Abstract
Background
We aimed to examine both the diagnostic modalities used to identify breast lesions and their surgical management in an Ontario community hospital.
Methods
We conducted a retrospective chart review of the preoperative diagnostic tools used by 6 general surgeons for palpable and nonpalpable breast lesions and considered the types of surgical procedures performed. Patients who underwent noncosmetic breast surgery in the year 2000 were included in the study (n = 180).
Results
Of the 182 breast lesions, 89 (49%) were malignant. Of the 100 palpable lesions removed, fine needle aspiration biopsy (FNAB) was performed on 48. Positive FNABs in this study were highly predictive of malignancy (100%). Only 1 core needle biopsy was performed on a palpable lesion. Of the 78 mammograms obtained for nonpalpable lesions, the PPV (positive predictive value) of malignancy for “suggestive” lesions was 100%, 75% for “suspicious” lesions, 40% for “probably benign” lesions, 0% for “benign” lesions and 37% for lesions categorized as “needs additional imaging.” Other preoperative diagnostic tools used were ultrasonography (n = 44) and stereotactic biopsies (n = 3). Of the initial operations performed, 76 were lumpectomies and 88 were needle-localized biopsies. Only 15 patients underwent initial definitive procedures, and of these 5 had positive margins and 8 had close (≤ 1-mm) margins. Positive margins were found in 35% of the needle-localized lumpectomies (61% had a close margin), in 60% of lumpectomies (75% had a close margin) and in 2 of the 5 lumpectomies with axillary node dissections done as first operations. Six frozen sections were obtained. Only 11% of surgical specimens were oriented for pathology. Reoperations were performed on 91% of women with malignancies (or 67% with a close margin).
Conclusions
Considerable variation existed between surgeons with regard to the types of preoperative diagnostic procedure used and operations performed. The rate of positive margins was high, which resulted in many reoperations.
Abstract
Contexte
Nous voulions analyser à la fois les méthodes de diagnostic utilisées pour identifier les lésions du sein et leur traitement chirurgical dans un hôpital communautaire de l'Ontario.
Méthodes
Nous avons procédé à une étude rétrospective sur dossier des outils de diagnostic préopératoires utilisés par six chirurgiens généraux dans le cas de lésions palpables et non palpables du sein et analysé les types d'interventions chirurgicales pratiquées. Nous avons inclus les patientes qui ont subi une intervention chirurgicale non esthétique du sein en 2000 (n = 180).
Résultats
Sur les 182 lésions du sein, 89 (49 %) étaient malignes. Sur les 100 lésions palpables enlevées, on a procédé à une ponction-aspiration à l'aiguille fine dans 48 cas. Dans cette étude, des résultats positifs à la ponction-aspiration étaient très prédicteurs de la présence de cancer (100 %). On a procédé à une seule biopsie à l'aiguille creuse sur une lésion palpable. Sur les 78 mammographies obtenues dans le cas de lésions non palpables, la VPP (valeur prédictive positive) de la présence de cancer s'établissait à 100 % dans le cas des lésions «évocatrices», à 75 % dans celui des lésions «suspectes», à 40 % dans celui des lésions «probablement bénignes», à 0 % dans celui des lésions «bénignes» et à 37 % dans celui des lésions «nécessitant une imagerie supplémentaire». Parmi les autres outils diagnostiques préopératoires, on a utilisé aussi l'échographie (n = 44) et la biopsie stéréotaxique (n = 3). Parmi les interventions initiales, on a pratiqué 76 tumorectomies et 88 biopsies localisées à l'aiguille. Quinze patientes seulement ont subi une intervention initiale définitive : cinq présentaient des bordures positives et huit, des bordures rapprochées (≤ 1 mm). On a constaté la présence de bordures positives dans 35 % des tumorectomies localisées à l'aiguille (61 % comportaient une bordure rapprochée), dans 60 % des tumorectomies (75 % avaient une bordure rapprochée) et dans 2 des 5 tumorectomies conjuguées à une dissection des ganglions axillaires pratiquées comme première intervention. On a obtenu six coupes congelées. Seulement 11 % des spécimens chirurgicaux étaient orientés en vue de la pathologie. On a pratiqué une nouvelle intervention chez 91 % des femmes qui avaient une lésion cancéreuse (ou 67 % qui présentaient une bordure rapprochée).
Conclusions
Il existait une variation importante entre chirurgiens quant aux modes de diagnostic préopératoire utilisés et aux types d'interventions pratiquées. Le nombre de bordures positives était élevé, entraînant un grand nombre de nouvelles interventions.
