obvious application is for the delivery of antibiotics during surgical procedures. There are many reports of the encapsulation and release of antibiotics from fibrin sealant.7,8,12–16 Generally, the drugs incorporated into the fibrin sealant were fairly water soluble, and the release rate from sealants occurred over hours rather than days. This has led to studies using more hydrophobic antibiotics, which may release over periods of a week or more.5,12
There has been only limited use of fibrin sealants in orthopedic surgery. The sealants have been used as a composite to hold bone fragments or ceramic implants in place in tendon repair and in periodontal repair.6,21 More recently they have been successfully deployed in knee arthroplasty and hip replacement operations. However, antibiotic-loaded fibrin sealants have great potential for use in many other orthopedic operations that currently require postoperative systemic administration of antibiotics. For example, in spinal realignment procedures, it is often necessary to expose large areas of bone and tissue and to implant structural devices over an extended surgical time period, thus leaving large wound lengths at risk for bacterial infection. Further, these surgical sites are often associated with a poor blood supply. Because the tissues receive a reduced exposure to antibiotics delivered via the blood stream, the broad-spectrum antibiotic cefazolin is often prescribed at doses as high as 3 g/d during the first 24–36 hours to ensure effective concentrations at the site of action. It is particularly important to expose surgical sites containing implants to high concentrations of antibiotics immediately after surgery, because even the smallest bacterial infection at the implant–tissue interface may cause an inflammatory response and possible rejection of the implant. Since the use of fibrin sealants in orthopedic procedures is growing, it seems logical that this material should be used to deliver antibiotics. Another potential application might be to replace the antibiotic impregnated cement beads used in compound fractures, which must eventually be removed.
Although the development of drug resistance is of great concern to surgeons, we do not believe that this method of local application of a controlled-release formulation of cefazolin would exacerbate this problem. The drug would be applied as a single dose, and all of it would be released at the surgical site over 24–48 hours. It is likely that this method would augment existing antibacterial strategies, and so the likelihood of additional problems related to drug resistance would be small.
In this study, we have shown that the water-soluble antibiotic cefazolin is released from the fibrin sealant over 48 hours, and most of the drug is released in the first 24 hours. There might be some differences in release rates between the sample groups shown in Figure 1 and Figure 3. However, we did not try to overinterpret the data, which might have attached too much significance to minor effects. Therefore, significance testing between drug loadings or disc thickness in these experiments has far less importance than this overall finding of a 48-hour release profile for all samples.
These in-vitro release profiles demonstrate the same rapid release problems encountered in other studies with different water-soluble antibiotics. However, it is likely that, in vivo, the antibiotic may be released more slowly than indicated from in vitro studies performed in relatively large volumes of aqueous media, owing to poor vascularization of many orthopedic surgical sites. The same argument may also apply to the rate of clearance of the antibiotics from the site: the released drug may remain at the site for some time.
We found that the hydrophobic antibiotic erythromycin was released slowly from fibrin sealants but degraded rapidly in aqueous environments and was therefore eliminated as an antibiotic for use in a fibrin-based release system. However, cefazolin was found to retain more than 97% of its potency in PBS at 37°C at 48 hours and 93% at 5 days. Clearly, the 48-hour release profile and the acceptable stability of this drug suggest that cefazolin-loaded fibrin sealants may offer an effective route of postoperative antibiotic delivery to orthopedic surgical sites.
Acknowledgments
Funding for this project was provided by the Aventis Behring Canada Research and Education Fund.
Poster presentation at the 2003 Annual Meeting of the Canadian Orthopaedic Association.
Competing interests: None declared.
