Abstract
Huntington disease (HD) is a neurodegenerative disorder caused by a CAG repeat expansion in the gene coding for huntingtin protein. Several mechanisms have been proposed by which mutant huntingtin (mHtt) may trigger striatal neurodegeneration, including mitochondrial dysfunction, oxidative stress, and apoptosis. Furthermore, mHtt induces DNA damage and activates a stress response. In this context, p53 plays a crucial role in mediating mHtt toxic effects. Here we have dissected the pathway of p53 activation by mHtt in human neuronal cells and in HD mice, with the aim of highlighting critical nodes that may be pharmacologically manipulated for therapeutic intervention. We demonstrate that expression of mHtt causes increased phosphorylation of p53 on Ser46, leading to its interaction with phosphorylation-dependent prolyl isomerase Pin1 and consequent dissociation from the apoptosis inhibitor iASPP, thereby inducing the expression of apoptotic target genes. Inhibition of Ser46 phosphorylation by targeting homeodomain-interacting protein kinase 2 (HIPK2), PKCδ, or ataxia telangiectasia mutated kinase, as well as inhibition of the prolyl isomerase Pin1, prevents mHtt-dependent apoptosis of neuronal cells. These results provide a rationale for the use of small-molecule inhibitors of stress-responsive protein kinases and Pin1 as a potential therapeutic strategy for HD treatment.
Huntington disease is a dominantly inherited neurodegenerative disorder due to an expanded CAG repeat sequence in the HD gene that elongates a segment of glutamine residues in the protein huntingtin (Htt) (1). The most striking pathological manifestation of HD is a gradual loss of neurons, predominantly in the striatum, causing motor abnormalities and cognitive decline (2). Toxic properties of mutant huntingtin (mHtt) are believed to cause HD. Among them, mitochondrial dysfunction and generation of reactive oxygen species (ROS) lead to DNA lesions (3, 4). Interestingly, expression of full-length mHtt protein and N-terminal fragments containing the polyQ expansion elicit a DNA damage response, with activation of the ATM/ATR pathways (3, 4) and their downstream effectors, including the tumor suppressor p53 (3, 5, 6). p53 mediates mitochondrial dysfunction and cytotoxicity in HD cells and in transgenic animal models, whereas its inhibition prevents these phenotypes (5).
p53 governs a wide array of pathways involved in genomic stability, antioxidant activities, and energy metabolism in addition to promoting either cytostatic or cytotoxic responses to intrinsic and exogenous sources of cellular stress (7). Given the complexity of p53 functions within the cell, a better understanding of how signaling networks converge on this hub to modulate mHtt-dependent toxicity is required to shed light on the reduced ability of the brain neurons to survive. Regulation of p53 activities relies on a complex network of posttranslational modifications and protein interactions (8, 9), which ultimately determine the outcome of the p53 response. This process entails site-specific phosphorylation by several DNA damage-activated protein kinases, including, among others, ataxia telangiectasia mutated (ATM), ATM and Rad3-related (ATR), homeodomain-interacting protein kinase 2 (HIPK2), and PKCδ. The subsequent transduction of stress-dependent phosphorylation into specific conformational changes of p53 that fully unleash its apoptotic activity is performed by the prolyl isomerase Pin1. This enzyme catalyzes cis/trans isomerization of proline bonds preceded by phosphorylated serine or threonine residues (pSer/Thr-Pro), thereby altering structure and functions of its substrates (10, 11). Upon genotoxic insults, Pin1 binds multiple sites on p53, promoting its accumulation in stressed cells, the activation of its transcriptional functions, and the induction of its apoptotic activity (12–14).
Phosphorylation-dependent prolyl isomerization triggered by Pin1 represents an essential mechanism in modulating several signaling pathways involved in DNA damage and apopotosis. In the CNS, Pin1 is highly expressed and regulates several substrates, including the hyperphosphorylated form of tau in Alzheimer’s disease (15), whereas in Parkinson disease (PD) Pin1 facilitates formation of α-synuclein inclusions (16).
Based on these considerations, we reasoned that Pin1 could be critical for mediating p53-dependent mHtt toxicity. Therefore, by investigating its role in this context, we may highlight crucial upstream events involved in HD pathogenesis and unveil attractive targets for development of novel therapeutic options to counteract HD.
Results
Expression of Mutant Huntingtin Promotes the Interaction of p53 with Pin1.
