Abstract
Mounting evidence indicates that children who are reared in harsh families are prone to chronic diseases and premature mortality later in life. To shed light on the mechanisms potentially underlying this phenomenon, we evaluated the hypothesis that such families engender a pro-inflammatory phenotype, marked by exaggerated cytokine responses to bacterial stimuli and resistance to the anti-inflammatory properties of cortisol. A sample of 135 adolescent females were assessed four times over 1.5 years, with repeated measurements of psychological stress and inflammatory activity. To the extent that they were reared in harsh families, participants displayed an increasingly pro-inflammatory phenotype over the follow-up. This was marked by increasingly pronounced cytokine responses to in vitro bacterial challenge, and a progressive desensitization of the glucocorticoid receptor, which hampered cortisol’s ability to properly regulate inflammatory responses. If sustained, these tendencies may place children from risky families on a trajectory for the chronic diseases of aging.
It’s long been clear that children who are raised in risky families - marked by conflict, a lack of warmth, inadequate parenting, and household chaos – are prone to a broad array of adverse social and emotional outcomes (Cicchetti & Toth, 2005; Heim & Nemeroff, 2001). But recently, evidence has mounted to suggest that early family life may have even further-reaching consequences, and play a role in shaping people’s susceptibility to chronic diseases later in life (Repetti et al., 2002; Shonkoff et al., 2009). For example, the Adverse Childhood Experiences Study assessed the medical history of 17,000+ adults, and found that rates of cardiovascular disease, autoimmune disorders, and premature mortality were 1.5-2.0-fold higher in those exposed to familial violence, abuse, and neglect as children (Anda et al., 2009; Dong et al., 2004; Dube et al., 2009). These patterns have been substantiated in experimental studies with animals (e.g., Ader & Friedman, 1965; Avitsur et al., 2006; Barreau et al., 2004; Chida et al., 2007), suggesting they are likely to reflect causal effects of early experience on adult health (Cohen et al., 2007).
These findings raise a difficult mechanistic question. How does a harsh family climate “get under the skin” in a manner that is sufficiently persistent to affect vulnerability to diseases that arise many decades later (Miller, Chen, & Cole, 2009)? One hypothesis advanced to answer this question suggests that harsh family lives engender a phenotype characterized by exaggerated behavioral and biological responses to threatening stimuli (Cicchetti & Toth, 2005; Zhang et al., 2006). Biologically, this phenotype is thought to arise partly as a result of stressful early experiences desensitizing the glucocorticoid receptor, which in turn enables greater outflow from the hypothalamic-pituitary-adrenocortical (HPA) axis and the sympathetic nervous system (SNS), and hampers the ability of cortisol to regulate the magnitude of inflammatory responses to infection and injuries (G.E. Miller et al., 2009b). This response pattern is thought to serve adaptive functions in the context of acute threats to well-being, but if activated chronically may exact an allostatic toll on the body that ultimately contributes to chronic diseases of aging (McEwen, 1998; Shonkoff et al., 2009).
Evidence has been accruing to support the basic tenets of this hypothesis. To the extent that they were raised in harsher family climates, people display greater cardiovascular, neurohormonal, and inflammatory responses to stress as adults (e.g., Luecken & Lemery, 2004; Pace et al., 2006; Repetti et al., 2002). Consistent with the notion that this response pattern exacts a physiologic toll on the body, those from risky families also show higher blood pressure, worse metabolic profiles, and greater inflammatory activity (Danese et al., 2007; Lehman et al., 2005; Miller et al., 2009b; Taylor et al., 2006b), all of which would heighten their risk for later disease. While these studies are provocative, two design features complicate interpretation of the findings. First, they relate early family climate to biological processes assessed on a single occasion in adulthood. This makes it difficult to ascertain temporal precedence and, assuming that family climate has causal effects on biology, whether it has long-term consequences that grow with time. Second, those studies that assess responses to stress utilize lab paradigms, which are strong on internal validity, but don’t often correspond well with patterns seen in real-life contexts.
