Abstract
Persistent protein synthesis inhibition (PSI) is a robust predictor of eventual neuronal death following cerebral ischemia. We thus tested the hypothesis that persistent PSI inhibition and neuronal death are causally linked. Neuronal viability strongly correlated with both protein synthesis and levels of eukaryotic (translation) initiation factor 4G1 (eIF4G1). We determined that in vitro ischemia activated calpain, which degraded eIF4G1. Overexpression of the calpain inhibitor calpastatin or eIF4G1 resulted in increased protein synthesis and increased neuronal viability compared with controls. The neuroprotective effect of eIF4G1 overexpression was due to restoration of cap-dependent protein synthesis, as well as protein synthesis-independent mechanisms, as inhibition of protein synthesis with cycloheximide did not completely prevent the protective effect of eIF4G1 overexpression. In contrast, shRNA-mediated silencing of eIF4G1 exacerbated ischemia-induced neuronal injury, suggesting eIF4G1 is necessary for maintenance of neuronal viability. Finally, calpain inhibition following global ischemia in vivo blocked decreases in eIF4G1, facilitated protein synthesis, and increased neuronal viability in ischemia-vulnerable hippocampal CA1 neurons. Collectively, these data demonstrate that calpain-mediated degradation of a translation initiation factor, eIF4G1, is a cause of both persistent PSI and neuronal death.
Keywords: global ischemia, eukaryotic (translation) initiation factor 4G1 cleavage
Protein synthesis is a highly regulated process essential for normal growth, development, and maintenance of cells. Various cellular stressors including viral infection, nutrient deprivation, and heat shock inhibit protein synthesis (1). The characteristics of the stress are important in the eventual recovery of protein synthesis. A sublethal insult such as hypoglycemia initially inhibits protein synthesis, but translation returns upon nutrient restoration. In contrast, inhibition of protein synthesis due to infection or other stimuli can be irreversible and associated with cell death (2).
Stimuli inducing programmed cell death have been repeatedly shown to inhibit protein synthesis (2). Likewise, agents that inhibit protein synthesis induce cell death (3, 4). However, in no case have protein synthesis inhibition and cell death been directly linked. The relationship between persistent protein synthesis inhibition (PSI) and cell death is exemplified by neurons following an ischemic insult (5), and induction of ischemia provides a useful model to study the mechanisms leading to PSI and cell death.
PSI occurs in all neurons destined to die following global ischemia (5). In contrast to the rapid restoration of energy metabolism (6), recovery of protein synthesis is slower and may fail in ischemia-susceptible brain regions (7, 8). This is also demonstrated by focal ischemia models in which the brain regions where protein synthesis is inhibited following 1 h of ischemia predict the infarct volume at 3 d (9).
Ischemia (5) and other stimuli inducing programmed cell death (2) cause polysome disassociation, indicating inhibition of translation initiation. Examination of the levels and phosphorylation states of a number of critical translation initiation factors following cerebral ischemia suggests that depressed levels of the scaffolding protein responsible for delivering the mRNA to the ribosome for translation, eukaryotic initiation factor 4G1 (eIF4G1), are correlated with persistent PSI (10). Excitotoxicity caused by ischemia and neurodegenerative diseases is known to result in NMDA-induced calcium influx into neurons, resulting in activation of the protease calpain (11). Calpain is known to degrade eIF4G1 in vitro (12), and it has been implicated in ischemia-induced decreases in eIF4G1(13); inhibition of calpain also provides neuroprotection in a focal ischemia model (14).
The purpose of the present study was to determine the underlying mechanisms mediating persistent PSI and to determine whether persistent PSI is directly related to neuronal death in both in vitro and in vivo ischemia models. Our results indicate that eIF4G1 is degraded following both in vitro and in vivo ischemia in a calpain-dependent manner. Moreover, we demonstrate that maintenance of near normal levels of a translation initiation factor, eIF4G1, either by overexpression or calpain inhibition results in decreased PSI and increased neuroprotection against oxygen–glucose deprivation (OGD) in vitro and global ischemia in vivo.
Results
PSI and Viability Are Correlated with Duration of Ischemia.
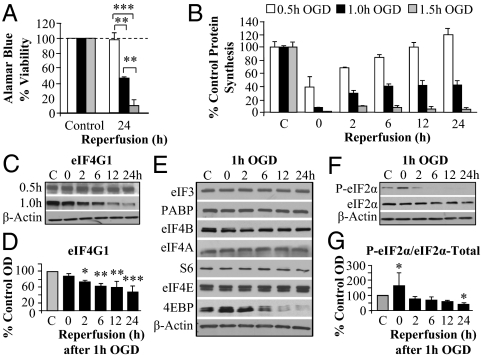
We initially sought to establish our pure (∼97%) primary neuronal culture system coupled with OGD as a viable model of persistent PSI observed following in vivo ischemia. To model both ischemia-sensitive and -resistant areas of the brain, we determined durations of OGD that were sublethal, moderately lethal (i.e., lethal to a fraction of the cultured cells), and severely lethal (i.e., lethal to most of the cells). Viability was assayed at 24 h after 0.5, 1, and 1.5 h OGD by Alamar Blue reduction (metabolic state), lactate dehydrogenase (LDH) release (membrane breakdown), and nuclear fragmentation (apoptosis). Sublethal (0.5 h) OGD did not result in significant loss of viability as determined by any of these assays. However, viability measured by Alamar Blue fluorescence after OGD for 1 h and 1.5 h decreased by 50% and 90%, respectively (Fig. 1A), with corresponding increases in LDH release (Fig. S1A) and increased nuclear fragmentation with Hoechst staining (Fig. S1 B and C). Sublethal OGD resulted in an initial inhibition of protein synthesis (∼60%) followed by a progressive recovery to control levels by 24 h reperfusion (Fig. 1B). Lethal levels of OGD caused a greater initial inhibition of protein synthesis but with recovery to 45% and 10% of control translation at 24 h reperfusion after 1 h and 1.5 h OGD, respectively. Thus, viability was positively correlated with protein synthesis levels.