Breast cancer continues to be the most common cancer diagnosed among Canadian women, representing 29% of total cancers and ranked first for every age group.1 Even though its incidence has increased steadily over the past 3 decades, and national and provincial guidelines have been developed to improve consistency of care, there remains considerable variability in how breast cancer is treated across Canada.2
Clinical guidelines have been developed regarding the management of palpable and nonpalpable breast lesions in terms of investigations and surgical management.2 Substantial research has been done in recent years regarding the accuracy of breast screening mammography, core needle biopsies (CNBs) and fine-needle aspiration biopsies (FNABs) of breast lesions. Regarding palpable lesions, the Canadian guidelines recommend that FNAB or CNB should be performed on all lesions preoperatively to establish a diagnosis and allow better planning of surgical intervention.2 As screening mammography has become more widespread, more and more needle-localized biopsies are being performed for benign lesions. Percutaneous needle biopsy guided by ultrasonography or stereotactic biopsy for preoperative diagnosis of nonpalpable breast lesions has become commonplace and has led to lower rates of positive margins at the time of first operations.3–6
It is recommended that when an open surgical biopsy is performed, and carcinoma is suspected, the procedure should be a lumpectomy resulting in uninvolved margins to prevent a second operation and to reduce local recurrence.3,7 Although there is presently no consensus on the extent of excision for resection, there is evidence that long-term local control over breast tumours is best achieved when a significant amount of breast tissue surrounding the tumour is removed.8 When margins are found to be positive after tumour excision, the involved margins should be re-excised, although again there is no consensus on what should be considered an adequate margin.9 In addition, surgical specimens should be submitted intact and should be oriented with sutures for pathological examination.7,9
This study has applied current recommendations and practice for the diagnosis and surgical management of breast lesions to a community hospital with a view to improving the future management of this disease.
Methods
A retrospective study of all women who had noncosmetic breast surgery in a community hospital in the year 2000 was carried out. The patients' mean age was 55.3 (range 21–92) years. A total of 180 patients were included in the study, 2 of whom had lesions in both breasts. Therefore, 182 breast lesions were removed.
Physical findings were obtained from the surgeon's consultation notes and included whether a lesion was palpable or nonpalpable, the side and size of lesion, the status of lymph nodes and the presence of skin changes. The results of diagnostic tests, including FNAB, ultrasonography, mammography, CNB and stereotactic biopsy, were taken from patients' computerized hospital charts. For FNAB, the results were recorded as “malignant,” “suspicious,” “benign,” “atypical” or “insufficient.” Information on the date and type of surgery was obtained from operative reports. Surgical pathology, as well as information on the orientation of specimens with sutures and margin status, was taken from operative and synoptic pathology reports. Surgery was performed by 6 general surgeons.
The results of mammography were categorized using the American College of Radiology Breast Imaging Reporting and Data System (BI-RADS). The BI-RADS categories for reporting include “negative,” “benign,” “probably benign,” “needs further imaging,” “suspicious” and “suggestive,” with these last 2 considered to be positive mammography results. There was no radiologist as an investigator on this paper.
The term positive predictive value (PPV) applied to the diagnostic tests in this study refers to the probability that a patient has the disease when considering only those patients who test positive. Negative predictive value (NPV) refers to the probability that a patient does not have the disease when considering only those patients with negative test results. The sensitivity of diagnostic tests refers to the probability that a test is positive when given to a group of patients with the disease. The specificity is the probability that a test will be negative among patients who do not have the disease.
For the purposes of this study, margins were considered positive if there was tumour tissue at the inked edges of the surgical specimens; however, margin status was also calculated using a “close” (that is, ≤ 1 mm) margin as reported by the pathology department.
Results
Of the 182 breast lesions that were operated on (from 180 patients), 89 (49%) were malignant. Various types of malignant lesions were identified on pathology (Table 1). Fifty-one percent of the lesions excised were benign, again with a variety of diagnoses on pathological examination (Table 1). Of the lesions studied, 80 were nonpalpable (44%), 100 were palpable (55%), and in 2 cases it was not stated whether the lump could be palpated.
