Abstract
DNA polymerase δ (Polδ) plays an essential role in replication from yeast to humans. Polδ in Saccharomyces cerevisiae is comprised of three subunits, the catalytic subunit Pol3 and the accessory subunits Pol31 and Pol32. Yeast Polδ exhibits a very high processivity in synthesizing DNA with the proliferating cell nuclear antigen (PCNA) sliding clamp; however, it has remained unclear how Polδ binds PCNA to achieve its high processivity. Here we show that PCNA interacting protein (PIP) motifs in all three subunits contribute to PCNA-stimulated DNA synthesis by Polδ, and mutational inactivation of all three PIP motifs abrogates its ability to synthesize DNA with PCNA. Genetic analyses of mutations in these PIPs have revealed that in the absence of functional Pol32 PIP domain, PCNA binding by both the Pol3 and Pol31 subunits becomes essential for cell viability. Based on our biochemical and genetic studies we infer that yeast Polδ can simultaneously utilize all three PIP motifs during PCNA-dependent DNA synthesis, and suggest that Polδ binds the PCNA homotrimer via its three subunits. We consider the implications of these observations for Polδ’s role in DNA replication.
DNA replication in eukaryotes is carried out by DNA polymerases (Pol) α, Polδ, and Polε. On the lagging strand, replication is initiated by RNA primers, synthesized by the primase subunits of Polα, which are extended by Polα to a total length of approximately 30_nt (1, 2). Pols δ and ε function in replication by binding proliferating cell nuclear antigen (PCNA), a ring-shaped sliding clamp, which increases the procesivity of these Pols (3, 4). On the lagging strand, PCNA-bound Polδ extends the DNA fragment synthesized by Polα to a total length of approximately 100–250 nt (1, 2). Whereas replication on the lagging strand occurs via synthesis of a series of short Okazaki fragments, the leading strand is synthesized continuously and in the direction of replication fork movement.
The roles of Polδ and Polε in the replication of leading and lagging strands have been subject to varying interpretations in the past. For example, the observations that Polδ replicates both the leading and lagging strands of the SV40 virus genome (5) and the fact that the DNA polymerase activity of Polδ is essential for survival of yeast cells (6–8), whereas the DNA polymerase domain of Polε can be deleted in yeast cells without a significant impairment of viability (9, 10), have suggested a crucial role for Polδ in the replication of both the leading and lagging DNA strands. On the other hand, more recent genetic studies with mutant alleles of Pol3 and Pol2, the catalytic subunits of yeast Polδ and Polε, respectively, have implicated a role for Polε in the replication of the leading strand and for Polδ in the replication of the lagging strand (11, 12).
Polδ from Saccharomyces cerevisiae consists of three subunits: Pol3 (125 kDa), Pol31 (55 kDa), and Pol32 (40 kDa). Pol3, the catalytic subunit, contains both the polymerase and 3′ → 5′ exonuclease proofreading activities (6–8). Pol31 forms a stable complex with Pol3, to which it binds via the second of the two C-terminal zinc finger modules, and Pol31 binds also the Pol32 subunit; thus, Pol31 acts as an intermediary in bridging the association of Pol3 with Pol32 (13–16). Both the Pol3 and Pol31 subunits are essential for viability, whereas pol32Δ cells are viable but display evidence of DNA replication defects (6–8, 13, 17).
A comparison of properties of S. cerevisiae Polδ comprised of the Pol3 and Pol31 subunits, referred to as Polδ* (18), with the three subunit Polδ has shown that Polδ* is less efficient in DNA synthesis than Polδ, and Polδ synthesizes DNA with PCNA with a higher processivity than Polδ*. Although the POL32 gene is not essential in S. cerevisiae, pol32Δ cells show cold sensitivity for growth and sensitivity to hydroxyurea (HU), and the pol32Δ mutation exhibits synthetic lethality with conditional mutations in the POL3 and POL31 genes (13).
A recent study with S. cerevisiae Polδ has shown that it synthesizes DNA with PCNA with a remarkably high processivity such that it can extend a single primer around an entire 5.4-kb, single-stranded circular DNA in a single binding event (19). The high processivity of Polδ is intriguing for a lagging strand polymerase, particularly in view of the fact that Okazaki fragments in yeast and other eukaryotes are quite small. Although the high processivity would be clearly advantageous for the replication of leading strand, that could present a hindrance to the replication of the lagging strand where the polymerase must dissociate from the DNA after extension of each Okazaki fragment. To circumvent this problem, Polδ utilizes a collision-release mechanism whereby the polymerase is released from PCNA upon colliding with the 5′ terminus of the downstream fragment (19).
There is relatively little information available on how Polδ binds PCNA to attain its high processivity. A conserved PCNA binding PIP motif has been identified in the C terminus of Pol32, but mutational inactivation of this domain in S. cerevisiae has only a modest effect on processive DNA synthesis by the Polδ-PCNA complex (13, 15). A similar conserved PCNA binding motif is present also in the C terminus of the Schizosaccharomyces pombe cdc27 subunit (20) as well as in the C terminus of the P66 subunit of human Polδ (21), which are the respective homologues of the S. cerevisiae Pol32 subunit. Extensive biochemical and genetics studies with the cdc27 encoded protein of S. pombe have shown that cdc27 is essential for growth and that deletion of its extreme C-terminal end that contains the PIP motif confers defects in PCNA binding, and S. pombe Polδ containing such C-terminally deleted cdc27 supports a less processive mode of DNA synthesis with PCNA than the wild-type protein (20). The C-terminal PCNA binding motif of cdc27 is essential for viability in S. pombe; however, overexpression of cdc27 protein deleted for the PCNA binding motif restores viability to cdc27Δ cells (20). In human Polδ also, the C-terminal PCNA binding motif in the P66 subunit promotes Polδ’s ability for PCNA binding (21). The X-ray structure of the PIP-box peptide derived from the P66 subunit of human Polδ (residues 452–466) has shown that the conserved hydrophobic residues in the PIP-box bind the interdomain connector loop (IDCL) of PCNA (22).
