Abstract
A great deal is known about the export of spliced mRNAs, but little is known about the export of mRNAs encoded by human cellular genes that naturally lack introns. Here, we investigated the requirements for export of three naturally intronless mRNAs (HSPB3, IFN-α1, and IFN-β1). Significantly, we found that all three mRNAs are stable and accumulate in the cytoplasm, whereas size-matched random RNAs are unstable and detected only in the nucleus. A portion of the coding region confers this stability and cytoplasmic localization on the naturally intronless mRNAs and a cDNA transcript, which is normally retained in the nucleus and degraded. A polyadenylation signal, TREX mRNA export components, and the mRNA export receptor TAP are required for accumulation of the naturally intronless mRNAs in the cytoplasm. We conclude that naturally intronless mRNAs contain specific sequences that result in efficient packaging into the TREX mRNA export complex, thereby supplanting the splicing requirement for efficient mRNA export.
Although the vast majority of pre-mRNAs in higher eukaryotes contain introns, 5% of human protein-coding genes do not contain introns (1). However, these naturally intronless mRNAs encode proteins of critical importance, including the histones, the c-Jun proto-oncoprotein, and the antiviral IFN proteins. In addition, many viral genes lack introns. Because most genes in higher eukaryotes contain introns, understanding the gene expression of naturally intronless genes has lagged behind that of intron-containing genes.
Studies over the past several years have revealed that splicing is not only essential for removing introns but also plays important roles in numerous other steps in gene expression because of functional coupling among the different steps. For example, splicing is coupled to RNA polymerase II (RNAP II) transcription, 3′ end formation, mRNA stability, mRNA export, translation, and cytoplasmic localization of mRNA (2–5). Several studies indicate that mRNAs generated by splicing are both more stable and more efficiently exported to the cytoplasm than their cDNA transcript counterparts (6, 7). This splicing-dependent enhancement of mRNA stability/export is because of, at least in part, recruitment of the conserved transcription/export (TREX) complex, which contains the multisubunit THO complex and the proteins UAP56, Aly, Tex1, and CIP29 (6–8). In vitro studies showed that the human TREX complex associates with the 5′ end of mRNAs during splicing through an interaction between Aly and the cap-binding complex (9, 10). Because splicing occurs cotranscriptionally, the human TREX complex is indirectly recruited to mRNA during transcription (4, 9). In contrast, in yeast, in which most genes lack introns, the TREX complex is directly recruited to mRNA during transcription and 3′ end formation (11–13). The interactions between the 3′ end formation machinery and the TREX complex are conserved from yeast to humans, indicating that a role for 3′ end formation in mRNA export is also likely in humans (13).
The RNA processing machinery and the TREX complex are evenly distributed throughout the nucleoplasm, with a portion concentrated in 25–50 discrete foci known as nuclear speckles (6, 14). Total polyA RNA has a similar distribution (14, 15). Although the details remain to be established, several studies are consistent with the proposal that splicing occurs in association with nuclear speckles (refs. 16 and 17 and references therein), and recent work revealed that TREX components play a role in releasing both polyA RNA and spliced reporter mRNAs from these domains for export (17). During the final nuclear steps in the export pathway, the mRNA export receptor TAP associates with the mRNA through interactions with Aly and functions to transport the mRNA to the cytoplasm (7, 18). It is currently not known whether naturally intronless cellular mRNAs associate with nuclear speckles, and the factors and mechanism of export of these mRNAs are not yet understood.
In higher eukaryotes, most of the work on export of mRNAs derived from naturally intronless genes has been done with viruses. A key conclusion from these studies is that viral intronless mRNAs interact with specific cellular or viral proteins to recruit the cellular export machinery (19–23). For example, in recent work, Boyne et al. (19) showed that a herpes virus protein mediates viral intronless mRNA export through the TREX/TAP pathway. For naturally occurring mammalian cellular intronless mRNAs, most studies have been carried out with histone H2A mRNA, which contains elements in the coding region that promote mRNA export (24–26). One of these elements binds to members of the SR family of splicing factors, which mediates conserved interactions with TAP (27, 28). However, H2A mRNA is unique relative to most naturally intronless mRNAs, because 3′ end formation of H2A requires a stem-loop structure and the mRNA lacks a polyA tail. Some work has also been carried out with naturally human cellular intronless mRNAs that contain polyA tails (21, 29). The work by Kimura et al. (29) found that IFN-α1 mRNA export is dependent on Exportin1, and the work by Guang et al. (21) found that specific portions of the coding region of c-Jun are required for cytoplasmic accumulation of mRNA. Finally, export studies have been carried out using cDNA transcripts (30–32). Although these transcripts are typically unstable and inefficiently exported (33–35), the length of the cDNA is a determinant of export (30) through the TREX/TAP pathway (36). The relationship between export of cDNA transcripts and mRNAs derived from naturally intronless cellular genes remains to be determined.
In this study, we show that three naturally intronless mRNAs (HSPB3, IFN-α1, and IFN-β1) are all initially evenly distributed throughout the nucleoplasm and do not associate with nuclear speckles. The mRNAs then accumulate in the cytoplasm. In contrast, size-matched transcripts of random sequence are detected only in the nucleoplasm and are highly unstable. Thus, transcript length is not a determinant for the stable accumulation of naturally intronless mRNAs in the cytoplasm. Consistent with this conclusion, we identified a portion of the coding region in each naturally intronless mRNA that is required for their cytoplasmic accumulation. We provide evidence that export of the naturally intronless mRNAs occurs through the TREX/TAP pathway and is transcription-independent but polyadenylation-dependent. Together, our data indicate that naturally intronless mRNAs are exported from the nucleus through coding region sequences that bypass splicing-dependent mRNA export.