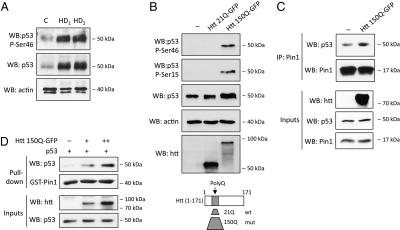
Analysis of postmortem brains of HD patients revealed high levels of p53 relative to healthy controls (Fig. 1A), in agreement with previous reports (5). To study the stress pathways responsible for p53 activation in HD neurons, we then analyzed p53 phosphorylation. Interestingly, in HD brains, p53 was phosphorylated on Ser46 (Fig.1A), a modification that has been associated with activation of its apoptotic function upon stress (14, 17). Nuclear accumulation of mHtt N-terminal fragments is observed in HD brains (18) and animal models (18). Expression of these truncated forms recapitulates many molecular and neurological HD phenotypes (19). The N-terminal fragment (residues 1–171) of either wild-type or mutant Htt (bearing 21 and 150 polyQ, respectively) were thus expressed in SH-SY5Y human neuroblastoma cells to verify whether p53 Ser46 phosphorylation was a consequence of mHtt expression. Interestingly, mutant but not wild-type Htt induced the phosphorylation of endogenous p53 on Ser46, in addition to the previously reported phosphorylation of Ser15 (6) (Fig. 1B). Because Ser46 phosphorylation generates a target site for the prolyl isomerase Pin1 (12, 14), we asked whether Pin1 might play a role in mediating activation of p53 upon mHtt expression in neuronal cells. Strikingly, expression of the mHtt-150Q fragment was sufficient to promote the interaction of endogenous p53 and Pin1 proteins in SH-SY5Y cells (Fig. 1C). Moreover, mHtt expression stimulated direct interaction of p53 with Pin1 as demonstrated by GST-Pin1 pull-down assays (Fig. 1D), and this effect was proportional to the amount of mHtt. Of note, Pin1 neither interacted with Htt (1–171) protein fragments (Fig. S1 A and B) nor affected mHtt protein levels (Fig. S1 C and D).
Fig. 1.
Expression of mutant huntingtin induces the interaction of p53 with Pin1. (A) Phosphorylation of p53 on Ser46 in postmortem brains was compared between HD patients (HD1 and HD2) and healthy controls (C) by Western blot. (Middle and Bottom) Levels of total p53 and actin as loading control. (B) SH-SY5Y cells were transfected with constructs expressing the N-terminal 1–171 Htt fragment with either 21Q or 150Q. p53 was immunoprecipitated from equal amounts of total cell lysates and analyzed by Western blot with antibodies specific for phosphorylated Ser46 and total p53. The levels of actin and Htt proteins in input lysates are shown. (C) SH-SY5Y were transfected with indicated constructs. Cell lysates normalized for p53 protein levels were subjected to coimmunoprecipitation to analyze interaction of endogenous p53 and Pin1. (D) H1299 cells were transfected with indicated constructs, and the interaction of p53 with recombinant Pin1 protein was analyzed by GST pull-down of cell lysates normalized for p53 levels.
Pin1 Mediates Activation of the p53 Pathway by mHtt.
It has been previously shown that expression of mHtt in SH-SY5Y cells triggers a p53-dependent response involving the activation of apoptotic genes, including Bax and PUMA (5). As shown in Fig. 2A, Pin1 potentiated the induction of p53 transcriptional activity by mHtt. Moreover, induction of endogenous PUMA in response to mHtt expression in these cells also required Pin1 (Fig. 2B).
Fig. 2.
Pin1 activity is required for induction of p53-dependent apoptosis by mHtt. (A) The cooperative effect of mHtt and Pin1 on p53 transcriptional activity was evaluated by transfecting SH-SY5Y cells with pG13-Luc reporter and vectors expressing mHtt(1–171)150Q and increasing amounts (+, ++) of Pin1-HA. The graph shows means and SD of three independent experiments. (B) SH-SY5Y cells were transfected with indicated constructs and siRNA oligonucleotides, and the expression of the p53 target PUMA was evaluated by Western blot after 48 h. (C) SH-SY5Y cells were transfected with the indicated combinations of constructs expressing mHtt(1–171)150Q-GFP, Pin1 siRNA oligonucleotides, and siRNA-resistant wild-type Pin1-HA (WT) or the catalytically inactive mutant S67E (CI). Apoptosis of mHtt GFP-expressing cells was evaluated by TUNEL assay after 48 h. The histograms show mean and SD of three independent experiments. (D) Expression of p21WAF1 mRNA was analyzed by qRT-PCR from the striatum of 12-mo-old mice of the indicated genotypes (at least three mice for each genotype), normalizing for expression of β-actin. A t test was performed using homoschedastic variance through groups and one tail parameter. p53 was immunoprecipitated from equal amounts of brain lysates of the same mice and analyzed by Western blot. Actin protein levels in input lysates are shown. (E) The expression of the p53 target PUMA in total brain lysates of 12-mo-old mice of the indicated genotypes (two mice for each genotype, 1 and 2) was analyzed by Western blot.
In agreement with previous reports (5), expression of mHtt in SH-SY5Y cells provoked apoptosis, which was reduced by 50% upon silencing p53 expression (Fig. 2C). The same effect was observed upon silencing the expression of Pin1. Importantly, mHtt-induced apoptosis could be reestablished in Pin1-depleted cells by overexpression of a siRNA-resistant Pin1 construct; this was not effective in cells depleted of p53 (Fig. 2C), suggesting that the effect of Pin1 relies on p53. It is noteworthy that expression of a catalytically inactive Pin1 mutant was unable to rescue knockdown of endogenous Pin1, proving that the prolyl isomerase activity is essential for transducing mHtt-dependent stress into p53 activation. Similar results were also observed in another neuroblastoma cell line, SK-N-SH (Fig. S2). These data indicate that the Pin1/p53 pathway plays a major role in neuronal apoptosis induced by mHtt.