Because the PIP motif in Pol32 is only minimally required for the highly processive DNA synthesis by yeast Polδ and because viability is not affected in cells lacking a functional Pol32 PIP, we postulated that additional PCNA binding sites exist in the Polδ holoenzyme and contribute to its replication function in vivo. In this study we identify functional PCNA binding motifs in both the Pol3 and Pol31 subunits and show that mutations in any of the PIP motifs affect Polδ’s proficiency for synthesizing DNA with PCNA. Genetic analysis of yeast mutants defective for the various Polδ PIPs has revealed that in the absence of functional Pol32 PIP, PCNA binding by both the Pol3 and Pol31 PIPs becomes essential for viability.
Results
DNA Synthesis Activity of Pol3, Polδ*, and Polδ.
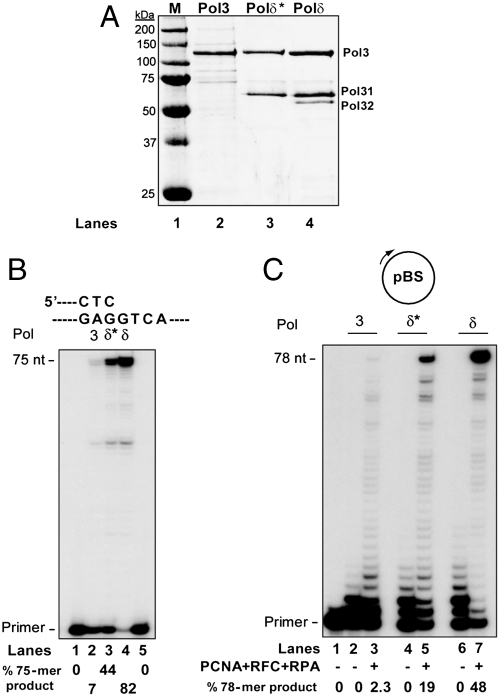
The catalytic subunit Pol3, Polδ*, and the three subunit Polδ holoenzyme comprised of Pol3, Pol31, and Pol32, were expressed in yeast cells and purified (Fig. 1A). The purification of these three forms of Polδ has allowed us to examine the contributions of the Pol31 and Pol32 subunits to the DNA synthetic activity of Pol3, and to analyze PCNA-dependent stimulation of DNA synthesis, providing insights in the functionality of different subunits in PCNA binding.
Fig. 1.
DNA synthetic activity of Pol3, Polδ*, and Polδ. (A) Different forms of Polδ. Proteins were purified to near homogeneity as described in SI Text and resolved on a SDS-12% polyacrylamide gel developed with Coomassie blue. Lane 1, molecular weight standards; Lane 2, Pol3 catalytic subunit; Lane 3, Polδ* (Pol3/Pol31); Lane 4, Polδ (Pol3/Pol31/Pol32). Pol32 does not stain as well as the other subunits. (B) DNA synthesis activity of Pol3, Polδ*, and Polδ. One nanomolar of each protein was incubated with the linear DNA substrate (10 nM) in the presence of all four dNTPs (100 μM) without salt for 10 min at 30 °C. Nucleotide sequence adjacent to the primer∶template junction is shown above, and the position of the primer and fully extended 75-nt product are indicated. The reaction products were resolved on a 10% polyacrylamide gel containing 8 M urea followed by autoradiography. Lanes 1 and 5, DNA substrate alone; Lane 2, Pol3; Lane 3, Polδ*; Lane 4, Polδ. (C) Effect of PCNA on DNA synthesis by Pol3, Polδ*, and Polδ. One nanomolar of Pol3 (lanes 2, 3), Polδ* (lanes 4, 5), or Polδ (lanes 6, 7) was incubated with the circular ssDNA substrate (10 nM) under standard reaction conditions but containing 150 mM NaCl and 250 μM ATP. DNA synthesis was limited to the addition of 36 nt by omitting dTTP from the reaction, which results in the formation of 78 nt full-length product. Reactions were carried out in the presence (+) or absence (−) of PCNA (100 ng), RFC (50 ng), and RPA (200 ng) as indicated. Lane 1 is the DNA substrate alone.
The DNA synthesis activities of Pol3, Polδ*, and Polδ were first examined on a linear duplex template∶primer in the presence of all four deoxynucleotides (dNTPs) in reactions that contained no salt. Pol3 alone synthesizes DNA quite inefficiently, but the addition of Pol31 greatly improves the proficiency of DNA synthesis by Pol3 as indicated from the increase in the amount of fully extended primer synthesized by Polδ* (Fig. 1B, compare lanes 2 and 3). A further increase in DNA synthesis occurs with the presence of the Pol32 subunit, generating Polδ holoenzyme, where almost all the primer has been extended to full-length product (Fig. 1B, lane 4). Hence, both accessory subunits accentuate the capacity of the catalytic subunit for DNA synthesis.
The Pol3 Subunit Alone is Stimulated by PCNA.
