Abstract
The conditioning regimen administered prior to allogeneic transplantation for acute myeloid leukemia (AML) must be sufficiently immunosuppressive to ensure engraftment and contributes to the antileukemic impact of the procedure. A broad spectrum of regimens have been studied, varying in their intensity, whether high-dose or reduced intensity, and in the agents used, containing total body irradiation (TBI) plus cyclophosphamide, fludarabine, busulfan, and/or antithymocyte globulin. Over the past two decades, research has influenced the way conditioning regimens are applied. Newer research shows that targeted radiotherapy using an anti-CD45 antibody should be able to reduce toxicity, improve tumor cell kill, and thereby improve results.
Keywords: transplant, acute myeloid leukemia, 131I-anti-CD45 antibody, fludarabine, busulfan, cyclophosphamide, antithymocyte globulin, total body irradiation
Introduction
From the earliest days of allogeneic hematopoietic cell transplantation (HCT), researchers have struggled to develop and define the optimal conditioning regimen for patients with acute myeloid leukemia (AML). In 1982, in the first published randomized trial of conditioning regimens, Thomas et al reported that a regimen of cyclophosphamide (CY) plus fractionated total body irradiation (TBI) (6 fractions of 2 Gy) was superior to CY plus single dose TBI (10 Gy) for patients with AML in first remission.1 After more than 25 years of subsequent research, it is unclear that a better regimen has been developed. But the trials that have been done over that interval have deepened our understanding and point the way to methods that should improve treatment outcome. The following brief review will summarize some of the key trials, discuss how they have influenced our thinking, and present our current approach to the optimization of the conditioning regimen for AML.
Early randomized trials of high-dose conditioning regimens
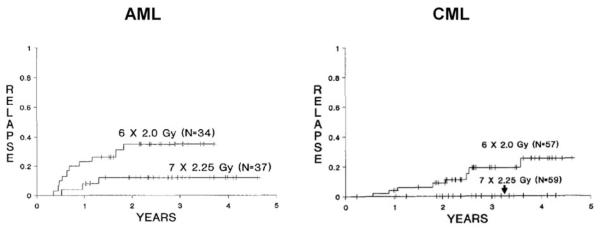
Three randomized trials comparing conditioning regimens for AML in first remission were published between 1988 and 1992. In one study, CY plus TBI was compared to melphalan plus TBI with no obvious difference in outcomes being detected.2 In a second trial, CY plus 12 Gy TBI (6 fractions of 2 Gy) was compared to CY plus 15.75 Gy TBI (7 fractions of 2.25 Gy).3 Relapse following transplantation was significantly reduced with the higher dose of TBI; however, the nonrelapse mortality was increased with the higher dose of TBI, and consequently, survival in the two arms was equivalent. Nonetheless, this study is of interest in that it remains perhaps the clearest demonstration that the dose of irradiation delivered to AML is of clinical relevance. This conclusion was bolstered by the results of a similar trial conducted in chronic myeloid leukemia that had a similar outcome (See Figure 1).4 A third trial compared CY and TBI (CYTBI) vs the commonly used regimen of busulfan and cyclophosphamide (BUCY).5 In this study, survival was superior with the CYTBI regimen. Following that publication, several other randomized trials were presented over the next few years questioning the superiority of CYTBI over BUCY.6-8 These subsequent studies either included chronic myeloid leukemia patients,6,7 or were of very small size8 and thus didn’t directly refute the original findings of superiority of CYTBI. However, in the initial comparison of CYTBI to BUCY, BU levels were not pharmacologically monitored and doses were not adjusted. Given data that, by targeting a specific plasma concentration of busulfan, toxicities can be avoided and relapse reduced, the lack of pharmacologic adjustment of busulfan might have accounted for some of the failures in that arm of the study. A subsequent nonrandomized registry analysis included 381 patients with AML in first remission treated with either CYTBI or BUCY and found a lower incidence of relapse with CYTBI (particularly extramedullary and central nervous system relapse) but no significant differences in treatment-related mortality, leukemia-free survival, or overall survival.9 Thus, in 2002, one could conclude that there was a dose response of AML to irradiation, other drugs could be substituted for cyclophosphamide, and that, although no regimen was clearly superior to CYTBI, almost equivalent results could be achieved with BUCY if one paid attention to the pharmacology of BU.
