Abstract
The world is currently undergoing an unprecedented decline in biodiversity, which is mainly attributable to human activities. For instance, nonnative species introduction, combined with the extirpation of native species, affects biodiversity patterns, notably by increasing the similarity among species assemblages. This biodiversity change, called taxonomic homogenization, has rarely been assessed at the world scale. Here, we fill this gap by assessing the current homogenization status of one of the most diverse vertebrate groups (i.e., freshwater fishes) at global and regional scales. We demonstrate that current homogenization of the freshwater fish faunas is still low at the world scale (0.5%) but reaches substantial levels (up to 10%) in some highly invaded river basins from the Nearctic and Palearctic realms. In these realms experiencing high changes, nonnative species introductions rather than native species extirpations drive taxonomic homogenization. Our results suggest that the “Homogocene era” is not yet the case for freshwater fish fauna at the worldwide scale. However, the distressingly high level of homogenization noted for some biogeographical realms stresses the need for further understanding of the ecological consequences of homogenization processes.
Keywords: β-diversity, conservation, nonnative species, uniqueness, differentiation
Human activities affect both abiotic (e.g., global warming, pollution) and biotic (e.g., overexploitation, species introduction) components of ecosystems (1–3). Anthropogenic stressors are responsible for changes in species assemblages through two complementary processes: extirpations and introductions (4). The resulting taxonomic changes affect both the number of species in each locality (α-diversity) and the similarity in species composition among localities (β-diversity). Although the way extirpations and introductions affect α-diversity patterns has been fairly well documented at the world scale (5, 6), their effect on β-diversity remains little known. However, local and regional studies report that introduced species can increase the taxonomic similarity of assemblages in terms of species composition (i.e., decreased β-diversity), a process called taxonomic homogenization (7–10). Taxonomic homogenization is a crucial component of the current biodiversity crisis throughout the world (11–15) because it may open the door to a new era that has been called the “Homogocene” or “New Pangea” (16). The end point of the Homogocene era is characterized by a planet where all previously independent regions become linked by human activities, lose their taxonomic distinctiveness, and share a common and uniform pool of species, which might lead to reduced resistance and resilience of ecosystems to perturbations (14, 16). Although local to regional measurements of change in taxonomic similarity caused by human activities have been made (8–10), empirical assessment of worldwide change in taxonomic similarity is scarce (cf. 17). Here, we use a global database on freshwater fish occurrences to measure historical and current taxonomic similarity of fish faunas at the world, realm, and river basin scales. We show that until now, the increase of taxonomic similarity (i.e., taxonomic homogenization) has been extremely low at the world scale but has reached substantial levels for highly invaded realms, such as the Nearctic and Palearctic, especially in some of their river basins.
We used a global database on historical and current freshwater fish occurrences over 1,054 river basins (6). These changes in species composition are largely attributable to human activities (6, 18, 19) and have mainly occurred during the past 2 centuries (20–22). We computed Jaccard’s similarity between each pair of river basins for historical and current fauna composition (9, 23).
At the world scale, we analyzed the distribution of pairwise taxonomic similarity for all basin pairs over the world for historical and current situations. Similarly, at the realm scale, we analyzed, for each of the six biogeographical realms, the distribution of pairwise similarity for all basin pairs belonging to the same realm. For these two scales of analysis, we additionally quantified historical and current taxonomic uniqueness as the proportion of basin pairs having no species in common at the realm and global scales (9).
In addition to assessing changes at the world and realm scales, we studied changes in taxonomic similarity at the basin scale by measuring the change in the average taxonomic similarity between each river basin and all the other basins belonging to the same realm, such that we obtained a single score of change in taxonomic similarity for each river basin. We tested the significance of the change observed in mean taxonomic similarity for each river basin against the null hypothesis that species introduction and extirpation were random processes independent of species identity (Methods).