For examining PCNA-dependent DNA synthesis, we utilized a circular single-stranded DNA primed with an oligonucleotide at a unique site, and PCNA was loaded onto DNA by replication factor C (RFC) in the presence of replication protein A (RPA). Assays were performed in the presence of 150 mM NaCl because this salt concentration approximates physiological ionic strength, and DNA synthesis was limited to the addition of 36 nt to the primer by omitting dTTP from the reaction, which resulted in 78-mer full-length product. The ability to purify Pol3 as a monomeric subunit, apart from the Pol31/Pol32 subunits, allowed us to first test whether the catalytic subunit alone could be stimulated by PCNA. Surprisingly, DNA synthesis by Pol3 alone was stimulated by PCNA, albeit weakly (Fig. 1C). To determine the region of Pol3 responsible for this stimulation, we next examined two deletion mutations of the Pol3 protein, lacking either the 68 N-terminal residues, or lacking the C-terminal 112 residues which contain the two zinc finger motifs (Fig. 2A). As shown in Fig. 2, whereas deletion of the N terminus had no effect on PCNA stimulation of Pol3, deletion of the C terminus resulted in complete loss of PCNA stimulation. Because the C terminus of Pol3 encompasses the Pol31 binding region, it was possible that the weak stimulation observed for Pol3 arose from an undetectable contamination of the Pol31/32 complex in the purified protein, which would have been absent from the C-terminally deleted Pol3. However, because examination of the amino acid sequence between the thumb region within the catalytic core and the first zinc finger in Pol3 revealed a potential PCNA binding motif (Q - - - L - - F I) between residues 993–1,005 (Fig. 2A), we tested whether PCNA-dependent stimulation of DNA synthesis by Pol3 depends on this motif. We therefore mutated the conserved L999, or the F1002 and I1003 residues to alanines, and examined these mutant proteins for PCNA stimulation. As shown in Fig. 2B, both the L999A and F1002A I1003A mutations resulted in loss of PCNA stimulation by Pol3. Moreover, PCNA exerts an inhibitory effect on DNA synthesis by the Pol3 pip mutant proteins, presumably reflecting a reduction in their ability to access the PCNA-bound template-primer junction in the absence of functional PIP. Because these Pol3 mutant proteins are still able to bind Pol31/32, as indicated from the copurification of these subunits in Polδ holoenzyme harboring the mutant Pol3 subunit (see below), but these mutations affect PCNA stimulation of Pol3, the stimulation of wild-type Pol3 must have arisen through its interaction with PCNA; hence the region containing residues 993–1,005 represents a bona fide PCNA binding PIP domain in Pol3.
Fig. 2.
Identification of a PCNA binding site in Pol3. (A) A schematic representation of Pol3 proteins used. Amino acid positions are shown in parentheses. Pol3ΔN encompasses residues 68–1,097 and Pol3ΔC harbors residues 1–985. The exonuclease (exo) and DNA polymerase domains are shown as boxes, and the ovals in the C terminus indicate the two zinc finger motifs. The sequence of the PIP motif is given, and the conserved residues in PIP changed to alanines in this study are underlined. (B) Stimulation of the Pol3 catalytic subunit by PCNA. One nanomolar of wild-type or mutant Pol3 protein was incubated with the circular ssDNA substrate (10 nM) under conditions used for PCNA stimulation in Fig. 1C. Lane 1, DNA substrate alone. In lanes 2–11, DNA synthesis by the wild-type or mutant Pol3 protein was examined in the presence or absence of PCNA (100 ng), RFC (50 ng), and RPA (200 ng), as indicated.
A PIP Motif in Pol31 Modulates Polδ Binding to PCNA.
Polδ* displays a substantial increase in stimulation of DNA synthesis with PCNA as indicated by the increase in the amount of full-length product formed compared to that observed with Pol3 alone (Fig. 1C, compare lanes 5 and 3). A further enhancement in PCNA-stimulated DNA synthesis was observed with Polδ as reflected by the increased formation of full-length product with Polδ than with Polδ* (Fig. 1C, compare lanes 7 and 5). The Pol31 subunit contains an inactive phosphodiesterase domain, and an oligonucleotide binding (OB) fold which could increase the affinity of Polδ for DNA (23, 24) (Fig. 3A), thereby resulting in an increase in DNA synthesis by Pol3 and stimulation of synthesis with PCNA. However, in our search for additional PIP motifs within the Polδ holoenzyme, we identified a region between residues 321–330 of Pol31 that harbors a sequence with similarity to the PIP motif (Fig. 3A). We therefore mutated the tyrosine 327 and phenylalanine 328 residues in this motif to alanines and examined the effects of the Y327A, F328A mutations in Pol31 on DNA synthesis by Polδ in the presence of PCNA. A comparison of DNA synthesis in the presence of PCNA by wild-type Polδ and by Polδ harboring the Pol31 Y327A F328A mutant subunit shows that the mutant Polδ protein is not as proficient in DNA synthesis with PCNA as the wild-type protein, as indicated by the lesser amount of full-size product formed by the mutant protein than by Polδ (Fig. 3C, compare lanes 7 and 3). These results suggested that Pol31 also harbors a functional PIP domain (Fig. 3C).
Fig. 3.