Table 1
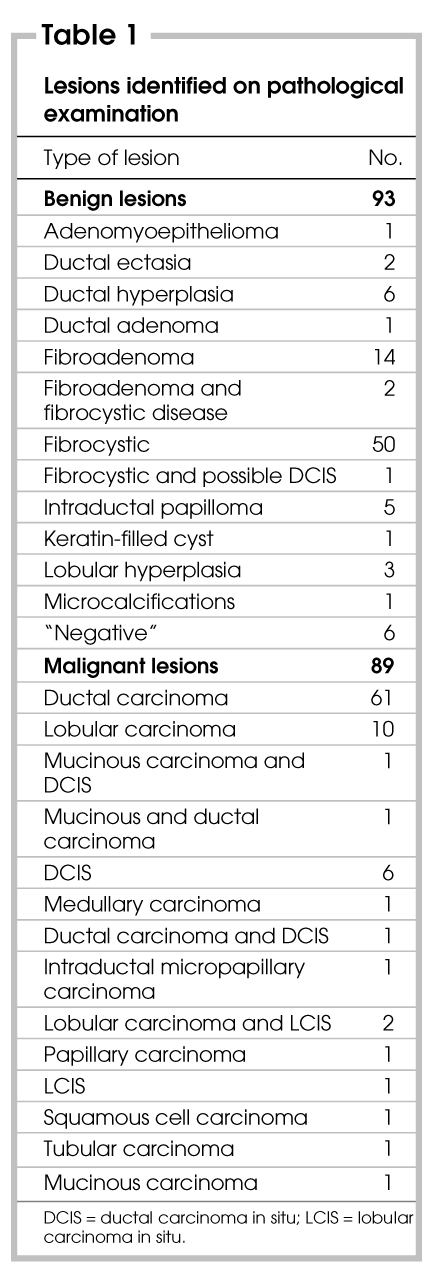
Forty-eight of the patients with palpable lesions had an FNAB (48%). Of these, 17 were positive for malignancy, and all 17 were malignant on final pathological examination. Six of the FNABs were suspicious for malignancy, and all 6 were found to be malignant. Ten of the FNABs were benign, with 8 of these confirmed benign at surgery. Three of the FNABs showed atypical cells, and 2 of these were malignant. Of all of the FNABs performed, 12 had insufficient cells (25%) and only 1 of these lesions was malignant.
Mammography was performed on 145 of the 180 women (81%). Of the mammograms, 78 were obtained for nonpalpable lesions. Three of these were “suggestive” of malignancy, and all of these were malignant (PPV 100%). Twenty-eight lesions were “suspicious,” and 21 of these were malignant (PPV 75%). Ten lesions were “probably benign,” and 4 of these were malignant (PPV 40%). Two lesions were “benign” on mammography, and both were found to be benign (PPV 0%). There were 35 nonpalpable lesions in the “needs additional imaging” category and, of these, 13 were malignant (PPV 37%).
Mammograms were obtained for 67 of the 100 palpable lesions. Of these, 9 were “suggestive” of cancer on mammography, and all 9 were malignant (PPV 100%). Nineteen lesions were “suspicious” on mammography, and of these 16 were malignant (PPV 84%). Five lesions were “probably benign,” and all of these were benign (PPV 0%). Six were “benign,” with 1 of these being malignant at surgery (PPV 16%) and had a suspicious FNAB result. Eleven mammograms were in the category of “needs additional imaging,” with 6 of these being malignant at surgery (PPV 55%). Seventeen palpable lesions were “negative” on mammography, and of these 5 were malignant at surgery (PPV 29%). FNABs were performed on all of these lesions, 2 of which had positive findings, 2 of which had negative findings and 1 had insufficient cells.
Only 3 stereotactic biopsies were performed, 1 on a palpable lesion and 2 on nonpalpable lesions. One biopsy was positive for malignancy on a nonpalpable lesion, 1 showed sclerosing adenosis and 1 could not be completed for technical reasons. In the patient with positive findings on stereotactic biopsy, lumpectomy and axillary lymph node dissection (ALND) were performed as the first operation. In all patients, only 1 CNB was performed, and it demonstrated malignancy. This patient had a modified radical mastectomy based on the findings of this CNB.
Forty-four sonograms were obtained of 26 solid lesions, of which 14 were malignant (PPV 54%). Eight of the sonograms showed cystic lesions, and 2 of these were malignant (PPV 25%). Four of the sonograms showed negative findings, and 1 of these lesions (25%) was found to be malignant.
Intraoperatively, only 6 frozen sections were performed. Definitive surgeries were performed in 3 of these cases and, although the results of pathological examination were positive for invasive cancer in the other 3 cases, only lumpectomies were performed.
The types of initial operations in this study are listed in Table 2, using the nomenclature found in the operative reports. The terminology used by the surgeons for the operative procedures was quite variable.