Fig. 1.
Protein synthesis inhibition correlates with cell death and eIF4G1 levels. (A) Viability was measured with the Alamar Blue reduction assay. (B) Protein synthesis was measured as incorporation of [35S]methionine into nascent peptides for 15 min [cpm relative to total protein content (micrograms) of each well] following 0.5, 1.0, and 1.5 h OGD durations at indicated reperfusion times. (C and D) Western analysis and quantitation of eIF4G1 after OGD. Sublethal (0.5 h) OGD did not result in decreases. Moderately lethal (1 h) OGD caused a progressive decrease first measured at 2 h reperfusion. (E) Other translation initiation factors did not decrease after 1 h OGD at indicated reperfusion times. The decrease of the negative regulator 4EBP would not be expected to inhibit translation. (F and G) Western blot of the total and P-eIF2α levels with quantitation following 1 h OGD shows a transient rise in P-eIF2α after OGD. Bars represent mean ± SD values of n = 3–4 independent experiments. *P < 0.05, *P < 0.01, and ***P < 0.001 vs. control unless otherwise indicated. Abbreviations: 4EBP, 4E binding protein; PABP, poly(A) binding protein; OD, optical density; S6, small ribosomal subunit 6 protein.
Levels of eIF4G1 Correlate with Persistent PSI.
Continuing to validate our in vitro model of ischemia, we compared changes in initiation factor levels and phosphorylation states induced by in vitro OGD to the results in the in vivo literature. As seen in vivo (15–18), there were no changes in many of the regulatory initiation factors following 1 h OGD (Fig. 1E and Fig. S1 D–F). Lethal (1 h) but not sublethal OGD, however, resulted in the progressive decrease of eIF4G1 to ∼50% of control during 2–24 h reperfusion (Fig. 1 C and D). Notably, the decrease in the level of eIF4G1 correlates with decreased viability and PSI at 24 h reperfusion. This initial evidence supports our hypothesis that loss of critical levels of eIF4G1 is responsible for PSI following ischemia and ultimately cell death. Of note, total levels of 4EBP did decrease at 6–24 h, but because 4EBP is a negative regulator of translation, decreased 4EBP is not expected to contribute to PSI.
Phosphorylation of eIF2α (P-eIF2α) is a mechanism known to inhibit translation by blocking delivery of tRNAs to the ribosome (1). Transient increases in P-eIF2α with levels returning to baseline by 6 h of reperfusion have been repeatedly observed following in vivo ischemia (5). OGD (1 h) doubled the amount of P-eIF2α, but the level returned to baseline within 2 h of reperfusion (Fig. 1 F and G). These data validate our in vitro model as an adequate representation of in vivo ischemia. Furthermore, the onset and duration of the increase in P-eIF2α indicate its potential involvement in initial PSI, but the transiency of the phosphorylation and prior in vivo evidence demonstrating that blocking P-eIF2α has no effect on PSI (10) exclude its role in mediating persistent PSI.
Calpain Is Activated Following in Vitro Ischemia.
Neuronal death following OGD has been linked to both caspase- and calpain-dependent mechanisms (19, 20), and eIF4G1 has been shown to be a substrate of both caspase-3 (3) and calpain (12, 13). We thus attempted to determine whether these proteases are involved in the decrease in eIF4G1 following OGD.
We first evaluated the cleavage products of the caspase-3 and calpain substrate α-spectrin following OGD. Both proteases cleave the 240-kDa protein, leaving a 150-kDa fragment, but calpain further cleaves it to a 145-kDa fragment whereas caspase-3 produces a 120-kDa fragment. Following 1 h OGD there was a decrease in the full-length protein and a corresponding increase in the appearance of the calpain-induced 145-kDa fragment, although there was no change in the caspase-induced 120-kDa fragment (Fig. S2 A and B). This suggests that calpain rather than caspase was activated in our model, which was confirmed with caspase and calpain activity assays and by inhibition of caspases following 1 h OGD. Caspase activity did not increase above control, and caspase inhibition failed to provide neuroprotection following OGD (Fig. S2 C, E, and F). In contrast, calpain activity increased immediately after OGD and for 6 h of reperfusion (Fig. S2D). These findings are in agreement with previous studies of OGD in mixed glial and neuronal cultures (20).
Calpain Inhibition Blocks eIF4G1 Cleavage, Reduces PSI, and Increases Survival.
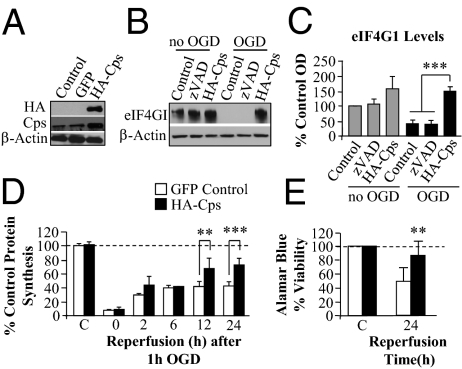
On the basis of the above observations, we examined the role of calpain on eIF4G1 levels, protein synthesis, and viability after OGD. Calpain inhibition was achieved by overexpression of calpastatin (Cps), the endogenous inhibitor of calpain (11), with an HA tag (Fig. 2A). Calpastatin overexpression increased full-length α-spectrin and decreased the 145-kDa band of α-spectrin following 1 h OGD and 24 h reperfusion. Treatment with zVAD did not alter the α-spectrin cleavage profile (Fig. S3 A and B).
