Abstract
Background
Investigation into the surgical application of robot technology continues to expand. We report on the first case series of robotic-assisted mitral valve (RAMV) repair in Canada with use of the da Vinci telemanipulation system (Intuitive Surgical, Sunnyvale, Calif.).
Methods
Between February 2004 and August 2004, 10 patients with normal left ventricular function and severe mitral valve regurgitation underwent RAMV repair with use of the da Vinci system. Peripheral cardiopulmonary bypass, transthoracic aortic cross-clamping and antegrade cardioplegia were used in all cases. A minithoracotomy in the fourth intercostal space and 2 ports in the third and fifth intercostal spaces allowed surgical access. All mitral valve valvuloplasties and band annuloplasties were done endoscopically with robotic assistance.
Results
Nine of 10 patients had successful valve repair, and 1 had conversion to mitral valve replacement due to persistent regurgitation. There were no deaths, strokes or need for sternotomy. One patient required re-exploration for bleeding.
Conclusion
Minimally invasive RAMV repair is feasible and safe with promising early postoperative results when performed by experienced surgical personnel accomplished in both mitral valve procedures and robotic techniques.
Abstract
Contexte
L'investigation des applications chirurgicales de la robotique continue de prendre de l'ampleur. Nous présentons un rapport sur la première série de cas de réparation de la valvule mitrale avec assistance robotique (VMAR) au Canada pratiquée avec le système de télémanipulation da Vinci (Intuitive Surgical, Sunnyvale, Calif.).
Méthodes
Entre février et août 2004, 10 patients dont le ventricule gauche fonctionnait normalement et qui avaient une régurgitation sévère de la valvule mitrale ont subi une réparation VMAR effectuée avec le système da Vinci. On a utilisé dans tous les cas une circulation extracorporelle, le clampage transthoracique de l'aorte et une cardioplégie antérograde. Une minithoracotomie pratiquée dans le quatrième espace intercostal et deux orifices pratiqués dans le troisième et le cinquième des espaces intercostaux ont assuré l'accès chirurgical. Toutes les valvuloplasties de la valvule mitrale et les annuloplasties ont été réalisées par endoscopie avec assistance robotique.
Résultats
Des 10 patients, 9 ont subi une réparation réussie de la valvule et il a fallu convertir l'intervention en remplacement dans un cas à cause d'une régurgitation persistante. Il n'y a eu aucun décès, aucun accident vasculaire cérébral et aucun patient n'a eu besoin de sternotomie. Il a fallu pratiquer une réexploration chez un patient à cause d'un saignement.
Conclusion
La réparation VMAR à effraction minimale est faisable et sécuritaire et promet des résultats postopératoires rapides lorsqu'elle est pratiquée par du personnel chirurgical expérimenté qui maîtrise à la fois les interventions sur la valvule mitrale et les techniques robotiques.
Cardiac surgery has undergone a technologic transformation over the last decade. Starting with Cohn,1 Cosgrove2 and their colleagues, it was established that the standard approach to valve surgery through a median sternotomy could be modified to allow smaller sternal and parasternal incisions with improved patient satisfaction, superior patient outcomes and decreased hospital costs. Further pioneering work from groups around the world has enabled cardiac surgeons to press forward with innovative advancements in a concerted effort to develop minimally invasive cardiac surgical techniques,3–5 while still upholding the high standards and excellent results of previous conventional procedures.6 Early skeptics of robotic surgery questioned the increased technical difficulties of performing cardiac surgery with reduced exposure and limited access;7,8 however, with the emergence of videoassistance and computer-enhanced telemanipulation, visual acuity and instrument dexterity within the thoracic cavity continues to improve. Since the introduction of the robotic-assisted cardiac surgery program at the London Health Sciences Centre in September 1998, exploration into minimally invasive mitral valve surgery has continued to advance.9 After starting with an initial minimally invasive approach through a right minithoracotomy using voice-activated endoscopic visualization (AESOP 3000; Computer Motion, Goleta, Calif.)10 and manual repair techniques, we now report on this centre's preliminary experience with robotic-assisted mitral valve (RAMV) repair with use of the da Vinci surgical system (Intuitive Surgical, Sunnyvale, Calif.). This surgical telemanipulation system allows 3-dimensional visualization of the operative field and improves instrument positioning and handling within the thoracic cavity. Our main objective for this case series was to demonstrate the feasibility of performing RAMV repair safely with acceptable postoperative results.
Methods
Between February 2004 and August 2004, 10 patients underwent RAMV surgery with use of the da Vinci system at the London Health Sciences Centre. Ten mitral valve repairs were performed, with 1 conversion to mitral valve replacement. A single surgeon performed all 10 procedures. Patients with severe pulmonary hypertension or extensive thoracic adiposity unsuitable to endoscopic visualization were excluded from the study.