Table 2
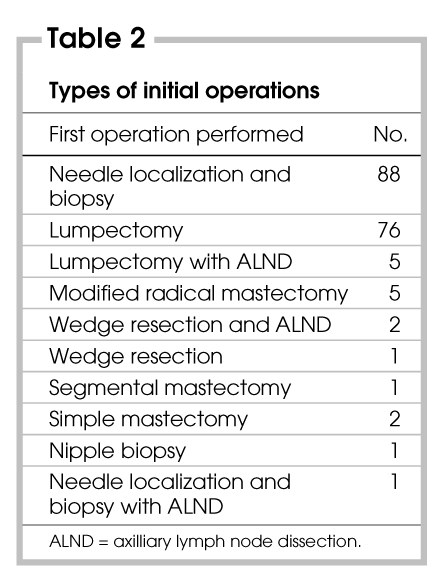
There were 14 definitive or single-stage first operations for palpable lesions (5 lumpectomies with ALND, 5 modified radical mastectomies, 2 simple mastectomies and 2 wedge resections with ALND), and all of these patients had malignant lesions on pathological examination. Wide variation existed in the preoperative diagnostic tools used by the surgeons in all of these cases. In 12 of these 14 cases, FNAB was performed and was positive for cancer. Mammography was performed in 11 of these 14 cases and showed positive findings in 9. A CNB was used in 1 case, and the findings were positive. Frozen sections were used in 3 cases. For 2 of these definitive surgeries, no preoperative or intraoperative investigations were used. Only 1 definitive procedure was performed on a nonpalpable lesion (needle-localized biopsy with lumpectomy and ALND) on the basis of stereotactic biopsy results. Of the 15 patients who underwent what were intended to be single-stage operations, 5 had positive margins after the procedures.
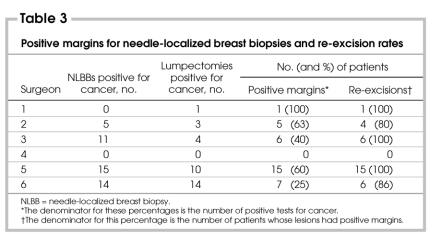
Of the surgical specimens obtained from the initial operations, only 11% were oriented and 2 specimens were sent to pathology in pieces. Of the 46 needle-localized biopsies that were done for malignancy, 16 had positive margins after the first operation (35%). This increased to 61% if a close (≤ 1-mm) margin was used. Of the 32 patients who had lumpectomies as first operations for malignant lesions, 19 of these had positive margins (60%). This increased to 75% if a close margin was used. Only 91% of the women with positive margins after their first operations had re-excisions, and re-excision rates varied among surgeons (Table 3). The re-excision rate was much lower for women with close margins, with only 67% having reoperations.
Table 3
Fifty-nine or 67% of the 88 women with malignancies had second operations. These reoperations included ALND, lumpectomy, lumpectomy and ALND, mastectomy, modified radical mastectomy, re-excision, re-excision and ALND, scar revision, and segmental mastectomy and ALND. Three of the women in this study went on to have third operations: 2 simple mastectomies and 1 skin nodule resection.
Discussion
The primary objective of the Clinical Practice Guidelines for the Care and Treatment of Breast Cancer is to “assist women and their physicians in making the most clinically effective and personally acceptable decision regarding the choice of primary surgery for potentially curable breast cancer.”9 As the diagnosis and surgical management of breast cancer is so critical to the prognosis of this disease, and because there is variation in the treatment of breast cancer in this country, it was our objective by conducting this timely retrospective study to determine whether current practice standards and guidelines are being followed by general surgeons in a community hospital.10 Although we analyzed all breast lesions operated on in the year 2000, practices had changed very little at this community hospital at the time of writing of this paper.
