Abstract
I- uptake in the thyroid, the first step in thyroid hormone biosynthesis, is mediated by the Na+/I- symporter (NIS) with an electrogenic 2Na+ : 1I- stoichiometry. We have obtained mechanistic information on NIS by characterizing the congenital I- transport defect-causing NIS mutant G93R. This mutant is targeted to the plasma membrane but is inactive. Substitutions at position 93 show that the longer the side chain of the neutral residue at this position, the higher the Km for the anion substrates. Unlike WT NIS, which mediates symport of Na+ and the environmental pollutant perchlorate electroneutrally, G93T/N/Q/E/D NIS, strikingly, do it electrogenically with a 2∶1 stoichiometry. Furthermore, G93E/Q NIS discriminate between anion substrates, a discovery with potential clinical relevance. A 3D homology model of NIS based on the structure of the bacterial Na+/galactose transporter identifies G93 as a critical player in the mechanism of the transporter: the changes from an outwardly to an inwardly open conformation during the transport cycle use G93 as a pivot.
Keywords: iodide transport defect, homology modeling, radioiodide therapy, sodium solute cotransporter family
The iodine-containing thyroid hormones T3 and T4 (triiodothyronine and thyroxine) are essential for development and maturation of the central nervous system, skeletal muscle, and lungs in the fetus and newborn, and they regulate intermediary metabolism (1). I- uptake into the thyroid, the first step in T3 and T4 biosynthesis is mediated by the Na+/I- symporter (NIS). By coupling the inward transport of Na+ down its electrochemical gradient to the translocation of I- against its electrochemical gradient, NIS avidly concentrates I- in the thyroid. NIS-mediated I- transport is electrogenic: Two Na+ ions are transported with each I- (2). Remarkably, NIS translocates different substrates with different stoichiometries, as NIS-mediated transport of perrhenate (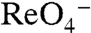 ) or perchlorate (
) or perchlorate (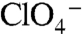 ) is electroneutral (
) is electroneutral (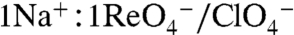 ) (3).
) (3).
NIS activity has long played a role in the diagnosis and treatment of thyroid disease, including the highly successful treatment of thyroid cancer with radioiodide after thyroidectomy (4). We cloned NIS and have since extensively characterized it (2, 5–9). Human NIS (hNIS) and rat NIS (rNIS) share 84% amino acid identity and 93% similarity (10). The secondary structure model of NIS shows 13 transmembrane segments (TMS), an extracellular amino terminus (Nt), and an intracellular carboxy terminus (Ct) (7) (Fig. S1).
Congenital hypothyroidism due to an I- transport defect (ITD) is a rare autosomal recessive disorder (11, 12); 13 ITD-causing NIS mutations have been reported to date (Fig. S1). The mutants that have been studied in detail have provided key mechanistic information on NIS (13–17). For example, substitutions at T354 have revealed that this position requires an -OH group at the β-carbon, which we showed is involved in Na+ binding/translocation, and proposed a structural homology between the Aquifex aeolicus Na+/leucine transporter (LeuT) (18) and NIS, despite a lack of sequence homology (19). Consistent with our prediction, the Vibrio parahaemolyticus Na+/galactose transporter (vSGLT), which belongs to the same family as NIS (Na+/solute cotransporter family 5A), has the same fold as LeuT (20–22).
The NIS mutation G93R was identified in a patient who developed goitrous hypothyroidism due to a compound heterozygous G93R/T354P NIS mutation (23). Having shown that NIS translocates different substrates with different stoichiometries (3), here we now report startling changes in Na+/anion stoichiometry and anion selectivity uncovered by the molecular characterization of various amino acid substitutions at position 93 in NIS.
Results
G93R NIS Is Targeted to the Plasma Membrane but Is Inactive.
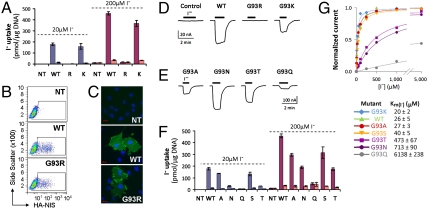
Whereas COS-7 cells transiently transfected with WT NIS avidly accumulated I- upon incubation with a subsaturating [I-] (20 μM) (Km(I-) of WT rNIS is approximately 30 μM), and this activity was inhibited by 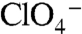 , G93R NIS was inactive (Fig. 1A, blue) even at near-saturating [I-] (200 μM) (Fig. 1A, red).
, G93R NIS was inactive (Fig. 1A, blue) even at near-saturating [I-] (200 μM) (Fig. 1A, red).
Fig. 1.
Analysis of G93 NIS substitutions in COS-7 cells and X. laevis oocytes. (A) Steady-state I- transport in WT, G93R, or G93K NIS-transfected COS-7 cells. Cells were incubated with 20 μM I- in the absence (blue bars) or presence (light blue bars) of 80 μM 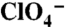 , or with 200 μM I- in the absence (red bars) or presence (pink bars) of 800 μM
, or with 200 μM I- in the absence (red bars) or presence (pink bars) of 800 μM 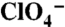 . Values shown (pmol I-/μg DNA ± SD) are from one of at least five different experiments and corrected for transfection efficiency. (B) Flow cytometry under nonpermeabilized conditions with an Ab against the extracellular HA epitope at the NIS Nt, showing WT (19%) and G93R NIS (15%). (C) HA immunostaining of WT and G93R NIS under nonpermeabilized conditions. NIS is stained with Alexa 488 (green) and nuclei with DAPI (blue). Red scale bar = 20 μm. (D and E) Current traces are shown in response to 5 mM I- in (D) control (water-injected) X. laevis oocytes or oocytes expressing WT, G93R, or G93K NIS, or (E) G93A, N, T, or Q NIS. The evoked inward currents represent NIS-mediated electrogenic Na+/I- cotransport into the cell. Vm = -50 mV. (F) I- transport as in A in COS-7 cells transfected with WT, G93A, N, Q, S, or T NIS cDNAs. (G) Kinetics of I- transport by WT and G93K, A, S, T, N, and Q NIS. [Na+]o = 100 mM and Vm = -50 mV. For each mutant, the current values were normalized to the maximum current obtained at saturating [I-]. The smooth lines are fits of the data to the Michaelis–Menten equation. Values represent the means ± SE from at least four oocytes.