We then analyzed activation of the p53 response in the brains of Hdh CAG knock-in mice in which the glutamine tract of mouse Htt is extended to 111 residues (HdhQ111) (20). These mice show striatal neurodegeneration, reactive gliosis, and gait abnormalities at older age (after 24 mo) (21). However, we observed stabilization of p53 in brain extracts and the consequent transcriptional induction of the p53 target gene p21WAF1 in the striatum of 12-mo-old HdhQ111 mice compared with their WT littermates, HdhQ7 (Fig. 2D). This finding suggests that activation of the p53 pathway by mHtt-associated stress is an early event in HD pathogenesis and could precede neurological symptoms. HdhQ111 mice were then crossed with Pin1KO mice (22) to verify whether Pin1 is required for p53 activation. The interaction between p53 and Pin1 was clearly detectable in protein extracts obtained from HdhQ111/Pin1WT mouse brains (Fig. S3A), confirming what was observed in human cells. Importantly, in contrast to HdhQ111/Pin1WT mice, p53 transcriptional activity was not induced in HdhQ111/Pin1KO mice (Fig. 2D), and the expression of the apoptotic p53 target PUMA in HdhQ111 mouse brains was indeed dependent on Pin1 (Fig. 2E). We therefore analyzed striatal neurodegeneration in 24-mo-old mice (Fig. S3 B and D). In agreement with published data (21), moderate neuronal loss was observed in HdhQ111 mice compared with WT Htt littermates on a Pin1 WT background (Fig. S3D). Pin1 KO mice also showed a similar reduction of striatal neurons number compared with WT littermates, which could possibly be ascribed to the reported age-dependent neurodegeneration of mice lacking Pin1 (23). Importantly, in Pin1 KO mice the numbers of striatal neurons did not further decrease on mHtt expression, suggesting that lack of p53 activation (Fig. 2 D and E) might indeed prevent Htt-dependent neurodegeneration in mice devoid of Pin1.
These results indicate that Pin1 plays a critical role for p53 activation in response to mHtt expression in striatal neurons of a mouse model of HD pathogenesis.
Phosphorylation of p53 on Ser46 by HIPK2 and PKCδ Is an Upstream Event in the mHtt-Pin1-p53 Pathway.
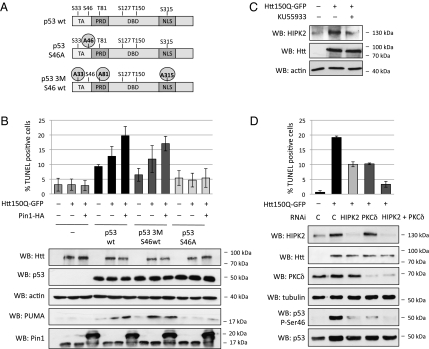
Our observations indicated that phosphorylation of p53 on Ser46 is triggered by mHtt (Fig. 1 A and B). To define whether this or other phosphorylations are responsible for activating p53 apoptotic response upon expression of mHtt, we used p53 phosphorylation mutants with single and multiple substitutions of Ser/Thr with Ala residues within Pin1 binding sites (14) (Fig. 3A). Expression of these proteins in p53-null H1299 cells demonstrated that Ser46 is required for mHtt-induced apoptosis, because a Ser46-Ala p53 mutant was unable to cause apoptosis in response to mHtt expression (Fig. 3B). In contrast, a p53 mutant (p53 3M-S46wt) that lacked the remaining three major Pin1 binding sites (i.e., Ser33, Thr81, and Ser315) could efficiently induce apoptosis downstream to mHtt expression. This protein was phosphorylated on Ser46 in cells expressing mHtt (Fig. S4A), and its apoptotic activity was potentiated by Pin1 (Fig. 3B). Both p53 WT and p53 3M-S46WT were then able to induce the proapoptotic p53 target PUMA upon transfection of mHtt, whereas p53 S46A was almost inactive (Fig. 3B). Of note, similar experiments performed in mouse neuroblastoma cells demonstrated that phosphorylation of Ser58, the mouse homolog of p53 Ser46, is essential for mHtt-induced apoptosis (Fig. S4B).