Correspondence to: Dr. Stephen Tredwell, BC Children's Hospital, A200–4480 Oak St., Vancouver BC V6H 3V4; fax 604 875-2275; stredwell@cw.bc.ca
References
- 1.Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg 2001;182:8S-14S. [DOI] [PubMed]
- 2.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg 2001;182:40S-44S. [DOI] [PubMed]
- 3.Jackson MR. Fibrin sealants in surgical practice: an overview. Am J Surg 2001;182:1S-7S. [DOI] [PubMed]
- 4.Mankad PS, Codispoti M. The role of fibrin sealants in hemostasis. Am J Surg 2001;182:21S-28S. [DOI] [PubMed]
- 5.Jackson MR, MacPhee MJ, Drohan WN, et al. Fibrin sealant: current and potential clinical applications. Blood Coagul Fibrinolysis 1996;7:737-46. [PubMed]
- 6.Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties, and clinical applications. J Biomater Appl 1993;7:309-52. [DOI] [PubMed]
- 7.Thompson DF, Davis TW. The addition of antibiotics to fibrin glue. South Med J 1997;90:681-4. [DOI] [PubMed]
- 8.Redl H, Schlag G, Stanek G, et al. In vitro properties of mixtures of fibrin seal and antibiotics. Biomaterials 1983;4:29-32. [DOI] [PubMed]
- 9.Kitazawa H, Sato H, Adachi I, et al. Microdialysis assessment of fibrin glue containing sodium alginate for local delivery of doxorubicin in tumour-bearing rats. Biol Pharm Bull 1997;20:278-81. [DOI] [PubMed]
- 10.Miura S, Mii Y, Miyauchi Y, et al. Efficacy of slow-releasing anticancer drug delivery systems on transplantable osteosarcomas in rats. Jpn J Clin Oncol 1995;25:61-71. [PubMed]
- 11.Zarge J, Husak IV, Huang P, et al. Fibrin glue containing fibroblast growth factor type 1 and heparin decreases platelet deposition. Am J Surg 1997;174:188-92. [DOI] [PubMed]
- 12.Woolverton CJ, Fulton JA, Salstrom S, et al. Tetracycline delivery from fibrin controls peritoneal infection without measurable systemic antibiotic. J Antimicrob Chemother 2001;48:861-7. [DOI] [PubMed]
- 13.Marone P, Monzillo V, Segu C, et al. Antibiotic-impregnated fibrin glue in ocular surgery: in vitro antibacterial activity. Ophthalmologica 1999;213:12-5. [DOI] [PubMed]
- 14.Kram HB, Bansal M, Timberlake O, et al. Antibacterial effects of fibrin glue-antibiotic mixtures. J Surg Res 1991;50:175-8. [DOI] [PubMed]
- 15.Park MS, Kim YB. Sustained release of antibiotic form a fibrin-gelatin-antibiotic mixture. Laryngoscope 1997;107:1378-81. [DOI] [PubMed]
- 16.Fujimoto K, Yamamura K, Osada T, et al. Subcutaneous tissue distribution of vancomycin from a fibrin glue/Dacron graft carrier. J Biomed Mater Res 1997;36:564-7. [DOI] [PubMed]
- 17.Osada T, Yamamura K, Yano K, et al. Distribution and serum concentration of sisomicin released from fibrin glue-sealed Dacron graft in the rat and human. J Biomed Mater Res 2000;52:53-7. [DOI] [PubMed]
- 18.Morikawa T. Tissue sealing. Am J Surg 2001;182:29S-35S. [DOI] [PubMed]
- 19.Schenk WG, Burks SG, Gagne PH, et al. Fibrin sealant improves hemostasis in peripheral vascular surgery: a randomized prospective trial. Ann Surg 2003;237:871-6. [DOI] [PMC free article] [PubMed]
- 20.Jackson MR. New and potential uses of fibrin sealants as an adjunct to surgical hemostasis. Am J Surg 2001;182:36S-39S. [DOI] [PubMed]
- 21.Soffer E, Ouhayoun JP, Anagnostou F. Fibrin sealants and platelet preparations in bone and periodontal healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:521-8. [DOI] [PubMed]