Figure 1.
Relapse rates in two prospective randomized trials of allogeneic transplantation from matched siblings following a preparative regimen of cyclophosphamide plus 12 Gy or 15.75 Gy of TBI in AML (left panel) or CML (right panel).3,4
Permission requested by BW on 10/1/09
Subsequent studies of high-dose conditioning regimens
In 2002-2003, several papers were published in which fludarabine (FLU) was substituted for CY.10,11 The studies clearly demonstrated that FLUBU combinations were sufficiently immunosuppressive to facilitate engraftment from HLA-matched siblings and unrelated donors, and that acute toxicities were probably reduced compared to what would be expected with BUCY. In some studies, antithymocyte globulin (ATG) was added to the conditioning regimen, while in others it was omitted.12 The general experience was that the addition of ATG did not impede engraftment and led to a somewhat reduced incidence of acute and chronic graft vs host disease (GVHD). Unfortunately, none of these studies were restricted to AML and none had randomized controls, deficiencies which continue to the present day. A retrospective matched controlled analysis comparing the experience in a variety of hematologic malignancies with FLUBU-ATG at one center with the Center for International Blood and Marrow Transplant Research data using BUCY found a lower risk of acute and chronic GVHD and transplant-related mortality with the FLUBU-ATG regimen but a higher risk of relapse and no significant difference in disease-free or overall survival.13 Currently, there is no clear consensus about the superiority of any single ablative transplant regimen for AML in first remission. In a recent study, we examined the outcomes of three consecutive phase II studies of BUCY-ATG, FLU120BU-ATG (fludarabine at 120 mg), and FLU200BU-ATG (fludarabine at 200 mg) in patients with AML.14 The best overall survival was found in the FLU120BU-ATG, but the limited size and nonrandomized nature of the analysis dictates caution in making any conclusions.
Reduced-intensity conditioning regimens
Initial transplants for AML were all conducted using high-dose conditioning regimens because of the need to administer doses of therapy that were sufficiently immunosuppressive to assure allogeneic engraftment. Two major developments allowed for the exploration of reduced-intensity conditioning. One was the realization that there were agents, such as fludarabine and ATG, that were sufficiently immunosuppressive that, if added to reduced doses of busulfan or similar agents, resulted in engraftment.15 The second observation, and one that continues to be somewhat counterintuitive, is that, by increasing the amount of immunosuppression given post transplant, one can assure engraftment with far reduced amounts of pretransplant immunosuppression.16 The initial trials of reduced-intensity conditioning were conducted largely to allow transplantation to be conducted in older, less fit patients. Two additional consequences of these studies were that they allowed for an estimation of the extent of the graft-vs-leukemia effect apart from the impact of the high-dose preparative regimen, and, as will be explained in greater detail, these studies have provided a foundation upon which higher dose, very specific antileukemia therapy can be added, thereby paving the way for the development of an improved conditioning regimen.
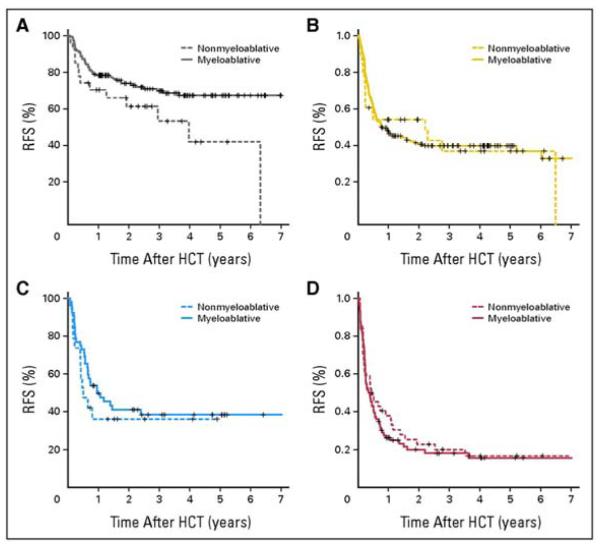
A considerable number of phase II trials of reduced-intensity conditioning regimens have been published and are reviewed by Sandmaier et al.17 The intensity of these regimens varies between those that contain substantial doses of busulfan and likely would lead to extremely prolonged if not lethal myelosuppression if stem cells were not transplanted, to very mild regimens, such as the fludarabine-2 Gy TBI regimens used in Seattle, which result in clearly reversible myelosuppression if cells are not transplanted. A comparison of results obtained with the Seattle reduced-intensity approach compared with outcomes seen with our high-dose regimens