Results and Discussion
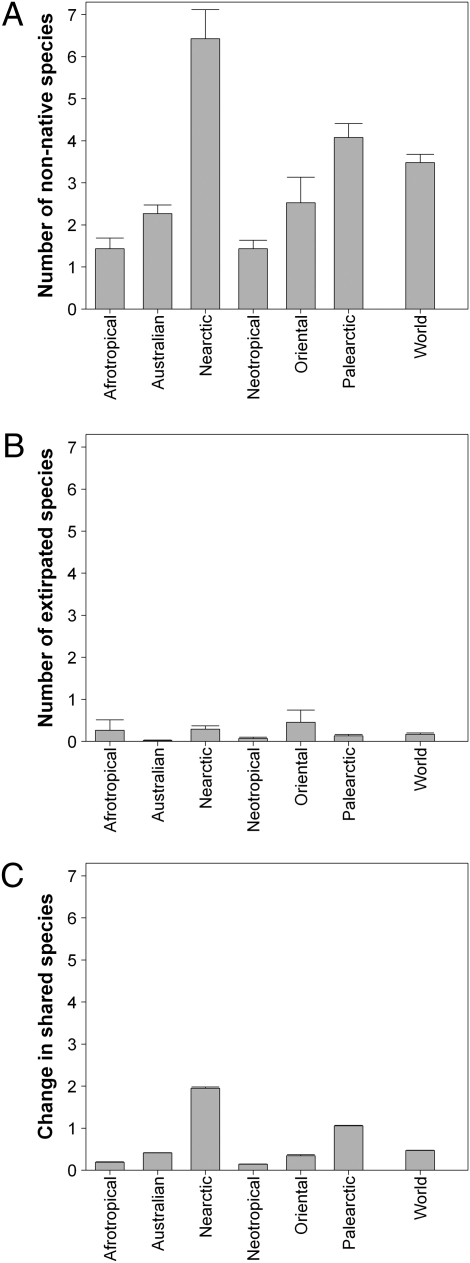
The Palearctic and Nearctic realms are the two most invaded realms (Fig. 1, Fig. 2A, and Table 1), with a mean of six and four nonnative species per basin, respectively, contrasting with the Afrotropical and Neotropical realms, where, on average, only two nonnative species have been introduced per basin (Fig. 2A). Extirpations were less frequent than introductions, with, on average, less than one species extirpated per basin (Fig. 2B). The number of species shared by two basins has increased slightly at the world scale and for most realms (i.e., less than 0.5 additional shared species; Fig. 2C), although the increase is higher for the Nearctic and Palearctic realms (i.e., up to 2 additional shared species; Fig. 2C).
Fig. 1.
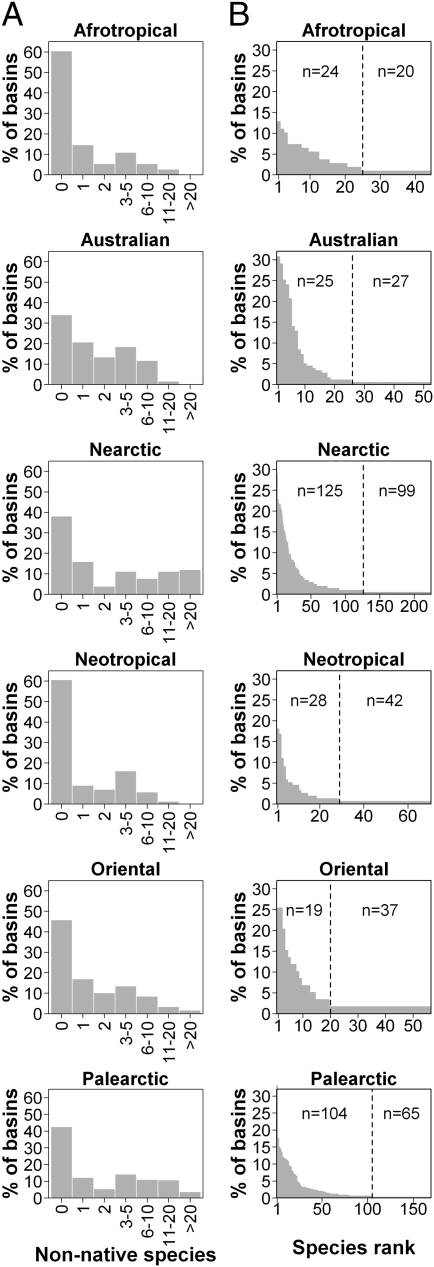
Patterns of nonnative species introductions in the six realms. (A) Distribution of the number of nonnative species in basins. (B) Percentage of occurrence of nonnative species in each realm. The vertical dashed line separates nonnative species that have been introduced in only one basin (Right) from nonnative species that have been introduced in at least two basins (Left).