. Values shown (pmol I-/μg DNA ± SD) are from one of at least five different experiments and corrected for transfection efficiency. (B) Flow cytometry under nonpermeabilized conditions with an Ab against the extracellular HA epitope at the NIS Nt, showing WT (19%) and G93R NIS (15%). (C) HA immunostaining of WT and G93R NIS under nonpermeabilized conditions. NIS is stained with Alexa 488 (green) and nuclei with DAPI (blue). Red scale bar = 20 μm. (D and E) Current traces are shown in response to 5 mM I- in (D) control (water-injected) X. laevis oocytes or oocytes expressing WT, G93R, or G93K NIS, or (E) G93A, N, T, or Q NIS. The evoked inward currents represent NIS-mediated electrogenic Na+/I- cotransport into the cell. Vm = -50 mV. (F) I- transport as in A in COS-7 cells transfected with WT, G93A, N, Q, S, or T NIS cDNAs. (G) Kinetics of I- transport by WT and G93K, A, S, T, N, and Q NIS. [Na+]o = 100 mM and Vm = -50 mV. For each mutant, the current values were normalized to the maximum current obtained at saturating [I-]. The smooth lines are fits of the data to the Michaelis–Menten equation. Values represent the means ± SE from at least four oocytes.
Flow cytometry (FC) with an anti-rNIS-Ct Ab showed that G93R NIS was synthesized and expressed at levels similar to those of WT NIS (Fig. S2A). To determine the amount of NIS at the cell surface, we engineered an extracellular HA tag at the Nt of NIS. FC using an anti-HA Ab showed that G93R NIS was targeted to the plasma membrane (Fig. 1B). This was further confirmed by immunofluorescence (Fig. 1C). Therefore, the lack of activity of G93R NIS was not due to impaired targeting.
Lysine Is Tolerated at Position 93.
Surprisingly, G93K NIS was as active as WT NIS (Fig. 1A). Thus, the positive charge of Arg was not the cause of G93R NIS lack of function, even though position 93 was predicted to be located within TMS III. To examine the electrophysiological correlates of the uptake experiments described above, we expressed WT and G93 NIS mutants in Xenopus laevis oocytes. In control oocytes, I- (≤ 5 mM) did not evoke an electrogenic response, whereas in WT NIS-expressing oocytes it evoked an inward current that represents NIS-mediated electrogenic Na+/I- symport (2). G93K NIS supported robust I--evoked currents, confirming that position 93 tolerates this residue (Fig. 1D).
G93A, N, S, and T were all active (Fig. 1 E and F). The magnitude of the currents reflects the level of expression of the relevant protein in a given oocyte and should not be taken to indicate the transport ability of a specific mutant as compared to any other or to WT NIS. Such comparisons are effectively made in the standardized kinetic analyses (Figs. 1G, 2A, 3 D and E, and 4D). Notably, G93Q NIS showed currents only at very high [I-] (5 mM) (Fig. 1E). Consistent with this observation, there was no I- accumulation in COS cells expressing G93Q NIS even at 200 μM I- (Fig. 1F), although the protein was targeted to the cell surface (Fig. S2B). Each of the other NIS mutants (A, S, T, and N) displayed different levels of I- transport activity upon incubation with 20 μM I- with a clear pattern: the longer the side chain, the lower the activity (Fig. 1F) and this was not due to different levels of expression (Fig. S2A). Interestingly, 200 μM I- resulted in a greater relative increase in transport by G93N and T as compared to WT NIS, suggesting a change in their Km(I-) (Fig. 1F).
Fig. 2.
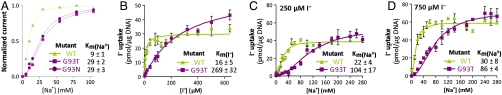
Kinetics of I- transport in X. laevis oocytes and MDCK cells. (A) Kinetics of Na+ dependence of I- transport by WT, G93T, and G93N NIS. [I-] = 5 mM and Vm = -50 mV. Current values were normalized to the maximum current obtained at saturating [Na+]. The smooth lines are fits of the data to the Hill equation. The Hill coefficient values were 1.9 ± 0.2 for WT, 2.0 ± 0.1 for G93T, and 1.9 ± 0.1 for G93N NIS. Values represent the mean ± SE from at least four oocytes. (B) Initial rates (2-min time points) of I- uptake were determined at the indicated [I-]s. Km(I-)s are indicated as an average ± SE (n = 5 experiments). The graph shown is a representative experiment. (C and D) Na+ dependence of I- uptake. Cells were incubated for 2 min with (C) 250 or (D) 750 μM I- and the indicated [Na+]s. The graph is representative of > 3 experiments. Isotonicity was kept constant with choline-Cl. In all flux experiments, the activity was standardized by expression levels at the cell surface, and background values obtained with nontransfected cells were subtracted (< 5 in B and C; and 12 pmol/μg DNA in D).
Fig. 3.
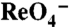 transport kinetics in MDCK cells and X. laevis oocytes. (A) Initial rates (2 min) of
transport kinetics in MDCK cells and X. laevis oocytes. (A) Initial rates (2 min) of 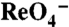 uptake in transduced MDCK cells were determined at the indicated [
uptake in transduced MDCK cells were determined at the indicated [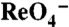 ]s. Background in nontransfected cells (< 2 pmol/μg DNA) was subtracted. (B) Na+ dependence of
]s. Background in nontransfected cells (< 2 pmol/μg DNA) was subtracted. (B) Na+ dependence of 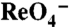 transport: Cells were incubated for 2 min with 180 μM
transport: Cells were incubated for 2 min with 180 μM 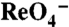 and the indicated [Na+]s.
and the indicated [Na+]s. 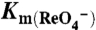 s are given as an average ± SE of all experiments. The graph is a representative experiment. Background obtained with NT cells (< 2 pmol/μg DNA) was subtracted. (C) Current traces in response to 1 mM
s are given as an average ± SE of all experiments. The graph is a representative experiment. Background obtained with NT cells (< 2 pmol/μg DNA) was subtracted. (C) Current traces in response to 1 mM 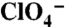 (Top Traces) or
(Top Traces) or 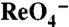 (Bottom Traces) in oocytes expressing WT, G93R, K, N, or T NIS. Vm = -50 mV. For each mutant,
(Bottom Traces) in oocytes expressing WT, G93R, K, N, or T NIS. Vm = -50 mV. For each mutant, 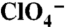 and
and 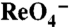 traces were obtained in the same oocyte. (D) Kinetics of
traces were obtained in the same oocyte. (D) Kinetics of 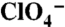 transport by G93T and N NIS in oocytes. [Na+]o = 100 mM and Vm = -50 mV. Data analyzed as in Fig. 1G. (E) Kinetics of Na+ dependence of anion transport by G93T and G93N NIS in oocytes with 1 mM
transport by G93T and N NIS in oocytes. [Na+]o = 100 mM and Vm = -50 mV. Data analyzed as in Fig. 1G. (E) Kinetics of Na+ dependence of anion transport by G93T and G93N NIS in oocytes with 1 mM 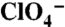 Vm = -50 mV. Data were processed as in Fig. 2A. The Hill coefficient values were 1.9 ± 0.2 for G93N, and 2.2 ± 0.2 for G93T NIS.