To gain further insights into these issues, this study followed a cohort of young women over 1.5 years, and repeatedly assessed their exposure to real-life stressors. At each visit we also assessed several processes involved with the regulation of inflammation and, using multi-level models, were able to estimate how much early family climate shaped their trajectories over time. We predicted that to the extent that they were reared in a harsh family environment, participants would display evidence of an increasingly pro-inflammatory phenotype over the follow-up. This would be marked by progressive desensitization of the glucocorticoid receptor, which would hamper cortisol’s ability to regulate cytokine responses to bacterial challenge, and thereby facilitate an increasingly pro-inflammatory environment. We also expected this phenotype to manifest in response to psychological stress, such that in participants from harsh families, life events would activate inflammatory processes in an accentuated fashion.
METHOD
Participants
Data were collected as part of a larger project on depression and atherosclerosis in young women at risk for affective disorders. The participants were recruited from the Vancouver, BC community through advertisements. Eligibility criteria included being (1) female, 15-19 years old, and fluent in English, (2) free of acute illness in the past 2 weeks, (3) without a history of chronic medical or psychiatric disorders, and (4) at high risk for developing an initial episode of depression. High-risk was defined as having a first-degree relative with a history of affective disorder, and/or scoring in the top quartile of the population on cognitive vulnerability to depression
This report focuses on 135 participants who completed assessments of early family climate. As Table 1 shows, they had a mean age of 17.00 years at study entry, were mainly of Asian or European descent, and generally came from well-educated families. The participants were re-assessed on 3 occasions, roughly 6, 12, and 18 months after study entry. The larger project was reviewed and approved by the University of British Columbia’s Research Ethics Board. Written consent was obtained from all participants. For those younger than 18, a parent or guardian also provided consent.
Table 1.
Characteristics of the sample (N = 135).
| Characteristic | Mean ± SD or Number (Percent) |
|---|---|
| Age | 17.00 ± 1.38 |
|
| |
| Caucasian | 68 (50.37%) |
|
| |
| East or South Asian | 56 (41.48%) |
|
| |
| Parental education (years) | 14.71 ± 2.97 |
|
| |
| Daily cigarette smoker | 2 (1.50%) |
|
| |
|
| |
| Body mass index (kg/m2) | 21.61 ± 2.57 |
|
| |
| Alcohol use (drinks/week) | 2.11 ± 4.29 |
|
| |
| Strenuous exercise (hours/week) | 2.07 ± 1.96 |
|
| |
| Subjective sleep quality (1-4) | 3.01 ± 0.51 |
|
| |
| Risky Families Questionnaire (1-5) | 1.93 ± 0.46 |
|
| |
| Circulating interleukin-6 (pg/ml) | |
| Visit 1 | 0.68 ± 0.66 |
| Visit 2 | 0.77 ± 0.81 |
| Visit 3 | 0.75 ± 0.92 |
| Visit 4 | 0.74 ± 0.71 |
| Production of interleukin-6 (pg/ml) | |
| Visit 1 | 43,235 ± 15,239 |
| Visit 2 | 48,077 ± 16,678 |
| Visit 3 | 46,174 ± 17,084 |
| Visit 4 | 48,013 ± 16,116 |
|
| |
| Resistance to glucocorticoids (log IC50) | |
| Visit 1 | −6.43 ± 0.33 |
| Visit 2 | −6.45 ± 0.27 |
| Visit 3 | −6.46 ± 0.28 |
| Visit 4 | −6.45 ± 0.27 |
Measures
Early family climate
To assess early familial experiences, we administered the Risky Families Questionnaire (Taylor et al., 2006a). This scale poses 13 questions tapping the harshness of the family climate. Sample items include “How often did a parent or other adult in the household swear at you, insult you, put you down, or act in a way that made you feel threatened?” and “How often would you say that a parent or other adult in the household behaved violently toward a family member or visitor in your home?” When responding, participants were instructed to focus on the time prior to their entry in the study (i.e., from birth to age 14), and respond on a scale ranging from 1 (not at all) to 5 (very often). This instrument is internally consistent, with Cronbach’s α = .78, and responses are stable over time, with 6-month retest correlations exceeding .90 (G.E. Miller, 2009, unpublished data).