Fig. 2.
Calpain inhibition following OGD results in higher eIF4G1 levels, protein synthesis, and viability. Rat primary cortical neurons were transfected with HA-calpastatin (HA-Cps) or GFP. (A) Transfection with HA-Cps was confirmed by immunoblotting for HA (Upper) and increased level of calpastatin relative to control cultures or to cultures transfected with GFP. (B) Western analysis showed greater eIF4G1 levels in cultures transfected with HA-Cps than in cultures transfected with GFP with or without zVAD treatment and no OGD. After 1 h OGD and 24 h reperfusion, levels of eIF4G1 were higher in HA-Cps–transfected cells (not treated with zVAD) than in GFP-transfected cells with or without treatment with zVAD (100 μM 1 h before, during 1 h OGD, and for 24 h after). (C) Quantitation of data in B. (D) Protein synthesis (cpm per microgram protein) was greater in HA-Cps– than in GFP-transfected controls after 1 h OGD and 12 and 24 h reperfusion. (E) Viability measured using Alamar Blue at 24 h reperfusion was greater in HA-Cps–transfected cells compared with GFP-transfected cells. Bars represent mean ± SD of n = 3–4 independent experiments. **P < 0.01; ***P < 0.001.
Overexpression of calpastatin, but not inhibition of caspases, resulted in significantly increased levels of eIF4G1 after 1 h OGD and 24 h reperfusion compared with GFP-transfected controls (Fig. 2 B and C). Analysis of protein synthesis following 1 h OGD in calpastatin and GFP-transfected neurons showed an initial inhibition of protein synthesis with increased recovery in the calpastatin-overexpressing neurons compared with time-matched GFP controls at 12 and 24 h reperfusion (Fig. 2D). The increase in protein synthesis was associated with increases in viability at 24 h reperfusion (Fig. 2E and Fig. S3C). This result provides further evidence that calpain is mediating eIF4G1 cleavage following OGD in cultured neurons and that decreases in eIF4G1 account for persistent PSI and, ultimately, neuronal death following ischemia.
eIF4G1 Is a Calpain Substrate.
To confirm that eIF4G1 is a substrate of calpain (12, 13), we used a cell-free assay where we mixed recombinant eIF4G1 with recombinant calpain I and activated the enzyme with calcium. Calpain I clearly cleaved eIF4G1 during the incubation. This action is a specific effect of calpain as both the endogenous calpain inhibitor calpastatin and the pharmacological calpain-specific inhibitor ALLN abrogated eIF4G1 cleavage (Fig. S4A).
We next examined the time course of eIF4G1 cleavage by calpain. Incubation of recombinant eIF4G1 with calpain for specified times resulted in progressive cleavage of eIF4G1 into size-specific products as shown by probing with eIF4G1 N- and C-terminal directed antibodies (Fig. S4 B and C). Notably, ∼50- and 66-kDa fragments were seen using the C-terminal antibody, which corroborates the findings in neuronal cultures following OGD (Fig. S4F) and in brain following focal ischemia (18).
In a further attempt to identify the cleavage sites of calpain on eIF4G1, we used recombinant protein fragments of eIF4G1. Recombinant calpain cleaved eIF4G1 fragments consisting of amino acids 197–674 and 675–1129 (Fig. S4E). Analysis of the cleavage products by mass spectrometry positively identified the bands as eIF4G1 and confirmed the molecular weights as 66, 50, and 16 kDa (Fig. S4D). Unfortunately, multiple attempts to identify the specific sequence targeted by calpain using Edman degradation failed. Together, these results suggest that calpain does not recognize a single site on eIF4G1, but progressively cleaves the protein to either modify or abolish its function.
Overexpression of eIF4G1 Is Neuroprotective.
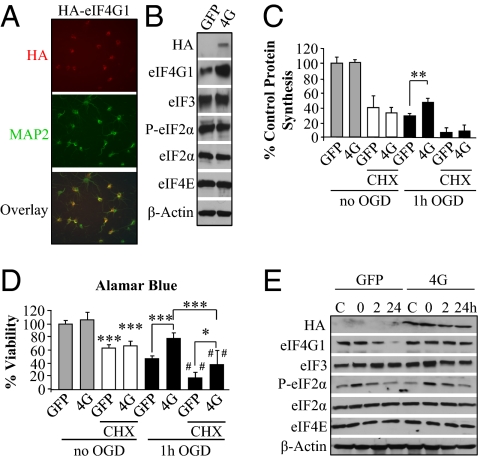
We hypothesized that neuronal death following ischemia is directly related to failure to preserve levels of eIF4G1. To test this hypothesis, we overexpressed eIF4G1 in primary neurons using a lentiviral vector. The lentivirus expressing human HA-eIF4G1 was present in ∼75–90% of neurons (Fig. 3A). Transfection of eIF4G1 did not affect the level of several other initiation factors or the phosphorylation state of eIF2α (Fig. 3B). Overexpression of eIF4G1 resulted in increased protein synthesis and neuronal viability 24 h after 1 h OGD compared with GFP control (Fig. 3 C and D). To determine whether the neuroprotective effect of eIF4G1 was directly due to protein synthesis, we inhibited protein synthesis using cycloheximide (CHX). A concentration response curve of CHX application demonstrates that concentrations of CHX that substantially inhibited protein synthesis for 24 h also resulted in neuronal death (Fig. S5A). Protein synthesis was inhibited to ∼30% of untreated levels following 24 h of exposure to CHX (50 μM) in both groups under control conditions and was nearly completely inhibited in both groups after OGD (Fig. 3C).