Fig. 3.
p53 Ser46 phosphorylation is promoted by mHtt through HIPK2 and PKCδ and is required for apoptosis induction by Pin1. (A) p53 scheme indicating Pin1 consensus sites (phospho–Ser/Thr-Pro). DBD, DNA binding domain; NLS, nuclear localization signal; TA, transactivation domain. p53 mutants have Ser/Thr-to-Ala substitutions in Pin1 consensus sites at residue 46 (p53 S46A) or at the three other major Pin1-binding sites at residues 33, 81, and 315 (p53 3M-S46wt). (B) p53-null H1299 cells were transfected with the indicated constructs (see also A). Apoptosis of mHtt GFP-expressing cells was evaluated by TUNEL assay after 48 h. The histograms show mean and SD of three independent experiments. (C) SH-SY5Y cells were transfected with mHtt(1–171)150Q-GFP and treated with the ATM inhibitor KU-55933 10 μM for 24 h. HIPK2 protein levels were evaluated by Western blot. (D) SH-SY5Y cells were transfected with the indicated combinations of mHtt(1–171)150Q-GFP expression construct and siRNA oligonucleotides for HIPK2 and PKCδ. Apoptosis of mHtt GFP-expressing cells was evaluated by TUNEL assay after 48 h. The histograms show mean and SD of three independent experiments.
Therefore, we concluded that phosphorylation of p53 on Ser46 is a crucial event in the pathway leading to neuronal death induced by mHtt in human and mouse cells. Our data also indicate that modification at this site is sufficient for Pin1 to enhance p53’s apoptotic function in cells expressing mHtt.
Among the protein kinases that catalyze phosphorylation of p53 on Ser46, HIPK2 plays a key role in unleashing p53’s apoptotic activity upon DNA damage (24). HIPK2 is induced by cytotoxic stimuli through the ATM/ATR pathway (25), which becomes activated upon mHtt-dependent stress (3). Interestingly, expression of mHtt was sufficient to up-regulate HIPK2 protein levels in SH-SY5Y cells, and this effect required ATM kinase activity because it was prevented by treatment with the ATM-specific inhibitor KU55933 (Fig. 3C).
We thus inhibited HIPK2 expression by RNAi, which dampened mHtt-dependent phosphorylation of p53 on Ser46 with concomitant decrease of apoptosis (Fig. 3D). This result indicates a major role for HIPK2 in the activation of p53 by mHtt. Interestingly, depletion of HIPK2 did not fully prevent Ser46 phosphorylation triggered by mHtt (Fig. 3D), implying the involvement of other kinases in inducing apoptosis downstream of mHtt. We postulated that PKCδ might concur to this effect, because its activation has been reported to regulate p53 during neuronal death (26). Knockdown of PKCδ by RNAi strongly decreased phospho-Ser46 (Fig. 3D), consistent with the notion that this enzyme phosphorylates Ser46 upon DNA damage (27). Moreover, mHtt-dependent apoptosis was also reduced. Importantly, concomitant knockdown of both HIPK2 and PKCδ synergized to inhibit both Ser46 phosphorylation and mHtt-induced apoptosis (Fig. 3D). The reduction of apoptosis exceeded that obtained by silencing p53, suggesting that HIPK2 and PKCδ might also regulate p53-independent apoptotic pathways downstream to mHtt.
Pin1 Unlocks p53 from iASPP in Response to mHtt Expression.
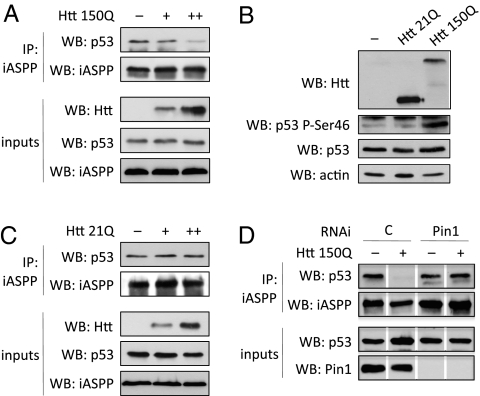
We have previously shown that Pin1-mediated isomerization of phosphorylated Ser46-Pro47 site unleashes p53’s apoptotic potential upon DNA damage, by leading to its dissociation from the apoptosis inhibitor iASPP (14). Because mHtt triggers both DNA damage (3) and Ser46 phosphorylation, we investigated whether it can induce p53 dissociation from iASPP. Coimmunoprecipitation experiments highlighted that the interaction between p53 and iASPP was progressively lost upon expression of increasing amounts of mHtt in H1299 cells (Fig. 4A). This effect correlated with and required Ser46 phosphorylation (Fig. 4B): indeed, WT Htt caused neither p53 Ser46 phosphorylation (Fig. 4B) nor iASPP detachment (Fig. 4C), and S46A mutation impaired the dissociation of p53 from iASPP on mHtt expression (Fig. S4C). Importantly, when Pin1 expression was silenced, p53 remained bound to iASPP regardless of mHtt expression (Fig. 4D). These data indicate that blocking Pin1 prevents p53 from inducing proapoptotic effectors due to the sustained inhibition by iASPP even in presence of mHtt-dependent stress.
Fig. 4.
mHtt induces dissociation of p53 from iASPP in a Ser46- and Pin1-dependent fashion. (A) H1299 cells were transfected with constructs expressing p53 and increasing amounts (+, ++) of mHtt(1–171)150Q; the interaction of p53 with endogenous iASPP protein was then analyzed by coimmunoprecipitation. (B) H1299 cells were transfected with constructs expressing p53 and Htt(1–171) fragments bearing either 21Q or 150Q. Total cell lysates were analyzed by Western blot with antibodies specific for Ser46-phosphorylated and for total p53. (C) The interaction between p53 and iASPP proteins after transfection of increasing amounts of wt Htt(1–171)21Q in H1299 cells was determined as in A. (D) The effect of RNAi-mediated knockdown of Pin1 on the dissociation of p53 from iASPP induced by overexpressed mHtt was determined as in A.