Inflammatory parameters
Peripheral blood was drawn at each visit to assess three aspects of inflammation. First, the extent of systemic inflammatory activity was quantified via serum levels of interleukin-6 (IL-6). This molecule plays a key role in orchestrating chronic inflammatory responses, and is often used to index ongoing immune activation (Miller, Maletic, & Raison, 2009). IL-6 levels were quantified in duplicate with commercially available high-sensitivity ELISA kits (#HS600B, R&D Systems; Minneapolis, MN), which have a minimum detection threshold of 0.039 pg/ml and inter- and intra-assay variability below 10%.
Second, to assess the capacity of participants’ white blood cells to respond to microbial challenge, we cultured them with a bacterial stimulus, lipopolysaccharide (LPS). When immune cells encounter LPS, they secrete proteins such as IL-6, which help clear infections and heal injuries. While this response is critical for survival, its magnitude and duration must be carefully regulated, because excessive inflammation contributes to chronic diseases. This assay quantifies how aggressively participants white blood cells respond to a fixed dose of LPS. Whole blood was drawn into lithium-heparin Vacutainers (Becton-Dickinson, Oakville, ON), diluted 10:1 with saline, and then incubated with LPS (50 ng/ml; Sigma; Saint Louis, MO) for 6 hours at 37°C in 5% CO2. The supernatants were collected and frozen at −80°C until analysis. IL-6 was assayed in duplicate by ELISA with kits that have a minimum detectable threshold of 0.7 pg/ml and inter- and intra-assay variability below 5% (DuoSet ELISA Development Systems; R&D Systems).
Finally, to measure sensitivity to signals that regulate inflammation, we quantified IL-6 production in cells that had been co-incubated with LPS and cortisol. Cortisol conveys anti-inflammatory messages to immune cells, and this assay measures their ability to dampen IL-6 production when signaled to do so. Blood was diluted 10:1 with saline and dispensed into 6-well culture plates with LPS (50 ng/ml), along with one of five doses of hydrocortisone (final concentrations: 0, 2.76*10−5, 2.76*10−6, 2.76*10−7, 2.76*10−8 M HC; Sigma Chemicals; St. Louis, MO, USA). After six hours of incubation at 37°C in 5% CO2, the supernatants were collected and frozen until analysis. IL-6 levels were quantified in duplicate using the same ELISA kits (DuoSet ELISA Development Systems; R&D Systems). Dose-response curves were later generated for each subject’s data. From them, we calculated the concentration of hydrocortisone needed to diminish IL-6 production by 50%. This value is called the log inhibitory coefficient-50 (log IC50), and is inversely proportional to glucocorticoid sensitivity, meaning that higher values indicate that immune cells are less sensitive to cortisol’s anti-inflammatory signals.
Episodic stressors
At each visit we administered the UCLA Life Stress Interview – Adolescent Version (Adrian & Hammen, 1991). This semi-structured interview covers acute and chronic forms of stress over the past six months. Interviewers ask a series of open-ended questions about family, friends, school, and health, and gather details about episodic stressors, which are defined as specific events with a discrete onset and offset. To judge the objective impact of episodic stressors, our team made a consensus rating, after it had been briefed on event details by the primary interviewer. Impact ratings ranged from 1 (no long-term impact) to 5 (severe long-term impact). Importantly, consensus ratings explicitly considered the context in which an event had occurred. For example, if a subject’s grandfather had a heart attack, the impact rating would depend on factors such as how close their relationship was, whether she visited him in the hospital, and whether she had previous experience coping with serious family illnesses. Following convention, we consider episodic stressors rated ≥ 2.5 (moderate long-term impact) to be major events (Miller & Chen, 2006).