Role of PIP motifs in Pol3, Pol31, and Pol32 in DNA synthesis by Polδ with PCNA. (A) Identification of the PIP box in Pol31 subunit. The schematic representation of yeast Pol31 shows the phosphodiesterase (PDE) domain and the OB-fold. The sequence of the PIP domain is shown, and the Y327 F328 residues in the Pol31 PIP box that were mutated to alanines are underlined. (B) Schematic representation of the Pol32 protein. The sequence of the PIP box is shown and the F344 F345 residues that were changed to alanines are underlined. The N-terminal approximately 104 residues of Pol32 are involved in binding to Pol31. (C) Mutational inactivation of PIP domains in each of the Polδ subunits affects DNA synthesis with PCNA. One nanomolar each of Polδ or its mutant derivatives was incubated with the circular ssDNA substrate (10 nM) in the presence of a mixture of 100 μM dGTP, dCTP, and dATP under standard PCNA stimulation assay conditions. For Polδ, WT indicates wild-type protein, and other designations represent the presence of the indicated mutant subunit in Polδ. Lane 1, DNA substrate alone. In lanes 2–19, DNA synthesis by wild-type or mutant Polδ was examined in the presence or absence of 100 ng PCNA, 50 ng RFC, and 200 ng RPA as indicated. The amount of full-length (78 nt) product formed by the various forms of Polδ is shown on the bottom.
PCNA Binding Modules in all Three Subunits Contribute to Polδ’s Proficiency in DNA Synthesis with PCNA.
The PIP motifs in the Pol3 and Pol31 proteins identified in this study, along with the known PIP motif in the C terminus of Pol32, has allowed us to examine the contribution of these various PIP motifs to PCNA stimulation within the context of the Polδ holoenzyme. The pip mutations did not affect Polδ complex formation as the mutant forms purified in an identical manner as the wild-type holoenzyme. We also verified by SDS-PAGE analyses that various mutant Polδ holoenzymes displayed the same subunit composition as the wild-type holoenzyme. In addition, our observation that the DNA synthetic activity of various mutant Polδ holoenzymes in the absence of PCNA, RFC, RPA, and without salt was the same as that of the wild-type holoenzyme (Fig. 1B, lane 4) provided further evidence that the subunit composition was not affected by any of the pip mutations.
To analyze the roles of PIP motifs individually, we purified Polδ complexes that carry either (1) the F1002A, I1003A mutations in the Pol3 PIP, (2) the Y327A, F328A mutations in the Pol31 PIP, or (3) where the PIP of Pol32 had been inactivated by deleting C-terminal residues 337–350 of the protein (1–336) or by changing the conserved F344, F345 residues to alanines (Fig. 3B). As shown in Fig. 3C, Polδ harboring a mutation in the Pol3 PIP (lane 5) or lacking the Pol32 PIP (lane 9) exhibits a reduction in PCNA-stimulated DNA synthesis and the formation of fully extended product was impaired by these mutations, similar to that observed for mutations in the Pol31 PIP (lane 7). Thus PCNA binding motifs in all three subunits affect Polδ’s ability to synthesize DNA with PCNA, and they all appear to affect it to about the same degree.
Next we examined PCNA-dependent DNA synthesis by Polδ in which the PIP domains of two or more of the subunits have been simultaneously inactivated. Mutational inactivation of the PIP domains in the Pol3 and Pol31 subunits of Polδ led to a further reduction in the formation of fully extended product with PCNA (Fig. 3C, lane 11). As indicated from the amount of fully extended product formed (78 nt), PCNA-dependent stimulation of DNA synthesis was also impaired for mutant Polδ proteins where mutations in either the Pol3 or the Pol31 PIP domain were combined with the Pol32 protein lacking the C-terminal PIP domain (Fig. 3C, lanes 13 and 15). However, because a considerable level of PCNA-dependent DNA synthesis still remains in Polδ proteins harboring PIP mutations in two of the three subunits, next we examined whether the simultaneous inactivation of all three PIP domains in the Pol3, Pol31, and Pol32 subunits confers a further reduction in Polδ’s proficiency for DNA synthesis with PCNA. Interestingly, PCNA has no stimulatory effect on DNA synthesis by such a mutant Polδ enzyme (Fig. 3C, lanes 17 and 19). We infer from these observations that each of the three subunits of Polδ harbors a PIP domain and each contributes to the proficient ability of Polδ to functionally interact with PCNA.
Synthetic Lethality of pol32Δ with Mutations in the POL3 or POL31 PIP Domain.
To examine the in vivo role of the POL3 and POL31 PIP domains, we generated yeast strains harboring the mutant form of the POL3 PIP, the POL31 PIP, or of both, at their respective sites in the yeast genome. Rather surprisingly, viability was not affected by mutations in the PIP domain of either the POL3 or the POL31 gene, or even upon the simultaneous inactivation of the PIP domains in both these genes (Fig. 4A). However, the pol3 pip mutant was hypersensitive to the replication inhibitor HU, as growth was quite adversely affected on yeast extract/peptone/dextrose (YPD) plates containing 10 mM HU. The pol31 pip mutation also affected growth on HU, but the effect of this mutation was much less severe than that of the pol3 pip mutation, whereas the simultaneous mutational inactivation of POL3 and POL31 PIP domains led to a further decline in growth on HU (Fig. 4A). As has been reported previously and shown in Fig. 4B, the pol32Δ mutant is highly sensitive to growth on HU, but HU sensitivity is not dependent upon PCNA binding, because we find that the pol32 pip mutation engenders no more adverse effect on growth in the presence of HU than that seen for the wild-type strain (Fig. 4B).
Fig. 4.