Results
Nucleocytoplasmic Distribution of Naturally Intronless Human Cellular mRNAs.
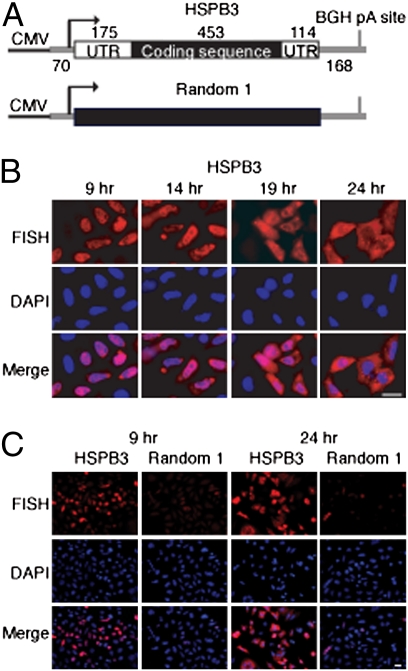
To investigate the nucleocytoplasmic distribution of naturally intronless mRNAs, we inserted three different human genes that lack introns, HSPB3, IFN-α1, and IFN-β1, into a vector containing the CMV promoter (Fig. 1A and Figs. S1 and S2). All of the constructs contained the natural 5′ and 3′ UTRs and full-length natural coding sequence. For comparison, we used size-matched random sequence constructs (designated CMV Random 1, 2, and 3, respectively) (Fig. 1A and Figs. S1 and S2). The bovine growth hormone polyA site and vector sequence are present in all of the constructs (Fig. 1A and Figs. S1 and S2). To examine the nucleocytoplasmic distribution of mRNA derived from these constructs, we transiently transfected equal amounts of each into HeLa cells and then carried out FISH 9, 14, 19, and 24 h after transfection.
Fig. 1.
Naturally intronless HSPB3 mRNA accumulates in the cytoplasm in a sequence-dependent manner. (A) Schematic of CMV HSPB3 and its size-matched counterpart CMV Random 1. Vector sequences are shown as gray lines. BGH pA, bovine growth hormone polyA site. (B) FISH was used to determine the nucleocytoplasmic distribution of HSPB3 mRNA after transiently transfecting HeLa cells for the times indicated. DAPI staining was used to identify the nucleus, and the merged images are shown in Bottom. (Scale bar: 10 μm.) (C) FISH comparison of HSPB3 and Random 1 transcripts at 9 or 24 h after transient transfection at lower magnification. (Scale bar: 10 μm.)
Significantly, the results obtained with the naturally intronless mRNAs were very different from the results obtained with the random sequence transcripts. Specifically, all three naturally intronless mRNAs were detected in the nucleus by 9 h after transfection but were mostly cytoplasmic by 19 and 24 h after transfection (Fig. 1B and Figs. S1 and S2). In contrast, with the Random 1, 2, and 3 constructs, only a few cells containing florescent signal were detected at the 9- or 24-h time point, and of these cells, the signal was both much weaker and mainly nuclear (Fig. 1C and Figs. S1 and S2). A comparison of the naturally intronless mRNAs vs. random constructs by RNase protection assays (RPAs) also showed that the overall level of RNA is much less for the random constructs (see Fig. 5 below and Fig. S8). This difference is not because of transfection efficiency (Fig. 5 and Fig. S8). In addition, the same levels of RNA were detected by FISH with all of the constructs 5 min after microinjection into HeLa nuclei (17) (Fig. S3), indicating that all of the CMV constructs are transcribed with similar efficiencies. Instead, the data indicate that the naturally intronless mRNAs are significantly more stable over time than the random RNAs and that only the naturally intronless mRNAs accumulate in the cytoplasm (Fig. 1C and Figs. S1 and S2). In addition, previous studies have shown that cDNA transcripts and defective transcripts are rapidly degraded in the nucleus (refs. 37 and 38 and references therein). Consistent with these results, our data indicate that the random RNAs are also degraded in the nucleus based on the observation that the WT RNAs are abundantly detected only in the nucleus at the 9-h time point at which time the random RNAs are already degraded. Thus, the most straightforward interpretation of our data is that the random RNAs are largely degraded in the nucleus and that the portion that is not degraded is mostly retained in the nucleus. Studies of mRNAs generated by splicing showed that they accumulate in nuclear speckles before export (refs. 16 and 17 and references therein and Fig. S5C). In contrast, neither cDNA transcripts nor the random RNAs associate with speckles. Consistent with this splicing dependency for localization to speckles, our data show that naturally intronless mRNAs also do not accumulate in speckles but are, instead, evenly distributed throughout the nucleoplasm (Fig. 1B and Figs. S1 and S2).