Fig. 2.
Changes in fish assemblage structure induced by human activities across biogeographical regions and at the world scale. The number of nonnative species introduced per river basin (A), number of native species extirpated per river basin (B), and increase in the number of species shared by river basin pairs between the historical and the current situations (C) are shown. The historical situation indicates assemblages before introductions and extirpations attributable to human activities and corresponds to the preindustrial period. The current situation indicates today’s assemblages (native species − extirpated species + nonnative species). Values are mean (±SEM) over basins from each biogeographical realm or over all the basins considered at the world scale. SEM bars, although present in the figure, are sometimes not visible because of their low value.
Table 1.
Number of native, nonnative, extirpated, and threatened species at the realm and world scales
| No. basins | No. native species | No. species occurring in basins outside their native range* | Translocated nonnative species, % | No. species encountering at least one extirpation | Species evaluated by the IUCN, % | No. threatened species | Threatened species among species evaluated, % | |
| Afrotropical | 109 | 2,333 | 44 | 59 | 29 | 40 | 237 | 25 |
| Australian | 179 | 404 | 52 | 37 | 4 | 19 | 30 | 40 |
| Nearctic | 207 | 694 | 224 | 79 | 47 | 23 | 96 | 60 |
| Neotropical | 155 | 3,900 | 70 | 61 | 11 | 2 | 35 | 43 |
| Oriental | 59 | 1,606 | 56 | 48 | 27 | 3 | 24 | 56 |
| Palearctic | 345 | 1,011 | 169 | 82 | 29 | 25 | 110 | 44 |
| World | 1,054 | 9,722 | 444 | 75† | 145 | 16 | 524 | 35 |
IUCN, International Union for Conservation of Nature.
*Translocated species (nonnative species introduced in river basins belonging to a realm where they are present as native) and exotic species (nonnative species introduced in a realm where they are not present as native).
†Only 25% (111 species) have been introduced in at least one realm where they historically did not occur.
Following species introductions and extirpations, uniqueness has decreased by 13% at the global scale (i.e., from a historical value of 81% to a current value of 68%) and by less than 7% in all realms except the Nearctic and Palearctic, which showed a 15% drop and a 9% drop, respectively (Fig. 3A). Historical pairwise taxonomic similarity across basins was low in the six realms (mean < 10%; Fig. 3B). Mean taxonomic similarity has increased over the past 2 centuries by less than 0.3% in the Afrotropical, Australian, Neotropical, and Oriental realms (Fig. 3B). In contrast, it increased by 1.1% in the Nearctic and 0.6% in the Palearctic (Fig. 3B). At the global scale, mean taxonomic similarity has increased by only 0.5%.
Fig. 3.
Changes in taxonomic uniqueness and taxonomic similarity among fish faunas between historical and current situations for the six realms and at the world scale. The taxonomic uniqueness (percentage of river basin pairs sharing no species) (A), mean taxonomic similarity between pairs of river basins (measured using Jaccard’s similarity index) (B), and proportion of river basin pairs showing taxonomic homogenization (“h,” red), no changes (“0”, white) or taxonomic differentiation (“d,” blue) between historical and current situations (C) are shown. The historical situation indicates assemblages before introductions and extirpations attributable to human activities and corresponds to the preindustrial period. The current situation indicates today’s assemblages (native species − extirpated species + nonnative species).