Effects of the pol3 pip, pol31 pip, and pol32 pip mutations on viability. (A) HU sensitivity of yeast strains lacking a functional PIP in either Pol3 (pol3 pip), Pol31 (pol31 pip), or both subunits. (B) HU sensitivity of pol32Δ cells transformed with the CEN plasmids containing either the wild-type POL32 gene, the pol32 pip (F344A, F345A) mutant gene, or with no POL32 gene. Cells were grown in liquid synthetic complete medium lacking uracil (SC-ura), serially diluted, and then spotted onto YPD plates containing the indicated amounts of HU. (C) The pol3 pip and pol31 pip mutations exhibit synthetic lethality with the pol32Δ mutation. Yeast strains harboring the genomic pol3 pip or pol31 pip mutations were transformed with the URA3-based vectors pPOL393 (YCpPOL3-URA3) and pPOL327 (YCpPOL31-URA3) which contain the wild-type POL3 and POL31 gene, respectively. The pol32Δ mutation was subsequently generated in these strains, and cells were then plated on media containing 5-FOA to counter-select for cells that had lost the wild-type POL3- or POL31-containing plasmids. Although 5-FOA-resistant colonies could be obtained in the wild-type POL32 strains, no such colonies were recovered in the strains carrying the pol32Δ mutation. Thus, the absence of POL32 (pol32Δ) in combination with either the pol3 pip or pol31 pip mutations results in inviability. (D) Synthetic lethality of the pol3 pip or the pol31 pip mutations with the pol32 pip (F344A, F345A) mutation. Genomic pol3 pip or pol31 pip mutant strains complemented by the URA3-based vectors pPOL393 (YCpPOL3-URA3) or pPOL327 (YCpPOL31-URA3) and harboring the pol32Δ mutation as in C were transformed with LEU2-based CEN vectors harboring either the wild-type POL32 gene, the pol32 pip mutation, or no POL32 insert. Cells were plated on 5-FOA-containing medium to counter-select for cells that lost the wild-type POL3 or POL31 plasmids. Colonies resistant to 5-FOA could be obtained in the strains carrying the wild-type POL32 plasmid, but not in the strains carrying either the pol32 pip mutation, or the empty vector control (YCpLac322). Thus, the pol32 pip mutation causes lethality in the presence of the pol3 pip or pol31 pip mutations.
Next we examined the effect of the pol32Δ mutation in combination with the pol3 or pol31 pip mutations on cell survival. Although the pol32Δ mutation can readily be made in wild-type cells, we were unable to obtain a pol32Δ mutation in cells carrying mutations in the PIP domain of either the POL3 or the POL31 gene. To verify the possibility that the pol3 pip and pol31 pip mutations confer lethality in pol32Δ cells, we utilized a plasmid shuffle system in an attempt to recover pol32Δ in a pol3 pip or pol31 pip mutant background. First, we introduced into yeast cells harboring the genomic pol3 pip or pol31 pip mutation, low copy CEN URA3-based plasmids that carried either the wild-type POL3 or the POL31 gene, respectively. The pol32Δ mutation was then generated in these strains (which was now possible because of the complementation of the genomic pip mutations by the plasmids carrying their respective wild-type POL3 or POL31 gene). To test whether loss of the wild-type POL3- or POL31-containing plasmids could be tolerated in such cells, we counter-selected for loss of the URA3 plasmid on media containing 5-fluoroorotic acid (5-FOA). However, we were unable to recover any cells that lost the wild-type POL3 or POL31 plasmids in the pol32Δ background, whereas they were easily obtained in the presence of POL32 (Fig. 4C). Hence, Pol32 becomes indispensable for the in vivo function of Polδ in cells lacking the functional PIP domain in either the Pol3 or Pol31 subunit of the polymerase.
Requirement of the PCNA Binding Motif in Pol32 for Restoring Viability to Cells Lacking a Functional Pol3 or Pol31 PIP Domain.
Next, using a similar plasmid shuffle system, we determined whether the POL32 gene mutated in its PIP domain could restore viability to pol3 pip pol32Δ or pol31 pip pol32Δ cells, where the pol3 pip or pol31 pip mutant gene is carried in the genome. For this purpose, we generated LEU2-based plasmids carrying either the wild-type POL32 gene or the pol32 F344A, F345A (pol32 pip) mutant gene and transformed them into the pol3 pip pol32Δ or pol31 pip pol32Δ yeast strains harboring the CEN/ARS URA3-based plasmid that carried the wild-type POL3 or POL31 genes, respectively. We then counter-selected for loss of the URA3-based wild-type POL3 or POL31 plasmids by plating cells on medium containing 5-FOA. As shown in Fig. 4D, cells that lost the wild-type POL3 URA3 plasmid could be obtained as 5-FOA-resistant colonies when the wild-type POL32 plasmid was present in the pol3 pip pol32Δ strain; however, no 5-FOA-resistant colonies could be recovered when the plasmid contained the pol32 pip mutation, or with a vector with no POL32 gene. Similarly for the pol31 pip pol32Δ strain, the wild-type POL32 gene was able to maintain viability in the absence of the wild-type POL31 plasmid, but the pol32 F344A, F345A pip mutation lacked this ability. We infer from these observations that in yeast cells lacking a functional PIP in either the Pol3 or Pol31 subunit, the ability of Pol32 to bind PCNA via its PIP motif becomes indispensable for in vivo function of Polδ in replication.