Vm = -50 mV. Data were processed as in Fig. 2A. The Hill coefficient values were 1.9 ± 0.2 for G93N, and 2.2 ± 0.2 for G93T NIS.
Fig. 4.
G93E NIS does not transport I- but transports 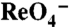 electrogenically. (A) Steady-state
electrogenically. (A) Steady-state  transport assays in nontransfected (NT) or COS-7 cells transfected with WT or G93K, D, or E NIS cDNAs. Cells were incubated for 1 h with 3 μM
transport assays in nontransfected (NT) or COS-7 cells transfected with WT or G93K, D, or E NIS cDNAs. Cells were incubated for 1 h with 3 μM 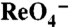 in the absence (light green bars) or presence (yellow bars) of 120 μM
in the absence (light green bars) or presence (yellow bars) of 120 μM 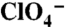 , or with 30 μM
, or with 30 μM 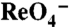 in the absence (dark green bars) or presence (olive bars) of 800 μM
in the absence (dark green bars) or presence (olive bars) of 800 μM 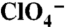 . (B) Steady-state I- transport activity was assayed in WT, G93D, or G93E NIS-expressing COS-7 cells as in Fig. 1A. (C) Current traces in response to 5 mM I- (Left) or 1 mM
. (B) Steady-state I- transport activity was assayed in WT, G93D, or G93E NIS-expressing COS-7 cells as in Fig. 1A. (C) Current traces in response to 5 mM I- (Left) or 1 mM 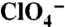 (Center) or
(Center) or  (Right) in oocytes expressing G93D (Top Traces), G93E (Middle Traces), or G93Q NIS (Bottom Traces). Vm = -50 mV. For each mutant, all current traces were obtained in the same oocyte. (D) Kinetics of anion transport by G93D, E, and Q NIS, as in Fig. 1G. (E) Kinetic parameters.
(Right) in oocytes expressing G93D (Top Traces), G93E (Middle Traces), or G93Q NIS (Bottom Traces). Vm = -50 mV. For each mutant, all current traces were obtained in the same oocyte. (D) Kinetics of anion transport by G93D, E, and Q NIS, as in Fig. 1G. (E) Kinetic parameters.
The Km Values of G93N and T NIS for I- and Na+ Are Significantly Higher than Those of WT NIS.
The Km(I-) values of G93A and K NIS were 30 ± 2 and 33 ± 2 μM, similar to that for WT NIS [Fig. S2C and refs. 13–17, 19)]. In contrast, G93T and N NIS had significantly higher Km(I-) values (282 ± 44 and 358 ± 69 μM, respectively) (Fig. S2D). Electrophysiologically, whereas G93K, A, and S exhibited Km(I-) values comparable to those of WT NIS, G93N and T NIS exhibited > 18-fold higher Km(I-), and G93Q NIS had an astonishingly > 200-fold higher estimated Km(I-) (6.1 mM) (Fig. 1G). Similarly, the mutants with a high Km(I-) also had a higher Km(Na+) (Fig. 2A). The Hill coefficient for Na+ activation of NIS-mediated inward currents remained unchanged at 2 for WT NIS and G93 mutants.
To eliminate the experimental variation in transient transfection, we transduced MDCK (Madin–Darby canine kidney) cells with a lentiviral vector to permanently express HA-tagged hNIS (WT or G93T). As G93N and T NIS behaved similarly (Fig. 1 F and G), further studies in MDCK cells focused on G93T hNIS. I- transport kinetics in MDCK cells recapitulated those in COS-7 cells: WT hNIS Km(I-) was 16 ± 5 μM (3, 17), whereas G93T hNIS Km(I-) was 269 ± 32 μM (Fig. 2B).
WT NIS-mediated Na+/I- symport is electrogenic with a 2Na+ : 1I- stoichiometry (2, 3) (Fig. 2A). At 250 μM I-—close to its Km(I-)—the G93T hNIS Km(Na+) was 104 ± 16 mM (Fig. 2C). Raising the [I-] to 750 μM, the G93T Km(Na+) decreased by approximately 20% (86 ± 4 mM) (Fig. 2D). Therefore, increasing the concentration of one substrate decreases the Km of the cosubstrate in G93T NIS, as reported for WT NIS (2) and other cotransporters (24, 25).
Asn and Thr Substitutions at Position 93 Convert NIS-Mediated 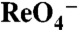 (and
(and 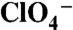 ) Transport from Electroneutral to Electrogenic.
) Transport from Electroneutral to Electrogenic.
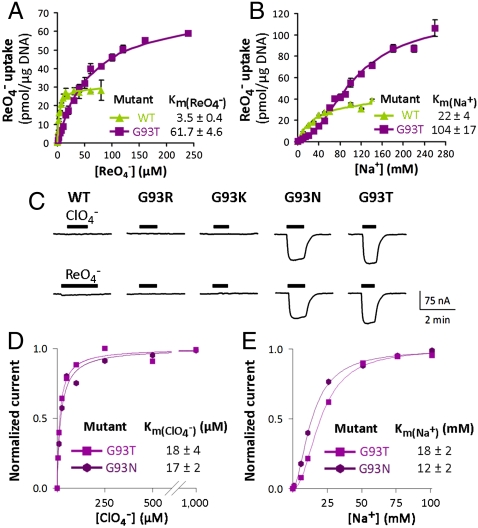
The Km of G93T NIS for 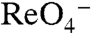 (61.7 ± 4.6 μM) was similarly approximately 17-fold higher than that of WT hNIS (3.5 ± 0.4 μM) (Fig. 3A and ref. 26); the levels of
(61.7 ± 4.6 μM) was similarly approximately 17-fold higher than that of WT hNIS (3.5 ± 0.4 μM) (Fig. 3A and ref. 26); the levels of 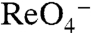 transport by G93T hNIS were considerably higher than those of WT hNIS (Fig. 3
A and B). Strikingly, the
transport by G93T hNIS were considerably higher than those of WT hNIS (Fig. 3
A and B). Strikingly, the 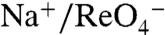 symport mediated by G93T NIS showed sigmoidal Na+ dependence (Hill coefficient = 2) (Fig. 3B), suggesting that the substitution converted the
symport mediated by G93T NIS showed sigmoidal Na+ dependence (Hill coefficient = 2) (Fig. 3B), suggesting that the substitution converted the 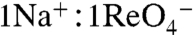 stoichiometry of WT NIS (3) to electrogenic (
stoichiometry of WT NIS (3) to electrogenic (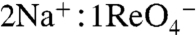 ). Furthermore, in X. laevis oocytes expressing G93T NIS,
). Furthermore, in X. laevis oocytes expressing G93T NIS, 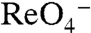 elicited inward currents, whereas there were no currents when WT NIS was expressed (Fig. 3C). Moreover, the environmental pollutant
elicited inward currents, whereas there were no currents when WT NIS was expressed (Fig. 3C). Moreover, the environmental pollutant 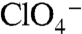 , which is structurally similar to
, which is structurally similar to 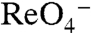 , also elicited currents in oocytes expressing G93T or N NIS (Fig. 3C), in contrast to WT NIS (2), implicating position 93 as a key Na+/anion coupling link. Kinetic analysis of G93T and N NIS-mediated
, also elicited currents in oocytes expressing G93T or N NIS (Fig. 3C), in contrast to WT NIS (2), implicating position 93 as a key Na+/anion coupling link. Kinetic analysis of G93T and N NIS-mediated 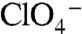 or
or 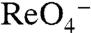 transport by electrophysiology revealed a
transport by electrophysiology revealed a 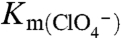 of 17 ± 2 μM for G93N and 18 ± 4 μM for G93T NIS and a
of 17 ± 2 μM for G93N and 18 ± 4 μM for G93T NIS and a 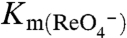 of 29 ± 2 μM for G93N and 24 ± 3 μM for G93T NIS (Fig. S3A).