Fig. 3.
Neuroprotection by eIF4G1 following ischemia is at least partially protein synthesis dependent. (A) Immunostaining for the HA tag was performed to assess transfection efficacy of lentiviral-mediated overexpression of HA-eIF4G1 (4G), and neuronal colocalization was demonstrated by costaining for MAP2. (B) Levels of initiation factors in primary neurons overexpressing HA-eIF4G1 compared with those in neurons expressing GFP are shown by Western blot. (C and D) Control (GFP-transfected) neurons and 4G-transfected neurons were untreated or exposed to 50 μM CHX for 24 h or subjected to 1 h OGD and 24 h reperfusion without or with 50 μM CHX. After 24 h, treated neurons were assessed for protein synthesis (cpm per microgram protein) (C) and viability (D). Both protein synthesis and survival were increased by 4G overexpression. (E) Western blot analysis showing the effect of GFP- or eIF4G1-transfection on the expression of regulatory translation initiation factors by neurons subjected to 1 h OGD at 0, 2, and 24 h reperfusion. Specifically, P-eIF2α was transiently increased at 0 and 2 h as in Fig. 1F, and eIF4G1 was decreased at 24 h in control neurons. Bars represent mean ± SD of n = 3–4 independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with control and where indicated, ##P < 0.01 compared with control CHX-treated neurons.
Examination of neuronal viability revealed that concentrations of CHX used to inhibit protein synthesis were mildly but significantly neurotoxic, causing ∼40% of neurons to die (Fig. 3D), which corroborates previous findings in primary neurons (4). The importance of protein synthesis in maintenance of viability following in vitro ischemia was further substantiated by the observation that CHX treatment significantly decreased neuronal viability following OGD in both the GFP- and eIF4G1-overexpressing cultures. Significantly reduced viability was also observed when comparing control cultures treated with CHX to OGD- and CHX-treated cultures (Fig. 3D, third and seventh bars). Importantly, inhibition of protein synthesis with CHX only partially blocked the neuroprotective effect of eIF4G1, as eIF4G1-overexpressing cultures treated with CHX had increased viability compared with OGD- and CHX-treated GFP neurons (Fig. 3D, seventh and eighth bars). This suggests that overexpression of eIF4G1 may confer neuroprotection via mechanisms other than by increasing global translation.
To further support our findings that persistent PSI is pathological, we examined the effect of eIF4G1 overexpression on the time course of protein synthesis following 1 h OGD. As seen with control cultures and calpastatin-overexpressing neurons (Fig. 2D), protein synthesis was immediately inhibited following 1 h OGD in both control and eIF4G1-overexpressing neurons (Fig. S5B). However, protein synthesis began to recover at 12 h reperfusion in eIF4G1-overexpressing neurons but not in controls.
Examination of protein levels following OGD revealed greater eIF4G1 levels at 2 and 24 h following OGD in eIF4G1- compared with GFP-overexpressing neurons (Fig. 3E and Fig. S5C). Furthermore, OGD resulted in a similar P-eIF2α profile in GFP- and eIF4G1-overexpressing neurons (Figs. 3E and 1E). Again, this suggests that P-eIF2α does not contribute to persistent PSI or delayed neuronal death. Instead, the data collectively support the inference that maintenance of critical levels of eIF4G1 is essential for neuronal survival following OGD and that the neuroprotection may be in part translation independent.
shRNA Silencing of eIF4G1 Exacerbates OGD-Induced Death.
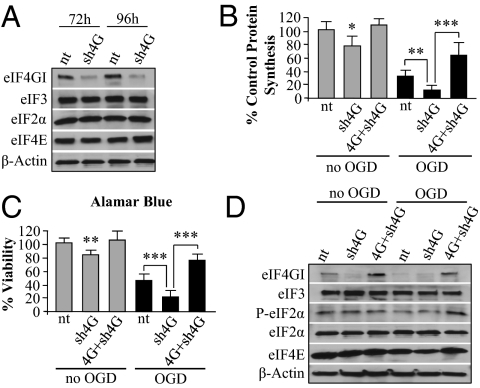
Our overexpression experiments demonstrate that maintenance or increase of eIF4G1 levels following OGD can restore protein synthesis and provide neuroprotection. The next step was to determine whether reducing the eIF4G1 level was sufficient to cause neuronal death. To determine this we transfected a lentiviral vector with shRNA targeting rat eIF4G1 into our primary cultures. Knockdown of eIF4G1 to ∼20% of control values was observed at 72 h and 96 h posttransfection without affecting levels of other initiation factors tested (Fig. 4A and Fig. S6A). Knockdown of eIF4G1 96 h posttransfection resulted in a 24% decrease in protein synthesis and a 17% decrease in neuronal viability relative to a control, non-targeting (nt) shRNA (Fig. 4 B and C). The decreases in translation are similar to the effects of eIF4G1 silencing observed in a recent study using a breast cancer cell line, and the incomplete loss of protein synthesis may be due to the presence of the eIF4G1 homologs eIFG2 and p97 (21). Using HeLa cell extracts and adding purified calpain, we found that all three proteins are equally vulnerable to cleavage by calpain (Fig. S7), and overexpression of eIF4G1 alone was sufficient to increase protein synthesis and provide neuroprotection following ischemia (Fig. 3 C and D). Ischemia-induced decreases in protein synthesis and viability were exacerbated by knockdown of eIF4G1 (Fig. 4 B and C). To determine whether the exacerbation of injury was related to loss of eIF4G1, we overexpressed human eIF4G1 in neurons depleted of endogenous eIF4G1; this treatment increased protein synthesis and neuronal viability (Fig. 4 B and C, fifth and sixth bars). Recovery of protein synthesis and viability was again associated with greater eIF4G1 levels, and there were no changes in levels of several other translation initiation factors (Fig. 4D and Fig. S6B).