Patients were given a paravertebral block and anesthetized. They were placed in a 30º left lateral decubitus position with the right arm at the side. A double-lumen endotracheal tube was inserted with left-sided single-lung ventilation. Cardiopulmonary bypass was established with cannulation of the right femoral artery and vein under transesophageal echocardiographic (TEE) guidance. A right-sided minithoracotomy 5–7 cm long was made through the fourth intercostal space. The fourth intercostal space was entered and the pericardium opened. A Chitwood–DeBakey transthoracic aortic cross-clamp (Scanlan International, Minneapolis, Minn.) was inserted through the second intercostal space laterally in the midaxillary line, and antegrade cardioplegic solution was instilled into the ascending aorta to maintain cardiac arrest and myocardial protection. A standard left atriotomy was performed, and mitral valve exposure was facilitated by retracting the left atrium with use of the Heartport retractor (Heartport Inc., Redwood City, Calif.). After inspection of the mitral valve, 2 additional ports were positioned for placement of the robotic instruments. A port was inserted in the fifth intercostal space anterior to the anterior axillary line for instruments used by the right arm of the surgical system while the other port was inserted in the third intercostal space of the midclavicular line for instruments used by the left arm of the system. There was a diagonal distance of 12 cm separating the 2 trocars inside the thoracic cavity, ensuring optimal movement without collision. The robotic camera was situated in the apex of the right-sided minithoracotomy.
After initial setup, the “console surgeon” was stationed at the surgical console away from the operating table while the “bedside surgeon” monitored, changed and retrieved operative instruments and materials through the minithoracotomy. The 3-dimensional high-resolution image viewed from the console, along with the 7° of freedom, tremor-free, scaled control of the robotic arms, enabled precise surgical tasks in an ergonomically favourable environment.5 All 10 band annuloplasties were completed robotically with the da Vinci telemanipulation system. Nitinol U-CLIP de-vices (Medtronic Inc., Minneapolis, Minn.)11 were used in place of conventional suture knots. The da Vinci system was then moved away from the operating table and the left atrium was closed under direct vision. Standard removal of air and weaning procedures were performed. Two ventricular pacing wires were brought out from the right ventricle, and the pericardium was reapproximated. Two chest tubes were inserted into the right pleural space for drainage. Before the chest was closed, an intercostal nerve block 1 intercostal space above and below the incision was given with cryotherapy or bupivacaine to provide further postoperative pain control.
Results
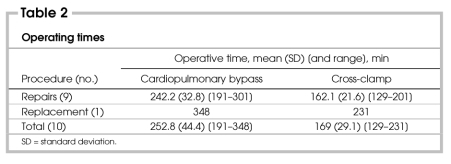
Preoperative demographic characteristics for all patients are shown in Table 1. Mitral valve insufficiency was due to degenerative myxomatous disease in 8 patients and endocarditis in 2 patients. Nine of 10 patients had torn chordae tendinae. Five patients had posterior mitral valve prolapse, 4 patients had bileaflet prolapse, and 1 patient had lone anterior leaflet prolapse with anterior leaflet perforation. Posterior prolapse was corrected with quadrangular resection and direct reapproximation, sliding annuloplasty or rotational annuloplasty. Triangular resections, chordae tendinae transposition or neochordae insertions were used for anterior leaflet prolapse. All 10 flexible annuloplasty bands (Duran AnCore Bands; Medtronic Inc., Minneapolis, Minn.) were inserted robotically. Each annuloplasty band required between 10 and 14 Nitinol clips.
Table 1
One patient underwent a combined procedure of mitral valve repair and pulmonary vein isolation with cryotherapy for preoperative paroxysmal atrial fibrillation. Two patients had adjunctive closure of a patent foramen ovale.
The patient requiring mitral valve replacement had bileaflet prolapse and thickened leaflets that did not come into apposition on static valve testing. No abnormalities of chordae tendinae were noted, and valvuloplasty was not required. The annuloplasty band was secured without complication. Although the patient was markedly improved from the preoperative state after weaning from bypass, TEE still revealed unacceptable moderate regurgitation. As heart function improved, mitral regurgitation became more pronounced. The decision was made to proceed with valve replacement using a mechanical valve. This procedure was done manually through the original minithoracotomy.