Discussion
To evaluate the effects of Pol31 and Pol32 subunits on DNA synthesis by Polδ, we have purified Pol3, Polδ*, and Polδ and compared their synthetic activity in the absence and presence of PCNA. In the absence of PCNA, we find that the Pol31 subunit augments the DNA synthesis activity of Pol3, and that the addition of Pol32 further increases the capacity of Pol3 catalytic activity. As has been shown previously, Polδ*, comprised of Pol3/Pol31, is stimulated by PCNA, and the addition of Pol32, constituting the Polδ holoenzyme, further increases the processivity of DNA synthesis with PCNA (18). Because the OB domain in Pol31 and the winged helix-turn-helix domain present in the N-terminal region of Pol32 represent DNA binding regions (24, 25), these subunits could have contributed to PCNA-stimulated DNA synthesis by simply enhancing the affinity of Polδ for DNA. However, because the only known PCNA binding domain (PIP), located in the Pol32 subunit, is only minimally required for processive DNA synthesis by Polδ, we surmised that other PCNA binding sites reside in one or more of the Polδ subunits. Interestingly, we find that the Pol3 catalytic subunit alone is stimulated by PCNA, and the stimulation can be attributed to a canonical PIP motif located in a linker peptide between the catalytic core thumb domain and the C-terminal zinc finger motifs involved in Pol31 binding. We also show that the increased PCNA stimulation of the Polδ* complex over that observed for the Pol3 subunit is dependent upon a PIP motif located in the Pol31 subunit. From our analysis of PCNA-stimulated DNA synthesis by Polδ harboring various combinations of mutations in one or more of the PIP domains of Pol3, Pol31 and Pol32, we conclude that all these PIP domains affect Polδ’s binding to PCNA and contribute to its processivity. Furthermore, the observation that Polδ lacking all three PIPs is completely devoid of any PCNA stimulation could suggest that all interactions between Polδ and PCNA are initiated through these PIPs.
Although our biochemical studies indicate a role for PIP motifs in all three Polδ subunits in its ability to synthesize DNA with PCNA, it would be important to verify their direct binding to PCNA from structural analyses of Polδ bound to PCNA at the template-primer junction in the presence of an incoming dNTP. In addition to providing proof for multisubunit PCNA binding by Polδ via these PIP motifs, such structural studies could help determine how the pip mutations in different subunits affect Polδ’s binding to PCNA.
Requirement of PCNA Binding Motifs in Polδ Subunits for Viability of Yeast Cells.
We have shown that S. cerevisiae cells lacking any one of the Polδ PIPs remain viable. However, although S. cerevisiae cells remain viable in the absence of Pol32, mutational inactivation of the PIP domain in either Pol3 or Pol31 confers lethality to pol32Δ cells. Moreover, the viability of cells lacking functional PIPs in either Pol3 or Pol31 is dependent upon a functional PCNA binding PIP domain in Pol32. In contrast, yeast cells still remain viable in the absence of both PIP domains of Pol3 and Pol31. Hence, our genetic analyses indicate that each PIP functions in the cell, but although Polδ can carry on its essential role in DNA replication through interaction with PCNA via the Pol32 PIP domain alone, in the absence of functional Pol32 PIP, PCNA binding by both the Pol3 and Pol31 subunit are required for promoting the essential function of Polδ. However, even though the ability of Polδ to bind PCNA via only the Pol32 PIP supports viability, the elevated HU sensitivity of Pol3 and Pol3 Pol31 pip mutants (Fig. 4A) would suggest that PCNA binding by these subunits modulates the action of Polδ in replication in yeast cells with a functionally intact Pol32 PIP.
Evolutionary Conservation of PIP Domains in Polδ Subunits Among Eukaryotes.
To consider the possibility that multisubunit binding of Polδ to PCNA has been conserved evolutionarily, we examined whether the PCNA binding motifs have been conserved among the Polδ subunits in other eukaryotes. As shown in Fig. S1A, a conserved sequence similar to the PIP motif present in the Pol3 subunit of S. cerevisiae Polδ exists also in the Polδ catalytic subunit from S. pombe, Drosophila melanogaster, Mus musculus, and Homo sapiens, and the motif is positioned similarly between the thumb and the first zinc binding finger. Moreover, in addition to the conservation of the PIP motif sequence, overall, this region shares a high degree of sequence similarity. The C-terminal PIP motif of S. cerevisiae Pol32 is highly conserved among the cognate subunits from other eukaryotes (Fig. S1B), and biochemical studies with human Polδ have indicated a role of P66 in interaction with PCNA (21). In contrast to the evolutionary conservation of PIP domains present in the Pol3 and Pol32 subunits, we have been unable to identify a conserved PIP motif in the Pol31 counterparts from other eukaryotes. However, Polδ from these other eukaryotes contains an additional subunit, called Cdm1 in S. pombe and P12 in humans, and a PCNA binding motif has been identified in both subunits (26, 27). Human P12 harbors a PCNA binding domain (K R L I T D S Y) between amino acids 4 and 11, and biochemical studies have indicated a role for this domain in PCNA binding and in PCNA-stimulated DNA synthesis activity of Polδ (26). Thus, in humans and other higher eukaryotes, the loss of PIP in the Pol31 B-subunit counterpart may have been substituted for by the addition of a PIP in a fourth subunit.
Model for PCNA Binding by Polδ.
From our biochemical and genetic analyses of mutations in the PIP domains in Pol3, Pol31, and Pol32, we conclude that all these modules can assist in Polδ’s binding to PCNA. Because mutational inactivation of any of the three PIP motifs results in a clear reduction in PCNA-dependent DNA synthesis, evidently all three PIP motifs are utilized by Polδ during DNA synthesis with PCNA. Small angle X-ray scattering analysis of yeast Polδ complex comprised of Pol3, Pol31, and Pol32N proteins, wherein the Pol32 subunit contains the first 103 N-terminal residues, has indicated that the globular Pol3 catalytic subunit is linked to an elongated tail structure comprised of the Pol31 and Pol32N subunits. The Pol31-Pol32N complex exhibits considerable conformational variability with respect to Pol3, and extends far away from the globular domain such that Pol32 in these structures is placed > 50 Å away from the Pol3 globular domain (28). In addition, the C-terminal portion of Pol32 missing from these structures is predicted to exist in an elongated form as predicted from its larger than expected stokes radius (14). The flexible positioning of the Pol31 and Pol32 subunits, and presumably of their respective PIP motifs, within the Polδ complex raises the possibility that two or more of these subunits could bind to different monomers of one PCNA clamp simultaneously. Such a multiple subunit binding has been observed for yeast RFC, in which the RFC–PCNA crystal structure has shown that both RFC1 and RFC3 of the five subunit complex bind the IDCLs of two adjacent monomers of the closed PCNA clamp (29). In addition, RFC1 contains a second, nonessential PIP at its extreme N terminus, which may represent a PIP of unknown function. After the loading of PCNA onto DNA, however, RFC dissociates from the clamp allowing other proteins, such as Polδ, access to PCNA.