Fig. 4.
Silencing of eIF4G1 reduces protein synthesis in neurons and exacerbates OGD-induced death. (A) Neurons were transfected with either shRNA lentivirus targeting rat eIF4G1 (sh4G) or a nontargeting shRNA virus (nt) for 72 and 96 h. Cell lysates were analyzed by Western blot and probed to determine eIF4G1 protein level. eIF4G1 silencing was effective, but other translation initiation factors were not altered. (B) After 1 h OGD and 24 h reperfusion, protein synthesis was reduced in control (nt) and more so in sh4G cultures. Cotransfection of neurons for 72 h with human HA-eIF4G1 and sh4G (4G+sh4G) significantly restored protein synthesis relative to sh4G under control conditions and after 1 h OGD and 24 h reperfusion. (C) Viability was greater in 4G+sh4G cotransfected neurons compared with control (nt) and sh4G transfected neurons following OGD; the data parallel the changes in protein synthesis shown in B under the same conditions. (D) Western blot showed eIF4G1 silencing in sh4G transfected neurons and expression at greater than control levels in 4G+sh4G transfected neurons under control conditions (nt) and after 1 h OGD and 24 h reperfusion with little change in other initiation factors. Bars represent mean ± SD of n = 3–4 independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Cap-Dependent Translation Is Inhibited Following OGD.
Cap-dependent translation via translation initiation factor binding of the 7-methyl-guanosine cap is the predominant means for endogenous cellular mRNA translation (1). We thus wanted to determine whether cleavage of eIF4G1 by calpain following OGD would affect cap-dependent translation following OGD. The solely cap-dependent cellular β-globin 5′-UTR was inserted into Renilla luciferase expression vectors (SI Materials and Methods) and transfected into the neuronal cultures for 2 h at the times indicated. The viral encephalomyocarditis virus (EMCV) 5′-UTR was also inserted upstream of a Firefly luciferase vector and transfected into all cells as a transfection control. Following incubation, the cells were lysed and luciferase activities were measured.
Under control conditions, the capped β-globin was robustly translated (Fig. S8A). Following OGD, β-globin translation was severely inhibited at all times measured (3, 7, and 25 h). We also wanted to determine whether the restoration of protein synthesis due to eIF4G1 overexpression was due to reinstatement of cap-dependent translation. As in the previous experiment, capped β-globin mRNA was transfected into eIF4G1- and GFP- transfected neurons at the indicated times after OGD. Luciferase activity indicating cap-dependent translation was inhibited following OGD in GFP-transfected neurons for at least 24 h (Fig. S8B). Overexpression of eIF4G1, however, increased cap-dependent translation to near control levels by 25 h after the insult. These results indicate that the increase in protein synthesis caused by increasing eIF4G1 levels was, at least in part, due to increased cap-dependent translation.
Calpain Inhibition Reduces Ischemia-Induced Persistent PSI and Increases Neuronal Viability in Vivo.
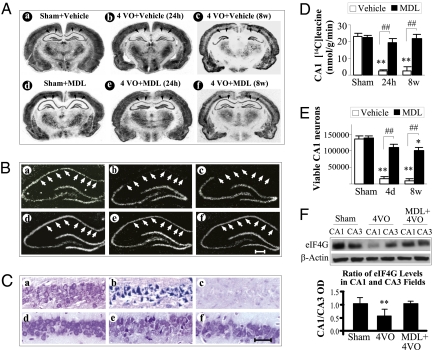
We wished to determine whether PSI and neuronal death following in vivo ischemia were associated with calpain activation and loss of eIF4G1; to this end we used the rat four-vessel occlusion model of global ischemia. In this model, a brief period of global ischemia induces delayed neurodegeneration preferentially in CA1 pyramidal neurons of the hippocampus (22). Twenty-four hours after ischemia induced by 12-min four-vessel occlusion (23), α-spectrin in CA1 was largely cleaved to the 145-kDa fragment. This effect was prevented by the calpain inhibitor MDL 28170 (see SI Materials and Methods for details on drug administration), which also increased full-length α-spectrin 24 h after ischemia (Fig. S9 A and B). Protein synthesis determined by autoradiography and quantification of [14C]leucine uptake was markedly depressed in CA1 at 24 h and 8 wk after 12 min ischemia (Fig. 5A). In contrast, there was no decrease in protein synthesis in hippocampal CA3 or cortex at these times. Calpain inhibition prevented the depression of protein synthesis at both 24 h and 8 wk (Fig. 5 A, B, and D and Table S1). Importantly, neuronal viability was also increased in the CA1 field of rats treated with the calpain inhibitor at 24 h and 8 wk (Fig. 5 C and E), as was recently shown with shRNA-mediated knockdown of μ-calpain (24). Levels of eIF4G1 in CA1 were also reduced in the vulnerable CA1 but not in the resistant CA3; the reduction in CA1 was largely prevented by the calpain inhibitor MDL 28170 (Fig. 5F). In total, these results recapitulate our in vitro findings demonstrating that increased calpain activity following ischemia in vivo is responsible for decreased levels of eIF4G1, persistent PSI, and ultimately delayed neuronal death.