Alternative explanations
We assessed several other variables that might provide alternative explanations for any associations. They included demographic (age, ethnicity, socioeconomic status) and biobehavioral (smoking, total adiposity, physical activity, alcohol use, sleep quality) factors known to covary with or directly modulate inflammatory processes (O’Connor et al., in press), as well as symptoms of depression. Socioeconomic status (SES) was indexed by the highest educational degree achieved by the subject’s mother or father. Smokers were classified as persons consuming > 10 cigarettes daily, and adiposity was measured as body mass index (BMI; kg/m2), Physical activity was measured as minutes each week engaged in “regular activity akin to brisk walking, jogging, bicycling, etc, long enough to work up a sweat” (Paffenbarger et al., 1993). Alcohol use was quantified as drinks per week, and subjective sleep quality was rated on a 1-4 scale, using a well-validated instrument (Buysse et al., 1989). In preliminary analyses we established that all of these factors, except body mass, were stable over the follow-up period. Hence, we averaged values across visits and used this aggregate as a covariate. Because it increased significantly over the study (F = 4.85, p = .003), body mass was modeled as a time-varying covariate. Finally, depressive symptoms were assessed at each visit using the Beck Depression Inventory (Beck et al., 1961), and used to examine whether low mood was inflating the observed associations.
RESULTS
Statistical Approach
To determine whether early-life family climate presaged changes in inflammatory processes, we estimated a series of growth-curve models with HLM 6.03 (Raudenbush et al., 2006). In the within-person (or level-1) model we estimated outcomes as a function of months from study entry, BMI, and a residual term. These models yielded a series of person-specific intercepts reflecting outcomes at study entry (β0i) and person-specific trajectories reflecting the rate of change over the 1.5-year follow-up (β1i). In the between-person (or level-2) models, we estimated β0i and β1i values for each subject as a function of age, ethnicity, SES, physical activity, alcohol use, sleep quality, and early-life family climate. (Smoking was not included as a covariate because only 2 participants engaged in it regularly.) The models also included random variables specifying the amounts by which each subject deviated from the sample’s average β0i and β1i
Preliminary Analyses
We began by estimating simple level-1 models to describe patterns of change. During the follow-up, the sample as a whole displayed a significant increase in stimulated IL-6 production (β10 = 215, SE = 76, p = .006), but the other two outcomes, sensitivity to cortisol inhibition and the extent of systemic inflammation, remained stable over time (p’s = .66 and .24, respectively). However, analyses revealed significant variability around these sample-wide estimates (p’s < .005), suggesting that participants differed reliably in their trajectories over time. In the next set of models, we added BMI to level-1 equations. It covaried with systemic inflammation over time such that, as a woman’s BMI increased, so did her level of IL-6 in circulation (β10 = 0.06, SE = 0.02, p = .01). BMI was not associated with stimulated production of IL-6 or sensitivity to cortisol inhibition (p’s < .49).
Early Family Climate
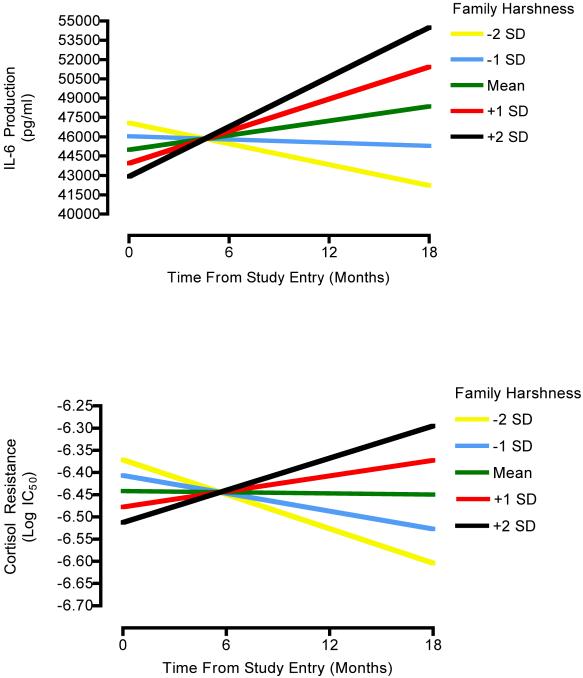
Based on these findings we estimated another series of models, exploring whether early-life family climate predicted variability in inflammatory trajectories, above and beyond the effects of potential confounds (i.e., BMI at level-1 and age, ethnicity, SES, physical activity, alcohol use, and sleep quality at level-2). The results of these analyses are displayed in Table 2. As can be seen, the confounds were not consistently associated with inflammatory parameters at study entry (upper half of table), or with the rate at which they changed over follow-up (lower half of table). However, early-life family climate was a significant predictor of IL-6 production (p = .01) and cortisol sensitivity (p = .04) trajectories. To the extent that they were reared in a harsh family environment, participants displayed increasing stimulated IL-6 production over the follow-up, and became less sensitive to cortisol’s anti-inflammatory properties (see Figure 1). In terms of effect size, family climate accounted for 24.70% of the between-participants variance in trajectories of IL-6 production, net of the contribution of demographic and biobehavioral factors. The parallel figure for cortisol sensitivity trajectories was 7.48%. Family climate was not associated with trajectories of systemic inflammation as reflected in circulating IL-6 (p = .75), or with any of the outcomes at the time of study entry (p’s > .13).