Palpable breast lesions
Although current practice in the management of palpable breast lesions is to ascertain a preoperative diagnosis from biopsy, only 48% of the women with palpable lesions in this study had FNABs and only 1 had a CNB. It is well documented that if an excisional biopsy is done as a diagnostic procedure, the surgeon will likely not perform a wide resection of normal tissue around the lesion and, therefore, margins are more likely to be positive than with a preoperative diagnosis of cancer. It would seem intuitive that obtaining a tissue diagnosis preoperatively would allow women and their surgeons to better plan their breast and axillary surgery as a single-stage procedure.6
Of the FNABs performed on the patients in this study, the sensitivities are in accordance with those reported in the literature.2 For FNABs that show malignant cells, a sensitivity of 99.2%–100% is reported in the literature, and these were 100% sensitive in this study. For FNABs that are “suspicious or atypical,” a 50%–70% sensitivity of malignancy has been reported, compared with 89% in this study. “Nondiagnostic” FNABs have a reported sensitivity of 5%–22% for malignancy and were 8% sensitive in this study. “Benign” FNABs have a reported false-negative rate of 25%–30% and a 20% false-negative rate in this study.6 Only 48% of the women with palpable lesions in our study had FNABs performed on their lesions. Of these FNABs, 25% had insufficient cells for pathology, which is consistent with the 33% described in the literature.11
It is interesting that although CNB is considered commonplace for palpable lesions in current practice, only 1 CNB was performed in the group described here. Reports in the literature confirm that the false-negative rates of 25%–30% for FNAB are reduced to 10% with CNB.6 In addition, CNB tissue can be analyzed pathologically to differentiate between invasive and in situ carcinoma.12 The reasoning for the use of CNB in the 1 patient in this study was not clearly stated and was performed after an FNAB showed suspicious results. The lack of CNBs may be because all the FNABs in this study were performed in the surgeons' clinics, and none of the surgeons have core needle devices in their offices. CNBs can be done using ultrasonographic guidance; however, these are only performed by radiologists in the hospital and surgeons may not have wanted to delay time to diagnosis by arranging these biopsies. In many cases in this study, diagnosis was confirmed through excisional biopsy and not preoperatively through CNB or FNAB.
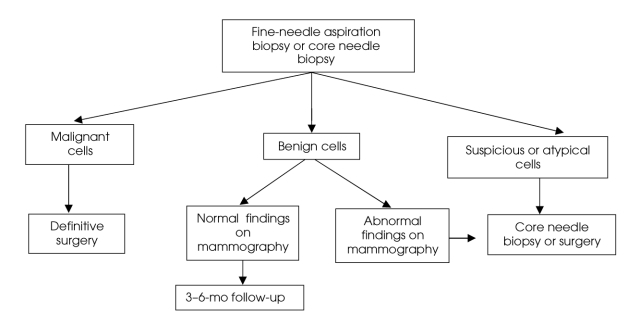
It is recommended in the case of palpable lesions that diagnosis be confirmed preoperatively with FNAB or CNB to reduce the number of surgical procedures, as well as to facilitate better planning of breast and axillary surgery for cancer.2,6,13 CNB reduces false-negative rates and should be performed when possible. If FNAB or CNB results show malignant cells, definitive surgery can take place. If FNAB cells are “suspicious or atypical,” CNB or excisional biopsy should be performed. If FNAB results are benign, and mammographic findings are normal, then follow-up should take place in 3–6 months. However, if findings on mammography are positive, then CNB or excisional biopsy should take place2 (Fig. 1).
FIG. 1. Management of palpable lesions.2
Mammography
An important part of this study involved correlating the mammographic interpretation with the final pathology. Orel and colleagues14 set out to determine the PPV for malignancy of each of the BI-RADS categories in 1312 mammograms performed between 1991 and 1996 before needle localizations in these patients. The PPV for “suggestive” lesions was 97%, which is comparable to the 100% for nonpalpable lesions in our study. For “suspicious” lesions, the PPV was 30%, which was much lower than the 75% in our study. In a study by Ball and coworkers,13 the PPV for “suggestive” lesions was only 77.4% and for “suspicious” lesions, 26.5%. For “probably benign” lesions, the PPV was 2% versus 40% in our study. These lesions had a PPV of 0% in the study reported by Ball and colleagues.13 “Benign” lesions had a PPV of 0% in the study by Orel and coworkers and a PPV of 0% in our study. Finally, for lesions that “need additional imaging,” the PPV was 13% compared with 37% in our study.14
About 15% of women with palpable breast cancer will have mammography that shows no evidence of cancer.11 In our study, 21% of the women with palpable lumps and benign findings on mammography had malignancies. Less has been written about the PPV of mammography using the BI-RADS system for palpable breast lumps. The PPV of diagnostic mammography overall for palpable lumps should be higher than that for nonpalpable lesions (26.8% v. 17.5%).11 The PPV of positive findings (“suspicious” or “suggestive”) on mammography for palpable lesions in our study was 82%, which was high, but of the benign and negative findings on mammograms, 25% were malignant on surgical pathological examination.