Interfering with p53 Activation by Pin1 Prevents Apoptosis Downstream of mHtt.
Given that p53 activation mediates the cytotoxic effects of mHtt, we hypothesized that pharmacologic inhibition of catalytic activity of either Pin1 or of the kinases that phosphorylate p53 on the Pin1 target site Ser46 could prove effective in preventing mHtt-induced apoptosis. In fact, treatment with the specific Pin1 inhibitor PiB (28) reduced mHtt-dependent apoptosis (Fig. 5A) with efficiency similar to knockdown of Pin1 or p53 (Fig. 2C). Intriguingly, inhibition of Pin1 also appeared to reduce Ser46 phosphorylation. Treatment of SH-SY5Y cells with rottlerin, a compound widely used to inhibit PKCδ activity, also reduced mHtt-dependent cellular toxicity and dampened Ser46 phosphorylation triggered by mHtt (Fig. 5B) similar to PKCδ knockdown (Fig. 3D). Because specific inhibitors of HIPK2 are not available, we attempted to pharmacologically interfere with this pathway by inhibiting the upstream kinases. Treatment of SH-SY5Y cells with caffeine, a well-known inhibitor of ATM/ATR activities, strongly reduced mHtt-dependent cellular toxicity and Ser46 phosphorylation (Fig. 5C). We then focused on ATM, which, besides inducing HIPK2, directly phosphorylates p53 on Ser46, in addition to Ser15 (29). Strikingly, the ATM-specific inhibitor KU55933 was effective in preventing mHtt-induced apoptosis by reducing phosphorylation of p53 on both Ser46 and Ser15 (Fig. 5D).
Fig. 5.
Inhibition of p53 phosphorylation on Ser46 or of Pin1-mediated isomerization reduces mHtt-dependent apoptosis. (A–D) SH-SY5Y cells were transfected with a construct expressing mHtt(1–171)150Q-GFP and treated with the Pin1 inhibitor PiB, 5 μM (A), the PKCδ inhibitor rottlerin, 5 μM (B), the ATM/ATR inhibitor caffeine, 3 mM (C), the ATM-specific inhibitor KU-55933, 10 μM (D), or with same amount of solvent as a control (–). Apoptosis of mHtt GFP-expressing cells was evaluated by TUNEL assay after 24 h. Graphs show means and SD of three independent experiments. To detect p53 phosphorylation, p53 was immunoprecipitated from equal amounts of total cell lysates and analyzed by Western blot. The protein levels of Pin1, actin, and mHtt in input lysates are shown. (E) Model for regulation of p53 by Pin1 upon cellular stress generated by mutant huntingtin. In cells expressing mHtt, the activities of ATM, HIPK2, and PKCδ lead to phosphorylation of p53 on Ser46. Subsequent prolyl isomerization of the phospho–Ser46-Pro47 site by Pin1 then unlocks p53 from the apoptosis inhibitor iASPP, leading to induction of apoptotic genes. Pharmacologic interference with this pathway can be accomplished by use of small-molecule inhibitors that target ATM, PKCδ, or Pin1, thereby preventing p53 cytotoxic activity.
Together, our experimental evidences support a model (Fig. 5E) where stress generated by mHtt triggers activation of ATM, HIPK2, and PKCδ kinases, which lead to phosphorylation of p53 on Ser46. This process preludes Pin1-dependent prolyl isomerization and consequent dissociation of p53 from iASPP as a prerequisite for taking the apoptotic route.
Discussion
Despite the huge amount of data accumulated so far, a cure for HD is not yet available. Though attention has been especially devoted to the roles of downstream effectors, such as caspases, in neurodegeneration, the identity of druggable upstream mediators of polyQ-dependent toxicity still remains elusive. In this work we have focused on an emerging model of HD pathogenesis, where mHtt evokes a canonical DNA damage response in neuronal cells, with induction of ATM/ATR kinases and consequent activation of p53 (3). We have observed that an important mark of p53 activation, i.e., Ser46 phosphorylation, is induced downstream of mHtt expression and is also evident in the brains of HD patients. Ser46 phosphorylation is specifically triggered by severe or persistent stress and represents a major event in shifting the p53 response from cell-cycle arrest to apoptosis (17, 30, 31). Here we have shown that this modification is a prerequisite for execution of apoptosis downstream of mHtt. We have previously demonstrated that recognition of phosphorylated Ser46 by the prolyl isomerase Pin1 leads to dissociation of p53 from the apoptosis inhibitor iASPP (14). Our results indicate that in cells expressing mHtt, isomerization by Pin1 is an essential step for unleashing the apoptotic potential of p53 from iASPP, thus allowing induction of apoptotic effectors.