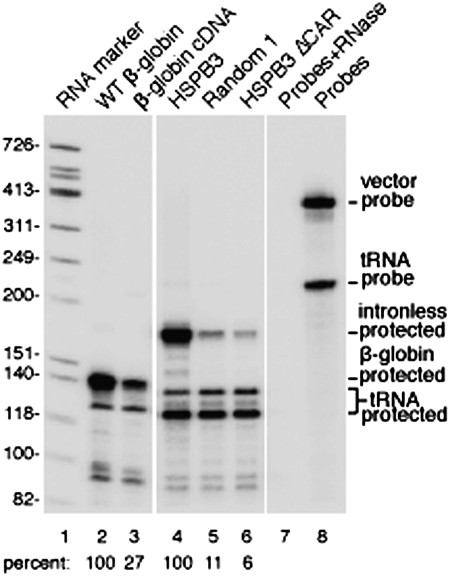
Fig. 5.
HSPB3 CAR is required for mRNA stability. The indicated CMV constructs (1 μg) were cotransfected into HeLa cells together with 1 μg of a tagged tRNA construct. Cells were harvested 9 h after transfection for RPA, and protected bands were separated on a 6.5% denaturing polyacrylamide gel. Quantitation was carried out by phosphoimager. The tRNA RPA products were used for normalization of the data. The levels of RPA products for WT β-globin and HSPB3 were adjusted to 100% and compared with the related constructs.
Nucleocytoplasmic Distribution of Naturally Intronless mRNAs Is Transcription-Independent but Polyadenylation-Dependent.
In yeast, the mRNA export machinery is recruited to intronless mRNAs during transcription (7). Thus, we asked whether the nucleocytoplasmic distribution of our human intronless mRNAs was affected by transcription. For this analysis, we microinjected nuclei with either the CMV-DNA constructs encoding the naturally intronless mRNAs or RNA synthesized in vitro with T7 RNA polymerase and containing an artificial polyA tail. Consistent with our transfection data, all three mRNAs from the CMV constructs were mostly cytoplasmic, whereas the Random 1, 2, and 3 transcripts were degraded or only detected in the nucleus by 3 h (Fig. S4). When RNAs synthesized in vitro were microinjected, we detected them in the nucleus at the 0-h time point and mostly in the cytoplasm by 3 h (Fig. S4). We conclude that the nucleocytoplasmic distribution of these naturally intronless mRNAs is transcription-independent.
We next investigated the role of polyadenylation in the nucleocytoplasmic distribution of these mRNAs. Accordingly, the AATAAA in the polyA signal was mutated to GCGGCG (Fig. 2A and Fig. S5), and constructs were microinjected into nuclei followed by FISH. As shown in Fig. 2B and Fig. S5, mRNAs with the WT polyA signal accumulated in the cytoplasm, whereas mRNAs lacking the polyA signal were detected mainly in discrete nuclear foci (Fig. 2B and Fig. S5). The nuclear foci did not colocalize with SC35 (Fig. S5) and thus, are not nuclear speckles. The identity of these foci remains to be determined. In contrast to the naturally intronless mRNAs, human β-globin containing the mutated polyA site was detected in nuclear speckles that colocalized with SC35 (Fig. S5). We conclude that polyadenylation is required for cytoplasmic accumulation of both naturally intronless mRNAs and spliced mRNAs. These data are consistent with previous work in both yeast and mammalian cells, indicating a role for 3′ end formation and polyadenylation in mRNA export (39–43).
Fig. 2.
Cytoplasmic accumulation of naturally intronless mRNA is polyadenylation-dependent. (A) Schematic of the CMV HSPB3 construct containing a mutant polyA signal (AATAAA was changed to GCGGCG). (B) CMV HSPB3 or CMV HSPB3 with mutant polyA signal were microinjected into HeLa cell nuclei; transcription was blocked by adding α-amanitin 20 min after injection, and FISH was carried out 3 h later. (Scale bar: 10 μm.)
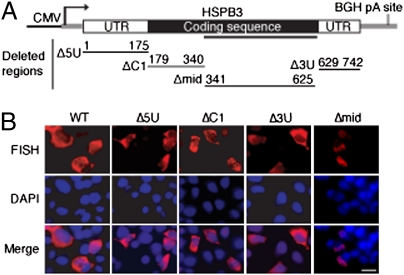
Naturally Intronless mRNAs Contain Cytoplasmic Accumulation Regions in Their Coding Sequence.
To determine whether a specific element is present in naturally intronless mRNAs that is required for their cytoplasmic accumulation, we deleted regions along the entire gene (Fig. 3A and Fig. S6). For HSPB3, deletion of the 5′ UTR, 3′ UTR, or 5′ portion of the coding region (constructs Δ5U, Δ3U, and ΔC1) had no significant effect on cytoplasmic accumulation of the mRNA (Fig. 3B). In contrast, when a 285-nt region in the coding region was removed (construct Δmid), very little FISH signal was detected at 24 h, and of this signal, the RNA was present in the nucleus (Fig. 3B). A deletion analysis was also carried out for IFN-α1 and IFN-β1, and a region of 183 or 162 nt that is required for their cytoplasmic accumulation was identified (Fig. S6). For the purposes of this study, we have designated these regions cytoplasmic accumulation regions (CARs).
Fig. 3.
Identification of a coding region sequence CAR required for cytoplasmic accumulation of naturally intronless HSPB3. (A) Schematic showing HSPB3 construct and deletion mutants. Deleted regions and corresponding nucleotides are indicated. The solid line under the coding region indicates the position of the sequence required for cytoplasmic accumulation of HSPB3. (B) FISH was carried out to localize WT or mutant HSPB3 mRNAs. (Scale bar: 10 μm.)