These patterns were confirmed by the analysis of complementary distribution parameters (Table 2). For instance, the median value of pairwise taxonomic similarity remains low (<0.2%) at the world scale and for the Afrotropical, Neotropical, and Oriental realms. In contrast, for the Nearctic and Palearctic realms, the median values increased by 2.56% and 0.99%, respectively (Table 2). For all six realms, the distribution became less right-skewed and less peaked (Table 2), confirming the tendency toward a loss of uniqueness and an increase in taxonomic similarity. Nevertheless, it is noteworthy that the proportion of basin pairs becoming less similar (i.e., experiencing taxonomic differentiation) exceeds 17% in the six realms (Fig. 3C). In the Afrotropical and Neotropical realms, more than 70% of basin pairs have not experienced change in their taxonomic similarity and more than half of the remaining basin pairs experienced taxonomic differentiation (Fig. 3C). Furthermore, the two realms with the highest frequency of differentiation (25.4% in the Nearctic and 27.3% in the Palearctic) are also those where homogenization is the most frequent (36% and 28%, respectively; Fig. 3C).
Table 2.
Distribution parameters of pairwise taxonomic similarity among river basins in the six biogeographical realms and at the world scale for the historical and current situations
| Median, % | Skewness | Kurtosis | ||
| Afrotropical | Historical | 0 | 3.31 | 14.8 |
| Current | 0 | 2.89 | 10.0 | |
| Australian | Historical | 0 | 3.11 | 10.3 |
| Current | 0 | 2.96 | 9.7 | |
| Nearctic | Historical | 1.32 | 2.64 | 8.1 |
| Current | 3.88 | 2.46 | 7.4 | |
| Neotropical | Historical | 0 | 4.55 | 26.8 |
| Current | 0.16 | 4.19 | 22.1 | |
| Oriental | Historical | 0 | 4.06 | 17.7 |
| Current | 0 | 1.00 | 17.4 | |
| Palearctic | Historical | 2.04 | 2.75 | 8.6 |
| Current | 3.03 | 2.55 | 7.4 | |
| World | Historical | 0 | 6.35 | 50.4 |
| Current | 0 | 5.68 | 42.0 |
The historical situation indicates assemblages before introductions and extirpations attributable to human activities and corresponds to the preindustrial period. The current situation indicates today’s assemblages (native species − extirpated species + nonnative species).
Overall, these results indicate a weak homogenization at the global and realm scales, although it is stronger in the Nearctic and Palearctic. Previous studies at the regional scale reported a greater increase of taxonomic similarity (i.e., 7–20%) over a wide range of plant and animal phyla, which led to the forecast of a similarly high increase of taxonomic similarity at the global scale (7, 9, 11, 17, 18, 24, 25). Our results are in contrast to this last idea, because in our study, the taxonomic similarity of fish faunas remains largely unchanged at the world scale (i.e., 0.5%).
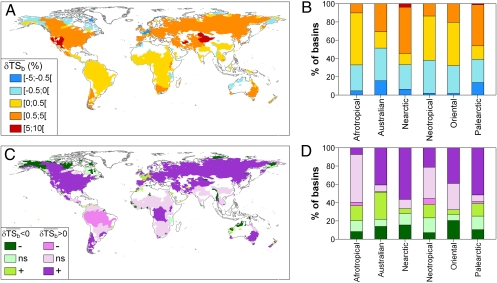
Analyses at the basin scale confirmed the trends observed at the world and realm scales. Indeed, taxonomic homogenization occurred in most of the basins, even though a few displayed differentiation (Fig. 4 A and B). The null model revealed that most of the homogenizations observed are significantly higher than expected, except in the Afrotropical and Neotropical realms (Fig. 4 C and D). Likewise, most of the differentiations are of lower magnitude than expected. This is the case for the basins of Western Europe (Fig. 4 C and D); given the high number of nonnative species they received, if nonnative species introduction had been random, the intensity of differentiation would have been higher than observed.
Fig. 4.