The PCNA binding motifs in the three subunits could affect Polδ function in different ways. Because the Pol3 PIP lies just C-terminal to the thumb domain of Polδ, and presents the Polδ catalytic core in very close proximity to the DNA passing through PCNA, Pol3 PIP could be important for proficient DNA synthesis and contribute to the processivity of the enzyme. The location of Pol32 and Pol31 PIP on an extended flexible part of Polδ would suggest that they act more as tethers in keeping Polδ bound on PCNA. Such a mode of PCNA binding, in addition to conferring high processivity, would provide Polδ the flexibility whereby other proteins could access PCNA, but Polδ still remains bound to PCNA. Thus in situations when other proteins need to bind PCNA, as for example Fen1 during lagging strand replication, the Pol3 subunit could become dislodged from PCNA whereas the Pol31 and Pol32 subunits would keep Polδ attached to one or more of the PCNA monomers.
Implications and Conclusions.
An important implication of multisite PCNA binding is that it allows Polδ to remain bound to PCNA while still permitting access of other proteins to PCNA. Such a mode of PCNA binding could be important for ensuring the integrity and stability of the replisome not only during normal replication, but also when the fork stalls at naturally occurring impediments or at DNA lesions in the template strand because then Polδ could still remain bound to PCNA while allowing for access of repair proteins to PCNA as well as to DNA. The increased HU sensitivity of pol3 pip and pol31 pip mutants is in keeping with a role of Polδ PIP motifs in stabilizing stalled replication forks.
In conclusion, we show here that yeast Polδ can bind the PCNA homotrimer via its three subunits and suggest that although different in some details, human Polδ also binds PCNA through three of its four subunits. Our observations on multisite PCNA binding by Polδ, when viewed in the context of Polδ’s other properties that include its ability to synthesize DNA with PCNA in a highly processive manner (19), and the fact that Polδ is essential for replication whereas Polε is not, make it rather difficult to understand why Polδ is restricted to replicating only the lagging DNA strand and not both.
Materials and Methods
The details for protein purification, for DNA substrates used, and for genetic studies are given in SI Text. For DNA synthesis assays on the linear DNA substrate, reactions contained 40 mM Tris·HCl, pH 7.5, 5 mM MgCl2, 1 mM DTT, 10% glycerol, 100 μg/mL bovine serum albumin, and 100 μM each of dGTP, dATP, dTTP, and dCTP. Reactions were carried out for 10 min at 30 °C. For experiments on circular DNA, reaction conditions were the same as on linear DNA except for the addition of 150 mM NaCl and 250 μM ATP, and the omission of dTTP to limit synthesis to the addition of 36 nt. PCNA stimulation assays were assembled on ice by mixing PCNA (100 ng), RFC (50 ng), RPA (200 ng) with the substrate DNA (10 nM) in reaction buffer. The reaction mixture was then shifted to 30 °C for 2 min prior to the addition of 1 nM DNA polymerase and further incubated for 10 min. Reactions were stopped by the addition of loading buffer (40 μL) containing 20 mM EDTA, 95% formamide, 0.3% bromphenol blue, and 0.3% cyanol blue. The reaction products were resolved on 10% polyacrylamide gels containing 8 M urea. Visualization of the results was done using Molecular Dynamics STORM PhosphoImager and ImageQuant software.
Supplementary Material
Acknowledgments.
This study was supported by National Institutes of Health Grant CA107650.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109981108/-/DCSupplemental.