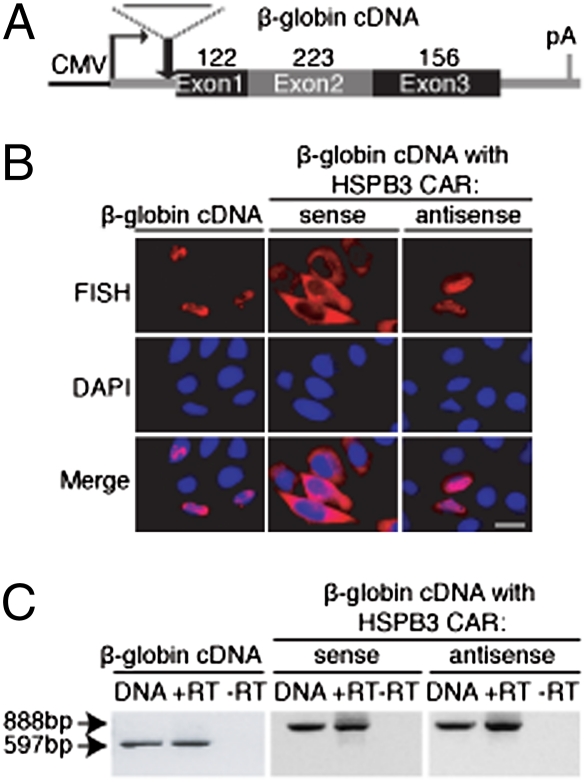
To determine whether the CARs in each intronless mRNA could affect the cytoplasmic accumulation of a heterologous RNA that is normally retained/degraded in the nucleus, we inserted each CAR into the 5′ end of β-globin cDNA. As a control, we also inserted the antisense of each CAR into the same site (Fig. 4A and Fig. S7). These fusion constructs were transfected into HeLa cells followed by FISH. Significantly, in all cases, the CARs in the sense orientation led to a dramatic enhancement of the cytoplasmic accumulation of the β-globin cDNA transcript, whereas the antisense of these regions had no effect (Fig. 4B and Fig. S7). To exclude the possibility that enhanced cytoplasmic accumulation was because of cryptic splicing from inserting the CARs, RT-PCR of total RNA isolated from the transfected cells was carried out. As a positive control, PCR was carried out using the plasmid as a template, and a band of the expected size was detected (Fig. 4C, lanes DNA, and Fig. S7). As a negative control, reverse transcriptase was omitted, and no bands were observed (Fig. 4C, lanes −RT and Fig. S7). As shown in Fig. 4C, lanes +RT and Fig. S7, all constructs generated RT-PCR products of the expected size, indicating no detectable cryptic splicing. We conclude that the CARs can rescue the cytoplasmic accumulation of the otherwise largely nuclear β-globin cDNA transcript. Together, our data indicate that naturally intronless mRNAs contain specific regions that function in the stable accumulation of mRNA in the cytoplasm.
Fig. 4.
CAR from HSPB3 rescues cytoplasmic accumulation of β-globin cDNA transcript. (A) Schematic of CMV-β-globin cDNA construct indicating the position where the 285-nt CAR from HSPB3 was inserted in both sense and antisense orientation. (B) The indicated CMV constructs were transfected into HeLa cells followed by FISH 24 h later. (Scale bar: 10 μm.) (C) PCR analysis to assay for cryptic splicing. Arrows indicate the PCR products of expected size for the full-length RNA. Lane DNA, PCR using plasmid DNA to generate a marker for the full-length product expected for no cryptic splicing; lane +RT, RT-PCR products of indicated constructs after transfection into HeLa cells; lane −RT, negative control for RT-PCR showing that plasmid DNA was degraded after DNase I treatment.
CARs Stabilize Naturally Intronless mRNAs.
Our FISH data showed significantly higher levels of fluorescence signal with all three naturally intronless mRNAs compared with sized-matched random RNAs (Fig. 1 and Figs. S1 and S2). These data raised the possibility that the CARs are not only required for cytoplasmic accumulation of mRNA but also play a role in stability of the mRNAs. To verify the FISH data and directly determine the levels of RNA from the different constructs, we carried out RPAs. Each construct was cotransfected with a modified tRNA construct driven by an RNAP III promoter as a control of transfection efficiency. Cells were harvested after 9 h, and RPA was carried out. Significantly, this analysis revealed that all three naturally intronless mRNAs (Fig. 5, lane 4 and Fig. S8A, lanes 4 and 7) were present at much higher levels (ranging from 8- to 50-fold) than the random RNA counterparts (Fig. 5, lane 5, and Fig. S8A, lanes 5 and 8) or intronless constructs lacking the CARs (Fig. 5, lane 6, and Fig. S8A, lanes 6 and 9). Consistent with previous studies (33–35, 37, 44), the level of spliced β-globin mRNA was also much greater than the level of the β-globin cDNA transcript (Fig. 5, lanes 2 and 3). Moreover, the CARs stabilize the β-globin cDNA transcript, although the effect was not as significant as splicing (Fig. S8B, compare lanes 4, 6, and 8 with lanes 2 and 3). Together, this analysis indicates that both the random RNAs and the naturally intronless mRNAs lacking the CARs are much less stable than the naturally intronless mRNAs. We conclude that the CARs function in mRNA stability and cytoplasmic accumulation of the mRNAs.
Role for the TREX/TAP Pathway in Cytoplasmic Accumulation of Naturally Intronless mRNAs.