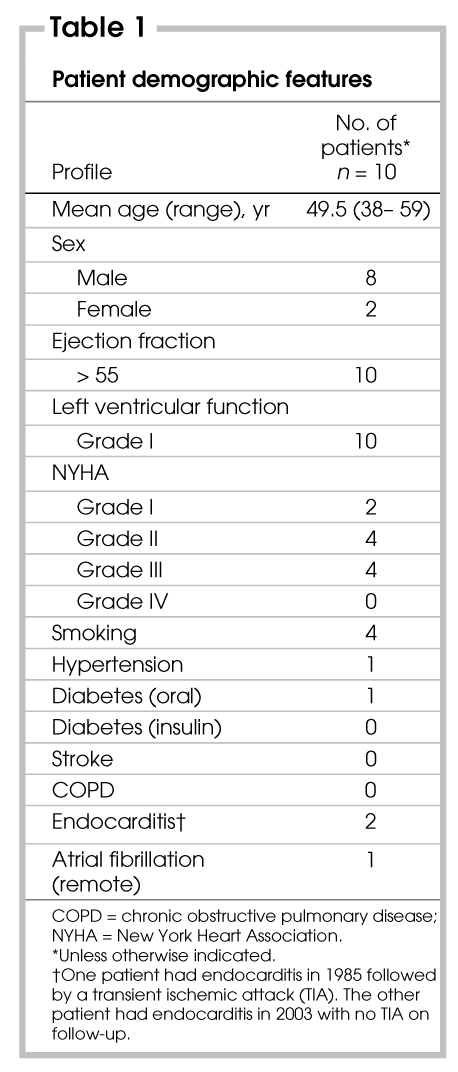
Operative times for cardiopulmonary bypass are summarized in Table 2. Postoperative length of stay ranged from 5 to 19 (median 6) days. The patient who required mitral valve replacement had an extended 19-day stay because of difficulty achieving therapeutic anticoagulation. Seven patients were transferred to the ward from the intensive care unit (ICU) within 24 hours (range from 1–4 d, median 1 d). Of those who had an extended stay in the ICU — 3 days for 1 patient who had persistent right pleural effusion, 4 days for a patient with insufficient cardiac output, and 2 days for a patient requiring re-exploration for bleeding at the dome of the diaphragm at the site of one of the retraction sutures. This was controlled with no further complications.
Table 2
There were no deaths or strokes and no sternotomy was needed. Eight patients (80%) had no mitral regurgitation on postoperative TEE, whereas mild regurgitation was noted in 2 patients (20%). No thoracic wound infections occurred, but 1 groin infection was recorded. Five patients were given blood transfusions. One patient with normal variant mild leukopenia and cytopenia preoperatively required 5 units of platelets for postoperative thrombocytopenia. Finally, atrial fibrillation developed postoperatively in 2 patients. One patient converted to sinus rhythm with anti-arrhythmic therapy in hospital and the other was cardioverted to sinus rhythm at a follow-up appointment.
Discussion
When compared with surgical specialties such as general surgery, orthopedics, gynecology or urology, technologic advances in endoscopy have had little effect to date on the practice of cardiac surgery.
Casselman and associates12 give credence to the potential role of endoscopic techniques in cardiac surgery with a recently reported large, successful series of endoscopic mitral valve repairs using a minithoracotomy. However, the widespread acceptance of this approach may prove difficult. The requirement for superior endoscopic skill, unfamiliar to most practising cardiac surgeons and considerably estranged from standard cardiac surgical manoeuvres, makes the dissemination of this approach to training at the resident level challenging, as the volume of cardiac procedures enhanced by its use may not merit the efforts needed to foster such training.
In this paper, we have reported on our initial experience with computer-enhanced telemanipulation, or robotic-assisted mitral valve repair. Unlike long-shafted endoscopic instruments, robotic-assisted surgery uses hand motions like those of traditional surgery performed at a distant console. The ability of this technology to enable the surgeon to perform minimally invasive procedures while conforming to the surgeon's traditional training is formidable, and does not force the surgeon to acquire new endoscopic skills. Furthermore, the enhanced dexterity of robotic instrumentation compared with endoscopic instruments broadens the applicability of robotics beyond that of valve surgery to more extensive procedures like coronary artery surgery. This increases the likelihood of robotics being accepted as a necessary new advance by the cardiac surgical community.
Our preliminary experience suggests that in highly selected patients, cardiac surgeons at specialized centres committed to robotics training can perform RAMV surgery safely with satisfactory results. Although this series shows that the operative times are longer than in traditional mitral valve surgery, other studies have shown that both cardiopulmonary bypass times and aortic cross-clamp times decrease steadily toward those of traditional techniques after the first 10 cases.3,5,13 More experience at this centre using the da Vinci telemanipulation system for mitral valve surgery is required to comment further on this issue.
Although the sample size in this preliminary study is too small to be of statistical value, both the median ICU and overall hospital stays (1 and 6 d) were comparable to those recorded for isolated mitral valve surgery through a sternotomy at our institution (median ICU stay of 1 d and hospital stay of 7 d).