Table 2.
Level-2 predictors of inflammatory parameters at study entry and over time.
| IL-6 Production |
Cortisol Sensitivity |
IL-6 Circulating |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | Coefficient | SE | p | Coefficient | SE | p | Coefficient | SE | p |
| Baseline: | |||||||||
| Constant | 42.99 | 17.8 | .01 | −645 | 5.00 | .01 | 0.62 | 0.09 | .01 |
| Age | −1.33 | 1.06 | .21 | 0.09 | 3.43 | .97 | 0.00 | 0.10 | .98 |
| Race | 4.31 | 3.04 | .16 | 2.01 | 8.53 | .81 | 0.25 | 0.15 | .10 |
| SES | 0.41 | 1.24 | .74 | 0.42 | 2.29 | .86 | −0.01 | 0.06 | .99 |
| Exercise | 0.68 | 0.63 | .28 | 0.65 | 1.23 | .60 | −0.08 | 0.06 | .13 |
| Alcohol Use | −0.02 | 0.26 | .94 | 0.76 | 0.56 | .18 | 0.29 | 0.25 | .26 |
| Sleep Quality | 1.37 | 2.68 | .61 | 1.66 | 6.55 | .80 | 0.11 | 0.08 | .17 |
| Harsh Family | −1.04 | 1.22 | .39 | −3.65 | 3.25 | .27 | −0.08 | 0.05 | .13 |
| Trajectory: | |||||||||
| Constant | 0.26 | 0.98 | .01 | 0.01 | 0.37 | .36 | 0.01 | 0.01 | .29 |
| Age | 0.02 | 0.05 | .79 | 0.04 | 0.03 | .20 | 0.01 | 0.01 | .45 |
| Race | −0.10 | 0.15 | .35 | −0.04 | 0.06 | .56 | −0.00 | 0.02 | .94 |
| SES | −0.12 | 0.07 | .11 | −0.05 | 0.03 | .09 | −0.01 | 0.01 | .74 |
| Exercise | −0.03 | 0.03 | .34 | 0.01 | 0.01 | .34 | 0.00 | 0.01 | .97 |
| Alcohol Use | −0.01 | 0.02 | .71 | 0.00 | 0.00 | .99 | −0.02 | 0.02 | .31 |
| Sleep Quality | −0.35 | 0.17 | .05 | −0.44 | 0.53 | .41 | 0.01 | 0.01 | .39 |
| Harsh Family | 0.23 | 0.09 | .01 | 0.60 | 0.29 | .04 | 0.00 | 0.01 | .75 |
Note. In level-1 models, the outcomes were predicted from time, coded in months from study entry, and body mass index. Race is coded as 1 = white and 0 = other. All other variables are grand-mean centered.
Figure 1.
Early-life family climate and inflammatory trajectory in adolescence. To the extent that they were reared in a harsh family climate, participants had increasing stimulated IL-6 production over the follow-up (upper panel), and became less sensitive to cortisol’s anti-inflammatory properties (lower panel).