References
- 1.Murakami Y, Eki T, Hurwitz J. Studies on the initiation of simian virus 40 replication in vitro: RNA primer synthesis and its elongation. Proc Nat Acad Sci USA. 1992;89:952–956. doi: 10.1073/pnas.89.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 3.Tsurimoto T, Stillman B. Replication factors required for SV40 DNA replication in vitro II. Switching of DNA polymerase alpha and delta during initiation of leading and lagging strand synthesis. J Biol Chem. 1991;266:1961–1968. [PubMed] [Google Scholar]
- 4.Yuzhakov A, Kelman Z, Hurwitz J, O'Donnell M. Multiple competition reactions for RPA order the assembly of the DNA polymerase δ holoenzyme. EMBO J. 1999;18:6189–6199. doi: 10.1093/emboj/18.21.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waga S, Stillman B. Anatomy of a DNA replication fork revealed by reconstitution of SV40 DNA replication in vitro. Nature. 1994;369:207–212. doi: 10.1038/369207a0. [DOI] [PubMed] [Google Scholar]
- 6.Boulet A, Simon M, Faye G, Gauer GA, Burgers PMJ. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 1989;8:1849–1854. doi: 10.1002/j.1460-2075.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartwell LH. Sequential function of gene products relative to DNA synthesis in the yeast cell cycle. J Mol Biol. 1976;104:803–817. doi: 10.1016/0022-2836(76)90183-2. [DOI] [PubMed] [Google Scholar]
- 8.Simon M, Giot L, Faye G. The 3′ to 5′ exonuclease activity located in the DNA polymerase delta subunit of Saccharomyces cerevisiae is required for accurate replication. EMBO J. 1991;10:2165–2170. doi: 10.1002/j.1460-2075.1991.tb07751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng W, D'Urso G. Schizosaccharomyces pombe cells lacking the amino-terminal catalytic domains of DNA polymerase epsilon are viable but require the DNA damage checkpoint control. Mol Cell Biol. 2001;21:4495–4504. doi: 10.1128/MCB.21.14.4495-4504.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kesti T, Flick K, Keranen S, Syvaoja JE, Wittenberg C. DNA polymerase ε catalytic domains are dispensable for DNA replication, DNA repair, and cell viability. Mol Cell. 1999;3:679–685. doi: 10.1016/s1097-2765(00)80361-5. [DOI] [PubMed] [Google Scholar]
- 11.Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PMJ, Kunkel TA. Division of labor at the eukaryotic replication fork. Mol Cell. 2008;30:137–144. doi: 10.1016/j.molcel.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pursell ZF, Isoz I, Lundstrom E-B, Johansson E, Kunkel TA. Yeast DNA polymerase ε participates in leading-strand DNA replication. Science. 2007;317:127–130. doi: 10.1126/science.1144067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerik KJ, Li X, Pautz A, Burgers PM. Characterization of the two small subunits of Saccharomyces cerevisiae DNA polymerase δ. J Biol Chem. 1998;273:19747–19755. doi: 10.1074/jbc.273.31.19747. [DOI] [PubMed] [Google Scholar]
- 14.Johansson E, Majka J, Burgers PM. Structure of DNA polymerase delta from Saccharomyces cerevisiae. J Biol Chem. 2001;276:43824–43828. doi: 10.1074/jbc.M108842200. [DOI] [PubMed] [Google Scholar]
- 15.Johansson E, Garg P, Burgers PMJ. The Pol32 subunit of DNA polymerase δ contains separable domains for processive replication and proliferating cell nuclear antigen (PCNA) binding. J Biol Chem. 2004;279:1907–1915. doi: 10.1074/jbc.M310362200. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez Garcia J, Ciufo LF, Yang X, Kearsey SE, MacNeill SA. The C-terminal zinc finger of the catalytic subunit of DNA polymerase delta is responsible for direct interaction with the B-subunit. Nucleic Acids Res. 2004;32:3005–3016. doi: 10.1093/nar/gkh623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto K, Nakashima N, Ohara T, Maki S, Sugino A. The second subunit of DNA polymerase III (delta) is encoded by the HYS2 gene in Saccharomyces cerevisiae. Nucleic Acids Res. 1998;26:477–485. doi: 10.1093/nar/26.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burgers PMJ, Gerik KJ. Structure and processivity of two forms of Saccharomyces cerevisiae DNA polymerase δ. J Biol Chem. 1998;273:19756–19762. doi: 10.1074/jbc.273.31.19756. [DOI] [PubMed] [Google Scholar]
- 19.Langston LD, O'Donnell M. DNA polymerase δ is highly processive with proliferating cell nuclear antigen and undergoes collision release upon completing DNA. J Biol Chem. 2008;283:29522–29531. doi: 10.1074/jbc.M804488200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermudez WP, MacNeill SA, Tappin I, Hurwitz J. The influence of the Cdc27 subunit on the properties of the Schizosaccharomyces pombe DNA polymerase δ. J Biol Chem. 2002;277:36853–36862. doi: 10.1074/jbc.M202897200. [DOI] [PubMed] [Google Scholar]
- 21.Ducoux M, et al. Mediation of proliferating cell nuclear antigen (PCNA)-dependent DNA replication through a conserved p21Cip-1-like PCNA-binding motif present in the third subunit of human DNA polymerase δ. J Biol Chem. 2001;276:49258–49266. doi: 10.1074/jbc.M106990200. [DOI] [PubMed] [Google Scholar]
- 22.Bruning JB, Shamoo Y. Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-δ p66 subunit and flap endonuclease-1. Structure. 2004;12:2209–2219. doi: 10.1016/j.str.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Aravind L, Koonin EV. Phosphodiesterase domains associated with DNA polymerases of diverse origins. Nucleic Acids Res. 1998;26:3746–3652. doi: 10.1093/nar/26.16.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baranovskiy AG, et al. X-ray structure of the complex of regulatory subunits of human DNA polymerase δ. Cell Cycle. 2008;7:3026–3036. doi: 10.4161/cc.7.19.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arunkumar AI, Stauffer ME, Bochkareva E, Bochkarev A, Chazin WJ. Independent and coordinated functions of replication protein A tandem high affinity single-stranded DNA binding domains. J Biol Chem. 2003;278:41077–41082. doi: 10.1074/jbc.M305871200. [DOI] [PubMed] [Google Scholar]
- 26.Li H, et al. Functional roles of p12, the fourth subunit of human DNA polymerase δ. J Biol Chem. 2006;281:14748–14755. doi: 10.1074/jbc.M600322200. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds N, Watt A, Fantes PA, MacNeill SA. Cdm1, the smallest subunit of DNA polymerase δ in the fission yeast Schizosaccharomyces pombe, is non-essential for growth and division. Curr Genet. 1998;34:250–258. doi: 10.1007/s002940050394. [DOI] [PubMed] [Google Scholar]
- 28.Jain R, et al. Structural insights into yeast DNA polymerase δ by small angle X-ray scattering. J Mol Biol. 2009;394:377–382. doi: 10.1016/j.jmb.2009.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowman GD, O'Donnell M, Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature. 2004;429:724–730. doi: 10.1038/nature02585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.