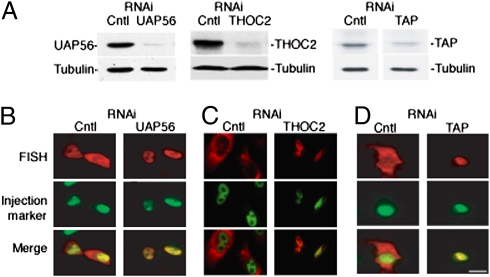
To determine whether the TREX complex and mRNA export receptor TAP are required for stability/export of naturally intronless cellular mRNAs, we knocked down TAP and the TREX components, THOC2 and UAP56 (Fig. 6A). Consistent with a previous study (45), knockdown of UAP56 resulted in nuclear retention of naturally intronless mRNAs (Fig. 6B and Fig. S9). In addition, knockdown of THOC2 or TAP also led to nuclear retention of all three naturally intronless mRNAs (Fig. 6 C and D and Fig. S9). Stability/export was not affected in the control knockdown cells (Fig. 6 B–D and Fig. S9). We conclude that TREX components (UAP56 and THOC2) and TAP are essential for cytoplasmic accumulation of these naturally intronless mRNAs. The THO complex is highly stable (9, 10), and thus, our observation that THOC2 knockdown inhibits cytoplasmic accumulation of naturally intronless mRNA raises the possibility that the entire THO complex may be involved. To further address the role of the TREX components in packaging/exporting the intronless mRNAs, we carried out RT-PCR using total RNA immunoprecipitated with antibodies against TREX components after transfection of the HSPB3 construct into HeLa cells. Significantly, a specific band was detected using antibodies to UAP56, ALY, THOC2, THOC5, THOC1, and CIP29, whereas no band was detected after RNA immunoprecipitation with a negative control antibody (α-HA) or in reactions without reverse transcriptase (Fig. S9) (we note that this analysis was not carried out with the random RNAs, because they are degraded). Together, our results indicate that the TREX complex packages the intronless mRNAs for export to the cytoplasm.
Fig. 6.
RNAi of TREX components (UAP56 and THOC2), or TAP blocks export of naturally intronless HSPB3 mRNA. (A) Western blot showing levels of UAP56, THOC2, or TAP proteins after siRNAs targeting UAP56 (and its relative URH49), THOC2, or TAP were transfected into HeLa cells. Cells were harvested 2 (UAP56), 3 (THOC2), or 1 d (TAP) after siRNA treatment. Tubulin was used as a loading control. (B–D) CMV construct encoding HSPB3 mRNA was microinjected into the nucleus of UAP56 (B), THOC2 (C), or TAP (D) knockdown cells. (Scale bar: 10 μm.)
Discussion
A great deal of progress has been made in determining the mechanisms and factors required for packaging and exporting mRNA from the nucleus to the cytoplasm, largely based on studies in yeast, human cells, and viruses (6, 7, 18, 46, 47). For human cellular mRNAs, most of the work has focused on spliced mRNAs and their cDNA counterparts. In the case of spliced mRNAs, a growing body of evidence indicates that the conserved TREX complex is recruited to the mRNA during splicing and that TAP functions in translocating the mRNA through the nuclear pore complex to the cytoplasm. In contrast, many cDNA transcripts are extremely unstable in the nucleus and also inefficiently accumulate in the cytoplasm (refs. 33–35, 37, and 44 and references therein). Both this instability and inefficient export may be attributed to inefficient recruitment of the TREX complex in the absence of splicing. Because mRNAs derived from naturally intronless genes also lack introns, we sought to determine why stability/export of these mRNAs was distinguished from the stability/export of cDNA or random RNA transcripts. Significantly, our data revealed that naturally intronless mRNAs are readily distinguished from cDNA/random transcripts. One of the most striking differences is that the three naturally intronless mRNAs that we examined (HSBP3, IFN-α1, and IFN-β1) were all stable and also accumulated in the cytoplasm over time.
In seeking to determine why there was such a striking difference between the naturally intronless mRNAs and the random RNAs/cDNA transcripts, we carried out deletion analysis of the constructs encoding the three naturally intronless mRNAs. In each case, we identified a portion of the coding region that conferred cytoplasmic accumulation on not only the naturally intronless mRNA itself but also a heterologous cDNA transcript that is normally largely degraded in the nucleus. We referred to these regions as CARs. The CARs in different mRNAs range from 162 to 285 nt, which seems to be similar to the c-Jun enhancer (CJE), a 201-nt region that was identified in naturally intronless c-Jun that confers cytoplasmic accumulation on a cDNA transcript that is normally degraded (48). Additional studies are needed to determine whether these regions have any properties in common. Because cDNA transcripts and random RNAs are so rapidly degraded in the nucleus, it is also not clear whether the CARs function in stabilizing the mRNAs or directly exporting the mRNAs, properties that are not mutually exclusive. In previous work, Conrad and Steitz (33) identified a Kaposi’s sarcoma viral RNA element, known as the expression and nuclear retention element (ENE). Insertion of this element into a cDNA transcript resulted in its stabilization, but the transcript remained nuclear (33). Additional studies showed that the ENE contains a U-rich element, which interacts with the polyA tail in the transcript and protects it from degradation (37, 49, 50). Thus, whereas the ENE rescues the stability of otherwise rapidly degraded cDNA transcripts, it does not allow cytoplasmic accumulation of the transcript (33). In this regard, the ENE differs from the CARs, because the CARs not only stabilize the mRNA but also result in their accumulation in the cytoplasm. These observations raise the possibility that the CARs function to recruit packaging proteins that stabilize the mRNA and also function in recruiting the export machinery.