for patients with AML is revealing. This analysis included 169 patients treated with reduced-intensity conditioning (RIC) vs 534 treated with high-dose conditioning (HDC). Donors included matched siblings in 29%, matched unrelated donors in 57% and mismatched unrelated donors in 15%, and the distribution was similar for RIC and HDC patients. For patients with AML in first remission, the 5-year survival was 45% with RIC vs 62% with HDC, and for patients in second remission, the figures were 30% vs 47%, respectively. Of course, patients were not randomly assigned to RIC vs HDC, and thus there were significant differences between the groups. A further analysis looked at the impact of RIC vs HDC according to patient comorbidities and disease risk.18 In patients with low comorbidities and low disease risk, there was no difference in nonrelapse mortality with the two approaches, but there was less relapse and improved survival with HDC (see Figure 2). In none of the other three combinations (low comorbidities, high-risk disease; high comorbities, low-risk disease; and high comorbidities, high-risk disease) did we find a difference in relapse-free or overall survival. In all four situations, HDC conditioning was associated with a reduced risk of relapse, but this was generally balanced by increased nonrelapse mortality in patients with higher-risk disease or greater comorbidities (see Table 1). Several conclusions can be drawn from these data: 1) the intensity of the conditioning regimen definitely impacts the risk of disease recurrence; 2) there is a substantial antileukemia effect of allogeneic transplantation even with very reduced conditioning; 3) the best choice for conditioning of any given patient, using current widely available regimens, depends on the status of the patient’s disease as well as their underlying health.
Figure 2.
Kaplan-Meier curves of relapse-free survival comparing nonmyeloablative and myeloablative transplant results among patients with low comorbidities and lower-risk disease (A), low comorbidities and higher-risk disease (B), high comorbidities and lower-risk disease (C), and high comorbidities and higher-risk disease (D).18
Sorror, ML et al: J Clin Oncol 25(27), 2007: 4246-4254). Reprinted with permission. © 2008 American Society of Clincal Oncology. All rights reserved.
Table 1.
Two-year nonrelapse mortality, relapse, and relapse-free survival with reduced-intensity conditioning based on comorbidity index and disease risk.
| NRM (%) | Relapse (%) | RFS (%) | ||
|---|---|---|---|---|
| Group 1 (low CI, low DR) | HDC | 11 | 14 | 75 |
| RIC | 4 | 33 | 63 | |
| Group 2 (low CI, high DR) | HDC | 24 | 34 | 43 |
| RIC | 3 | 42 | 56 | |
| Group 3 (high CI, low DR) | HDC | 32 | 27 | 41 |
| RIC | 27 | 37 | 36 | |
| Group 4 (high CI, high DR) | HDC | 46 | 34 | 20 |
| RIC | 29 | 49 | 23 |
Abbreviations: CI, comorbidity index; DR, disease-risk; HDC, high-dose conditioning; NRM, nonrelapse mortality; RIC, reduced-intensity conditioning; RFS, relapse-free survival
Radioimmunotherapy (RIT) to improve transplant conditioning
At least in theory, a possible way to increase the dose of irradiation administered to AML cells without substantially increasing systemic toxicity would be to target locally acting radioisotopes to hematopoietic tissues using monoclonal antibodies. Our group has explored the use of a 131-I-labelled anti-CD45 antibody (131-I-BC8). In prior studies, we found that 131-I-BC8 can deliver between 2- and 3-fold more irradiation to the bone marrow, spleen, lymph nodes, and other sites of leukemia than to any normal organ.19 We further found that using 131-I-BC8, a standard high-dose BUCY regimen plus at least 10 Gy of supplemental irradiation to the marrow could be administered to patients less than age 55 without undue toxicity, and that survival following such a regimen was superior to historical experience using standard BUCY alone.20
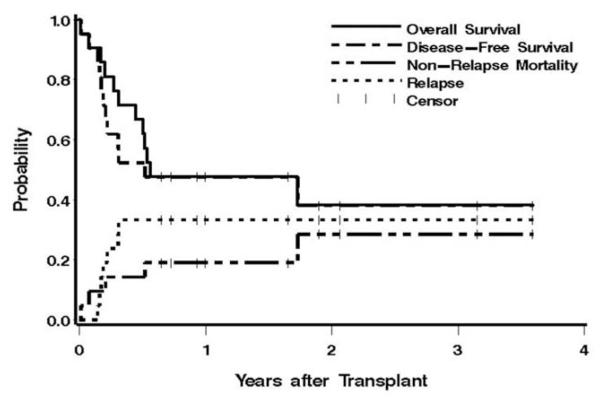