There is still the question however: Does minimally invasive RAMV surgery confer any real advantages beyond improved cosmesis when compared with the traditional approach via a sternotomy? Results from medical centres with a larger experience in RAMV repair suggest that there is indeed an advantage to using this technology. Felger and associates3 reported that patients with robotic mitral valve repair had decreased ventilator times, decreased numbers of reoperations for bleeding and a decreased length of hospital stay compared with a cohort who had mitral valve surgery via a sternotomy. To investigate further the likelihood of decreased ventilator times and fewer pulmonary complications, Tatooles and colleagues13 set up a planned anesthetic regimen to extubate patients in the operating room after robotic mitral valve repair. They were successful in extubating more than 80% of their patients immediately postoperatively with no reintubations. This allowed for a speedier recovery and earlier consideration for hospital discharge. Total port-access mitral valve repair with the da Vinci system was also reported recently.14 A transition in mitral valve surgery from sternotomy and minithoracotomy incisions to a port-access approach would likely reduce thoracic wound infections significantly. Early results pertaining to quality of life assessment have also been positive. Morgan and colleagues15 reported that patients who underwent RAMV surgery scored higher in 8 categories of quality of life assessment than those subjected to minimally invasive manual mitral valve repair through a minithoracotomy. Two categories, body pain and mental health, were statistically significant, despite an increase in cross-clamp and cardiopulmonary bypass times for the robotic procedures. Taken together, these reports suggest that the primary goals of RAMV repair: (a) to reduce surgical morbidity, (b) to reduce hospital length of stay and (c) to improve patient satisfaction and quality of life merit further investigation.
Conclusions
RAMV surgery is both feasible and safe, resulting in good early postoperative patient outcomes. Larger studies to evaluate the true impact of this technology on patient care are warranted.
Presented at the Canadian Cardiovascular Congress, Calgary, Alta., Oct. 23–27, 2004.
Competing interests: None declared.
Correspondence to: Dr. Scott McClure, Canadian Surgical Technologies & Advanced Robotics, University Hospital, 339 Windermere Rd., London ON N6A 5A5; fax 519 663-8401; scott.mcclure@c-star.ca
References
- 1.Cohn LH, Adams DH, Couper GS, et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg 1997;226:421-8. [DOI] [PMC free article] [PubMed]
- 2.Cosgrove DM, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg 1998;65:1535-9. [DOI] [PubMed]
- 3.Felger JE, Chitwood R, Nifong LW, et al. Evolution of mitral valve surgery: toward a totally endoscopic approach. Ann Thorac Surg 2001;72:1203-9. [DOI] [PubMed]
- 4.Mohr FW, Falk V, Diegler A, et al. Computer-enhanced ‚robotic' cardiac surgery: experience with 148 patients. J Thorac Cardiovasc Surg 2001;121:842-53. [DOI] [PubMed]
- 5.Nifong LW, Chu VF, Bailey M, et al. Robotic mitral valve repair: experience with the da Vinci system. Ann Thorac Surg 2003;75:438-43. [DOI] [PubMed]
- 6.Mohty D, Orszulak TA, Schaff HV, et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation 2001;104(12 suppl 1):I1-7. [DOI] [PubMed]
- 7.Robicsek F. Robotic cardiac surgery: Quo vadis? [editorial]. J Thorac Cardiovasc Surg 2003;126:623-4. [DOI] [PubMed]
- 8.Baldwin JC. Editorial (con) re minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:563-4. [DOI] [PubMed]
- 9.Menkis AH, Kodera K, Kiaii B, et al. Robotic surgery, the first 100 cases: Where do we go from here? Heart Surg Forum 2004;7:1-4. [PubMed]
- 10.Menkis AH, Fox S, Kiaii B, et al. Advances in fully robotic mitral valve repair. Can J Cardiol 2004;20(D Suppl):99D.
- 11.Reade CC, Bower CE, Maziarz DM, et al. Sutureless robot-assisted mitral valve repair: an animal model. Heart Surg Forum 2003;6:254-7. [PubMed]
- 12.Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [DOI] [PubMed]
- 13.Tatooles AJ, Pappas PS, Gordon PJ, et al. Minimally invasive mitral valve repair using the da Vinci robotic system. Ann Thorac Surg 2004;77:1978-84. [DOI] [PubMed]
- 14.Mehmanesh H, Henze R, Lange R. Totally endoscopic mitral valve repair. J Thorac Cardiovasc Surg 2002;123:96-7. [DOI] [PubMed]
- 15.Morgan JA, Argenziano M, Smith CR. Robotic valve surgery: How does the future look? Adv Cardiol 2004;41:157-63. [DOI] [PubMed]