Using RNAi, we found that cytoplasmic accumulation of all three of the naturally intronless mRNAs require TREX components (UAP56 and THOC2) and TAP. In addition, our data indicate that intronless mRNA is packaged by the TREX complex. These results are consistent with studies showing that export of naturally intronless viral mRNAs (19–23) and histone H2A mRNA (27) are mediated by TAP and that intronless mRNAs in yeast are mediated by the TAP homolog, Mex67 (7, 28). In the case of naturally intronless viral mRNAs, a virally encoded protein recruits the TREX complex (19–23). Binding of CARs to a cellular counterpart of this type of protein to recruit the TREX complex is one possibility. In contrast to yeast, in which the TREX complex is recruited to naturally intronless mRNAs cotranscriptionally and through interactions with the 3′ end formation machinery, our data show that the accumulation of naturally intronless human mRNAs in the cytoplasm is independent of transcription but is polyadenylation-dependent. Similar transcription independence was observed with spliced mRNA in humans, indicating that packaging for export can occur posttranscriptionally. Together, the data indicate that 3′ end formation and proper mRNA packaging by TREX are prerequisites for mRNA stability and efficient export of the spliced and naturally intronless mRNAs that we have examined.
Materials and Methods
Plasmids and Antibodies.
HSPB3, IFN-α1, and IFN-β1 were amplified from DNA purified from HeLa cells. HSPB3 was cloned into pcDNA3 vector (Invitrogen) at the KpnI and ApaI sites. IFN-α1 was cloned into the same vector at the HindIII site. IFN-β1 was cloned into vector pEGFP-N2 at the AflII and HindIII sites and then subcloned into pcDNA3 at the HindIII site. Deletion constructs were made using two-step PCR (51), and in all constructs, the start and stop codons were maintained in frame. For the heterologous CAR-β-globin cDNA constructs, CARs were inserted into the KpnI (CAR from HSPB3) or HindIII site (CARs from IFN-α1 and IFN-β1) immediately upstream of the β-globin cDNA. Constructs were verified by sequencing. Sequences of primers are available on request. β-globin WT and cDNA constructs were described (44). Polyclonal antibodies were used against UAP56, THOC2 (9), and TAP (gift from S. Masuda, Kyoto, Japan), and a monoclonal antibody was used against tubulin (Sigma). Antibodies for Westerns were used at dilutions of 1/1,000, 1/700, 1/500, and 1/10,000, respectively, for UAP56, THOC2, TAP, and tubulin.
Cell Culture, Transfection, and Microinjection.
HeLa cells were cultured in DMEM supplemented with 10% FBS. Transfection was carried out in MatTek plates using 500 ng of each plasmid and Lipofectamine 2000. For siRNA transfection, 1.25 μL 40 μM siRNA was added after mixing with 1 μL lipofectamine 2000. For DNA microinjection, a 10-μL mixture containing 2 μL 70-kDa Dextran (Molecular Probes) and 50 ng/μL each DNA was microinjected into HeLa cell nuclei. For RNA microinjection, a 10-μL mixture containing 2 μL 70-kDa Dextran (Molecular Probes) and 200 ng/μL each T7 RNA polymerase transcript (containing a polyA tail) was microinjected into HeLa cell nuclei. For FISH, samples were rinsed one time with 1× PBS, fixed with 4% paraformamide in PBS for 15 min, and then, permeabilized in 0.1% triton in PBS. After three rinses with 1× PBS, the samples were rinsed two times with 1× saline sodium citrate (SSC)/50% formamide, fluorescent probes were added, and FISH was performed overnight at 37 °C. FISH probes were DNA oligos of 70 nt labeled with ULYSIS Alexa Fluor 546 Nucleic Acid Labeling Kit (Molecular Probes) or oligos prelabeled at the 5′ end with Alexa Fluor 546 NHS ester and then HPLC-purified. Probe sequences are available on request. Images were taken with a Nikon TE2000E Inverted Fluorescence Microscope.
RPA.
To construct the vector probe for RPA, a PCR product was amplified from pcDNA3 using forward primer 5′-AAAGGTACCGGGCCCTATT CTATAGTGT -3′ and reverse primer 5′-TTTGGTACCTTCCCAATCCTCCCCTTGCT-3′ and cloned into pcDNA3 at KpnI site. The plasmid was linearized with XbaI and in vitro transcribed with T7 RNA polymerase. The probe was gel-purified on a 6.5% denaturing polyacrylamide gel. To carry out RPA, HeLa cells were cotransfected with 1 μg reporter and 1 μg tagged tRNA construct driven by an RNA polymerase III promoter (44). Cells were harvested 9 h after transfection, and total RNA was purified with TRIzol (Invitrogen). RNA was treated with DNase before RPA, and 4 μg total RNA were used for RPA with a 24 fmol 32P-labeled vector probe and a 10 fmol tRNA probe. RPA was carried out using the RPA III kit (Ambion). RPA bands were separated on 6.5% denaturing polyacrylamide gels and detected by phosphoimager.
Supplementary Material
Acknowledgments
We thank E. Folco, B. Lee, K. Dufu, and other members of the R.R. laboratory for useful discussion and comments on the manuscript. We are grateful to the Nikon Imaging Center at Harvard Medical School for training and use of microscopic equipment. This work was funded by a National Institutes of Health Grant GM043375 (to R.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113076108/-/DCSupplemental.