We next hypothesized that higher doses of 131-I-BC8 might be tolerable if combined with RIC. We thus performed a phase I dose escalation study combining increasing doses of 131-I-BC8 with FLU and 2 Gy TBI followed by allogeneic transplantation in patients with advanced AML or myelodysplasia.21 Fifty-eight patients were studied, all of whom were over age 50. Twenty-two received transplants from matched related donors, and 36 from matched unrelated donors. Eighty-six percent had active AML or MDS with > 5% blasts at the time of transplant. Our prior experience using only FLU plus 2 Gy TBI in patients with active, relapsed leukemia was that regrowth of leukemia, often within the first month or two of transplant, was so common that we abandoned the approach. Thus, patients entered onto this dose escalation trial were not candidates for our traditional reduced-intensity approach and generally were either too old or too unfit to tolerate HDC. The dose escalation of supplemental irradiation was determined by the amount of radiation delivered to the liver, starting at 12 Gy and increasing by 2 Gy in cohorts of 3 patients. A total of 21 patients were then treated at the maximum tolerated dose (MTD) in an effort to gain greater insight into the clinical activity of the regimen. At the starting dose of 131-I-BC8 delivering 12 Gy to the liver, dosimetry estimates were that slightly more than twice that dose was delivered to the marrow, or 25 Gy. The MTD of 131-I-BC8 was estimated to be 24 Gy administered to the liver; above that dose, we began to see pulmonary toxicities suggestive of radiation-induced lung injury with acute respiratory distress. At the MTD, 131-I-BC8 delivered an average of 32 Gy of supplemental irradiation to the marrow. Among the 58 treated patients, there were no deaths within the first 30 days after transplant, all patients engrafted, and all achieved a complete remission. The estimated 1-year overall survival was 41% (see Figure 3). Disease recurred in 25 patients (43%), and 7 died of nonrelapse causes (12%). The low early mortality rate combined with the 1-year survival rate achieved in older patients with relapsed or refractory AML were sufficiently encouraging that this study is being followed by a phase II trial in older patients with AML in first remission.
Figure 3.
Estimates of the probability of overall survival, disease-free survival, transplant-related mortality, and relapse among patients treated at the maximum tolerated dose of 24 Gy of radiation delivered to the liver by 131-I-BC8, followed by FLU and 2 GY TBI.21
Permission requested by BW on 10/1/09
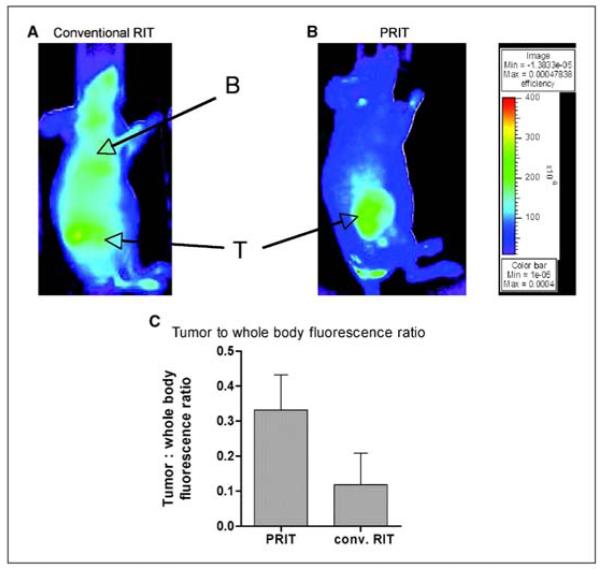
The specificity of irradiation delivered to marrow vs normal organs using directly labeled antibodies is limited and can likely be improved. One factor limiting the specificity of directly labeled antibodies is the slow antibody distribution phase immediately following antibody infusion. During this period, the antibody-isotope conjugate circulates in the blood delivering irradiation nonspecifically, and it is only after the antibody finally binds to the target organ that a degree of specificity is achieved. Pretargeted radioimmunotherapy (PRIT) is an approach designed to improve the specificity of radioimmunotherapy by first administering an antibody-streptavidin (SA) conjugate and allowing the antibody to accumulate at the target site. Only after the slow antibody distribution phase has been accomplished is the radionuclide delivered, using a radionuclide-biotin construct. Since the radionuclide-biotin construct is a relatively small molecule, tumor uptake and binding to the streptavidin is rapid and unbound radioactivity is rapidly cleared via renal excretion. To assess the possible utility of CD45 PRIT for AML, we conducted comparative in vivo imaging, biodistribution, and therapy experiments using human leukemia xenografts implanted in athymic mice.22In vivo imaging studies showed specific tumor images with minimal blood pool activity using PRIT, whereas directly labeled BC8 produced clear tumor imaging but with substantial and prolonged blood pool activity (see Figure 4). Therapy studies found that survival of mice bearing human myeloid tumors was