Considering these results, it is apparent that when mammographic results in our study were positive (BI-RADS categories of “suggestive” or “suspicious”), the PPV of this test was very high. Unfortunately, however, 33% of the nonpalpable lesions and 21% of the palpable lesions that were mammographically benign in this study were found to be malignant at surgery. We also found in our study that many more mammograms were categorized as “needs further imaging” (32%) as compared with the rate reported in the literature (6%–8%).11
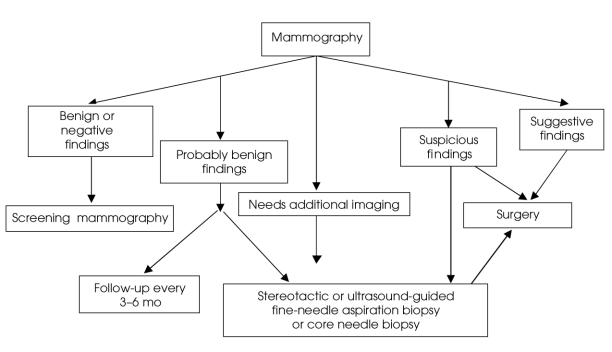
There remains considerable variability in management decisions made after positive findings on mammography, which leads to numerous unnecessary biopsies.2 In our study, 28% of the mammograms fell into benign categories, and all of these women still underwent a surgical procedure. Percutaneous biopsy has now become widely used for women with equivocal (“probably benign,” “needs further imaging”) mammographic results to avoid invasive excisional biopsy. Equivocal results can also be followed by repeat mammography every 3–6 months depending mostly on patient preference and anxiety, as well as risk factors.13 Women with positive findings on mammography may also benefit from CNB to possibly avoid unnecessary excisional biopsy or to allow a 1-stage procedure to be planned12 (Fig. 2).
Nonpalpable breast lesions
Needle-localized biopsy for nonpalpable lesions has been the standard of practice and in our study were found positive for malignancy in 52% of cases (compared with 9%–47% in the literature).13 However, as the use of screening mammography has increased, detection of more indeterminate nonpalpable lesions has led to large numbers of negative needle-localized biopsies. Percutaneous ultrasound-guided CNB and stereotactic biopsy are both now being used extensively as alternatives to needle localization to diagnose mammographically detected breast lesions. These modalities can spare as many as 80% of patients with suspicious image-detected abnormalities from undergoing a surgical procedure.12 Image-guided CNB has been shown to have a sensitivity of 93%–98% and specificity of 95%–100% for nonpalpable lesions.11 Minimally invasive ultrasound-guided or stereotactic CNBs result in less discomfort and decreased procedure time, complications and morbidity, as compared with needle-localized biopsy.4,13 When a diagnosis is obtained with one of these modalities, it usually eliminates one trip to the operating room.15
In our study only 2 stereotactic biopsies were performed on nonpalpable lesions, both of which were classified as “needed additional imaging” on mammography. One of these could not be completed for technical reasons and the other showed invasive cancer, which was confirmed on surgical excision. No other image-guided biopsies were performed on nonpalpable lesions before surgery.
Although ultrasonography of palpable and nonpalpable breast lesions has been shown to be 98%–100% accurate in the diagnosis of simple cysts, it was only accurate 75% of the time in this study, with 25% of what were called “cystic” lesions being malignant.2,11 Ultrasonography was used equally in this study for palpable and nonpalpable lesions and was used with a variety of other diagnostic modalities in all cases.
Surgical management of breast lesions
According to the Clinical Practice Guidelines for the Care and Treatment of Breast Cancer, “whenever an open biopsy is performed on the basis of even modest suspicion of carcinoma, the procedure should be, in effect, a lumpectomy, using wide local excision of the intact tumour surrounded by a cuff of tumour-free tissue.”9 Seventy-six first operations in our study were called lumpectomies; however, it is difficult to say retrospectively whether surgeons were in fact performing “excisional” biopsies instead, considering our high numbers of positive margins for lumpectomies. Generally for nonpalpable lesions, surgeons termed the first operations “needle localization and breast biopsy.” Although 6 intraoperative frozen sections were obtained in this study and all were positive, 3 of these women still only had lumpectomies as first operations.
When excisional biopsy of malignant lesions is performed instead of lumpectomy, a second operation is required to excise the tumour and reassess margins.2,9 In this situation, blood is often present in the biopsy cavity, there can be reactive induration and excision is more difficult, as is pathological evaluation of the re-excised tissue.9 In our study, 60% of the lumpectomies and 35% of the needle-localized biopsies performed at first operation had positive margins, and 2 of the 5 lumpectomies done as single-stage operations with ALND had positive margins as well. Only 91% of the women with positive margins had re-excisions of their tumours. Although not examined in this study, it would be interesting to see whether the rate of recurrences was higher in the women who had positive or close (≤ 1 mm) margins whose tumours were not re-excised.