We considered 2 alternative explanations for these findings. The first was that low mood was inflating the observed associations, by biasing participants’ recall of their early family life, and at the same time activating pro-inflammatory circuits (Miller & Blackwell, 2006; A.H. Miller et al., 2009). To evaluate this possibility we conducted another wave of analyses, in which symptoms of depression were added to level-1 models as a time-varying covariate. Early-life family climate continued to be a significant predictor of IL-6 production trajectories (p = .02) and a marginal predictor of cortisol sensitivity trajectories (p = .02) and a marginal predictor of cortisol sensitivity trajectories (p = .08) in these circumstances, suggesting that low mood played little role in these associations. The second alternative explanation had to with the timing of assessment. Because the Risky Families Questionnaire was added to the project after subject accrual had begun, there was some between-person variability in the timing of its administration. However, the timing of administration was unrelated to actual scores on the instrument (r = .09, p = .33), and was not associated with trajectories of any of the inflammatory parameters (p’s > .39). There was also no evidence of interactions between scores on the instrument and timing of administration for any of the inflammatory trajectories (p’s > .27).
Responsivity to Stress
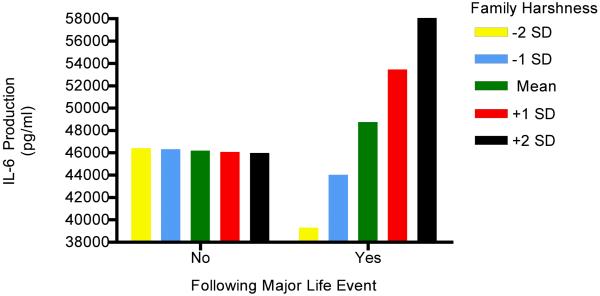
The final models explored whether being reared in a harsh family climate accentuated participants’ inflammatory responses to life stress. This hypothesis was evaluated in another series of growth-curve models, identical in structure to those above, except that a binary, person-centered, variable was added to level-1 reflecting exposure to a major event over the past 6 months. The analyses revealed a significant interaction between harsh families and life stress in predicting IL-6 production (γ21 = 4.68, SE = 1.21, p = .001). As Figure 2 illustrates, participants who were raised in harsher family climates had greater stimulated production of IL-6 at times when they had recently been exposed to a major life event, relative to times when they had not. By contrast, participants raised in non-harsh climates showed little change in IL-6 production (and perhaps even a small decline) after being exposed to a life event. These effects were above and beyond the contribution of demographic and biobehavioral confounds. They also were not a function of participants from harsh family climates simply having more frequent or impactful events, as scores on the Risky Families Questionnaire were only weakly related to the number and severity of episodic stressors over the study (r’s = .14, p’s = .10). Because participants from harsh climates will by definition experience more conflict, violence, etc. in their families, we also reran these analyses excluding any events that centered around family difficulties. The interaction for IL-6 production persisted under these conditions, (γ21 = 3.70, SE = 1.26, p = .004), suggesting that it reflected a broader tendency towards enhanced reactivity among participants from harsh family climates. There no were significant interactions for the other inflammatory outcomes (p’s > .47).
Figure 2.
Participants who were raised in harsher family climates had greater stimulated production of IL-6 at times when they had been exposed to a major life event, relative to times when they had not. By contrast, participants raised in warmer family climates showed little change in IL-6 production (and perhaps even a small decline) after being exposed to a life event.
DISCUSSION
Mounting evidence indicates that children who are raised in risky families environments are vulnerable to chronic disease when they reach the later decades of life (Repetti et al., 2002; Shonkoff et al., 2009). While the mechanisms underlying this phenomenon are not well understood, one attractive hypothesis is that harsh family climates engender a pro-inflammatory phenotype, which over time takes an allostatic toll on the body (Cicchetti & Toth, 2005; Miller et al., 2009b; Zhang et al., 2006). Our data provide support for this notion by showing that over a 1.5-year period, young women who were raised in risky families display increased IL-6 responses to 2 different types of threatening stimuli, an in vitro challenge with bacterial products and a real-life psychological stressor. Over this period they also show progressive desensitization of the glucocorticoid receptor, such that their immune cells exhibit increasing resistance to anti-inflammatory signals from cortisol.