significantly prolonged using CD45 PRIT compared with the results using directly labeled antibody. The interpretation of results of the use of an antihuman antibody to treat a human tumor in a murine model is complicated by the fact that murine tissues are devoid of human CD45, and therefore there is no specific binding of the test antibody to normal murine tissues. To overcome this limitation, we tested the relative specificity of a murine anti-CD45 administered after direct conjugation or using PRIT. After 24 hours, about 27% of the directly labeled antibody was found in target tissue, compared to 40% using PRIT. These data, admittedly from an imperfect murine model, suggest that PRIT should further improve the specificity of radioimmunotherapy.
Figure 4.
Fluorescent images of HEL xenograft-bearing athymic mice treated with either conventionally labeled anti-CD45 (A) or pretargeted anti-CD45. Images are shown of same camera intensity settings at 12 hours after injection. T indicates the tumor and B indicates the blood pool. The tumor to total body flourescence ratios are shown in panel C.22
Permission requested by BW on 10/1/09: Denied (10/7/09; re-requested)
Prior to initiating trials of PRIT in humans, we next chose to test the hypothesis that PRIT should improve the specificity of radio-immunotherapy in a nonhuman primate model. Unlike the murine studies, we examined PRIT using exactly the same reagents we plan to use in humans and in a model where the antibody crossreacts with normal tissues. In these studies, PRIT resulted in superior target to normal tissue ratios in the blood, lung, and liver (10.3:1, 18.9:1, and 9.9:1) compared to directly labeled antibody (2.6:1, 6.4:1, and 2.9:1).23 Given that with the use of standard directly labeled anti-CD45 we are able to deliver supplemental irradiation in excess of 32 Gy to the marrow without undue toxicity, these data suggest that we should be able to deliver at least 3-fold that dose (100 Gy) to the marrow and spleen without undue toxicity the liver or lungs. However, at these doses of irradiation to the marrow space, we may start to see damage to the marrow microenvironment, and ultimately, with such specific targeting, it may be that marrow microenvironmental damage rather than any extramedullary toxicity will prove to be dose-limiting. Indeed, this is what we found using a bisphosphonate to deliver high-dose beta-emitting particles (166-Ho) to bone and surrounding marrow as an alternative strategy.24
Summary
The development of methods to enable engraftment of donor hematopoietic cells with very low-dose regimens has allowed for the separation of the immunosuppressive and antitumoral elements of the transplant conditioning regimen. One consequence of this separation is the ability to conduct transplants and thereby gain a graft-vs-tumor effect in patients who would not be able to tolerate a high-dose regimen. A second, and arguably more important consequence, is that the separation allows for the development of transplant regimens in which the antitumoral and immunosuppressive elements can be individually optimized. Our clinical results using directly labeled anti-CD45 antibody combined with fludarabine and low-dose TBI already show considerable promise, and preclinical studies in mice and primates give us every reason to believe that, with the use of pretargeting, these results can be further improved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thomas ED, Clift RA, Hersman J, et al. Marrow transplantation for acute nonlymphoblastic leukemic in first remission using fractionated or single-dose irradiation. Int J Radiat Oncol Biol Phys. 1982;8:817–821. doi: 10.1016/0360-3016(82)90083-9. [DOI] [PubMed] [Google Scholar]
- 2.Helenglass G, Powles RL, McElwain TJ, et al. Melphalan and total body irradiation (TBI) versus cyclophosphamide and TBI as conditioning for allogeneic matched sibling bone marrow transplants for acute myeloblastic leukaemia in first remission. Bone Marrow Transplant. 1988;3:21–29. [PubMed] [Google Scholar]