HD neuropathology is characterized by massive loss of medium spiny neurons in the striatum. The influence of p53 in HD pathogenesis in vivo has been clearly shown by using mHtt-transgenic fly and mouse models (5). Here we demonstrate that genetic ablation of Pin1 in HdhQ111 KI mice prevents precocious activation of p53, suggesting that Pin1 is required for induction of the p53 response, at least in early stages of HD. In fact, we observed that p53 transcriptional activity is induced in striatal neurons in vivo more than 1 y before cell death, implying that these cells possess the ability to deal with chronic stress for long periods of time. Analysis of older animals also suggested that interference with p53 activation by targeting Pin1 might reduce Htt-induced neurodegeneration.
Our findings also imply that p53-independent pathways may concur to HD-related toxicity. The p53 family member p73 has been found relevant for mHtt-induced neuronal death (32), and the ability of Pin1 to potentiate the apoptotic activity of both p53 and p73 (33) might be critical in this respect.
By describing how mHtt stimulates the activation of p53 by triggering its phosphorylation-induced, Pin1-dependent isomerization, we suggest that this polyQ-expanded protein causes neurotoxicity by acting as an upstream inducer rather than through direct interaction with p53 (5, 34); this implies that p53 activation might represent a general pathogenic mechanism for polyglutamine diseases sharing the occurrence of DNA lesions (4). In the CNS, p53 mediates neuronal death in response to excitotoxicity and oxidative stress (35), and its activity has been implicated in other neurodegenerative diseases, such as PD (36). Intriguingly, p53 has been shown to increase mHtt expression (37). Therefore, by activating p53, Pin1 might enforce a noxious loop triggered by Htt mutation, enhancing its toxic effects. In this respect, common genetic polymorphisms (SNPs) affecting stress-induced p53 activation (38) might impinge on HD pathogenesis (39). However, SNPs in the Pin1 promoter region have also been described as affecting protein expression (40, 41), and it would be thus interesting to investigate whether Pin1 may act as a modifier of HD pathogenesis.
Identification of upstream pathogenic events in HD is crucial for designing therapeutic interventions, and the vast knowledge available on the p53 pathway provides an advantageous standpoint to highlight regulatory nodes suitable for manipulation. The model emerged from our data (Fig. 5E) details potential pharmacological targets. First, we demonstrated that small-molecule inhibitors of Pin1 can protect neuronal cells from mHtt-induced apoptosis in vitro and may therefore be effective as a therapeutic strategy for treatment of HD. Although it is arguable that development of clinically useful inhibitors of Pin1 awaits further improvement, our results may also indicate stress-induced p53 kinases as druggable therapeutic targets. The dissociation of p53 from iASPP and the induction of apoptotic effectors can indeed be prevented by interfering with phospho-Ser46 isomerization by Pin1 (14). Here we have succeeded in reducing mHtt-dependent apoptosis of neuronal cells by inhibiting the activity of PKCδ and ATM kinases, which lead to Ser46 phosphorylation either directly or indirectly. Protein kinases are a growing drug target class for diseases of peripheral tissues, and several candidate therapeutics targeting CNS kinases are now in various stages of preclinical and clinical development. For instance, specific inhibitors of PKCδ have shown preclinical in vivo efficacy in treatment of PD (42).
Because clinical trials of molecules that may restore functionality to a single cellular pathway have failed, special attention has been devoted to the identification of drugs that may interfere with different pathways at the same time. To this purpose, caffeine seems particularly interesting for its neuroprotective properties as a blocker of adenosine A2A receptors (ADORA2A) (43). Recently, a genetic variant of ADORA2A has been identified as a modifier of age at onset in HD (44). The combined actions as inhibitor of p53-mediated apoptotic pathway as well as neuroprotective molecule acting at adenosine receptors may increase caffeine’s chances as a pharmacological intervention for HD.
Methods
Cell Lines and HD Tissues.
SH-SY5Y and SK-N-SH human neuroblastoma cells were cultured in 1:1 Eagle’s minimal essential medium (MEM)/F12 Ham’s medium with 15% FBS, 0.5% GlutaMAX (Gibco), 1% nonessential amino acids, and antibiotics. Neuro-2a mouse neuroblastoma cells were cultured in MEM with 10% FBS, 1% GlutaMAX, 1% nonessential amino acids, and antibiotics. H1299 human lung carcinoma cells were cultured in Roswell Park Memorial Institute medium with 10% FBS and antibiotics. PPIase-Parvulin Inhibitor was from Calbiochem; Rottlerin and caffeine were from Sigma. Plasmids and siRNA oligonucleotides (Table S1) are described in SI Methods.
HD and control postmortem brain tissues were collected by the Harvard Brain Tissue Resource Center, McLean Hospital (Belmont, MA). HD brains were assigned Vonsattel grade-3 pathology. Postmortem intervals were from 12 to 32 h for controls and from 8 to 30 h for HD brains.
In Vitro Binding and Western Blot.