Fig. 5.
Calpain inhibition prevents ischemia-induced protein synthesis inhibition and neuronal death in vivo. (A) Autoradiography of [14C]leucine incorporation in brains of rats that received either vehicle or treatment with MDL 28170 (a calpain inhibitor) for 6 h by tail vein perfusion after global ischemia and intraperitoneally at 24, 48, and 72 h after ischemia (see SI Materials and Methods for details on drug administration). At 24 h and 8 wk after global ischemia (4VO), the [14C]leucine labeling was greatly reduced specifically in CA1 (arrows) and not in the adjacent CA3 in the vehicle-treated animal. (A, b and c; sham control in A, a). MDL treatment to block calpain largely prevented the depression of translation in all regions (A, e and f; sham control in A, d). (B) Magnification of the hippocampal region corresponding to sections in A showing absence of radiolabeling in CA1 (between arrowheads) at 24 h (B, b) and 8 wk (B, c) after ischemia. After MDL treatment, labeling was like that in sham (B, a and b) at both 24 h (B, e) and 8 wk after ischemia. (Scale bar, 35 μm.) (C) Cresyl violet staining of CA1 fields in sections corresponding to those in A but at 4 d and 8 wk after ischemia. Neurons appear to be degenerating at 4 d (C, b) and are absent at 8 wk (C, c). Calpain inhibition did not affect neurons at 8 wk after sham operation (C, b) and prevented death at 4 d (C, e) and 8 wk (C, f). (Scale bar, 35 μm.) (D) Quantification of [14C]leucine incorporation into proteins in the hippocampal CA1 field 24 h and 8 wk after ischemia in vehicle- and MDL-treated animals. Protein incorporation was reduced in vehicle-treated postischemic animals and not significantly different from sham controls in MDL-treated animals. (E) Viable neurons in the CA1 field were counted in stained brain sections as detailed in SI Materials and Methods. Whereas CA1 neurons were present at 24 h in postischemic animals, they were dying by 4 d and were not replaced at 8 wk (C, b and c). In MDL-treated rats the number of viable CA1 neurons was greater than in vehicle treated at both 4 d and 8 wk of reperfusion. MDL and sham groups were not statistically different at 4 d. (F) Four days following 4VO, eIF4G1 was decreased in CA1 but not in CA3. The eIF4G1 decrease in CA1 was prevented by MDL treatment. The ratio of eIF4G1 in CA1 to that in CA3 was significantly decreased in 4VO-only rats. Histology studies represent n = 8 for each of the four ischemia groups and n = 6 for the two sham groups. Bars represent mean ± SD of n = 6–8 animals per group. **P < 0.01, ischemia and vehicle vs. sham; *P < 0.05, ischemia and MDL vs. sham; ##P < 0.01, ischemia and vehicle vs. ischemia and MDL. Abbreviations: 4VO, four-vessel occlusion; OD, optical density.
Discussion
It has been known for nearly 40 y that protein synthesis is inhibited in the entire forebrain following global ischemia (7) and that persistent inhibition of protein synthesis is a robust predictor of eventual neuronal death in both global (8) and focal (9) ischemia. Despite this knowledge, protein synthesis inhibition has yet to be directly linked to neuronal death. In fact, persistent PSI was thought to be an epiphenomenon of ischemic injury that persisted due to repeated peri-infarct depolarizations or to be secondary to energy failure (9).
Here we provide compelling evidence of a direct link between PSI and ischemic neuronal death. The proposed mechanistic connection between ischemia and PSI is the pathological degradation of eIF4G1 mediated by ischemia-induced calpain activation. Calpain inhibition largely prevented ischemia-induced decreases in eIF4G1, allowing for recovery of protein synthesis. Maintenance of eIF4G1 levels by overexpression or calpain inhibition resulted in increased neuronal viability that was associated with increased cap-dependent protein synthesis.
To our knowledge, this is a unique finding demonstrating that degradation of a translation initiation factor is directly involved in neuronal death. The decrease in overall translation rate caused by multiple death stimuli is known to correlate with eIF4G1 levels (25). Decreases in eIF4G1 have also been implicated in neuronal death in previous in vivo studies that demonstrated decreased levels of eIF4G1 for up to 4 h of reperfusion in global ischemia models (5) and for up to 24 h following focal ischemia (18). Importantly, the loss of eIF4G1 at 24 h after focal ischemia (18) is highly correlated with the final infarction volume (9).
In the present study, we confirmed that calpain was activated following OGD and that inhibition of calpain by overexpression of its endogenous inhibitor calpastatin was sufficient to block decreases in eIF4G1. Calpain has been demonstrated to degrade eIF4G1 in a cell-free-assay (12), and it has been implicated in the degradation of eIF4G1 in brain following ischemia (13), both of which we confirm here. Importantly, our findings show that calpain inhibition by calpastatin not only blocks decreases in eIF4G1, but also reduces ischemia-induced PSI and increases neuronal viability.
Numerous attempts to identify preferential calpain cleavage sites have been unsuccessful (26); therefore, we used a cell-free system in an attempt to identify the calpain-mediated cleavage products of eIF4G1. Our cell-free assay demonstrated that calpain readily cleaves eIF4G1 into progressively smaller fragments with increased time. This result was unexpected as calpain activation generally results in cleavage at discrete sites on its substrates (26). Instead, the progressive cleavage of eIF4G1 is more reminiscent of degradation as seen with 20S proteasomal processing of eIF4G1 than of the site-specific cleavage by calpain (27).