World maps showing the mean change in taxonomic similarity between each basin and the other basins belonging to the same realm (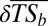 ). The direction and intensity of change from the historical situation to the current situation (A) and the proportion of basins in each class of
). The direction and intensity of change from the historical situation to the current situation (A) and the proportion of basins in each class of 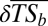 per realm (B) are shown. (C) Significance of the observed taxonomic homogenization or taxonomic differentiation against a null model testing random native species extirpation and nonnative species introduction. The “+” symbol indicates that the changes observed in taxonomic similarity are significantly greater than expected under the null hypothesis (P > 0.975), whereas the “−” symbol indicates that the changes observed in taxonomic similarity are significantly lower than expected under the null hypothesis (P < 0.025). ns indicates that the changes observed in taxonomic similarity are not significantly different from the values expected under the null hypothesis. (D) Per realm proportion of basins in each level of significance of
per realm (B) are shown. (C) Significance of the observed taxonomic homogenization or taxonomic differentiation against a null model testing random native species extirpation and nonnative species introduction. The “+” symbol indicates that the changes observed in taxonomic similarity are significantly greater than expected under the null hypothesis (P > 0.975), whereas the “−” symbol indicates that the changes observed in taxonomic similarity are significantly lower than expected under the null hypothesis (P < 0.025). ns indicates that the changes observed in taxonomic similarity are not significantly different from the values expected under the null hypothesis. (D) Per realm proportion of basins in each level of significance of 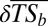 .
.
Finally, using generalized least squares models, we showed that the factor primarily correlating with change in mean taxonomic similarity per river basin is the number of nonnative species introduced (Table 3): The higher the nonnative species richness, the greater is the increase in mean taxonomic similarity. This positive relationship also varies according to the historical taxonomic similarity as well as the historical number of native species (significant interactions terms in Table 3). Specifically, these interactions indicate that the increase in taxonomic similarity with species richness is more marked for basins with historically low native richness and low taxonomic similarity.
Table 3.
Drivers of the observed change in mean taxonomic similarity from the historical situation to the current situation in the 1,054 basins
| Coefficients (±SE) | df | F value | P value | |
| Historical taxonomic similarity | 4.5 × 10−3 (±6.8 × 10−3) | 1; 1048 | 2.3 | 0.13 |
| Historical species richness | 9.3 × 10−6 (±3.5 × 10−6) | 1; 1048 | 0.4 | 0.55 |
| No. nonnative species introduced | 2.2 × 10−3 (±7.5 × 10−5) | 1; 1048 | 1,247.2 | <0.001 |
| Historical taxonomic similarity × no. nonnative species introduced | −9.8 × 10−3 (±8.2 × 10−4) | 1; 1048 | 125.1 | <0.001 |
| Historical species richness × no. nonnative species introduced | −4.2 × 10−6 (±4.4 × 10−7) | 1; 1048 | 90.1 | <0.001 |
An AIC-based selection (cutoff: ∆AIC = 4) was computed on all the generalized least squares models accounting for all the possible pairwise interactions between five factors: historical taxonomic similarity, historical species richness, number of native species extirpated, number of nonnative species introduced, and identity of the realm to which the basin belongs. The best model is presented. It has an AIC of −7,256.
Overall, human preference for a small number of species and the resulting uneven frequency of introduction of nonnative species (Fig. 1B) either caused taxonomic homogenization or at least mitigated the intensity of taxonomic differentiation. These changes in β-diversity are roughly consistent with the global distribution of nonnative fish richness (α-diversity) (6). Of the six introduction hotspots defined by Leprieur et al. (6), five have experienced marked taxonomic homogenization, namely, the Pacific Coast of North and Central America, southern South America, central Eurasia, South Africa and Madagascar, southern Australia, and New Zealand (Fig. 4A). This confirms that nonnative species are responsible for changes in both α- and β-diversity, a trend also confirmed by the strong contribution of the nonnative species to the change in taxonomic similarity (Table 3). The last introduction hotspot, Western and Southern Europe, exhibits a particular pattern because of differentiation in some basins (Fig. 4 A and B), even though the overall Western and Southern European trend is toward homogenization. This trend was consistent with previous regional studies showing that nonnative species identity (exotic or translocated) can have an opposite effect on change in taxonomic similarity (11). It should, however, be noted that although we detected a general trend toward homogenization at global, regional, and basin scales (Figs. 3 and 4), the homogenization strength remained lower than that reported in the literature (7, 9, 11, 17, 18, 24, 25), testifying that the Homogocene or New Pangea era (16) is still not the case for most of the earth’s freshwater fish faunas. However, the relatively high levels of homogenization and differentiation noted for the Palearctic and Nearctic realms indicate a need for a better understanding of the ecological consequences of human alterations in β-diversity.