- 3.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 4.Clift RA, Buckner CD, Appelbaum FR, et al. Allogeneic marrow transplantation in patients with chronic myeloid leukemia in the chronic phase: a randomized trial of two irradiation regimens. Blood. 1991;77:1660–1665. [PubMed] [Google Scholar]
- 5.Blaise D, Maraninchi D, Archimbaud E, et al. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: a randomized trial of a busulfan-Cytoxan versus Cytoxan-total body irradiation as preparative regimen: a report from the Group d’Etudes de la Greffe de Moelle Osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Thomas ED, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–2043. [PubMed] [Google Scholar]
- 7.Devergie A, Blaise D, Attal M, et al. Allogeneic bone marrow transplantation for chronic myeloid leukemia in first chronic phase: a randomized trial of busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: a report from the French Society of Bone Marrow Graft (SFGM) Blood. 1995;85:2263–2268. [PubMed] [Google Scholar]
- 8.Ringden O, Ruutu T, Remberger M, et al. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- 9.Litzow MR, Perez WS, Klein JP, et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–1124. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 10.Russell JA, Tran HT, Quinlan D, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8:468–476. doi: 10.1053/bbmt.2002.v8.pm12374451. [DOI] [PubMed] [Google Scholar]
- 11.Bornhauser M, Storer B, Slattery JT, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood. 2003;102:820–826. doi: 10.1182/blood-2002-11-3567. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. 2005;35:225–231. doi: 10.1038/sj.bmt.1704758. [DOI] [PubMed] [Google Scholar]
- 13.Bredeson CN, Zhang MJ, Agovi MA, et al. Outcomes following HSCT using fludarabine, busulfan, and thymoglobulin: a matched comparison to allogeneic transplants conditioned with busulfan and cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:993–1003. doi: 10.1016/j.bbmt.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell PV, Woolfrey AE, Storer B, et al. Effect of substituting fludarabine and thymoglobulin for cyclophosphamide in busulfan-based conditioning regimens on T-cell chimerism and outcomes after allogeneic hematopoietic cell transplantation (HCT) Biol Blood Marrow Transplant. 2008;14:112–113. [Google Scholar]
- 15.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 16.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 17.Sandmaier BM. Reduced-intensity conditioning followed by hematopoietic cell transplantation for hematologic malignancies. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ hematopoietic cell transplantation. Wiley-Blackwell; Oxford, UK: 2009. pp. 1043–1058. [Google Scholar]
- 18.Sorror ML, Sandmaier BM, Storer BE, et al. Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DC, Appelbaum FR, Eary JF, et al. Phase I study of (131)I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood. 1999;94:1237–1247. [PubMed] [Google Scholar]
- 20.Pagel JM, Appelbaum FR, Eary JF, et al. 131I-anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pagel JM, Gooley TA, Rajendran J, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I-anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009 Sep 28; doi: 10.1182/blood-2009-03-213298. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagel JM, Matthews DC, Kenoyer A, et al. Pretargeted radioimmunotherapy using anti-CD45 monoclonal antibodies to deliver radiation to murine hematolymphoid tissues and human myeloid leukemia. Cancer Res. 2009;69:185–192. doi: 10.1158/0008-5472.CAN-08-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green DJ, Pagel JM, Nemecek ER, et al. Pretargeting CD45 enhances the selective delivery of radiation to hematolymphoid tissues in nonhuman primates. Blood. 2009;114:1226–1235. doi: 10.1182/blood-2009-03-210344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appelbaum FR, Brown PA, Sandmaier BM, et al. Specific marrow ablation before marrow transplantation using an aminophosphonic acid conjugate 166Ho-EDTMP. Blood. 1992;80:1608–1613. [PubMed] [Google Scholar]