These findings converge with other studies that have observed greater inflammatory activity in adults who were reared in unfavorable circumstances (Danese et al., 2007; Miller et al., 2009b; Miller & Chen, 2007; Taylor et al., 2006b). They also extend previous research by showing that the magnitude of this disparity grows with time, such that youth from harsh families are set upon a trajectory of exaggerated pro-inflammatory responding and partial resistance to glucocorticoid signaling. It is not clear why the disparities were not apparent at study entry, but gradually accumulated as the follow-up progressed. Because the women in our sample were aged 15-18 at study entry, menarche-induced changes in immune functions are unlikely to be responsible. However, puberty sets into motion hormonal cascades that continue to promote maturation throughout adolescence (Dahl, 2004), and it is conceivable that they underlie the findings observed herein. Identifying these cascades and how they modulate immunity needs to be a focus of future research.
This work also highlights glucocorticoid receptor desensitization as one mechanism potentially underlying the pro-inflammatory phenotype. Cortisol is a powerful regulator of the transcriptional-control pathways that orchestrate immune responses to infection and injuries (Webster et al., 2002). To the extent that familial harshness impairs the immune system’s capacity to transduce cortisol-mediated signals, e.g., through glucocorticoid receptor desensitization, it would be expected to mount larger inflammatory responses to challenge and be slower to terminate them. In the short term, this pattern might benefit health by protecting against infectious threats. However, over the long term it would foster a low-grade inflammatory state (Miller et al., 2002; Raison & Miller, 2003) that contributes to aging-related conditions like the metabolic syndrome, autoimmune disorders, and cardiovascular disease (Nathan, 2002).
The study also extends previous research by showing that even normative variations in the early family climate can shape the evolution of response tendencies in the immune system. We assessed harshness on a 5-point continuum. For each one-half point increment in harshness, there was a 10 percent increase in stimulated IL-6 production over time, and a 4 percent decline in sensitivity to cortisol. These patterns suggest that there is a good deal of plasticity in the response tendencies of monocytes (the cells that engage LPS and secrete IL-6) and that even mild exposure to a risky family in early life can shift the developmental trajectory towards a pro-inflammatory phenotype.
Though a harsh family climate presaged changes in stimulated cytokine production and sensitivity to cortisol, there was no evidence that it associated with the degree of ongoing inflammatory activity, as marked by serum IL-6. These findings suggest that harshness affects leukocytes’ response to microbial challenges, but not their production of cytokines under quiescent conditions. This discrepancy may reflect the fact that in young people, the immune system’s response to inhibitory molecules like cortisol is relatively intact, which prevents it from the kind of overshooting that fosters ongoing inflammation. However, our data suggest that as they age, women from harsh families will show larger cytokine responses to challenge, and become progressively more resistant to cortisol-mediated inhibition. Over time such tendencies could tip the balance in favor of the kinds of ongoing inflammatory activity seen in adults who faced early-life maltreatment (Danese et al., 2007; Taylor et al., 2006b).
Several limitations of this study need to be considered. First, it used retrospective self-reports of early family climate, whose veridicality was not ascertained through collateral sources. Thus, it is possible that our measure captured something different than what it was intended to, like participants’ reconstructed memories of their early family lives, or descriptions of them that had been enhanced to be more socially desirable. However, even if it did, this work would still be valuable, because reports like this have been linked to excess morbidity and mortality (Anda et al., 2009; Dong et al., 2004; Dube et al., 2009). Thus, the phenomena that they are capturing, whether accurate reflections of early life or reconstructed versions of it, seem to be important for long-term health. Second, the observational design makes it impossible to derive causal inferences about the effects of early family climate. Although we used covariance analyses to eliminate the most plausible alternative explanations, other (unassessed) factors may have contributed to the observed associations. Third, our findings come from young women at risk for affective disorders, who are more likely than the general population to be reared in a harsh family climate, and are probably more sensitive to its effects. Thus, further research is needed to determine how generalizable the findings are to the broader population. Fourth, the study focused on a narrow window of time in the life course, 1.5 years of adolescence, and it is unclear how representative the trajectories we observed are of other periods. Collectively, these limitations suggest that our results should be considered preliminary until they have been substantiated in studies with representative samples, long-term prospective designs, and more thorough assessment of potential confounders.