The results presented in this paper suggest that persistent PSI (>12 h) following OGD is related to delayed neuronal death. Accordingly, addition of CHX at concentrations that severely decrease protein synthesis for 24 h exacerbated ischemia-induced neuronal death, whereas overexpression of either calpastatin or eIF4G1 increased cap-dependent protein synthesis and resulted in significant neuroprotection. A finding of this paper is that although the neuroprotective proteins did not prevent initial PSI (likely due to transient P-eIF2α up-regulation), they eventually restored translation by preserving levels of eIF4G1. This result is an important distinction in terms of neuroprotective strategies, as the aim for clinical neuroprotection is to prevent the eventual loss of brain tissue after ischemia wherein loss of eIF4G1 and initial PSI has already occurred.
Restoration of protein synthesis is clearly important for maintenance of neuronal viability following ischemia. Neuroprotection provided by eIF4G1 overexpression in vitro and MDL 28170 in vivo significantly increased protein synthesis; however, neuroprotection by eIF4G1 may be only partially due to restoration of translation, as treatment with CHX did not completely block the neuroprotective effect. Other mechanisms may therefore be involved in the protective effect of eIF4G1. It was recently demonstrated that eIF4G1 was involved with preservation of bioenergetics and mitochondrial activity along with suppression of autophagy (21). The potential role of eIF4G1 in regulating mitochondrial biogenesis and autophagy via protein synthesis-dependent and -independent mechanisms is an enticing area for further study.
In conclusion, we provide evidence that levels of a translation initiation factor are directly linked to cell viability. We show that calpain-mediated degradation of eIF4G1 is a causal link between ischemia and persistent PSI leading to neuronal death. Preservation of eIF4G1 levels promotes recovery from ischemia-induced PSI and provides neuroprotection. Additional assessments of the types of mRNA translated and the mechanism of translation following ischemia are also warranted. Further understanding of how eIF4G1 regulates persistent PSI and contributes to the multitude of factors causing postischemic neurodegeneration may lead to the development of new neuroprotective strategies.
Materials and Methods
Details beyond the descriptions here are provided in SI Materials and Methods.
Neuronal Ischemia Models.
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rat primary cortical neuronal cultures were derived from E18 embryos of Sprague–Dawley rats, and in vitro ischemia was induced in neurons by oxygen and glucose deprivation. Global ischemia was induced for 12 min in anesthetized male Sprague–Dawley rats under isolflurane anesthesis using the four-vessel occlusion model (23). Cell death and protein synthesis of hippocampal CA1 neurons were quantified at 24 h, 4 d, or 8 wk after ischemia (23).
Cell Viability Assays.
Neuronal viability was assessed with Alamar Blue, Hoechst 33342 staining and lactate dehydrogenase (LDH) release as previously described (23) with slight modification (SI Materials and Methods). The amount of LDH release was presented as percentage of control.
Protein Synthesis Assay.
Protein synthesis in vitro was measured using a pulse–chase method with [35S]methionine for 15 min at indicated times. [14C]Leucine incorporation was used for in vivo studies (SI Materials and Methods). Incorporated radiolabeled protein was precipitated from cell lysates and measured by scintillation counting. Incorporation measured in cpm per microgram protein relative to control was calculated.
Gene Transfection of Calpastatin.
Generation of the calpastatin plasmid with the C-terminal HA tag has been described previously (23). Transfection of calpastatin was achieved using Nucleofection (Amaxa) according to the manufacturer's protocol.
Recombinant Protein Preparation.
Recombinant myc-tagged eIF4G1 preparation has been described previously (3). The production of truncated C-terminal His-tagged recombinant eIF4G1 peptides amino acids 179–674 and 675–1129 is described further in SI Materials and Methods.
Lentiviral Vectors.
The HA-eIF4G1 plasmid was generated as previously described (23). Lentiviral vectors to overexpress human full-length HA-eIF4G1 were constructed by inserting the HA-eIF4G1 sequence into the FUW transfer vector, which is under control of the ubiquitin promoter. A control vector with the EGFP ORF was also constructed and inserted into the FUW transfer vector. Lentiviral vectors carrying a proprietary shRNA directed against rat eIF4G1 and a control shRNA sequence were purchased from Santa Cruz Biotechnology.
Statistical Analysis.
All results are expressed as mean ± SD from at least three independent experiments. Multiple group comparisons were performed using ANOVA with post hoc Fisher's protected least significant difference (PLSD) correction testing, and P < 0.05 was accepted as statistically significant.
Supplementary Material
Acknowledgments
This project was supported by National Institutes of Health Grants NS36736, NS43802, and NS45048 (to J.C.) and by Chinese Natural Science Foundation Grants 30870794 and 30670642 (to Y.G.). P.S.V. was supported by a National Institutes of Health National Research Service Award predoctoral fellowship (1F30NS057886), and F.Z. was supported by a Young Investigator American Heart Association grant (10SDG2560122). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and is supported by National Institutes of Health Grant NS 55363.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112635108/-/DCSupplemental.