Compared with the number of positive margins reported in the literature, the rate found here was very high. In a trial conducted by the European Organization for Research and Treatment of Cancer, 14% of women with lumpectomies had positive margins after first excisions.9 Ghossein and colleagues showed that 41% of tumourectomy patients had positive margins after first excisions.8 Cowen and colleagues reported positive margins in 15% of their patients after first tumour excisions.3
In the studies described here, positive margins were considered to be any tumour cells beyond the inked edges of resection.16 There is presently no consensus as to what should be considered a positive margin in the literature.2,9 Guidelines simply state that margins should be free of tumour.2 Some studies use > 1 mm as a negative margin and ≤ 1 mm as a close margin, with positive margins being defined as any tumour cells at the inked edges of the specimen.17 In a study by Schnitt and colleagues,18 61% of women had close (≤ 1 mm) or positive margins after breast-conserving surgery, and in our study the findings were similar at 61% for needle-localized lumpectomies and 75% for lumpectomies. Many studies do report an increased local recurrence when specimen margins are positive, although the outcome for close margins (≤ 1 mm) is less clear.2,16
Also critical in evaluating margins are technical factors such as number of specimen pieces, as well as the orientation of the specimen. It is recommended that when surgical biopsy is performed, the whole lesion be removed in 1 piece with a surrounding cuff of normal tissue.2 It is also recommended that surgical specimens be oriented for pathology using radiolucent clips or sutures.9 In our study, some of the specimens were sent for pathological examination in many pieces, and only 11% of specimens were oriented with sutures.
One of the goals of this study was to look at the reoperation rates for breast cancer in this community hospital. Although there are no reported reoperation rates in the literature, 67% of the women in our study with malignancy underwent more than 1 operative procedure. This rate seems exceedingly high. Reoperations in our study seemed to have been because of positive margins in many cases, excisional biopsies being performed instead of lumpectomies as first operations by some surgeons, and the use of ALND and mastectomies as nondefinitive first operations instead of being second or third operations.
Only 15 of the women in our study had definitive or potentially single-stage procedures as first operations based on a variety of preoperative and intraoperative diagnostic modalities. Five of these women had positive margins after their definitive surgery, and 8 had close (≤ 1 mm) margins. No single diagnostic tool was used consistently by any of the surgeons before performing a definitive procedure at first operation, although FNAB and mammography were performed and had positive findings in many of the patients. Three of these patients had frozen sections obtained intraoperatively. Only 2 of these patients had either a CNB or stereotactic biopsy performed for preoperative diagnosis before their definitive surgeries, which is now current practice in many centres.
Conclusions
The goal of this study was to examine the status of breast cancer diagnosis and surgery in a community hospital and make recommendations for management based on guidelines and current practice.
This study is limited by the fact that patient preferences for treatment could not be documented. Regarding the 51% of lesions that were benign on pathological examination in this study, many of these women may have made the choice to have their breast lesions removed even when they were apparently benign on examination, mammogram or FNAB. In addition, surgeons' choices of type of surgery and diagnostic modalities used preoperatively and intraoperatively are difficult to investigate because of the retrospective nature of this study. Some surgeons also performed very few breast surgeries. Although PPVs were recorded for various BI-RADS categories, as well as for ultrasonography and FNAB results, the numbers of procedures in each category were small and make these results difficult to interpret.
Of the positive findings from this study, it was apparent that a high percentage of lesions that were surgically removed were malignant. Positive findings on both FNAB and mammography were highly predictive of malignancy. Of the needle-localized biopsies done, a high percentage were positive for malignancy.
Some improvements could be made in the management of breast cancer based on this study. Core breast biopsy is now described as commonplace in the literature for palpable lesions and should be used to obtain preoperative diagnosis. Benign findings on mammograms may need to be reviewed, because these lesions often turned out to be malignant (33% of nonpalpable and 21% of palpable lesions). Very few preoperative stereotactic or ultrasound-guided CNBs were done and should be used in more cases of nonpalpable lesions to reduce the numbers of needle-localized surgical biopsies performed.
The number of positive and close margins after first operation and the reoperation rate were high. Wider excisions need to be performed, and surgeons should consider lumpectomies instead of excisional biopsy as first surgeries. With positive preoperative diagnostic results, more definitive surgeries could be done as first operations.