of 29 ± 2 μM for G93N and 24 ± 3 μM for G93T NIS (Fig. S3A).
No NIS-mediated 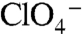 - or
- or 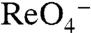 -evoked currents were observed in G93R or K NIS (Fig. 3C). In contrast, both G93T and N NIS exhibited sigmoidal Na+ dependence with
-evoked currents were observed in G93R or K NIS (Fig. 3C). In contrast, both G93T and N NIS exhibited sigmoidal Na+ dependence with 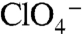 (Fig. 3E) or
(Fig. 3E) or 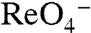 (Fig. S3B) (Hill coefficient = 2), corroborating the uptake data (Fig. 3B). Interestingly, although the
(Fig. S3B) (Hill coefficient = 2), corroborating the uptake data (Fig. 3B). Interestingly, although the  or
or 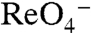 stoichiometry was different in G93T versus WT NIS, the relaxation kinetics of the presteady-state charge movements in G93N and G93T were similar to those of WT NIS, suggesting that the partial reactions corresponding to Na+ binding and binding-induced conformational changes were not significantly affected by the substitution (Fig. S4 and ref. 2). Furthermore, the transporter turnover rate, as determined by the ratio of the maximum I--evoked current (Imax) to the maximum presteady-state charge movements (Qmax), was approximately 35 s-1 in G93N and G93T mutants and was similar to that reported for WT NIS (2).
stoichiometry was different in G93T versus WT NIS, the relaxation kinetics of the presteady-state charge movements in G93N and G93T were similar to those of WT NIS, suggesting that the partial reactions corresponding to Na+ binding and binding-induced conformational changes were not significantly affected by the substitution (Fig. S4 and ref. 2). Furthermore, the transporter turnover rate, as determined by the ratio of the maximum I--evoked current (Imax) to the maximum presteady-state charge movements (Qmax), was approximately 35 s-1 in G93N and G93T mutants and was similar to that reported for WT NIS (2).
Glu93 and Gln93 Discriminate Between I- and 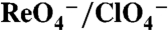 .
.
Even though 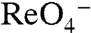 (or
(or 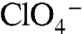 ) did not elicit currents in G93K NIS-expressing oocytes (Fig. 3C) at 3 μM
) did not elicit currents in G93K NIS-expressing oocytes (Fig. 3C) at 3 μM 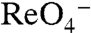 (the
(the  of WT hNIS), G93K transported
of WT hNIS), G93K transported 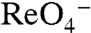 in MDCK cells (Fig. 4A), indicating that G93K transports
in MDCK cells (Fig. 4A), indicating that G93K transports 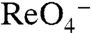 electroneutrally, just like WT NIS. In contrast, G93R, which did not transport I- in mammalian cells (Fig. 1A) or elicit currents in oocytes in the presence of I-,
electroneutrally, just like WT NIS. In contrast, G93R, which did not transport I- in mammalian cells (Fig. 1A) or elicit currents in oocytes in the presence of I-, 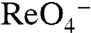 , or
, or 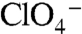 (Figs. 1D and 3C), did not translocate
(Figs. 1D and 3C), did not translocate 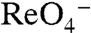 in MDCK cells either, even at high [
in MDCK cells either, even at high [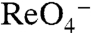 ]. Thus, it is not solely the presence of a positive charge at position 93 that renders NIS nonfunctional, but more specifically the Arg side chain.
]. Thus, it is not solely the presence of a positive charge at position 93 that renders NIS nonfunctional, but more specifically the Arg side chain.
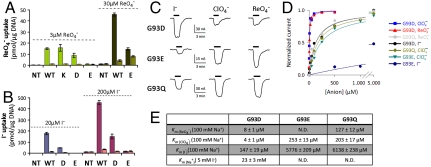
G93D NIS-expressing cells transported I-, although at significantly lower levels than WT NIS. On the other hand, G93E NIS-expressing cells showed no transport at 200 μM I- (Fig. 4B) or higher, though the protein was expressed at the plasma membrane (Fig. S5). I--evoked currents mediated by G93E NIS were extremely small even at exceedingly high [I-] (5 mM, > 160 times higher than the WT NIS Km(I-)) (Fig. 4C). Indeed, the G93E NIS estimated Km(I-) was extraordinarily high (> 6 mM), 220-fold higher than that of WT NIS (Fig. 4D), whereas G93D NIS-mediated robust I--evoked currents, albeit with a highly increased Km(I-) of approximately 150 μM (Fig. 4E).
G93D NIS-transfected cells transported 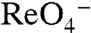 at 3 μM. In contrast, G93E NIS activity was barely detectable (Fig. 4A, light green). On the other hand, at a 10-fold higher [
at 3 μM. In contrast, G93E NIS activity was barely detectable (Fig. 4A, light green). On the other hand, at a 10-fold higher [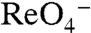 ], G93E NIS clearly exhibited
], G93E NIS clearly exhibited 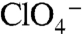 -sensitive
-sensitive 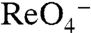 transport, although at lower levels than WT NIS (Fig. 4A, dark green). Electrophysiologically, both G93D and E exhibited
transport, although at lower levels than WT NIS (Fig. 4A, dark green). Electrophysiologically, both G93D and E exhibited 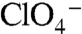 - and
- and 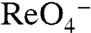 -evoked currents, suggesting a switch in transport stoichiometry leading to electrogenic
-evoked currents, suggesting a switch in transport stoichiometry leading to electrogenic 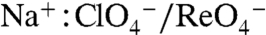 symport (Fig. 4C). Although the apparent affinity of G93D for
symport (Fig. 4C). Although the apparent affinity of G93D for 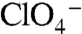 and
and 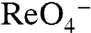 was high (Kms = 4 and 8 μM, respectively), that of G93E for
was high (Kms = 4 and 8 μM, respectively), that of G93E for 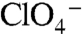 was significantly reduced (Km approximately 250 μM) (Fig. 4
D and E). G93Q also displayed
was significantly reduced (Km approximately 250 μM) (Fig. 4
D and E). G93Q also displayed 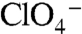 - and
- and  -elicited currents (Fig. 4C). The signal with G93E was too small for kinetic analysis; G93D (at 5 mM I-) yielded a sigmoidal relationship with a Km(Na+) of 23 ± 3 mM (Hill coefficient = 1.9) (Fig. S6). The striking observation that G93E and Q NIS transport
-elicited currents (Fig. 4C). The signal with G93E was too small for kinetic analysis; G93D (at 5 mM I-) yielded a sigmoidal relationship with a Km(Na+) of 23 ± 3 mM (Hill coefficient = 1.9) (Fig. S6). The striking observation that G93E and Q NIS transport 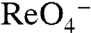 and
and 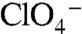 even though I- transport is severely impaired in these two mutants indicates that these two amino acids confer the ability to discriminate between substrates.