References
- 1.Shabalina SA, et al. Distinct patterns of expression and evolution of intronless and intron-containing mammalian genes. Mol Biol Evol. 2010;27:1745–1749. doi: 10.1093/molbev/msq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer P, et al. Coordination between transcription and pre-mRNA processing. FEBS Lett. 2001;498:179–182. doi: 10.1016/s0014-5793(01)02485-1. [DOI] [PubMed] [Google Scholar]
- 3.Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. doi: 10.1038/416499a. [DOI] [PubMed] [Google Scholar]
- 4.Perales R, Bentley D. “Cotranscriptionality”: The transcription elongation complex as a nexus for nuclear transactions. Mol Cell. 2009;36:178–191. doi: 10.1016/j.molcel.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. doi: 10.1016/j.cell.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Reed R, Hurt E. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell. 2002;108:523–531. doi: 10.1016/s0092-8674(02)00627-x. [DOI] [PubMed] [Google Scholar]
- 7.Köhler A, Hurt E. Exporting RNA from the nucleus to the cytoplasm. Nat Rev Mol Cell Biol. 2007;8:761–773. doi: 10.1038/nrm2255. [DOI] [PubMed] [Google Scholar]
- 8.Dufu K, et al. ATP is required for interactions between UAP56 and two conserved mRNA export proteins, Aly and CIP29, to assemble the TREX complex. Genes Dev. 2010;24:2043–2053. doi: 10.1101/gad.1898610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda S, et al. Recruitment of the human TREX complex to mRNA during splicing. Genes Dev. 2005;19:1512–1517. doi: 10.1101/gad.1302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng H, et al. Human mRNA export machinery recruited to the 5′ end of mRNA. Cell. 2006;127:1389–1400. doi: 10.1016/j.cell.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Zenklusen D, Vinciguerra P, Wyss JC, Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol. 2002;22:8241–8253. doi: 10.1128/MCB.22.23.8241-8253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abruzzi KC, Lacadie S, Rosbash M. Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. EMBO J. 2004;23:2620–2631. doi: 10.1038/sj.emboj.7600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson SA, Cubberley G, Bentley DL. Cotranscriptional recruitment of the mRNA export factor Yra1 by direct interaction with the 3′ end processing factor Pcf11. Mol Cell. 2009;33:215–226. doi: 10.1016/j.molcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao R, Bodnar MS, Spector DL. Nuclear neighborhoods and gene expression. Curr Opin Genet Dev. 2009;19:172–179. doi: 10.1016/j.gde.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molenaar C, Abdulle A, Gena A, Tanke HJ, Dirks RW. Poly(A)+ RNAs roam the cell nucleus and pass through speckle domains in transcriptionally active and inactive cells. J Cell Biol. 2004;165:191–202. doi: 10.1083/jcb.200310139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall LL, Smith KP, Byron M, Lawrence JB. Molecular anatomy of a speckle. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:664–675. doi: 10.1002/ar.a.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias AP, Dufu K, Lei H, Reed R. A role for TREX components in the release of spliced mRNA from nuclear speckle domains. Nat Commun. 2010 doi: 10.1038/ncomms1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MJ. From birth to death: The complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 19.Boyne JR, Colgan KJ, Whitehouse A. Herpesvirus saimiri ORF57: A post-transcriptional regulatory protein. Front Biosci. 2008;13:2928–2938. doi: 10.2741/2898. [DOI] [PubMed] [Google Scholar]
- 20.Chen IH, Sciabica KS, Sandri-Goldin RM. ICP27 interacts with the RNA export factor Aly/REF to direct herpes simplex virus type 1 intronless mRNAs to the TAP export pathway. J Virol. 2002;76:12877–12889. doi: 10.1128/JVI.76.24.12877-12889.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guang S, Felthauser AM, Mertz JE. Binding of hnRNP L to the pre-mRNA processing enhancer of the herpes simplex virus thymidine kinase gene enhances both polyadenylation and nucleocytoplasmic export of intronless mRNAs. Mol Cell Biol. 2005;25:6303–6313. doi: 10.1128/MCB.25.15.6303-6313.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toth Z, Stamminger T. The human cytomegalovirus regulatory protein UL69 and its effect on mRNA export. Front Biosci. 2008;13:2939–2949. doi: 10.2741/2899. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, et al. Imaging and characterizing influenza A virus mRNA transport in living cells. Nucleic Acids Res. 2008;36:4913–4928. doi: 10.1093/nar/gkn475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Carmichael GG. The mouse histone H2a gene contains a small element that facilitates cytoplasmic accumulation of intronless gene transcripts and of unspliced HIV-1-related mRNAs. Proc Natl Acad Sci USA. 1997;94:10104–10109. doi: 10.1073/pnas.94.19.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y, Wimler KM, Carmichael GG. Intronless mRNA transport elements may affect multiple steps of pre-mRNA processing. EMBO J. 1999;18:1642–1652. doi: 10.1093/emboj/18.6.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erkmann JA, Sànchez R, Treichel N, Marzluff WF, Kutay U. Nuclear export of metazoan replication-dependent histone mRNAs is dependent on RNA length and is mediated by TAP. RNA. 2005;11:45–58. doi: 10.1261/rna.7189205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Y, Gattoni R, Stévenin J, Steitz JA. SR splicing factors serve as adapter proteins for TAP-dependent mRNA export. Mol Cell. 2003;11:837–843. doi: 10.1016/s1097-2765(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert W, Guthrie C. The Glc7p nuclear phosphatase promotes mRNA export by facilitating association of Mex67p with mRNA. Mol Cell. 2004;13:201–212. doi: 10.1016/s1097-2765(04)00030-9. [DOI] [PubMed] [Google Scholar]