Analysis of p53-Pin1 interaction by GST pull-down and coimmunoprecipitation was done as described (12). p53-iASPP coimmunoprecipitation was performed as described (14). More details are provided in SI Methods.
Antibodies were anti-Pin1 polyclonal (12) and monoclonal (G-8 Santa Cruz); anti-p53 Pab240, FL-393, and DO-1 (Santa Cruz); anti-iASPP pAbiASPPN1 and mAbiASPP49.3 (14); anti-Huntingtin MAB5490 (Millipore); anti–phospho-Ser15-p53 (Cell Signaling Technology); anti–phospho–Ser46-p53 (BD Pharmingen); anti-PUMA ab-9643 (Abcam); anti-actin and anti-tubulin (Sigma), and anti-HSP90 F-8 (Santa Cruz). Anti-HIPK2 monoclonal antibody was a gift of L. Schmitz (University of Giessen, Giessen, Germany).
Mice Strains.
HdhQ111 knock-in mice expressing the complete endogenous Htt gene with 111 polyQ (20) and their wild-type littermates (HdhQ7) in C57BL background were provided by M. MacDonald (Massachusetts General Hospital, Boston). Pin1 KO mice in C57BL background (22) were provided by A. Means (Duke University, Durham, NC). HdhQ7/Q111:Pin1WT/KO mice were intercrossed to give double-homozygous and heterozygous littermates. Genotyping for both loci was performed by PCR on tail DNA as described (20, 22).
RT-PCR.
Total RNA was extracted with QIAzol, and cDNA was transcribed using QuantiTect Reverse Transcription Kit (Qiagen). RT-PCR was performed with QuantiFast SYBR Green PCR Kit (Qiagen). Primer sequences are reported in Table S2.
Apoptosis Assays.
TUNEL assays were performed with TMR Red in Situ Cell Death Detection Kit (Roche) following manufacturer’s instructions.
Supplementary Material
Acknowledgments
We thank colleagues at Laboratorio Nazionale Consorzio Interuniversitario per le Biotecnologie for advice, G. Pastore and M. Maurutto for technical support, S. Soddu, X. Lu, and T. Hofmann for reagents, and G. Leanza for mouse immunohistochemistry. This work was supported by Telethon Grant GGP07185 (to G.D.S. and F.P.) and Associazione Italiana per la Ricerca sul Cancro (G.D.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106198108/-/DCSupplemental.
References
- 1.The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Illuzzi J, Yerkes S, Parekh-Olmedo H, Kmiec EB. DNA breakage and induction of DNA damage response proteins precede the appearance of visible mutant huntingtin aggregates. J Neurosci Res. 2009;87:733–747. doi: 10.1002/jnr.21881. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni A, et al. Early and late events induced by polyQ-expanded proteins: Identification of a common pathogenic property of polyQ-expanded proteins. J Biol Chem. 2011;286:4727–4741. doi: 10.1074/jbc.M110.156521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae BI, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Illuzzi JL, Vickers CA, Kmiec EB. Modifications of p53 and the DNA damage response in cells expressing mutant form of the protein huntingtin. J Mol Neurosci. 2011;45:256–268. doi: 10.1007/s12031-011-9516-4. [DOI] [PubMed] [Google Scholar]
- 7.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collavin L, Lunardi A, Del Sal G. p53-family proteins and their regulators: Hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 10.Yeh ES, Means AR. PIN1, the cell cycle and cancer. Nat Rev Cancer. 2007;7:381–388. doi: 10.1038/nrc2107. [DOI] [PubMed] [Google Scholar]
- 11.Lu KP, Zhou XZ. The prolyl isomerase PIN1: A pivotal new twist in phosphorylation signalling and disease. Nat Rev Mol Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 12.Zacchi P, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature. 2002;419:853–857. doi: 10.1038/nature01120. [DOI] [PubMed] [Google Scholar]
- 13.Zheng H, et al. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature. 2002;419:849–853. doi: 10.1038/nature01116. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani F, et al. The prolyl isomerase Pin1 orchestrates p53 acetylation and dissociation from the apoptosis inhibitor iASPP. Nat Struct Mol Biol. 2007;14:912–920. doi: 10.1038/nsmb1306. [DOI] [PubMed] [Google Scholar]
- 15.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784–788. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 16.Ryo A, et al. Prolyl-isomerase Pin1 accumulates in Lewy bodies of Parkinson disease and facilitates formation of alpha-synuclein inclusions. J Biol Chem. 2006;281:4117–4125. doi: 10.1074/jbc.M507026200. [DOI] [PubMed] [Google Scholar]
- 17.Mayo LD, et al. Phosphorylation of human p53 at serine 46 determines promoter selection and whether apoptosis is attenuated or amplified. J Biol Chem. 2005;280:25953–25959. doi: 10.1074/jbc.M503026200. [DOI] [PubMed] [Google Scholar]
- 18.DiFiglia M, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 19.Schilling G, et al. Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet. 1999;8:397–407. doi: 10.1093/hmg/8.3.397. [DOI] [PubMed] [Google Scholar]