This paper and its results have been presented to the Quality Assurance Committee and Operations Committee at this community hospital, as well as to the hospital radiologists and surgeons. Improving the access to and skill level in performing stereotactic and ultrasound-guided CNBs within this hospital has been proposed and has begun. Some of the general surgeons have begun to use core biopsy instruments for palpable breast lesions within the hospital and office setting. The radiology department has reviewed their reporting of mammographic lesions. We have submitted a proposal for a study of overall breast health in this community to the Canadian Breast Cancer Foundation. We intend to repeat this study in 5 years to determine whether practices have changed in this community and to look at recurrence rates in our study population.
Competing interests: None declared.
Correspondence to: Dr. Catherine Hanley, McMaster University Family Medicine Residency Programme, 28 Southampton Dr., St. George ON N0E 1N0; cathyhanley@rogers.com
References
- 1.Chiarelli AM, Theis B, Holowaty E, et al. Breast Cancer in Ontario 1971–1996, Incidence and Mortality. Surveillance Unit and The Ontario Breast Screening Program Division of Preventive Oncology, Cancer Care Ontario. Toronto: Cancer Care Ontario; 2000.
- 2.The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer. CMAJ 1998;158 (Suppl 3):S1-S40. [PubMed]
- 3.Cowen D, Houvenaeghel G, Bardou V, et al. Local and distant failures after limited surgery with positive margins and radiotherapy for node-negative breast cancer. Int J Radiat Oncol Biol Phys 2000;47(2):305-12. [DOI] [PubMed]
- 4.Klimberg V. Advances in the diagnosis and excision of breast cancer. Am Surg 2003; 69:11-4. [PubMed]
- 5.Crowe JP, Patrick RJ, Rybicki LA, et al. Does ultrasound core breast biopsy predict histologic finding on excisional biopsy? Am J Surg 2003;186:397-9. [DOI] [PubMed]
- 6.Newman LA, Sabel M. Advances in breast cancer detection and management. Med Clin North Am 2003;87:997-1028. [DOI] [PubMed]
- 7.Members of the Breast Cancer Disease Site Group. Surgical management of early stage invasive breast cancer. Practice Guideline Report #1-1 Version 2. Toronto: Cancer Care Ontario; 2003.
- 8.Ghossein NA, Alpert S, Barba J, et al. Importance of adequate surgical excision prior to radiotherapy in the local control of breast cancer in patients treated conservatively. Arch Surg 1992;127:411-5. [DOI] [PubMed]
- 9.Scarth H, Cantin J, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: 3. Mastectomy or lumpectomy? The choice of operation for clinical stages I and II breast cancer (2002 update). CMAJ 2002;167:154-5. [PMC free article] [PubMed]
- 10.Porter GA, McMulkin-Tait H. Practice patterns in breast cancer surgery: Canadian perspective. World J Surg 2004;28: 80-6. [DOI] [PubMed]
- 11.Kerlikowske K, Smith-Bindman R, Ljung B, et al. Evaluation of abnormal mammography results and palpable breast abnormalities. Ann Intern Med 2003;139: 274-84. [DOI] [PubMed]
- 12.Schwartz GF, Feig SA. Nonpalpable breast lesions: biopsy methods and patient management. Obstet Gynecol Clin North Am 2002;29:137-57. [DOI] [PubMed]
- 13.Ball CG, Butchart M, MacFarlane JK. Effect on biopsy technique of the breast imaging reporting and data system (BI-RADS) for nonpalpable mammographic abnormalities. Can J Surg 2002;45:259-63. [PMC free article] [PubMed]
- 14.Orel SG, Kay N, Reynolds C, et al. BI-RADS categorization as a predictor of malignancy. Radiology 1999;211:845-50. [DOI] [PubMed]
- 15.Logan-Young W, Dawson AE, Wilbur DC, et al. The cost effectiveness of fine-needle aspiration cytology and 14-gauge core needle biopsy compared with open surgical biopsy in the diagnosis of breast carcinoma. Cancer 1998;82:1867-73. [DOI] [PubMed]
- 16.Spivack B, Khanna M, Tafra L, et al. Margin status and local recurrence after breast-conserving surgery. Arch Surg 1994;129: 952-7. [DOI] [PubMed]
- 17.Gage I, Schnitt SJ, Nixon AJ, et al. Pathologic margin involvement and the risk of recurrence in patients treated with breast-conserving therapy. Cancer 1996;78:1921-8. [DOI] [PubMed]
- 18.Schnitt SJ, Abner A, Gelman R, et al. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer 1994;74:1746-51. [DOI] [PubMed]