Even though policies have been implemented to prevent new introductions, we should be aware that the increase of global exchanges will certainly promote new introductions through ballast water, fish farming, and the aquarium trade (6, 22, 26, 27). In this context, predicting consecutive future changes in taxonomic similarity is a challenging issue because the identity of the future nonnative species may either induce taxonomic homogenization or differentiation (11, 28). In addition, the impact of nonnative species introductions on ecosystem functioning has been mostly addressed through species-centered approaches on a limited number of invasive species (29), whereas the ecological consequences of taxonomic homogenization remain largely unknown to date (cf. 13, 14, 30). Forthcoming studies should therefore focus on assessing how changes in β-diversity affect ecological and evolutionary processes from local to global scales (31). Based on our results, we appeal for studies to focus on the areas that experience high homogenization trends. Because most of these areas are also recognized as gathering a high number of threatened species and facing a large range of human-induced physical disturbances (3, 6), local and detailed studies are urgently needed to decide whether it is appropriate or not to set up efficient control, remediation, and eradication procedures (32). In addition to ecological concerns, further studies are needed to answer societal questions, such as how taxonomic homogenization will modify our perception of biodiversity through the loss of cultural landmarks.
Methods
Database.
We used a uniquely comprehensive database of six freshwater fish species distributions (available at http://data.freshwaterbiodiversity.eu). It contains freshwater fish species lists from 1,054 river basins scattered throughout the world covering more than 80% of the earth’s continental surface. For each of the 39,704 occurrences of 9,722 fish species, the native, extirpated, or established nonnative (i.e., nonnative species occurrences attributable only to human stocking were not considered) status is given. We studied changes in taxonomic similarity among fish assemblages at the world scale and in the six biogeographical realms: Afrotropical, Australian (including Oceania), Nearctic, Neotropical, Oriental, and Palearctic. Considering realms independently was motivated by their distinct history, fauna, and human activities (5, 6). A basin scale analysis was also performed to visualize how introductions and extirpations affect taxonomic similarity in each basin compared with all the basins belonging to the same realm.
Measuring Change in Taxonomic Similarity.
We assessed how the change in Jaccard’s similarity before and after species introductions and extirpations affected the taxonomic composition of assemblages (9, 23). Jaccard’s taxonomic similarity index (TSJ) is as follows: 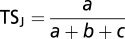 , where a is the number of species shared by two assemblages and b and c are the number of species present only in the first and second assemblages, respectively. TSJ equals zero when the two assemblages share no species (a = 0) and equals unity when they have identical species composition (b = c = 0).
, where a is the number of species shared by two assemblages and b and c are the number of species present only in the first and second assemblages, respectively. TSJ equals zero when the two assemblages share no species (a = 0) and equals unity when they have identical species composition (b = c = 0).
For each pair of river basins (i.e., pair of fish assemblages), taxonomic similarity was computed for “historical” and “current” species composition. Historical refers to the past fauna with only native species, and thus roughly corresponds to the preindustrial period (i.e., before the 18th century), because industrialization and associated goods exchanges are recognized as the main driver of the introduction of fish (as well as of other animals) mainly for aquaculture, fishing, and ornamental purposes (6). Current refers to present fauna with the nonnative species and without the native ones that have been extirpated.
We investigated changes in taxonomic similarity at three complementary scales (11). At the global scale, we studied the change in the distribution of taxonomic similarity values obtained for all basin pairs over the world from the historical situation to the current situation, using four parameters (mean, median, skewness, and kurtosis). We also computed the proportion of basin pairs that show homogenization (i.e., a rise in taxonomic similarity between the historical situation and the current situation) or differentiation (i.e., a decline in taxonomic similarity between the historical situation and the current situation).
For each of the six biogeographical realms, we conducted the same analyses by only considering changes in taxonomic similarity between pairs of basins belonging to the same realm.