References
- 1.Sonenberg N, Hershey JWB, Mathews M. Translational Control of Gene Expression. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2000. [Google Scholar]
- 2.Morley SJ, Coldwell MJ, Clemens MJ. Initiation factor modifications in the preapoptotic phase. Cell Death Differ. 2005;12:571–584. doi: 10.1038/sj.cdd.4401591. [DOI] [PubMed] [Google Scholar]
- 3.Bushell M, et al. Cleavage of polypeptide chain initiation factor eIF4GI during apoptosis in lymphoma cells: Characterisation of an internal fragment generated by caspase-3-mediated cleavage. Cell Death Differ. 2000;7:628–636. doi: 10.1038/sj.cdd.4400699. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa K, Estus S, Fu W, Mark RJ, Mattson MP. Neuroprotective action of cycloheximide involves induction of bcl-2 and antioxidant pathways. J Cell Biol. 1997;136:1137–1149. doi: 10.1083/jcb.136.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeGracia DJ. Acute and persistent protein synthesis inhibition following cerebral reperfusion. J Neurosci Res. 2004;77:771–776. doi: 10.1002/jnr.20225. [DOI] [PubMed] [Google Scholar]
- 6.Kleihues P, Hossmann KA, Pegg AE, Kobayashi K, Zimmermann V. Resuscitation of the monkey brain after one hour complete ischemia. III. Indications of metabolic recovery. Brain Res. 1975;95:61–73. doi: 10.1016/0006-8993(75)90207-3. [DOI] [PubMed] [Google Scholar]
- 7.Kleihues P, Hossmann KA. Protein synthesis in the cat brain after prolonged cerebral ischemia. Brain Res. 1971;35:409–418. doi: 10.1016/0006-8993(71)90484-7. [DOI] [PubMed] [Google Scholar]
- 8.Thilmann R, Xie Y, Kleihues P, Kiessling M. Persistent inhibition of protein synthesis precedes delayed neuronal death in postischemic gerbil hippocampus. Acta Neuropathol. 1986;71:88–93. doi: 10.1007/BF00687967. [DOI] [PubMed] [Google Scholar]
- 9.Hata R, Maeda K, Hermann D, Mies G, Hossmann KA. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 10.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 11.Vosler PS, Brennan CS, Chen J. Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol Neurobiol. 2008;38:78–100. doi: 10.1007/s12035-008-8036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wyckoff EE, Croall DE, Ehrenfeld E. The p220 component of eukaryotic initiation factor 4F is a substrate for multiple calcium-dependent enzymes. Biochemistry. 1990;29:10055–10061. doi: 10.1021/bi00495a007. [DOI] [PubMed] [Google Scholar]
- 13.Neumar RW, et al. Calpain mediates eukaryotic initiation factor 4G degradation during global brain ischemia. J Cereb Blood Flow Metab. 1998;18:876–881. doi: 10.1097/00004647-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Frederick JR, et al. Neuroprotection with delayed calpain inhibition after transient forebrain ischemia. Crit Care Med. 2008;36(11, Suppl):S481–S485. doi: 10.1097/ccm.0b013e31818a8ec8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeGracia DJ, Neumar RW, White BC, Krause GS. Global brain ischemia and reperfusion: Modifications in eukaryotic initiation factors associated with inhibition of translation initiation. J Neurochem. 1996;67:2005–2012. doi: 10.1046/j.1471-4159.1996.67052005.x. [DOI] [PubMed] [Google Scholar]
- 16.García L, et al. Ischaemic preconditioning in the rat brain: Effect on the activity of several initiation factors, Akt and extracellular signal-regulated protein kinase phosphorylation, and GRP78 and GADD34 expression. J Neurochem. 2004;88:136–147. doi: 10.1111/j.1471-4159.2004.02188.x. [DOI] [PubMed] [Google Scholar]
- 17.Martín de la Vega C, et al. Possible mechanisms involved in the down-regulation of translation during transient global ischaemia in the rat brain. Biochem J. 2001;357:819–826. doi: 10.1042/0264-6021:3570819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mengesdorf T, Proud CG, Mies G, Paschen W. Mechanisms underlying suppression of protein synthesis induced by transient focal cerebral ischemia in mouse brain. Exp Neurol. 2002;177:538–546. doi: 10.1006/exnr.2002.8002. [DOI] [PubMed] [Google Scholar]
- 19.Malagelada C, Xifró X, Miñano A, Sabriá J, Rodríguez-Alvarez J. Contribution of caspase-mediated apoptosis to the cell death caused by oxygen-glucose deprivation in cortical cell cultures. Neurobiol Dis. 2005;20:27–37. doi: 10.1016/j.nbd.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Newcomb-Fernandez JK, et al. Concurrent assessment of calpain and caspase-3 activation after oxygen-glucose deprivation in primary septo-hippocampal cultures. J Cereb Blood Flow Metab. 2001;21:1281–1294. doi: 10.1097/00004647-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol. 2008;181:293–307. doi: 10.1083/jcb.200710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 23.Cao G, et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci. 2007;27:9278–9293. doi: 10.1523/JNEUROSCI.2826-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bevers MB, et al. RNAi targeting micro-calpain increases neuron survival and preserves hippocampal function after global brain ischemia. Exp Neurol. 2010;224:170–177. doi: 10.1016/j.expneurol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantinou C, Clemens MJ. Regulation of translation factors eIF4GI and 4E-BP1 during recovery of protein synthesis from inhibition by p53. Cell Death Differ. 2007;14:576–585. doi: 10.1038/sj.cdd.4402045. [DOI] [PubMed] [Google Scholar]
- 26.Cuerrier D, Moldoveanu T, Davies PL. Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: The importance of primed side interactions. J Biol Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- 27.Baugh JM, Pilipenko EV. 20S proteasome differentially alters translation of different mRNAs via the cleavage of eIF4F and eIF3. Mol Cell. 2004;16:575–586. doi: 10.1016/j.molcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.