Despite these limitations, the study provides insights into mechanisms through which early family climate might come to shape later risk for chronic disease. In future research it will be important to further characterize the elements of the phenotype we observed, and ascertain how they might together influence habitual patterns of responding to threatening stimuli. Particularly relevant to this endeavor are data showing that children from riskier families are vigilant for cues that connote anger and threat (Chen et al., 2004; Pollak, 2008), and seem to remain so into adulthood, perhaps as a result of experience-dependent remodeling of the amygdala and/or prefrontal circuitry that regulates it (Gianaros et al., 2008; Taylor et al., 2006a). Research that explicates how these tendencies modulate the function of other bodily systems, e.g., metabolic, cardiovascular, immune, and what implications that has for disease, would be especially valuable. With data like these in hand, we should soon be able to construct multi-level models that depict how the social context a child is reared in “gets under” the skin to shape long-term health.
Acknowledgements
This research was supported by grants from the Canadian Institutes of Health Research (67191) and the National Institute of Child Health and Human Development (058502).
REFERENCES
- Ader R, Friedman SB. Social factors affecting emotionality and resistance to disease in animals. V: Early separation from the mother and response to a transplanted tumor in the rat. Psychosomatic Medicine. 1965;27:119–122. doi: 10.1097/00006842-196503000-00004. [DOI] [PubMed] [Google Scholar]
- Adrian C, Hammen C. Stress exposure and stress generation in children of depressed mothers. Journal of Consulting and Clinical Psychology. 1991;61:354–359. doi: 10.1037//0022-006x.61.2.354. [DOI] [PubMed] [Google Scholar]
- Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, Valerie EJ, Dube SR. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471-2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain, Behavior, and Immunity. 2006;20(4):339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;5:462–467. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Development. 2004;75(4):1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. American Journal of Respiratory and Critical Care Medicine. 2007;175(4):316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. Child maltreatment. Annual Review of Clinical Psychology. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts DL, Miller GE. Psychological stress and disease. Journal of the American Medical Association. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relation of childhood socioeconomic status and family environment to adult metabolic functioning in the CARDIA study. Psychosomatic Medicine. 2005;67:846–854. doi: 10.1097/01.psy.0000188443.48405.eb. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Lemery KS. Early caregiving and physiological stress responses. Clinical Psychology Review. 2004;24:171–191. doi: 10.1016/j.cpr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Blackwell E. Turning up the heat: Inflammation as a mechanism linking chronic stress, depression, and heart disease. Current Directions in Psychological Science. 2006;15:269–272. [Google Scholar]
- Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta(2)-adrenergic receptor in children with asthma. Proceedings of the National Academy of Sciences. 2006;103:5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosomatic Medicine. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Cole SW. Health psychology: developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009a;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok A, Walker H, Lim A, Nicholls EP, Cole SW, Kobor MS. Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009b;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of proinflammatory cytokines: A glucocorticoid resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity. doi: 10.1016/j.bbi.2009.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Blair SN, Lee I, Hyde RT. Measurement of physical activity to assess health effects in a free-living population. Medicine and Science in Sports and Exercise. 1993;25:60–70. doi: 10.1249/00005768-199301000-00010. [DOI] [PubMed] [Google Scholar]
- Pollak SD. Mechanisms linking early experience and the emergence of emotions: Illustrations from the study of maltreated children. Current Directions in Psychological Science. 2008;17:370–375. doi: 10.1111/j.1467-8721.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much:The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon RT. HLM Version 6.03. Scientific Software International; Chicago, IL: 2006. [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. Journal of the American Medical Association. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006a;60:296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biological Psychiatry. 2006b;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annual Review of Immunology. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Bagot R, Parent C, Nesbitt C, Bredy TW, Caldji C, Fish E, Anisman H, Szyf M, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. Biological Psychology. 2006;73:72–89. doi: 10.1016/j.biopsycho.2006.01.009. [DOI] [PubMed] [Google Scholar]