even though I- transport is severely impaired in these two mutants indicates that these two amino acids confer the ability to discriminate between substrates.
The NIS Homology Model Reveals Close Contact Between Gly93 and Trp255.
The structures of four bacterial Na+-driven transporters have been determined by X-ray crystallography: LeuT, a homologue of eukaryotic neurotransmitter transporters such as the norepinephrine, dopamine and serotonin, γ-aminobutyrate, and glycine transporters (18, 27); vSGLT (20), a homologue of the eukaryotic Na+/glucose transporter (SGLT-1); the benzylhydantoin transporter (Mhp1) from Moraxella liquefaciens (28), of the nucleobase-cation-symport-1 family of transporters; and the Na+/betaine symporter (BetP) from Corynebacterium glutamicum, of the betaine/choline/carnitine transporter family (29). The structure of LeuT was determined at the highest resolution (1.6 Å) (18). Surprisingly, although all four proteins share little sequence homology (< 17%), all have the same fold—an inverted topology repeat and unwound helices in regions critical for substrate binding—and a similar way of coordinating Na+.
Using as a template the X-ray structure of vSGLT (20), we generated a 3D homology model of NIS (Fig. 5A). Alignment of the NIS and vSGLT sequences using BLAST shows significant identity (27% for NIS residues 50 to 476), a value comparable to that between vSGLT and its eukaryotic homologue SGLT1 (31%). Critically, the extent of the identity between NIS and vSGLT is similar to that between LeuT and mammalian neurotransmitter transporters (20–25%) (18); even at this level of identity the LeuT structure has become a major model for elucidating mechanistic information on mammalian neurotransmitter transporters. Similarly, the NIS homology model provides vital information on the protein’s mechanism.
Fig. 5.
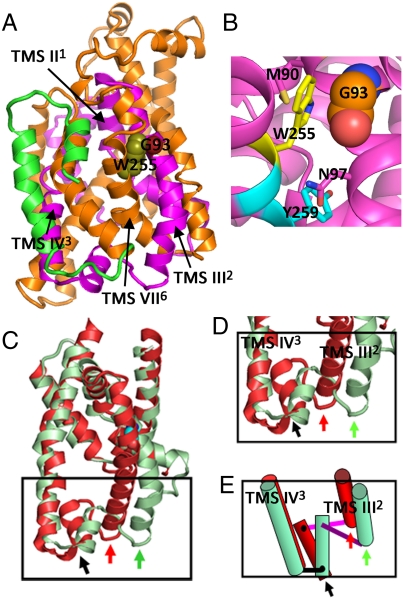
NIS model based on the vSGLT structure. The homology model, including NIS residues 50 to 476, was generated as described in SI Text. (A) Overall structure. TMS III–VI2–5 are colored magenta and TMS VII–XI6–10 orange. G93 (red) abuts the indole ring of W255 (olive). The rest of the model is colored green. (B) Close-up of the NIS cavity depicting G93 and W255 opposite the unwound portion of TMS VII6. N97 and Y259 also face each other at the bottom end of the cavity. M90 forms part of the upper roof of the cavity. Transition between two conformations of NIS: (C) Superposition of the inwardly open and outwardly open conformations showing the movement of the hairpins and the connecting residues. The inwardly open conformation (vSGLT-like) is colored light green and the outwardly open conformation (LeuT-like) red. The green arrow shows the position of TMS III2 in the inwardly open conformation and the red arrow the position of the outwardly open conformation. The black arrow indicates the point around which the connecting short helix pivots to allow the rotation of the two helical hairpins. (D) Close-up of boxed region in C. (E) Schematic representation of D; TMS III and IV2–3 are depicted as cylinders, the short helix as a rectangle, the unstructured connections between TMS III and IV2–3, and the rectangle as rods. Color scheme as in C and D.
In this model, TMS III2 and X9 (superscript Arabic numerals indicate the LeuT nomenclature) are at the edge of the molecule, defining the outer wall of a cavity that in vSGLT contains the galactose substrate (20). TMS VII6 crosses TMS III2 at an angle in such a way that G93 and W255 are in close contact, with the Cα of Gly abutting the indole ring of W255. They are at the opposite side of the cavity from the unwound portion of TMS VII6 (Fig. 5B), a common essential characteristic of Na+-driven symporters. Residues in the next helical turns (N97 and Y259) also face each other, at the bottom end of the cavity. M90, from the previous turn of TMS III2, is inside the cavity and forms part of its “upper” roof.
In vSGLT, galactose is bound in a cavity at the center of the core, shielded from the extracellular milieu by hydrophobic residues (20). The equivalent cavity in the NIS model may contain I- or I-/Na+ in one of the conformations of NIS during the transport cycle. To determine whether the NIS residues (W255 and Y259) play the key roles suggested by the model, we generated various HA-tagged hNIS mutants. W255A was inactive even at high concentrations of I-; W255Y accumulated I-, albeit less than WT NIS. In contrast, Y259A and Y259F were both functional, although less than WT NIS (Fig. S7A). Cells expressing all these mutants, except W255A, accumulated 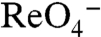 (Fig. S7B). Differences in transport were not due to varying levels of expression or plasma membrane targeting (Fig. S7 C and D).
(Fig. S7B). Differences in transport were not due to varying levels of expression or plasma membrane targeting (Fig. S7 C and D).
Gly93 and Trp255 Form a Ball-and-Socket Joint.