For these two scales, we additionally quantified historical and current taxonomic uniqueness as the proportion of basin pairs having no species in common over realm and global extents, respectively (9).
The two latter approaches summarize taxonomic similarity over large extents but do not permit us to evaluate changes at the basin scale. We hence designed a basin-scale approach by measuring the change in the average pairwise taxonomic similarity between each river basin and all the other basins belonging to the same realm (11).
We then used a spatial generalized least squares model to test the effect of the realm to which the basin belongs, the historical taxonomic similarity, the historical species richness, the number of nonnative species introduced, and the number of native species extirpated in each basin on the mean changes in similarity calculated for each basin. We consider a full model, including all simple terms as well as all two-term interactions. The model was simplified by investigating change in the Akaike information criterion (AIC) after removing terms one by one. We conserved and interpreted the model that displayed the lowest AIC.
Testing Observed Changes in Taxonomic Similarity at the River Basin Scale.
We tested the significance of the observed change in mean taxonomic similarity for each river basin against the null hypothesis that species introduction and extirpation were random processes. To this aim, for each river basin, starting from historical fish species composition, we computed current composition by randomly deleting the observed number of extirpated species and then randomly adding the current observed number of nonnative species. The simulated extirpated species were randomly drawn among the pool of native species listed as vulnerable by the International Union for Conservation of Nature (33) in the realm to which the focal basin belongs (Table 1). The nonnative species added were randomly drawn among the pool of nonnative species in the realm to which the basin belongs (Table 1). This procedure ensures that the observed current species richness is kept constant during the random process while preventing unrealistic introductions or extirpations.
The procedure was carried out 999 times for each river basin. For each iteration, the mean taxonomic similarity with the other river basins from the same realm was computed and was then compared with the historical value to provide simulated change in taxonomic similarity. The value observed was finally compared with the distribution of these simulated values to provide a P value for each river basin. A P value lower than 0.025 indicated a change in taxonomic similarity significantly lower than expected under the null hypothesis, whereas a P value greater than 0.975 indicated a change in taxonomic similarity significantly higher than expected.
Acknowledgments
We thank Alexis Chaine and David Mouillot for comments on this manuscript and Peter Winterton for correcting the English. We are grateful to two anonymous reviewers and to the editor for their constructive comments, which have improved the manuscript. This work was supported by the National Agency for Research on Freshwater Fish Diversity (Grant ANR-06-BDIV-010) and by the European Union Biodiversity of Freshwater Ecosystems (BIOFRESH) project (Seventh Framework European Program, Contract 226874).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. F.R. is a guest editor invited by the Editorial Board.
References
- 1.Butchart SHM, et al. Global biodiversity: Indicators of recent declines. Science. 2010;328:1164–1168. doi: 10.1126/science.1187512. [DOI] [PubMed] [Google Scholar]
- 2.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. Human domination of Earth’s ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 3.Vörösmarty CJ, et al. Global threats to human water security and river biodiversity. Nature. 2010;467:555–561. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 4.Sala OE, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- 5.Blanchet S, et al. Broad-scale determinants of non-native fish species richness are context-dependent. Proc Biol Sci. 2009;276:2385–2394. doi: 10.1098/rspb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008;6:e28. doi: 10.1371/journal.pbio.0060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKinney ML, Lockwood JL. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol Evol. 1999;14:450–453. doi: 10.1016/s0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- 8.Olden JD, Poff NL, McKinney ML. Forecasting faunal and floral homogenization associated with human population geography in North America. Biol Conserv. 2006;127:261–271. [Google Scholar]