- 20.White JK, et al. Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nat Genet. 1997;17:404–410. doi: 10.1038/ng1297-404. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler VC, et al. Early phenotypes that presage late-onset neurodegenerative disease allow testing of modifiers in Hdh CAG knock-in mice. Hum Mol Genet. 2002;11:633–640. doi: 10.1093/hmg/11.6.633. [DOI] [PubMed] [Google Scholar]
- 22.Atchison FW, Capel B, Means AR. Pin1 regulates the timing of mammalian primordial germ cell proliferation. Development. 2003;130:3579–3586. doi: 10.1242/dev.00584. [DOI] [PubMed] [Google Scholar]
- 23.Liou YC, et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature. 2003;424:556–561. doi: 10.1038/nature01832. [DOI] [PubMed] [Google Scholar]
- 24.Di Stefano V, Rinaldo C, Sacchi A, Soddu S, D’Orazi G. Homeodomain-interacting protein kinase-2 activity and p53 phosphorylation are critical events for cisplatin-mediated apoptosis. Exp Cell Res. 2004;293:311–320. doi: 10.1016/j.yexcr.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Winter M, et al. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat Cell Biol. 2008;10:812–824. doi: 10.1038/ncb1743. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Kim DC, Choi BH, Ha H, Kim KT. Regulation of p53 by activated protein kinase C-delta during nitric oxide-induced dopaminergic cell death. J Biol Chem. 2006;281:2215–2224. doi: 10.1074/jbc.M509509200. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida K, Liu H, Miki Y. Protein kinase C delta regulates Ser46 phosphorylation of p53 tumor suppressor in the apoptotic response to DNA damage. J Biol Chem. 2006;281:5734–5740. doi: 10.1074/jbc.M512074200. [DOI] [PubMed] [Google Scholar]
- 28.Uchida T, et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem Biol. 2003;10:15–24. doi: 10.1016/s1074-5521(02)00310-1. [DOI] [PubMed] [Google Scholar]
- 29.Kodama M, et al. Requirement of ATM for rapid p53 phosphorylation at Ser46 without Ser/Thr-Gln sequences. Mol Cell Biol. 2010;30:1620–1633. doi: 10.1128/MCB.00810-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oda K, et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–862. doi: 10.1016/s0092-8674(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 31.Smeenk L, et al. Role of p53 serine 46 in p53 target gene regulation. PLoS ONE. 2011;6:e17574. doi: 10.1371/journal.pone.0017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino M, et al. Transcriptional repression induces a slowly progressive atypical neuronal death associated with changes of YAP isoforms and p73. J Cell Biol. 2006;172:589–604. doi: 10.1083/jcb.200509132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantovani F, et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol Cell. 2004;14:625–636. doi: 10.1016/j.molcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Steffan JS, et al. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci USA. 2000;97:6763–6768. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mattson MP, Duan W, Pedersen WA, Culmsee C. Neurodegenerative disorders and ischemic brain diseases. Apoptosis. 2001;6:69–81. doi: 10.1023/a:1009676112184. [DOI] [PubMed] [Google Scholar]
- 36.Alves da Costa C, Checler F. Apoptosis in Parkinson’s disease: Is p53 the missing link between genetic and sporadic Parkinsonism? Cell Signal. 2011;23:963–968. doi: 10.1016/j.cellsig.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 37.Feng Z, et al. p53 tumor suppressor protein regulates the levels of huntingtin gene expression. Oncogene. 2006;25:1–7. doi: 10.1038/sj.onc.1209021. [DOI] [PubMed] [Google Scholar]
- 38.Grochola LF, Zeron-Medina J, Mériaux S, Bond GL. Single-nucleotide polymorphisms in the p53 signaling pathway. Cold Spring Harb Perspect Biol. 2010;2:a001032. doi: 10.1101/cshperspect.a001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chattopadhyay B, Baksi K, Mukhopadhyay S, Bhattacharyya NP. Modulation of age at onset of Huntington disease patients by variations in TP53 and human caspase activated DNase (hCAD) genes. Neurosci Lett. 2005;374:81–86. doi: 10.1016/j.neulet.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Segat L, et al. PIN1 promoter polymorphisms are associated with Alzheimer’s disease. Neurobiol Aging. 2007;28:69–74. doi: 10.1016/j.neurobiolaging.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, et al. A novel functional variant (−842G>C) in the PIN1 promoter contributes to decreased risk of squamous cell carcinoma of the head and neck by diminishing the promoter activity. Carcinogenesis. 2009;30:1717–1721. doi: 10.1093/carcin/bgp171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prediger RD. Effects of caffeine in Parkinson’s disease: From neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis. 2010;20(Suppl 1):S205–S220. doi: 10.3233/JAD-2010-091459. [DOI] [PubMed] [Google Scholar]
- 44.Dhaenens CM, et al. Huntington French Speaking Network A genetic variation in the ADORA2A gene modifies age at onset in Huntington’s disease. Neurobiol Dis. 2009;35:474–476. doi: 10.1016/j.nbd.2009.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.