As substitutions at and around position 93 have such dramatic effects on NIS activity, we used the NIS homology model to shed light on the participation of this position in the mechanism of NIS. Structural alignment of the NIS model with the four experimental structures, excluding vSGLT (the template for modeling NIS), showed large deviations reflecting that the crystal structures, as described by the authors, captured different states in the transport cycle: whereas vSGLT, and by extension the NIS homology model, are in the inwardly open conformation (20), the others are in various substates of the outwardly open conformation (18, 29). This analysis also shows that the coordination of the second Na+ (Na2) in NIS is equivalent to that in LeuT (19), but the first Na+ (Na1) must be coordinated differently in NIS. Among other changes, the helical hairpins formed by TMS II1 and III2, and TMS IV3 and V4, significantly change their relative orientations (Fig. S8 A and B). In this rearrangement, the structure of the IV–V3–4 pair remains highly conserved, whereas there are significant changes in the other pair. Alignment of TMS IV3 and V4 of the NIS model with those of LeuT shows a low rmsd between the aligned Cα carbons (1.9 Å for 77 Cα). When this alignment—optimizing the superposition of TMS IV3 and V4 between LeuT and NIS—is applied to the portion of the NIS model that includes TMS II–V1–4, the change in the relative orientation between the two helical hairpins becomes evident (Fig. S8 A and B). The conformational change is best described as a 25–30° rotation of the TMS II–III1–2 helical hairpin (Fig. 5C), which, strikingly, pivots around the contact between G93 and the indole ring of W255 (Fig. 5B). Thus, the G93/W255 pair can be described as a ball-and-socket joint—the Cα H of Gly, the ball; the six-member ring of Trp, the socket. Residues 118–132, which connect to the helical hairpins, provide a key structural element that permits the rotation of the two hairpins with small variations in their respective structures. This region consists of a short helix (Fig. S8 A and B, in olive) flanked by two short loops. The change in distance between the ends of the two hairpins is easily accommodated by a rotation of the short helix with small changes in the connecting pieces acting as hinges [Fig. 5 C and D (close-up), and E (schematic representation)]. The large displacement of the Ct of III2 “pushes,” via the connecting rod, the short helix, resulting in a large displacement of its Nt portion, whereas its Ct acts as a hinge for the rotation of the helix and does not move significantly, allowing TMS IV3 and V4 to remain close to their original positions. TMS VII6 (in the second half of NIS), which encompasses W255, the residue against which G93 rests, runs at an angle with respect to TMS IV3. As the rotation takes place, TMS VII6 has to move away as part of the overall conformational change.
Thus, the nature of the side chain at position 93 of NIS may control the transition between the outwardly and the inwardly open conformations and play a role in the kinetics as well as the stoichiometry. This transition involves a change in not only the relative sizes of the cavities open to either the cytosol or the extracellular milieu but also the volume of the substrate-binding cavity at the center of the molecule (Fig. S8 C and D).
Discussion
Here we have characterized the NIS mutation G93R, which occurs in TMS III2 of the symporter. The lack of activity of this mutant protein is not due to its having a positively charged residue within the membrane, as G93K results in an active transporter (Figs. 1 A, D, and G and 4A). In contrast to the many NIS mutants we have studied previously (13–17, 19), substitutions at position 93 show a significant change in the Km for I-. This is true for not only the neutral residues Thr, Asn, and Gln (Fig. 1G) but also Asp and Glu (Fig. 4D), indicating that NIS tolerates both, a strong basic (Lys) and a strong acidic residue (Asp) in the middle of TMS III2. Whether the side chains of these residues bear a charge at physiological pH depends on their respective pKas, a function of the electrostatic properties of their microenvironment. Lys, and even more likely Arg, remains positively charged, as the energetic cost of deprotonating these two side chains at the experimental pH of 7.5 is 4.12 and 6.77 kcal/mol, respectively. It is also probable that Asp and Glu exist as charged species, given the steep energy required for protonation at neutral pH (4.95 and 4.42 kcal/mol, respectively). If some of these residues are neutral in the environment of the protein, the energetic cost of protonation/deprotonation will be “paid” by a decrease in the overall stability of the mutant protein that may result in changes in the protein’s structure, ranging in severity from small local distortions to total misfolding.
Further kinetic analyses in G93T hNIS-transduced MDCK cells showed an approximately 18-fold higher Km for both I- (Fig. 2B) and  (Fig. 3A), and an approximately 5-fold higher Km for Na+ (Fig. 2C). This change in the Km for Na+ is larger than those observed in substitutions of β-OH-containing residues in TMS IX8 (19). Our 3D NIS model provides a rationale for the effects of the substitutions at position 93 on NIS activity. This site appears to be a pivot around which occurs one of the major components of the conformational change between the inwardly and the outwardly open conformations: the rotation of the helical hairpin formed by TMS III and IV2and3 (Fig. 5). Because it lacks a side chain, Gly, the WT residue, is ideally suited for this position. Nevertheless, other substitutions at position 93 result in proteins that not only are active but also in some cases produce additional changes in key properties of the transporter. The conformational changes we propose are fully compatible with the model proposed by Gouaux’s group (22, 30, 31) and by Forrest et al. (32), but they highlight the importance of position 93. Faham et al. also identified a Gly residue (G99 of vSGLT) as an important participant in the vSGLT transport cycle (20).
(Fig. 3A), and an approximately 5-fold higher Km for Na+ (Fig. 2C). This change in the Km for Na+ is larger than those observed in substitutions of β-OH-containing residues in TMS IX8 (19). Our 3D NIS model provides a rationale for the effects of the substitutions at position 93 on NIS activity. This site appears to be a pivot around which occurs one of the major components of the conformational change between the inwardly and the outwardly open conformations: the rotation of the helical hairpin formed by TMS III and IV2and3 (Fig. 5). Because it lacks a side chain, Gly, the WT residue, is ideally suited for this position. Nevertheless, other substitutions at position 93 result in proteins that not only are active but also in some cases produce additional changes in key properties of the transporter. The conformational changes we propose are fully compatible with the model proposed by Gouaux’s group (22, 30, 31) and by Forrest et al. (32), but they highlight the importance of position 93. Faham et al. also identified a Gly residue (G99 of vSGLT) as an important participant in the vSGLT transport cycle (20).
In the NIS model, the Cβ of nonglycine residues points toward the inside of the cavity (Fig. 5), which is occupied by galactose in vSGLT. By analogy, the substrates of NIS also likely occupy this cavity and may interact with the side chains of all substitutions at position 93. Arg is probably too large and rigid and may fill the cavity and interfere with substrate binding. Lys, although longer than Arg, is more flexible and may extend upward toward the extracellular space. Thr, Asn, and Asp, being smaller, allow the substrates to enter the cavity and be transported, although with a higher Km (Figs. 1G and 2 B–D).