- 9.Rahel FJ. Homogenization of fish faunas across the United States. Science. 2000;288:854–856. doi: 10.1126/science.288.5467.854. [DOI] [PubMed] [Google Scholar]
- 10.Winter M, et al. Plant extinctions and introductions lead to phylogenetic and taxonomic homogenization of the European flora. Proc Natl Acad Sci USA. 2009;106:21721–21725. doi: 10.1073/pnas.0907088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leprieur F, Beauchard O, Hugueny B, Grenouillet G, Brosse S. Null model of biotic homogenization: A test with the European freshwater fish fauna. Divers Distrib. 2008;14:291–300. [Google Scholar]
- 12.Lockwood JL, McKinney ML. Biotic Homogenization. New York: Kluwer; 2001. [Google Scholar]
- 13.Olden JD. Biotic homogenization: A new research agenda for conservation biogeography. J Biogeogr. 2006;33:2027–2039. [Google Scholar]
- 14.Olden JD, Leroy Poff N, Douglas MR, Douglas ME, Fausch KD. Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol. 2004;19:18–24. doi: 10.1016/j.tree.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Rahel FJ. Homogenization, differentiation, and the widespread alteration of fish faunas. In: Gido K, Jackson D, editors. Community Ecology of Stream Fishes. Bethesda: American Fisheries Society; 2010. [Google Scholar]
- 16.Rosenzweig ML. The four questions: What does the introduction of exotic species do to diversity? Evol Ecol Res. 2001;3:361–367. [Google Scholar]
- 17.Spear D, Chown SL. Taxonomic homogenization in ungulates: Patterns and mechanisms at local and global scales. J Biogeogr. 2008;35:1962–1975. [Google Scholar]
- 18.Olden JD, Poff NL. Ecological processes driving biotic homogenization: Testing a mechanistic model using fish faunas. Ecology. 2004;85:1867–1875. [Google Scholar]
- 19.Taylor BW, Irwin RE. Linking economic activities to the distribution of exotic plants. Proc Natl Acad Sci USA. 2004;101:17725–17730. doi: 10.1073/pnas.0405176101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine JM, D’Antonio CM. Forecasting biological invasions with increasing international trade. Conserv Biol. 2003;17:322–326. [Google Scholar]
- 21.Pysek P, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA. 2010;107:12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Zanden MJ. The success of animal invaders. Proc Natl Acad Sci USA. 2005;102:7055–7056. doi: 10.1073/pnas.0502549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olden JD, Rooney TP. On defining and quantifying biotic homogenization. Glob Ecol Biogeogr. 2006;15:113–120. [Google Scholar]
- 24.Qian H, Ricklefs RE. The role of exotic species in homogenizing the North American flora. Ecol Lett. 2006;9:1293–1298. doi: 10.1111/j.1461-0248.2006.00982.x. [DOI] [PubMed] [Google Scholar]
- 25.Rooney TP, Wiegmann SM, Rogers DA, Waller DM. Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv Biol. 2004;18:787–798. [Google Scholar]
- 26.Leprieur F, et al. Scientific uncertainty and the assessment of risks posed by non-native freshwater fishes. Fish Fish. 2009;10:88–97. [Google Scholar]
- 27.Strecker AL, Campbell PM, Olden JD. The aquarium trade as an invasion pathway in the Pacific Northwest. Fisheries (Bethesda, Md) 2011;36:74–85. [Google Scholar]
- 28.Lockwood JL. Life in a double-hotspot: The transformation of Hawaiian passerine bird diversity following invasion and extinction. Biol Invasions. 2006;8:449–457. [Google Scholar]
- 29.Cucherousset J, Olden JD. Ecological impacts of nonnative freshwater fishes. Fisheries (Bethesda, Md) 2011;36:215–230. [Google Scholar]
- 30.France KE, Duffy JE. Diversity and dispersal interactively affect predictability of ecosystem function. Nature. 2006;441:1139–1143. doi: 10.1038/nature04729. [DOI] [PubMed] [Google Scholar]
- 31.Whittaker RJ, et al. Conservation biogeography: Assessment and prospect. Divers Distrib. 2005;11:3–23. [Google Scholar]
- 32.Simberloff D, Parker IM, Windle PN. Introduced species policy, management, and future research needs. Front Ecol Environ. 2005;3:12–20. [Google Scholar]
- 33.Baillie JEM, Hilton-Taylor C, Stuart S. 2004 IUCN Red List of Threatened Species: A Global Species Assessment. Switzerland: International Union for Conservation of Nature, Gland; 2004. [Google Scholar]