- 29.Kimura T, Hashimoto I, Nagase T, Fujisawa J. CRM1-dependent, but not ARE-mediated, nuclear export of IFN-alpha1 mRNA. J Cell Sci. 2004;117:2259–2270. doi: 10.1242/jcs.01076. [DOI] [PubMed] [Google Scholar]
- 30.Masuyama K, Taniguchi I, Kataoka N, Ohno M. RNA length defines RNA export pathway. Genes Dev. 2004;18:2074–2085. doi: 10.1101/gad.1216204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nojima T, Hirose T, Kimura H, Hagiwara M. The interaction between cap-binding complex and RNA export factor is required for intronless mRNA export. J Biol Chem. 2007;282:15645–15651. doi: 10.1074/jbc.M700629200. [DOI] [PubMed] [Google Scholar]
- 32.Ullman KS. RNA export: Searching for mRNA identity. Curr Biol. 2002;12:R461–R463. doi: 10.1016/s0960-9822(02)00946-6. [DOI] [PubMed] [Google Scholar]
- 33.Conrad NK, Steitz JA. A Kaposi’s sarcoma virus RNA element that increases the nuclear abundance of intronless transcripts. EMBO J. 2005;24:1831–1841. doi: 10.1038/sj.emboj.7600662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nott A, Meislin SH, Moore MJ. A quantitative analysis of intron effects on mammalian gene expression. RNA. 2003;9:607–617. doi: 10.1261/rna.5250403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu S, Cullen BR. Analysis of the stimulatory effect of splicing on mRNA production and utilization in mammalian cells. RNA. 2003;9:618–630. doi: 10.1261/rna.5260303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi I, Ohno M. ATP-dependent recruitment of export factor Aly/REF onto intronless mRNAs by RNA helicase UAP56. Mol Cell Biol. 2008;28:601–608. doi: 10.1128/MCB.01341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conrad NK, Mili S, Marshall EL, Shu MD, Steitz JA. Identification of a rapid mammalian deadenylation-dependent decay pathway and its inhibition by a viral RNA element. Mol Cell. 2006;24:943–953. doi: 10.1016/j.molcel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Wyers F, et al. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725–737. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Lei EP, Silver PA. Intron status and 3′-end formation control cotranscriptional export of mRNA. Genes Dev. 2002;16:2761–2766. doi: 10.1101/gad.1032902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Libri D, et al. Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol Cell Biol. 2002;22:8254–8266. doi: 10.1128/MCB.22.23.8254-8266.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilleren P, McCarthy T, Rosbash M, Parker R, Jensen TH. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature. 2001;413:538–542. doi: 10.1038/35097110. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, Carmichael GG. Role of polyadenylation in nucleocytoplasmic transport of mRNA. Mol Cell Biol. 1996;16:1534–1542. doi: 10.1128/mcb.16.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuke H, Ohno M. Role of poly (A) tail as an identity element for mRNA nuclear export. Nucleic Acids Res. 2008;36:1037–1049. doi: 10.1093/nar/gkm1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valencia P, Dias AP, Reed R. Splicing promotes rapid and efficient mRNA export in mammalian cells. Proc Natl Acad Sci USA. 2008;105:3386–3391. doi: 10.1073/pnas.0800250105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hautbergue GM, et al. UIF, a new mRNA export adaptor that works together with REF/ALY, requires FACT for recruitment to mRNA. Curr Biol. 2009;19:1918–1924. doi: 10.1016/j.cub.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rougemaille M, Villa T, Gudipati RK, Libri D. mRNA journey to the cytoplasm: Attire required. Biol Cell. 2008;100:327–342. doi: 10.1042/BC20070143. [DOI] [PubMed] [Google Scholar]
- 47.Saguez C, Olesen JR, Jensen TH. Formation of export-competent mRNP: Escaping nuclear destruction. Curr Opin Cell Biol. 2005;17:287–293. doi: 10.1016/j.ceb.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Guang S, Mertz JE. Pre-mRNA processing enhancer (PPE) elements from intronless genes play additional roles in mRNA biogenesis than do ones from intron-containing genes. Nucleic Acids Res. 2005;33:2215–2226. doi: 10.1093/nar/gki506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conrad NK, Shu MD, Uyhazi KE, Steitz JA. Mutational analysis of a viral RNA element that counteracts rapid RNA decay by interaction with the polyadenylate tail. Proc Natl Acad Sci USA. 2007;104:10412–10417. doi: 10.1073/pnas.0704187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitton-Fry RM, DeGregorio SJ, Wang J, Steitz TA, Steitz JA. Poly(A) tail recognition by a viral RNA element through assembly of a triple helix. Science. 2010;330:1244–1247. doi: 10.1126/science.1195858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lei H, Vorechovsky I. Identification of splicing silencers and enhancers in sense Alus: A role for pseudoacceptors in splice site repression. Mol Cell Biol. 2005;25:6912–6920. doi: 10.1128/MCB.25.16.6912-6920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.