The significance of G93 is underscored by the manner in which WT NIS and the NIS mutants handle 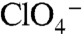 , a transported competitive inhibitor of NIS. The environmental and health impact of
, a transported competitive inhibitor of NIS. The environmental and health impact of 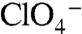 has acquired a new sense of urgency, as the anion has been detected as a contaminant in public water supplies (33). We have demonstrated that NIS actively transports
has acquired a new sense of urgency, as the anion has been detected as a contaminant in public water supplies (33). We have demonstrated that NIS actively transports 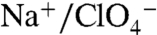 , including translocating it into milk, and that the
, including translocating it into milk, and that the 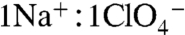 transport stoichiometry is electroneutral (
transport stoichiometry is electroneutral (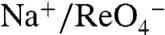 ) (3). Here we show that the stoichiometry of
) (3). Here we show that the stoichiometry of 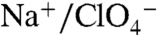 and
and 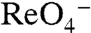 mediated by G93N/T/D/E/Q NIS is 2∶1 and thus electrogenic, in stark contrast to the electroneutral 1∶1 stoichiometry of their transport by WT NIS. The reason for this change may be that in WT NIS, both
mediated by G93N/T/D/E/Q NIS is 2∶1 and thus electrogenic, in stark contrast to the electroneutral 1∶1 stoichiometry of their transport by WT NIS. The reason for this change may be that in WT NIS, both 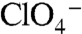 and
and  interfere with Na+ binding to the Na1 site. G93T/N/Q/D/E, rather than relieving this interference (Fig. S9), probably provide an additional Na+-coordinating group that shifts the position of Na1 away from the bound
interfere with Na+ binding to the Na1 site. G93T/N/Q/D/E, rather than relieving this interference (Fig. S9), probably provide an additional Na+-coordinating group that shifts the position of Na1 away from the bound 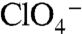 or
or 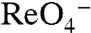 , but still participates in transport. G93A, which cannot contribute to this site, elicited currents barely detectable with
, but still participates in transport. G93A, which cannot contribute to this site, elicited currents barely detectable with 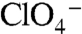 and
and 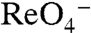 (Fig. S10). A possible explanation is that this substitution allows a small leak current that occurs only when transport takes place but is not thermodynamically coupled to it.
(Fig. S10). A possible explanation is that this substitution allows a small leak current that occurs only when transport takes place but is not thermodynamically coupled to it.
The lack of I- transport activity by G93R/Q/E NIS in mammalian cells probably results from the side chains being either too large or incompatible with the chemical requirements. G93E and Q exhibit the most surprising behavior: Although G93D transports I-, G93E and Q do so only at extremely high [I-] (Fig. 4
C–E), probably a consequence of the size difference between the side chains. Strikingly, G93E and Q not only transport 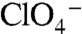 and
and 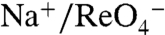 but also do so with a 2∶1 Na+∶anion stoichiometry. Although this observation is difficult to explain, it is interesting that Asp, Glu, and Gln have the chemical characteristics necessary for coordinating Na+, as we propose for the G93T and N substitutions, whereas G93K displays electroneutral
but also do so with a 2∶1 Na+∶anion stoichiometry. Although this observation is difficult to explain, it is interesting that Asp, Glu, and Gln have the chemical characteristics necessary for coordinating Na+, as we propose for the G93T and N substitutions, whereas G93K displays electroneutral 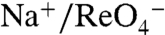 or
or 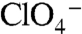 stoichiometry, just like WT NIS (Figs. 3C and 4A).
stoichiometry, just like WT NIS (Figs. 3C and 4A).
The selective transport of 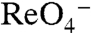 (Fig. 4) by G93E and Q NIS, which do not transport I-, may prove to be particularly useful for NIS-based gene therapy. NIS has been used clinically for > 60 y to successfully treat thyroid cancer with radioiodide, and efforts to make non-NIS-expressing tumors susceptible to radiotherapy by introducing NIS are currently under way (34). Given that NIS is endogenously expressed in the thyroid, thyroid NIS must be selectively down-regulated prior to radiotherapy by administering either high concentrations of T4 or high doses of I-. However, I- cannot be used to down-regulate thyroid NIS if the NIS molecule exogenously transfected into cancer cells also transports I-. Thus, introducing G93E or Q NIS into tumors instead of WT NIS would allow the down-regulation of thyroid NIS by I- without affecting NIS function in the tumor, as neither G93E nor Q NIS transports I-. This may in turn permit successful imaging with
(Fig. 4) by G93E and Q NIS, which do not transport I-, may prove to be particularly useful for NIS-based gene therapy. NIS has been used clinically for > 60 y to successfully treat thyroid cancer with radioiodide, and efforts to make non-NIS-expressing tumors susceptible to radiotherapy by introducing NIS are currently under way (34). Given that NIS is endogenously expressed in the thyroid, thyroid NIS must be selectively down-regulated prior to radiotherapy by administering either high concentrations of T4 or high doses of I-. However, I- cannot be used to down-regulate thyroid NIS if the NIS molecule exogenously transfected into cancer cells also transports I-. Thus, introducing G93E or Q NIS into tumors instead of WT NIS would allow the down-regulation of thyroid NIS by I- without affecting NIS function in the tumor, as neither G93E nor Q NIS transports I-. This may in turn permit successful imaging with 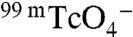 and treatment with
and treatment with  .
.
The data here presented indicate that the side chain at NIS position 93 has a major effect on the size and chemical characteristics of the ion cavities as they undergo the transition from the outwardly to the inwardly open conformation and also plays a role in the kinetics and the stoichiometry of transport. The positions equivalent to NIS G93 in other transporters may have a similar function.
Materials and Methods
Vesicular stomatitis virus glycoprotein (VSV-G) pseudotyped, human immunodeficiency virus-1-based, third-generation lentiviruses (35), one bearing WT hNIS and the other G93T hNIS, were generated using calcium phosphate-mediated cotransfection of 293T cells with four plasmids: a CMV promoter-driven packaging construct expressing the gag and pol genes, a Rous sarcoma virus promoter-driven construct expressing rev, a CMV promoter-driven construct expressing the VSV-G envelope, and a self-inactivating transfer construct driven by the CMV promoter containing the human immunodeficiency virus-1 cis-acting sequences and an expression cassette for either WT or G93T hNIS. MDCK cells (105) were transduced by adding 500 μL of viral supernatant per well in a six-well plate. Transduced cells were analyzed using flow cytometry. More details and associated references are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank the members of the Carrasco laboratory and Dr. Myles Akabas for critical reading of the manuscript and helpful suggestions. M.P.B. was supported by Medical Scientist Training Program Training Grant 5T32GM002788. M.J.M. was supported in part by the Howard Hughes Medical Institute–Cal Poly Pomona Undergraduate Research Apprentice Program, and by a National Institutes of Health (NIH) training grant (2R25GM061190). O.D. was supported in part by the American Thyroid Association. A.F. was supported in part by a grant from Ricerca Finalizzata Sanitaria Regione Piemonte. This work was supported by NIH Grants SC1GM086344 (to S.E.), NS-061827 (to L.M.A.), and DK-41544 and CA-098390 (to N.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108278108/-/DCSupplemental.
References
- 1.Dohan O, et al. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 2.Eskandari S, et al. Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. J Biol Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 3.Dohan O, et al. The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci USA. 2007;104:20250–20255. doi: 10.1073/pnas.0707207104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiners C, Hanscheid H, Luster M, Lassmann M, Verburg FA. Radioiodine for remnant ablation and therapy of metastatic disease. Nat Rev Endocrinol. 2011;7:589–595. doi: 10.1038/nrendo.2011.134. [DOI] [PubMed] [Google Scholar]
- 5.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 6.Levy O, et al. Characterization of the thyroid Na+/I- symporter with an anti-COOH terminus antibody. Proc Natl Acad Sci USA. 1997;94:5568–5573. doi: 10.1073/pnas.94.11.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy O, et al. N-linked glycosylation of the thyroid Na+/I- symporter (NIS). Implications for its secondary structure model. J Biol Chem. 1998;273:22657–22663. doi: 10.1074/jbc.273.35.22657. [DOI] [PubMed] [Google Scholar]
- 8.Tazebay UH, et al. The mammary gland iodide transporter is expressed during lactation and in breast cancer. Nat Med. 2000;6:871–878. doi: 10.1038/78630. [DOI] [PubMed] [Google Scholar]
- 9.Riedel C, Levy O, Carrasco N. Post-transcriptional regulation of the sodium/iodide symporter by thyrotropin. J Biol Chem. 2001;276:21458–21463. doi: 10.1074/jbc.M100561200. [DOI] [PubMed] [Google Scholar]
- 10.Smanik PA, et al. Cloning of the human sodium lodide symporter. Biochem Biophys Res Commun. 1996;226:339–345. doi: 10.1006/bbrc.1996.1358. [DOI] [PubMed] [Google Scholar]
- 11.Nicola JP, et al. Iodide transport defect: Functional characterization of a novel mutation in the Na+/I- symporter 5′-untranslated region in a patient with congenital hypothyroidism. J Clin Endocrinol Metab. 2011;96:E1100–1107. doi: 10.1210/jc.2011-0349. [DOI] [PubMed] [Google Scholar]
- 12.Spitzweg C, Morris JC. Genetics and phenomics of hypothyroidism and goiter due to NIS mutations. Mol Cell Endocrinol. 2010;322:56–63. doi: 10.1016/j.mce.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed-Tsur MD, De la Vieja A, Ginter CS, Carrasco N. Molecular characterization of V59E NIS, a Na+/I- symporter mutant that causes congenital I- transport defect. Endocrinology. 2008;149:3077–3084. doi: 10.1210/en.2008-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De La Vieja A, Ginter CS, Carrasco N. The Q267E mutation in the sodium/iodide symporter (NIS) causes congenital iodide transport defect (ITD) by decreasing the NIS turnover number. J Cell Sci. 2004;117:677–687. doi: 10.1242/jcs.00898. [DOI] [PubMed] [Google Scholar]
- 15.Levy O, Ginter CS, De la Vieja A, Levy D, Carrasco N. Identification of a structural requirement for thyroid Na+/I- symporter (NIS) function from analysis of a mutation that causes human congenital hypothyroidism. FEBS Lett. 1998;429:36–40. doi: 10.1016/s0014-5793(98)00522-5. [DOI] [PubMed] [Google Scholar]
- 16.Dohan O, Gavrielides MV, Ginter C, Amzel LM, Carrasco N. Na(+)/I(-) symporter activity requires a small and uncharged amino acid residue at position 395. Mol Endocrinol. 2002;16:1893–1902. doi: 10.1210/me.2002-0071. [DOI] [PubMed] [Google Scholar]
- 17.De la Vieja A, Ginter CS, Carrasco N. Molecular analysis of a congenital iodide transport defect: G543E impairs maturation and trafficking of the Na+/I- symporter. Mol Endocrinol. 2005;19:2847–2858. doi: 10.1210/me.2005-0162. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 19.De la Vieja A, Reed MD, Ginter CS, Carrasco N. Amino acid residues in transmembrane segment IX of the Na+/I- symporter play a role in its Na+ dependence and are critical for transport activity. J Biol Chem. 2007;282:25290–25298. doi: 10.1074/jbc.M700147200. [DOI] [PubMed] [Google Scholar]
- 20.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugarx symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abramson J, Wright EM. Structure and function of Na(+)-symporters with inverted repeats. Curr Opin Struct Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosugi S, Inoue S, Matsuda A, Jhiang SM. Novel, missense and loss-of-function mutations in the sodium/iodide symporter gene causing iodide transport defect in three Japanese patients. J Clin Endocrinol Metab. 1998;83:3373–3376. doi: 10.1210/jcem.83.9.5245. [DOI] [PubMed] [Google Scholar]
- 24.Parent L, Supplisson S, Loo DD, Wright EM. Electrogenic properties of the cloned Na+/glucose cotransporter: I. Voltage-clamp studies. J Membr Biol. 1992;125:49–62. doi: 10.1007/BF00235797. [DOI] [PubMed] [Google Scholar]
- 25.Loo DD, Eskandari S, Boorer KJ, Sarkar HK, Wright EM. Role of Cl- in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J Biol Chem. 2000;275:37414–37422. doi: 10.1074/jbc.M007241200. [DOI] [PubMed] [Google Scholar]
- 26.Zuckier LS, et al. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J Nucl Med. 2004;45:500–507. [PubMed] [Google Scholar]
- 27.Rudnick G. What is an antidepressant binding site doing in a bacterial transporter? ACS Chem Biol. 2007;2:606–609. doi: 10.1021/cb7001818. [DOI] [PubMed] [Google Scholar]
- 28.Weyand S, et al. Structure and molecular mechanism of a nucleobase-cation-symport-1 family transporter. Science. 2008;322:709–713. doi: 10.1126/science.1164440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ressl S, Terwisscha van Scheltinga AC, Vonrhein C, Ott V, Ziegler C. Molecular basis of transport and regulation in the Na(+)/betaine symporter BetP. Nature. 2009;458:47–52. doi: 10.1038/nature07819. [DOI] [PubMed] [Google Scholar]
- 30.Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- 32.Forrest LR, et al. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci USA. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Committee to Assess the Health Implications of Perchlorate Ingestion, National Research Council. Health Implications of Perchlorate Ingestion. Washington, DC: Natl Academic Press; 2005. pp. 75–114. [Google Scholar]
- 34.Hingorani M, et al. The biology of the sodium iodide symporter and its potential for targeted gene delivery. Curr Cancer Drug Targets. 2010;10:242–267. doi: 10.2174/156800910791054194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.